Abstract
Despite the clinical regression that typifies the initial response of advanced prostate cancer to gonadal testosterone depletion, tumors eventually progress. However, evidence supports the concept that signaling via the androgen receptor (AR) is important in progression to castration-resistant prostate cancer (CRPC).
Steroid hormones are synthesized from cholesterol in a series of tightly regulated steps involving the cleavage of carbon-carbon bonds, the introduction of functional groups derived from activated molecular oxygen, and the oxidation and reduction of carbon-carbon and carbon-oxygen bonds. In the adrenal cortex and gonads, steroidogenesis is tightly regulated, very efficient, and highly directional. In contrast, steroid metabolism in peripheral tissues is characterized by competing enzymes and pathways, low efficiency, and great variability. Many steps are mechanistically and functionally irreversible, but some are not, and the repertoire of specific enzymes, intracellular redox state, and access to hormone precursors all contribute to steroid flux and accumulation.
The investigation of steroid metabolizing enzymes in CRPC often assumes that the pathways and patterns of metabolism mirror those defined in the adrenals and gonads and validated by human deficiency syndromes. Unfortunately, several potential pathways using different enzymes might contribute substantially to androgen synthesis in CRPC. Finally, a number of mechanisms have been reported by which the AR is activated independent of ligand. Recent observations have suggested that AR forms with constitutive activity occur in CRPC, stimulating transcription without a requirement for ligand. This overview outlines a broad view of how the mechanisms by which the AR may be activated, whether by alternate pathways of androgen synthesis or the production of alternate forms of the AR, with an emphasis on what aspects must be accounted for when utilizing model systems to explore the biology of human prostate cancer.
Introduction
The definition of the biochemical pathways effecting the synthesis of steroid hormones has identified the enzymes and cofactors necessary to catalyze the synthesis of an array of steroid hormones. Pathway schematics, such as that depicted in Figure 1, represent a roadmap by which the flux of steroid precursors can flow from precursors to products and by which steroid hormones can be interconverted. Such maps do not adequately convey two important features of these pathways. The first is that although multiple enzymes may be capable of converting one steroid into another, the histological and subcellular compartmentalization of these enzymes represents a critical determinant of the final steroid product secreted by the steroidogenic tissues. The second is that in addition to substrate availability, the levels of enzymes catalyzing competing alternate pathways or otherwise modifying intracellular steroid concentrations can profoundly alter the patterns of steroid synthesis. A prostate cancer cell can be bathed in high concentrations of an immediate precursor to an active androgen, but if the cell cannot acquire the steroid and metabolize the precursor to androgen—before competing pathways expel, inactivate, or degrade the steroid—the steroids will never activate the androgen receptor (AR).
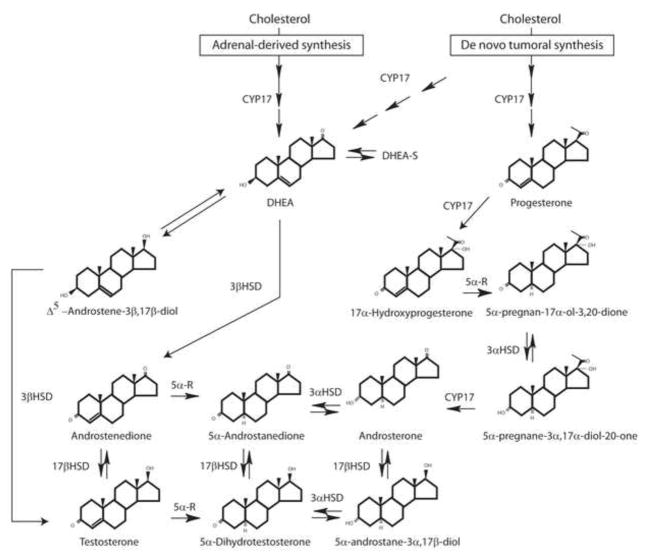
Figure 1.
Steroidogenic pathways to testosterone and dihydrotestosterone. Adrenal DHEA may be converted to testosterone and dihydrotestosterone via conversion through androstenedione or androstenediol as the initial step. Alternative, de novo steroidogenesis from cholesterol through the “backdoor pathway” may occur through progesterone → 17α-hydroxyprogesterone → 5α-pregnan-17α-ol-3,20-dione → 5α-pregnane-3α,17α-diol-20-one → androsterone → 5α-androstane-3α,17β-diol → dihydrotestosterone.
These features are particularly relevant when considering androgen biosynthesis. In this process, based upon pathways defined in androgen-secreting tissues, certain steroids are considered to be essential intermediates, and if a cell does not posses the enzyme(s) to make such intermediates, it might appear impossible to synthesize androgens. In reality, alternate routes may be possible using different intermediates permitting the production of androgen vital for growth - and treatments such as androgen deprivation therapy may well represent a selection for cells possessing the enzymes necessary for synthesizing androgens via these alternate pathways.
Normal steroidogenesis
Appropriately, most of the research in steroidogenesis has focused on the tissues that are the most active in terms of steroidogenesis: the adrenals, the gonads (testes and ovaries), and the placenta. All steroid hormones are synthesized from cholesterol, and only a few cells express biochemically significant amounts of the cholesterol side chain cleavage enzyme (P450scc, CYP11A1), the mitochondrial enzyme that catalyzes the conversion of cholesterol to pregnenolone, the first intermediate committed to steroid synthesis. The cells comprising the three zones of the adrenal cortex, the Leydig cells of the testis, the theca and granulosa cells of the ovary, and the trophoblastic cells of the placenta all possess sufficient amounts of CYP11A1 to contribute significantly to the pools of steroids in the circulation. In addition to CYP11A1, two electron transfer proteins, ferredoxin and ferredoxin reductase, are required to effect the synthesis of pregnenolone. In addition, the acute regulation of steroidogenesis is mediated by the steroidogenic acute regulatory protein (StAR), which stimulates the transfer of cholesterol from the outer to inner mitochondrial membranes, where CYP11A1 and ferredoxin reductase are found. It is possible that other cells, such as those in the brain and heart as well as castration-resistant prostate cancer (CRPC), might express smaller amounts of CYP11A1 sufficient for local generation of intracellular steroids, assuming the other requisite mitochondrial proteins are also present.
The normal adrenal gland as a model of ordered, efficient hormone synthesis
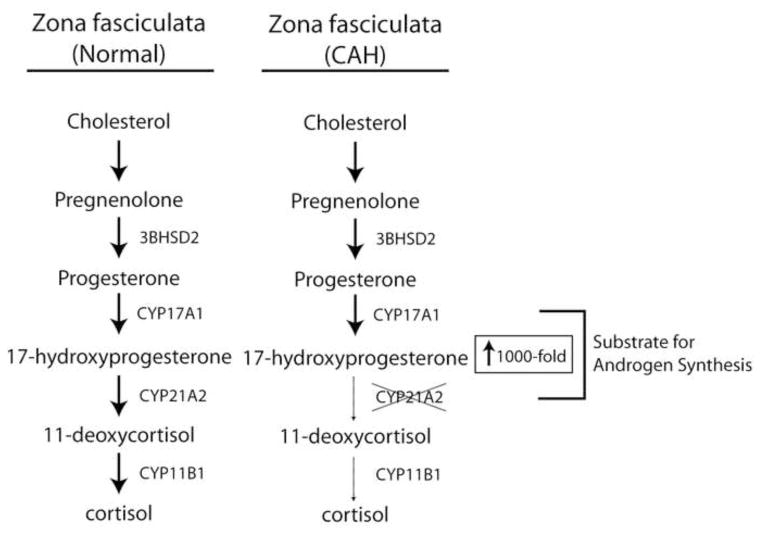
Consider the biosynthesis of cortisol in the zona fasciculata of the adrenal cortex (Figure 2). Nascent pregnenolone is metabolized by abundant and efficient 3β-hydroxysteroid dehydrogenase-Δ4/Δ5-isomerase type 2 (3βHSD2)1, yielding progesterone, which is a good substrate for steroid 17-hydroxylase (P450c17, CYP17A1) and affords 17-hydroxyprogesterone (17OHP)2. The steroid 21- (P450c21, CYP21A2) and 11-hydroxylases (P450c11β, CYP11B1) then sequentially oxygenate the steroid backbone to yield cortisol. All of the reactions are ordered, irreversible, and efficient, which is evidenced by the high circulating concentrations of cortisol (10–30 μg/dL, up to 1 μM), while concentrations of the intermediates are 10–100 times lower than those of cortisol. The biosynthesis of dehydroepiandrosterone sulfate (DHEAS) in the zona reticularis is likewise prolific, directional, and efficient, yielding circulating concentrations of DHEAS over 10 μM during much of adult life3. In the adrenal cortex and other endocrine cells for which production of circulating steroids is their major physiologic function, there is no redundancy or competition for multiple pathways. This orderly array of enzymes, positioned to metabolize precursors produced within a single cellular environment, coupled with an absence of competing enzymatic reactions leads to the synthesis of a predominant steroid product and minimal concentrations of other steroid products. Disruption of this normal orderly array leads to marked alterations in the patterns of steroid synthesis. This principle is demonstrated by human genetic deficiency states such as the congenital adrenal hyperplasias, in which a mutation in the cognate gene encoding a steroidogenic enzyme, most commonly CYP21A2 (21-hydroxylase deficiency, 21OHD), impairs cortisol biosynthesis (Figure 2). In 21OHD, 17OHP accumulates prior to the defective enzymatic step, and circulating concentrations of 17OHP rise—not 2 or 5-fold but up to 1,000-fold. This alteration also gives rise to a host of atypical steroid hormone products as well.
Figure 2.
Normal and abnormal adrenal steroidogenesis in the zona fasciculata. The normal pathway is shown on the left. Loss of function mutations in CYP21A2 (steroid 21-hydroxylase) lead to congenital adrenal hyperplasia (CAH; right), resulting in a massive increase in 17-hydroxyprogesterone. This 17-hydroxyprogesterone and upstream precursors are metabolized to androgens, probably via several distinct pathways.
Peripheral metabolism of steroid hormones
Once the steroid products exit the orderly steroidogenic “assembly lines” of the adrenal cortex, placenta, or gonads, pathways of steroid conversion become much more diverse. In some instances, peripheral metabolism of a steroid derived from a steroidogenic tissue and critical to distinct biological actions has been traced to the actions of specific enzymes—such as aromatization of testosterone (T) to estradiol and 5α-reduction of T to 5α-dihydrotestosterone (DHT). In most instances, however, activation, inactivation, and catabolic steps all compete for the same steroid and may produce radically different products in different cells. During genital development in the fetal male, T is the major androgen produced by the fetal testis from androstenedione (AD) by the enzyme 17β-hydroxysteroid dehydrogenase type 3 (17βHSD3). Circulating T is converted to the more potent androgen DHT in the genital tissues and prostate. The type 2 isoform of steroid 5α-reductase (SRD5A2) catalyzes this conversion. The critical nature of this conversion is evident in patients harboring mutations in the SRD5A2 gene. In these individuals, a second isoform (type 1) is unable to compensate for the deficiency of the type 2 enzyme, and such individuals exhibit male pseudohermaphroditism, emphasizing this strict requirement for SRD5A2 in male genital development4. Mutations in the HSD17B3 gene encoding 17βHSD3 cause a similar defect in male phenotypic development.5 In patients with deficiencies of steroid 5α-reductase (SRD5A2) and 17βHSD3, the precursor/product ratio (T/DHT or AD/T, respectively) is elevated, attesting to the mechanistic importance of the enzyme deficiencies. Paradigms such as these are responsible for the codification of pathways, which are readily found in textbooks, such as the pathway DHEA to AD to T to DHT.
While this paradigm is clearly relevant to male genital development, it does not explain the synthesis of all androgens. Of particular note is that normal females have significant levels of circulating T, despite the absence of testes and no expression of the 17βHSD3 enzyme. Most of T in the majority of women derives from adrenal precursors, not from direct secretion of T by the ovary, and the concentrations of T measured in women vary tremendously between individuals. The relevant pathways and enzymes for T synthesis in women remain elusive, although at least one alternate enzyme (17βHSD5) has been shown to catalyze the conversion of AD to T, albeit inefficiently6. In fact, the majority of situations in which steroids are metabolized in peripheral tissues are poorly characterized, and assumptions based on the classical pathways are likely to be incorrect.
Steroidogenesis in Pathologic States
In pathologic states such as 21OHD, disruption of the normal, organized flux of steroid metabolism permits alternate pathways of steroid synthesis to become evident. Although high 17OHP concentrations are used to diagnose 21OHD, 21-deoxycortisol—an unusual steroid normally only found in trace amounts—also rises and is a more specific marker of 21OHD7. A second feature of 21OHD is the shift of steroid flux to androgens rather than cortisol, due to the massive accumulation of precursors and the presence of enzymes in the androgen biosynthesis pathways (Figure 2). Consequently, girls with severe 21OHD are born with masculinized external genitals and a disorder of sexual differentiation (DSD)8. Based on the classical paradigm of virilization of the external genitalia, DHT must be produced, and the pathway has been assumed to involve the conversion of AD to T, and then to DHT.
In the preceding section, we noted that boys with 17βHSD3 deficiency make increased amounts of AD and show elevated circulating AD/T ratios. Despite high AD production, their genitals are under-masculinized, because no alternate 17βHSD enzyme can compensate and complete the synthesis of T and DHT. In this context, how is it possible that girls with 21OHD can masculinize when they do not express 17βHSD3? In addition, girls with 3βHSD2 deficiency produce similar levels of AD and its precursor, dehydroepiandrosterone (DHEA), than girls with 21OHD; yet these girls with 3βHSD2 deficiency are only minimally masculinized at birth. Finally, girls with 21OHD might have masculinized genitals, but their gender identification is still overwhelmingly as girls, although they exhibit some “tomboyish behavior.” These diverse observations suggest that the pathway to DHT in girls with 21OHD is not AD to T to DHT, because they lack a good enzyme to convert AD to T, and their brains do not show evidence of high T exposure.
One potential explanation for genital virilization in female patients with 21OHD derives from observations first made in studies of testicular androgen biosynthesis in the tammar wallaby. In this species, the testes produce 5α-androstane-3α,17β-diol (referred to hereafter as ‘Adiol’) as their major secretion during sexual differentiation. Adiol is taken up by the genital tissues and oxidized to DHT by one or more 3αHSD enzymes, a parallel with the human androgens and target tissue enzyme. The biosynthetic route to Adiol was elucidated by studying progesterone metabolism in the presence of a 5α-reductase inhibitor, 17β-N,N′-diethylcarbamoyl-4-methyl-4-aza-5α-androstan-3-one (4-MA), in which flux stopped at 17OHP, indicating that 17OHP, not T, was the key substrate for 5α-reductase—which was present in the testis itself. The product of 17-hydroxyprogesterone reduction, 5α-pregnan-17α-ol-3,20-dione, was metabolized by a reductive 3α-HSD to a new key intermediate, 5α-pregnane-3α,17α-diol-20-one (Pdiol). Pdiol is readily cleaved to the 5α-reduced, 19-carbon steroid androsterone (AST), which is the immediate precursor to Adiol 9. Adiol is converted in the prostate to DHT, completing this alternate pathway to DHT without ever using T as an intermediate10. It is very likely that this pathway contributes substantially to DHT production in girls with 21OHD, bypassing T entirely2.
Steroidogenesis in Prostate Cancer
DHT-driven AR function is indispensible for normal human prostate development11. The development of prostate cancer – from premalignant lesions to metastatic disease – similarly requires androgens and a functional AR12. This biology underlies the observation of Huggins and Hodges that depletion of gonadal T, or androgen deprivation therapy (ADT), causes tumor regression in the majority of men with prostate cancer13, 14. The mechanisms that drive the eventual development of “castration-resistant” prostate cancer (CRPC) have been the subject of intense study. The depletion of gonadal T would be expected to lead to a proportionate decline in intraprostatic and intratumoral T and DHT. However, ~95% declines in serum T only lead to 70–80% reductions in intraprostatic T and DHT.15, 16 Further, intratumoral concentrations of T and DHT in CRPC are sufficiently high to drive the transcription of AR-regulated genes17–19. The requirement for these intratumoral androgens in CRPC progression is demonstrated by clinical responses to secondary hormonal therapies, which either reduce the concentration of intratumoral androgens, or directly antagonize AR20–26. These clinical and experimental observations lead directly to a critical and hotly debated question regarding the origins of these intratumoral androgens. At least two possibilities exist for the source of these androgens: derivation from adrenal androgen precursors (mainly DHEA[S]) and synthesis via de novo intratumoral steroidogenesis from cholesterol.
The abundance of adrenal DHEA-S – generally found in micromolar concentrations in serum – makes this adrenal androgen precursor a prime suspect for the dominant 19-carbon precursor to T and/or DHT in CRPC27. After desulfation, DHEA may be converted to T and DHT in 2–3 steps, respectively28. The “other” Δ5 19-carbon steroid, Δ5-androstenediol (A5diol), is the 17β-hydroxyl equivalent of DHEA and does not decline in the prostate with ADT29. Two irreversible and 1 reversible steps are required for the conversion of DHEA to DHT. The 2 irreversible steps – conversion of 3-hydroxyl to 3-keto coupled with Δ5 → Δ4 isomerization by 3βHSD and Δ4,3-keto reduction by SRD5A – may occur sequentially starting from 17-keto (DHEA) or 17β-hydroxyl (A5diol) precursors. The reversible step is reduction of the 17-keto group of DHEA by a member of the 17βHSD isoenzymes en route to T and/or DHT. This step might occur in any of the 3 spaces determined by the partitions of the 2 irreversible modifications – proximal to 3βHSD (17-keto reduction of DHEA to A5diol), proximal to SRD5A (17-keto reduction of AD to T), or distal to SRD5A (17-keto reduction of 5α-androstanedione to DHT). Therefore, the “simple” conversion of adrenal precursors to DHT may follow or alternatively circumvent the “canonical” route from gonadal androgens to DHT. The dominant route – yet to be precisely determined – may depend on the expression and activity of specific isoenzymes in prostate cancer and possibly surrounding cells. As an example, one determinant of the dominant route is the expression levels of over a dozen 17βHSD isoenzymes, which each has oxidative/reductive and substrate preferences30–32.
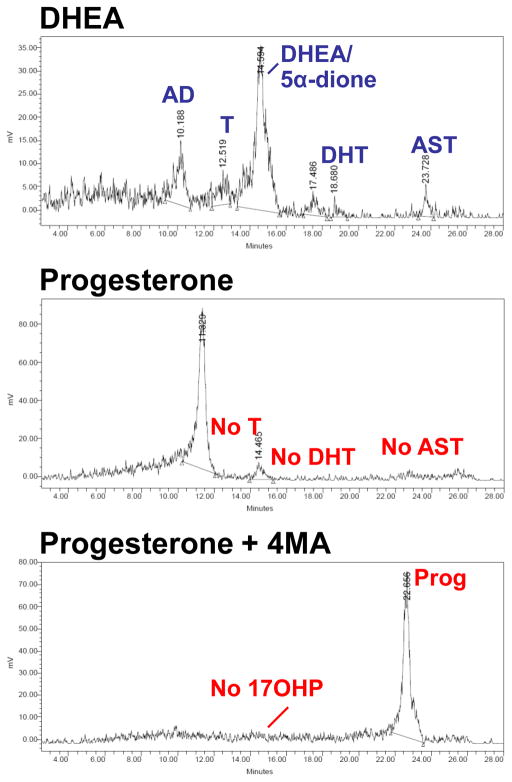
On the other hand, intratumoral de novo steroidogenesis from cholesterol has been proposed as an alternative to the genesis of T and DHT in CRPC33,34. Progesterone, an intermediate metabolite in de novo steroidogenesis, is found at biologically significant concentrations in the serum and tumors of mice bearing CRPC xenografts and there is evidence for the conversion of progesterone to other intermediate metabolites en route to T and DHT33. Although some studies have reported the expression and up-regulation of CYP17A1 in CRPC18, others have failed to detect CYP17A1 expression in the majority of tumors35. The relative contribution of de novo steroidogenesis, given the rate-limiting enzymes and abundance of adrenal precursors, remains to be defined; however, our findings comparing the metabolism of [3H]-DHEA versus [3H]-progesterone in the LNCaP model may offer some insights (Figure 3). DHEA is readily metabolized to androstenedione by 3βHSD28. In contrast, we cannot detect progesterone metabolism to 17OHP or AD by CYP17A1 over a 48 hour time frame. Furthermore, no T, AST, or DHT is detectable in these experiments starting with progesterone. Any 17OHP might not be detectable due to rapid 5α-reduction to 5α-pregnan-17α-ol-3,20-dione, as occurs in the tammar wallaby2. However, SRD5A inhibition with 4-MA still does not lead to detectable 17OHP in [3H]-progesterone treated LNCaP cells. Although these findings do not rule out de novo steroidogenesis in CRPC, these results, along with the abundance of adrenal androgens in serum, suggest that any contribution to DHT from de novo steroidogenesis would be overshadowed by flux from adrenal 19-carbon precursors. Findings of de novo steroidogenesis in other non-endocrine organs should also be noted. The genesis of neurosteroids in the brain is controversial, and the significance of low-level expression of CYP11A1 in glial cells, which is required for the initial and rate-limiting step in steroidogenesis, is unknown36. Although evidence for the de novo genesis of aldosterone has been reported in heart failure37, the suggestion of a significant contribution to cardiac pathophysiology by cardiac steroidogenesis has been met with some skepticism38.
Figure 3.
Steroid flux from DHEA versus progesterone. LNCaP cells were treated with [3H]-DHEA (100 nM), [3H]-progesterone (100 nM), and [3H]-progesterone (100 nM) pretreated with 4-MA (10 μM), for 48 hours. Steroids were extracted from the medium, treated with glucuronidase and analyzed by thin-layer chromatography (not shown) or high-performance liquid chromatography (HPLC). DHEA is readily metabolized to androstenedione (AD), testosterone (T), dihydrotestosterone (DHT) and androsterone (AST). In contrast, for cells treated with progesterone (Prog), there was no detectable 17OH-progesterone (17OHP), T, DHT, or AST. No 17OHP is detected with pretreatment with the SRD5A inhibitor 4-MA, and little Prog metabolism occurs. These results suggest that the major metabolic pathway of Prog in LNCaP cells begins with 5α-reduction, rather than 17-hydroxylation.
Genetic syndromes have helped define the fast lanes in the highways – or the dominant pathways in steroidogenic organs – which are robust, highly ordered, and efficient. However, defining the road – or roads - to T and/or DHT synthesis in peripheral tissues and CRPC, may require exploration of alternative, or parallel pathways, which take entirely different and unexplored paths to T and/or DHT compared to those that dominate in normal steroidogenic tissues.
Androgen Receptor Variants – another route to AR activation
The proceeding discussion is focused on the activation of the AR via the synthesis of active androgens by alternate steroidogenic pathways in CRPC. In addition, a number of reports have suggested that constitutively active AR variants, lacking the ligand-binding domain (LBD), are expressed in CRPC tumors39–45. Given their intrinsic activity in the absence of ligand, such AR variants would obviate the requirement for ligand, rendering ADT and AR antagonists ineffective. Given the molecular heterogeneity of tumor cells following treatment, it is likely that the presence of such AR variants identifies a subset of patients who either will be refractory to or have already exhausted all secondary hormonal therapies. Owing to the restriction of such studies to cell culture – and in a limited fashion to human samples – the genesis, activity, prevalence and function of these AR variants remain obscure. In addition, it seems plausible that the evolution of AR variants might be driven in part by the presence of certain steroids, which might select for tumors possessing the capacity to metabolize precursors to T, whereas the absence of specific steroids selects for tumor clones that express constitutively active AR variants. Furthermore, an understanding of AR variant expression might enable strategies to suppress their expression and thus restore responsiveness to secondary hormonal therapies.
Conclusions and Implications
The biological behavior of prostate cancer during progression to castration resistance demonstrates the continued importance of androgen synthesis, even in the individuals treated with medical or surgical castration and with agents designed to antagonize the actions of androgens. The routes by which such synthesis occurs remains a subject of intense research and the definition of the pathways responsible is critical for the design of more effective therapies. Finally, the identification of truncated forms of the AR that active in the absence of hormone emphasizes the need to define of the genesis of these truncated forms, establish their prevalence under treatment conditions effecting the inhibition of androgen synthesis or action, and identify methods to inhibit their activity.
Acknowledgments
This publication has been funded in part by a Howard Hughes Medical Institute Physician-Scientist Early Career Award, a Prostate Cancer Foundation Young Investigator Award, Grant Number UL1RR024982 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and from grant number PC080193 from the U.S. Army Medical Research and Materiel Command to NS. RJA is supported by a Clinical Scientist Award in Translational Research from the Burroughs-Wellcome Fund (1005954) and by the Charles A. and Elizabeth Ann Sanders Chair in Translational Research.
References
- 1.Simard J, Ricketts ML, Gingras S, Soucy P, Feltus FA, Melner MH. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005 Jun;26(4):525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 2.Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004 Nov;15(9):432–438. doi: 10.1016/j.tem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Semin Reprod Med. 2004 Nov;22(4):281–288. doi: 10.1055/s-2004-861545. [DOI] [PubMed] [Google Scholar]
- 4.Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991 Nov 14;354(6349):159–161. doi: 10.1038/354159a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson S, Geissler WM, Wu L, et al. Molecular genetics and pathophysiology of 17 beta-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996 Jan;81(1):130–136. doi: 10.1210/jcem.81.1.8550739. [DOI] [PubMed] [Google Scholar]
- 6.Penning TM, Burczynski ME, Jez JM, et al. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J. 2000 Oct 1;351(Pt 1):67–77. doi: 10.1042/0264-6021:3510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tonetto-Fernandes V, Lemos-Marini SH, Kuperman H, Ribeiro-Neto LM, Verreschi IT, Kater CE. Serum 21-Deoxycortisol, 17-Hydroxyprogesterone, and 11-deoxycortisol in classic congenital adrenal hyperplasia: clinical and hormonal correlations and identification of patients with 11beta-hydroxylase deficiency among a large group with alleged 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006 Jun;91(6):2179–2184. doi: 10.1210/jc.2005-1890. [DOI] [PubMed] [Google Scholar]
- 8.Speiser PW, White PC. Congenital adrenal hyperplasia. N Engl J Med. 2003 Aug 21;349(8):776–788. doi: 10.1056/NEJMra021561. [DOI] [PubMed] [Google Scholar]
- 9.Wilson JD, Auchus RJ, Leihy MW, et al. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003 Feb;144(2):575–580. doi: 10.1210/en.2002-220721. [DOI] [PubMed] [Google Scholar]
- 10.Shaw G, Renfree MB, Leihy MW, Shackleton CH, Roitman E, Wilson JD. Prostate formation in a marsupial is mediated by the testicular androgen 5 alpha-androstane-3 alpha,17 beta-diol. Proc Natl Acad Sci U S A. 2000 Oct 24;97(22):12256–12259. doi: 10.1073/pnas.220412297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McPhaul MJ. Androgen receptor mutations and androgen insensitivity. Mol Cell Endocrinol. 2002 Dec 30;198(1–2):61–67. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 12.Tomlins SA, Mehra R, Rhodes DR, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007 Jan;39(1):41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 13.Huggins C, Hodges CV. Studies on prostate cancer, I: the effect of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. [Google Scholar]
- 14.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005 Jul 13;294(2):238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004 Nov 1;10(21):7121–7126. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 16.Page ST, Lin DW, Mostaghel EA, et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006 Oct;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 17.Geller J, Albert J, Loza D, Geller S, Stoeltzing W, de la Vega D. DHT concentrations in human prostate cancer tissue. J Clin Endocrinol Metab. 1978 Mar;46(3):440–444. doi: 10.1210/jcem-46-3-440. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008 Jun 1;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005 Jul 1;11(13):4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 20.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. Apr 24;375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifi N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert Opin Investig Drugs. Jun 4; doi: 10.1517/13543784.2010.494178. [DOI] [PubMed] [Google Scholar]
- 22.Attard G, Reid AH, A’Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009 Aug 10;27(23):3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan CJ, Smith MR, Fong L, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 2010 Mar 20;28(9):1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danila DC, Morris MJ, de Bono JS, et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 2010 Mar 20;28(9):1496–1501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid AH, Attard G, Danila DC, et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 2010 Mar 20;28(9):1489–1495. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009 May 8;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenspan’s Basic and Clinical Endocrinology. 8. McGraw-Hill; 2007. [Google Scholar]
- 28.Evaul K, Li R, Papari-Zareei M, Auchus RJ, Sharifi N. 3{beta}-Hydroxysteroid Dehydrogenase Is a Possible Pharmacological Target in the Treatment of Castration-Resistant Prostate Cancer. Endocrinology. Jun 9; doi: 10.1210/en.2010-0138. [DOI] [PubMed] [Google Scholar]
- 29.Mizokami A, Koh E, Fujita H, et al. The adrenal androgen androstenediol is present in prostate cancer tissue after androgen deprivation therapy and activates mutated androgen receptor. Cancer Res. 2004 Jan 15;64(2):765–771. doi: 10.1158/0008-5472.can-03-0130. [DOI] [PubMed] [Google Scholar]
- 30.Moeller G, Adamski J. Integrated view on 17beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2009 Mar 25;301(1–2):7–19. doi: 10.1016/j.mce.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 31.Penning TM, Jin Y, Rizner TL, Bauman DR. Pre-receptor regulation of the androgen receptor. Mol Cell Endocrinol. 2008 Jan 16;281(1–2):1–8. doi: 10.1016/j.mce.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan N, Sharma KK, Andersson S, Auchus RJ. Human 17beta-hydroxysteroid dehydrogenases types 1, 2, and 3 catalyze bi-directional equilibrium reactions, rather than unidirectional metabolism, in HEK-293 cells. Arch Biochem Biophys. 2004 Sep 1;429(1):50–59. doi: 10.1016/j.abb.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Locke JA, Guns ES, Lubik AA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008 Aug 1;68(15):6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 34.Leon CG, Locke JA, Adomat HH, et al. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 2009 Oct 28; doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 35.Hofland J, van Weerden WM, Dits NF, et al. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. Feb 1;70(3):1256–1264. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 36.Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu EE, Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987 Sep 4;237(4819):1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- 37.Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab. 2001 Nov;86(11):5121–5126. doi: 10.1210/jcem.86.11.7925. [DOI] [PubMed] [Google Scholar]
- 38.Gomez-Sanchez CE, Gomez-Sanchez EP. Editorial: Cardiac steroidogenesis--new sites of synthesis, or much ado about nothing? J Clin Endocrinol Metab. 2001 Nov;86(11):5118–5120. doi: 10.1210/jcem.86.11.8102. [DOI] [PubMed] [Google Scholar]
- 39.Tepper CG, Boucher DL, Ryan PE, et al. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002 Nov 15;62(22):6606–6614. [PubMed] [Google Scholar]
- 40.Steinkamp MP, O’Mahony OA, Brogley M, et al. Treatment-dependent androgen receptor mutations in prostate cancer exploit multiple mechanisms to evade therapy. Cancer Res. 2009 May 15;69(10):4434–4442. doi: 10.1158/0008-5472.CAN-08-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009 Jan 1;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009 Mar 15;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008 Jul 1;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Libertini SJ, Tepper CG, Rodriguez V, Asmuth DM, Kung HJ, Mudryj M. Evidence for calpain-mediated androgen receptor cleavage as a mechanism for androgen independence. Cancer Res. 2007 Oct 1;67(19):9001–9005. doi: 10.1158/0008-5472.CAN-07-1072. [DOI] [PubMed] [Google Scholar]
- 45.Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. Aug 2;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]