Abstract
Background
Approximately 800 thousand patients require mechanical ventilation in the United States annually with an in-hospital mortality rate of over 30%. The majority of patients requiring mechanical ventilation are over the age of 65 and advanced age is known to increase the severity of ventilator-induced lung injury (VILI) and in-hosptial mortality rates. However, the mechanisms which predispose aging ventilator patients to increased mortality rates are not fully understood. Ventilation with conservative fluid management decreases mortality rates in acute respiratory distress patients, but to date there has been no investigation of the effect of conservative fluid management on VILI and ventilator associated mortality rates. We hypothesized that age-associated increases in susceptibility and incidence of pulmonary edema strongly promote age-related increases in ventilator associated mortality.
Methods
2 month old and 20 month old male C57BL6 mice were mechanically ventilated with either high tidal volume (HVT) or low tidal volume (LVT) for up to 4 hours with either liberal or conservative fluid support. During ventilation, lung compliance, total lung capacity, and hysteresis curves were quantified. Following ventilation, bronchoalveolar lavage fluid was analyzed for total protein content and inflammatory cell infiltration. Wet to dry ratios were used to directly measure edema in excised lungs. Lung histology was performed to quantify alveolar barrier damage/destruction. Age matched non-ventilated mice were used as controls.
Results
At 4hrs, both advanced age and HVT ventilation significantly increased markers of inflammation and injury, degraded pulmonary mechanics, and decreased survival rates. Conservative fluid support significantly diminished pulmonary edema and improved pulmonary mechanics by 1hr in advanced age HVT subjects. In 4hr ventilations, conservative fluid support significantly diminished pulmonary edema, improved lung mechanics, and resulted in significantly lower mortality rates in older subjects.
Conclusion
Our study demonstrates that conservative fluid alone can attenuate the age associated increase in ventilator associated mortality.
Keywords: Aging, Ventilator-Induced Lung Injury, Ventilator Associated Mortality, Pulmonary Edema, Fluid management, Respiratory Mechanics, Mechanical Ventilation
Introduction
Mechanical ventilation (MV) is a necessary and potentially life-saving clinical intervention. Many pathophysiological states such as Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS), pneumonia, asthma, and Chronic Obstructive Pulmonary Disease (COPD) can result in a person’s inability to adequately ventilate their lungs with spontaneous breathing (12)(31). It is estimated that annually there are 800 thousand hospitalizations in the United States requiring MV with an estimated in-hospital mortality rate of 34.5%. The largest population of patients requiring MV (53%) is the elderly. Incidence of MV and in-hospital mortality both sharply rise with patient age (12).
Advanced age is known to be associated with increased severity of ventilator-induced lung injury (VILI) (40). Setzer et al recently demonstrated that HVT (24 ml/kg) MV caused more lung injury in old rat subjects than in their younger counterparts. Lung wet to dry rations, lavage protein and cytokine concentrations, and systemic markers of inflammation were all elevated in the older HVT MV subjects (33). Additionally it has been shown that short-term mechanical ventilation with low tidal volume and hyperoxia increases pulmonary edema, lung inflammation, and decreases diaphragm function in old rats subjects compared to young adults (1). However, the exact mechanism(s) through which this relationship between aging and VILI is mediated remain ill defined. Consequently, preventative strategies for the age-associated increase in ventilator mortality are currently unknown. The changing mechanical properties of the aging lung lead to increases in lung compliance which can predispose the lung to over-distention and increase susceptibility to atelectasis during mechanical ventilation (2)(4)(14)(32). Further, the aging lung exhibits increasingly dysregulated immune/inflammatory responses to injury leading to pathological increases in pro-inflammatory behavior (33)(28).
Pulmonary edema is a hallmark of VILI(31). The incidence and in-hospital mortality rates of patients presenting with pulmonary edema is also known to increase with patient age(8)(6). Both the presence of pulmonary edema in ventilated patients and their overall survival rates can be affected by fluid management strategies (43). Recent studies show that conservative fluid management can decrease pulmonary edema, increase ventilator free days, and increase overall survival of patients with acute respiratory distress syndrome (ARDS) (22)(36). However patients undergoing mechanical ventilation with inadequate fluid support are also at risk of developing hypovolemia which overtime can lead to multiple organ system failure (30). Therefore administering optimal fluid balance is crucial for mechanical ventilator patient survival and recovery. Despite known benefits of conservative fluid protocols for patients with ARDS, its effects on VILI, interaction with patient age, and the role that they both may play in ventilator associated mortality rates has yet to be established.
We hypothesized that high tidal volume (HVT) mechanical ventilation would induce lung injury, degrade pulmonary mechanics, and increase pulmonary edema in older murine subjects. Further, we hypothesized that a greater sensitivity to these pathologies would result in an age-associated increase in ventilator associated mortality. Finally, we hypothesized that age associated increases in injury and mortality could be attenuated with a conservative fluid support strategy. To establish the role pulmonary edema plays in age associated increases in VILI and ventilator associated mortality and to evaluate fluid support protocols as a potential preventative treatment, we investigated the outcomes of both liberal and conservative fluid support strategies during high and low tidal volume mechanical ventilation in both young and old mice.
Materials and Methods
Animal Use
This study was approved by the VCU Institutional Animal Care and Use Committee (protocol number AD10000465). Male C57BL6 mice were used in these experiments. All animal experiments were carried out under IACUC University guidelines.
Age Groups
Age group I (Young): Young adult animals, 2–3 months of age, weighing 25 ± 3 g. Age group II (Old): Old animals, 20–22 months of age, weighing 35 ± 11 g. Ages of our young and old murine groups respectively were based on correlations between murine lifespan and known age-associated morphological changes in murine lung (17)(29).
Mechanical Ventilation
Young and old animal subjects were anesthetized with an intraperitoneal (IP) injection of 80mg/kg pentobarbital. In addition to anesthesia our MV regimen required that we arrest the subjects’ spontaneous breathing with administration of a paralytic. This was accomplished with IP injection of 0.8 mg/kg pancuronium bromide at the 0hr and 2hr time points. Depth of subject anesthesia was monitored continuously via EKG transducer monitors included in FlexiVent small animal ventilator (Scireq) hardware. To maintain an appropriate level of anesthesia over the course of ventilation pentobarbital redoses of 40mg/kg were administered as needed when subject heart rate exceeded 350 bpm. Each subject was randomized into the following treatment groups according to age: Young Low Tidal Volume (LVT), Young High Tidal Volume (HVT), Old LVT, and Old HVT. Following group assignment, subjects were mechanically ventilated with either LVT (8mL/kg, 125 BPM, 3cm H20 positive end expiratory pressure, [PEEP]) or HVT (25mL/kg for Young, 18mL/kg for Old, 90 BPM, 0cm H20 PEEP) ventilation protocol for either 1hr or 4hrs using a FlexiVent small animal ventilator (Scireq). LVT and HVT ventilation patterns and four-hour ventilation protocol were based on previously published in vivo models (29). Difference in tidal volumes of young and old HVT groups reflects a normalization of tidal volume across age based upon ideal body weight. Mice are considered to be adult at 2–3 months, but our measurements indicated that our 20–22 month old animal subjects were on average ~30% more massive than their younger counterparts. To better replicate clinical protocols the tidal volumes for our HVT ventilation were established using ideal/predicted body weights. The tidal volume for the old HVT group was therefore multiplied by 0.7, or the ratio of the average weight of the young subjects to that of the old. This gave an adjusted average tidal volume of 625ul±50ul and 630ul±62ul for the young and old HVT groups respectively.
Fluid Support Protocol
Animal subjects were administered either liberal (high) or conservative (low) fluid management protocols. Animals managed under high fluid received anesthesia with pentobarbital at a concentration of 10 mg/ml in saline and received IP infusions of saline at 100μL/hr. For a 25 gram subject requiring an hourly redose of pentobarbital the total 4hr saline infusion volume would be ~1ml. Animals managed under low fluid protocol were anesthetized with pentobarbital at 20 mg/ml and received no IP saline infusions. For a 25 gram subject requiring an hourly redose of pentobarbital the total 4hr saline infusion volume would be ~0.35ml.
Lung Mechanics
At the 0, 1, 2, 3, and 4hr time points the following forced inspiratory maneuvers: 1) Deep Inflation v7.0, 2) Snapshot-150 v7.0, and 3) PVs-V v7.0 were performed using the included FlexiWare software package (Scireq). Deep inflation acts as a recruitment maneuver and measures total lung capacity (TLC). Snapshot measures lung tissue compliance. The pressure volume (PV) maneuver inflates the lung to a sequence of linearly increasing then decreasing volumes while measuring the corresponding airway pressures. With these data the PV maneuver generates respiratory pressure-volume loops from which the PV-loop area (hysteresis) is calculated. For subjects who died during ventilation the last set of mechanical data points taken prior to death were included in analysis where possible.
Euthanasia
At the end of the 1hr and 4hr ventilations respectively each animal subject was fully exsanguinated and removed from mechanical ventilation.
Alveolar Lavage
Bronchoalveolar lavage was performed by instillation of saline using gravity feed driven flow at a height 30cm. This method was preferred over a forced installation to preserve lung architecture for histological analysis of alveolar airspace enlargement. Saline flowed freely into the lung until it stopped naturally. The installation tube and mouse were then inverted to allow the saline to flow freely back out and into a collection tube. This process was repeated three times. An average of 2.5 – 3.5mls of total bronchoalveolar lavage (BAL) fluid per subject was obtained using this technique.
Bronchoalveolar Lavage Fluid Cytology
BAL fluid was centrifuged at 300 x G, 4°C for 8 minutes. Supernatants were removed and cell pellets re-suspended in saline. Cells were then mounted onto glass slides using a cytospin device (Shandon). Cells were stained (3 Diff-Quik solutions staining kit) and cover-slipped. Cell populations were then analyzed using microscopy (Olympus) and the ratios of lymphocytes, leukocytes, and macrophages were determined.
Bronchoalveolar Lavage Fluid Protein Concentration
BCA assay analysis (Pierce) of protein concention was performed on all BAL fluid supernatants according to manufacturer’s instructions.
Lung Histology
Left lungs were held at constant pressure, fixed with 10% formalin, sectioned, and stained with H&E. Airspace enlargement, which quantifies relative differences in alveolar airspace area within lung histology sections, was measured and analyzed using in-house custom code written in MATLAB. This code quantified enlargement using a previously defined mean weighted enlargement metric (18). The weight of each airspace in the metric was dependent on the variance and skewness of its size. Stained slides were dehydrated and mounted using Permount mounting medium (Fisher) and imaged using an Olympus IX71 Microscope (Olympus).
Lung Wet to Dry Weight Ratios
From each animal subject, right lungs were excised, weighed, fully desiccated then lyophilized for 48 hours. Specimens were then weighed and a wet weight to dry weight ratio calculated (9). To prevent introduction of erroneous fluid to this measurement wet to dry ratios were not performed on subjects for whom bronchoalveolar fluid had been collected.
Non-Ventilated Controls
Measurements of each experimental variable except for pulmonary biomechanics was performed on non-ventilated control animals.
Statistical Analysis
All quantitative experimental studies were performed with a minimum of n=3 however the values vary between individual groups experiments. A more in-depth explanation of the variation in N values is given in the supplement. Survival statistics were performed with Kaplan-Meyer estimation. For wet to dry data analysis two way ANOVA was used to establish pairwise differences across age and one way ANOVA with Tukey tests were used to establish within group differences. All other statistics were performed using two way ANOVA. P values of <0.05 were considered significant. We used GraphPad Prism 5 statistical analysis software.
Results
Four Hour High Fluid Survival Rate
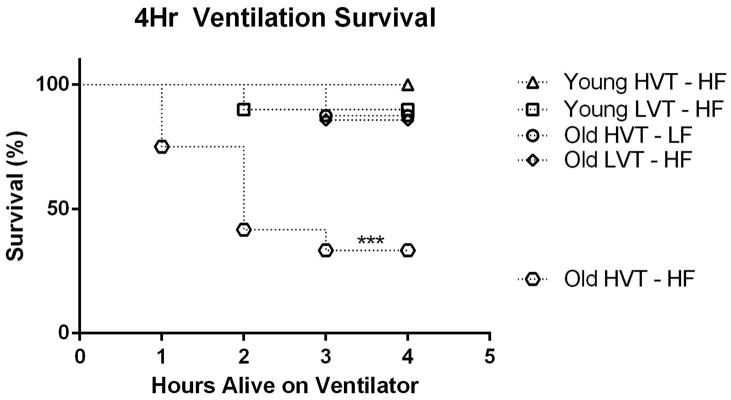
4hr survival curve for old subjects ventilated with HVT and high fluid support was statistically significantly lower than that observed in all other groups (Figure 1). In all, only four of twelve old HVT subjects survived the 4Hr duration of their respective ventilations. By contrast, only one old HVT subject on the low fluid support protocol died during mechanical ventilation. Additionally only one old LVT subject and one young LVT subject died during mechanical ventilation. No young HVT subjects died during mechanical ventilation (Figure 1).
Figure 1.
4Hr Survival Rate. Survival rate in the Old HVT-HF group was significantly less than all other groups. Data is presented as survival percentage N = 10 for Young LVT – HF, 10 for Young HVT – HF, 3 for Young HVT – LF, 7 for Old LVT – HF, 12 for Old HVT – HF, 8for Old HVT – LF ***p<0.001.
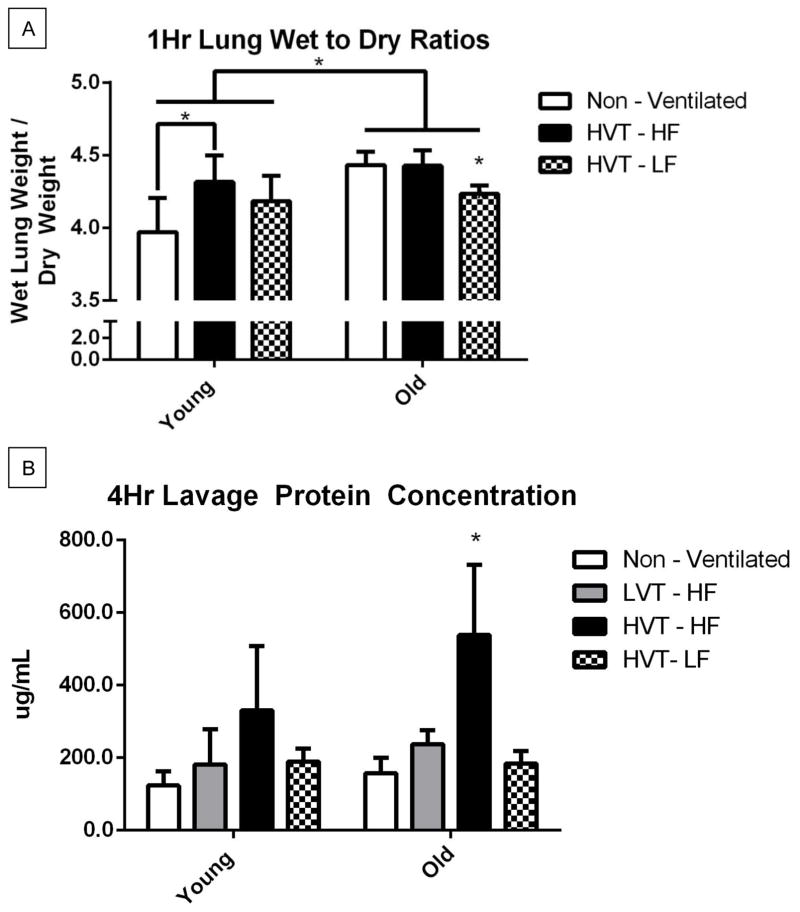
One Hour Lung Wet to Dry Ratios
Wet to dry ratios for each of the 1hr ventilation groups were measured (Figure 2A). A statistically significant group-wise difference across age was observed with the older murine groups exhibiting significantly greater wet to dry ratios than the young. The wet to dry ratios for young HVT subjects receiving high fluid protocol was significantly greater than that observed in the young non-ventilated group (Figure 2A). The wet to dry ratio of old HVT subjects receiving low fluid protocol was significantly lower than that observed in the old HVT subjects who received high fluid protocol and the old non-ventilated group (Figure 2A).
Figure 2.
A. 1Hr Lung Wet to Dry Ratios. There was a group wise statistically significant difference across age with old groups having significantly greater lung wet to dry ratio than young. The Young HVT-HF group was significantly greater than in the Young Non-Ventilated group. The Old HVT-LF group was significantly less than in all other old groups respectively. Data are presented as mean +/− st.dev N = 4 for Young Non-Vent, 4 for Young HVT – HF, 4 for Young HVT – LF, 4 for Old Non-Vent, 4 for Old HVT – HF, 5 for Old HVT – LF. B. 4Hr Lavage Protein Concentration. The Old HVT-HF group concentration was significantly greater than that of all other old groups respectively. N = 4 for Young Non-Vent, 4 for Young LVT – HF, 7 for Young HVT – HF, 3 for Young HVT – LF, 4 for Old Non-Vent, 4 for Old LVT – HF, 4 for Old HVT – HF, 5 for Old HVT – LF *p<0.05.
Four Hour Bronchoalveolar Lavage Fluid Protein Concentration
BAL fluid protein concentrations were measured for each animal subject surviving 4hr ventilation (Figure 2B). There was not a statistically significant group wise difference in lavage protein concentration across age. Within the old group, the lavage protein concentration of subjects ventilated with HVT and high fluid support was significantly greater than that of all other old groups respectively. Similar trends were seen between the young groups but none reached statistical significance.
Bronchoalveolar Lavage Fluid Cytology
Cytological differentials were measured on the BAL fluid of each subject surviving 4Hr ventilation (Table 1). The monocyte differentials of Old HVT high fluid group were significantly greater than those of both the Young Non-Ventilated and Old Non-Ventilated groups. No other statistically significant differences were observed.
Table 1.
Cellularity in BAL fluid. Data presented as absolute cell numbers in broncoalveolar lavage and as cell differentials (percentage of counted cells). Monocyte differential < Young Non-Ventilated and Old Non-Ventilated.
| Young Non-Vent | Young LVT-HF | Young HVT-HF | Young HVT-LF | Old Non-Vent | Old | Old | Old | |
|---|---|---|---|---|---|---|---|---|
| Lymphocytes (%) | 1.58±0.8 | 5.89±4.1 | 4.69±3.4 | 2.5±5 | 2.66±0.7 | 3.79±1 | 9±4.2 | 6.9±3.2 |
| Neutrophils (%) | 0.33±0.2 | 0.67±0.4 | 3.14±3.1 | 0 | 0.33±0.1 | 4.42±3 | 8.42±7.2 | 6.4±6.2 |
| Monocytes (%) | 98.08±0.8 | 93.33±4.6 | 92.22±5.6 | 97.5±5 | 97±0.7 | 91.7±3.4 | 79.55±9.1 * | 86.4±6.1 |
Data are presented as mean +/− st.dev N=4 for Young Non-Vent, 3 for Young LVT – HF, 6 for Young HVT – HF, 3 for Young HVT – LF, 6 for Old Non-Vent, 4 for Old LVT – HF, 4 for Old HVT – HF, 5 for Old HVT – LF
p<0.05.
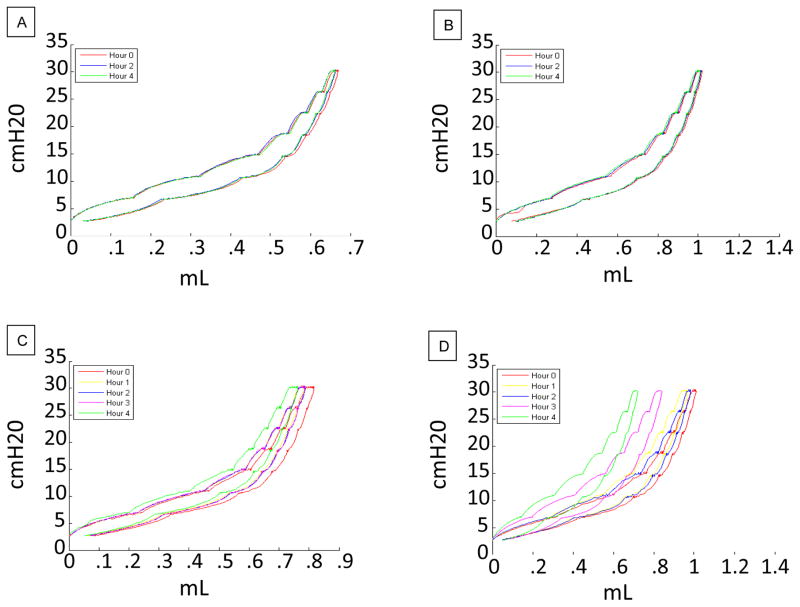
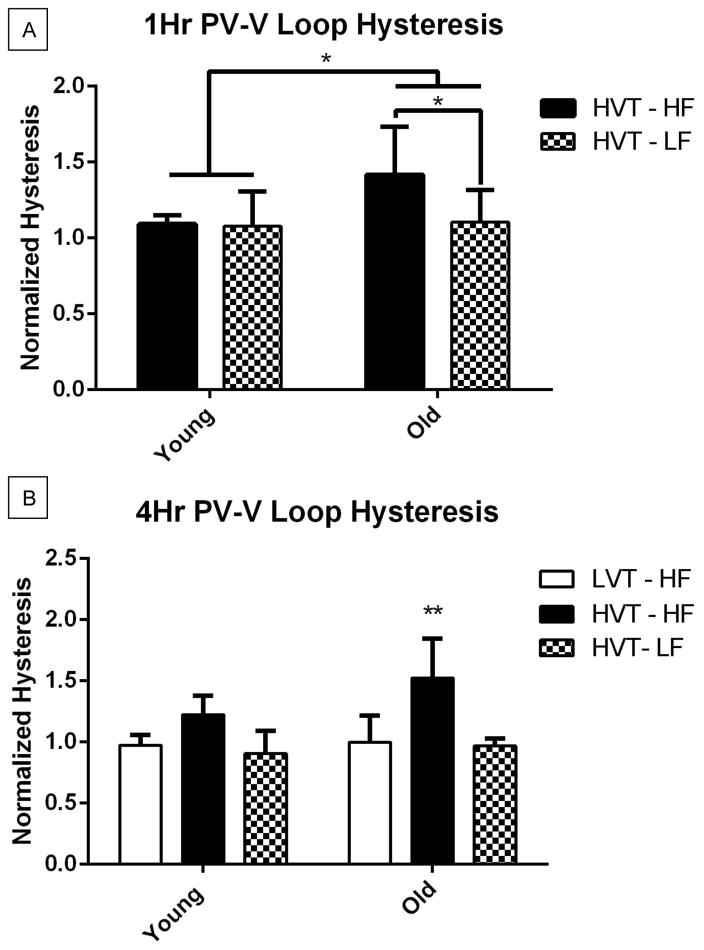
PV Loop Hysteresis
PV loops were generated hourly using the Flexivent software (Scireq) for each surviving animal subject. Representative PV loops are shown in Figure 3. The hysteresis values of each group were normalized with their respective 0hr values (Figure 4). There was a group wise difference across age with old groups having significantly greater 1Hr PV loop hysteresis than young. 1Hr PV loop hysteresis of Old HVT high fluid group was significantly greater than that of the Old HVT low fluid group (Figure 4A). The 4Hr PV loop hysteresis of the old HVT high fluid was significantly greater than that of both the old LVT high fluid and old HVT low fluid groups (Figure 4B). Similar trends were seen between the young groups but none reached statistical significance.
Figure 3.
A. Representative 4Hr PV Loop for a Young LVT-HF subject. B. Representative 4Hr PV Loop for an Old LVT-HF subject. C. Representative 4Hr PV Loop for a Young HVT-HF subject. D. Representative 4Hr PV Loop for an Old HVT-HF subject.
Figure 4.
A. 1Hr PV Loop Hysteresis. Hysteresis in the Old HVT-HF group was significantly greater than Young HVT-HF and Old HVT-LF groups. Data are presented as mean +/− st.dev N=7 for Young HVT – HF, 7 for Young HVT – LF, 9 for Old HVT – HF, 12 for Old HVT – LF. B. 4Hr PV Loop Hysteresis. Hysteresis of the Old HVT-HF group was greater than that of the Old LVT-HF and Old HVT-LF groups. N=5 for Young LVT – HF, 6 for Young HVT – HF, 3 for Young HVT – LF, 5 for Old LVT – HF, 4 for Old HVT – HF, 5 for Old HVT – LF *p<0.05, **p<0.01.
Lung Compliance
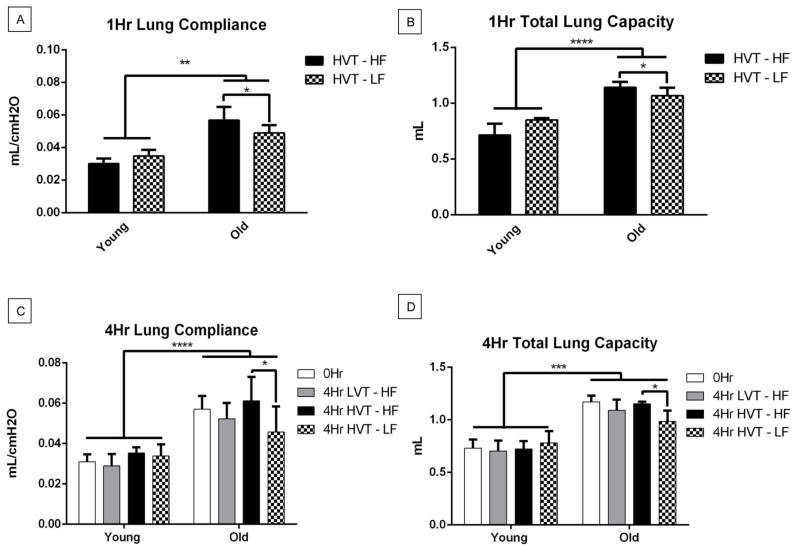
Lung compliance was measured hourly using the Flexivent software (Scireq) for each surviving animal subject (Figure 5). There was a significant group wise difference across age of both the 1Hr and 4Hr lung compliance measures with old groups having greater compliance than young. (Figure 5A, C). The 1Hr lung compliance of the old HVT high fluid group was significantly greater than that of the HVT low fluid group (Figure 5A). The 4Hr lung compliance of the old HVT high fluid group was greater than that of the old HVT low fluid group (Figure 5C). There was a strong non-significant trend with the old 4Hr HVT high fluid groups having a greater mean than the old 4Hr LVT low fluid group.
Figure 5.
A. 1Hr Lung Compliance. There was a significant group wise across age with the old groups having a greater compliance than the young. 1Hr lung compliance in the Old HVT-HF group was significantly greater that of the Old HVT-LF group. Data are presented as mean +/− st.dev N=11 for Young HVT – HF, 4 for Young HVT – LF, 14 for Old HVT – HF, 7 for Old HVT – LF. B. 1Hr Total Lung Capacity. There was a significant group wise difference across age with the old groups having a greater TLC than young. 1Hr TLC in the Old HVT-HF group was significantly greater that of the Old HVT-LF group. N=4 for Young HVT – HF, 4 for Young HVT – LF, 14 for Old HVT – HF, 14 for Old HVT – LF. C. 4Hr Lung Compliance. There was a significant group wise difference across age with the old groups having greater lung compliance than the young. 4Hr lung compliance in the Old HVT-HF group was significantly greater that of the Old 0Hr and Old HVT-LF groups. N=11 for 0Hr Young, 7 for Young LVT – HF, 8 for Young HVT – HF, 3 for Young HVT – LF, 16 for 0Hr Old, 6 for Old LVT – HF, 4 for Old HVT – HF, 6 for Old HVT – LF. D. 4Hr Total Lung Capacity. There was a significant group wise difference across age with the old groups having a greater TLC than young. 4Hr TLC in the Old HVT-HF group was significantly greater that of the Old HVT-LF group. N=11 for 0Hr Young, 7 for Young LVT – HF, 9 for Young HVT – HF, 3 for Young HVT – LF, 16 for 0Hr Old, 6 for Old LVT – HF, 4 for Old HVT – HF, 6 for Old HVT – LF, *p<0.05, **p<0.01, ***p<0.001, ****p<0.001.
Total Lung Capacity
Total Lung Capacity (TLC) was measured hourly using the Flexivent software (Scireq) for each surviving animal subject (Figure 5). There was a statistically significant group wise difference across age of both the 1Hr and 4Hr TLC with the old groups having greater TLC than young (Figure 5B, D). The 1Hr TLC of the old HVT high fluid group was significantly greater than that of the HVT low fluid group (Figure 5B). The 4Hr TLC of the old HVT high fluid group was significantly greater than that of the old HVT low fluid group (Figure 5D). There was a strong non-significant trend with the old 4Hr HVT high fluid group having a greater mean than the old 4Hr LVT high fluid group.
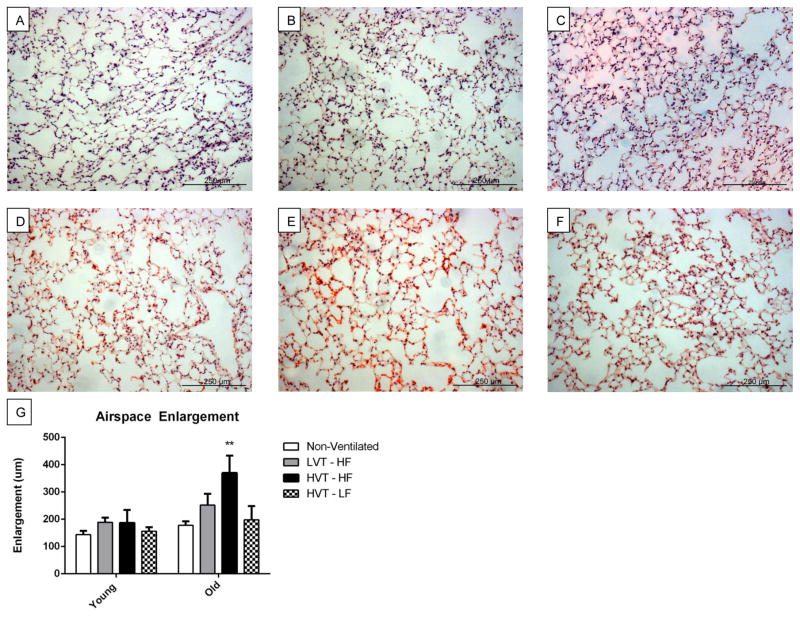
Airspace Enlargement
Representative histological images of lung stained with H&E of both young and old animal subjects ventilated with HV or LV are presented in Figure 6A–D. Airspace enlargement and an increased infiltration of erythrocytes could clearly be seen in images corresponding to both increased age and HVT ventilation. Low fluid protocols visually attenuated these effects. Airspace enlargement was quantified and calculated for each subject reaching 4hrs of mechanical ventilation. There was a significant group wise difference in airspace enlargement across age with old groups having a greater value that young. Airspace enlargement of old subjects ventilated with HVT and high fluid was significantly greater than that of all other groups (Figure 6E).
Figure 6.
A, B, C, D, E, F Representative 4Hr histological H&E images of A. Young LVT-HF, B. Young HVT-HF, C. Young HVT-LF, D. Old LVT-HF, E. Old HVT-HF, and F. Old HVT-LF lung sections respectively. G. Airspace Enlargement. There was a statistically significant groupwise difference across age with old groups having significantly greater enlargement than young. Enlargement was greater in the Old HVT-HF group than in all others. Data are presented as mean +/− st.dev N = 3 for Young Non-Vent, 6 for Young LVT – HF, 6 for Young HVT – HF, 3 for Young HVT – LF, 3 for Old Non-Vent, 3 for Old LVT – HF, 4 for Old HVT – HF, 3 for Old HVT – LF **p<0.01.
Discussion
The first aim of this study was to establish that older mouse subjects when subjected to HVT MV exhibit an increased severity of ventilator-induced lung injury as was recently reported in an aging rat model (33). Wilson et al established that significant stretch induced lung injury in young healthy mice requires mechanical ventilation with tidal volumes approaching 40mg/ml (38). Consequently most models of VILI use similarly high (>30mg/ml) VT and/or inspiratory pressures to induce injury. These settings however are generally considered significantly higher than those used in the clinical management of patients (38)(39). Setzer et al demonstrated that the increased sensitivity of the aging rat lung to mechanical ventilation allowed their group to achieve significant VILI with tidal volumes of 16–24mg/kg (33). So while a tidal volume of 40mg/kg may be more conventionally referred to as “High Tidal Volume”, our HVT protocol is both sufficient to cause ventilator induced injury and mortality in our older subjects and is closer to clinical relevance than other higher HVT standards.
Each of our experimental measures demonstrates a significant pattern of age-associated increase in inflammation and injury. Our second aim was to determine whether administration of a conservative fluid management strategy alone would attenuate these effects. While fluid management has long been an important issue in clinical medicine; the role of fluid management on the development and outcome of VILI had yet to be quantitatively studied in-vivo. The addition of conservative fluid support to our HVT ventilation protocol attenuated VILI and significantly decreased the mortality rate in older subjects.
Patient mortality is the gravest complication of mechanical ventilation. In our study neither advanced age nor HVT ventilation alone significantly increased subject mortality. Only with the combination of advanced age and HVT did our study yield a profound decrease in our subjects’ survival (Fig. 1). Considering the epidemiology of VILI the experimental validation of the age associated increase in ventilator mortality is already of paramount importance. Potentially even more meaningful however was that we were able to completely attenuate the age associated increase in our subject’s HVT mortality with the administration of a low fluid protocol. Pulmonary edema, pulmonary mechanics, lung histology, and BAL fluid cytology data all give a strong testimony that the resolution of VILI induced edema itself is a primary mechanism through which this low fluid protocol is able to enact such a dramatically protective effect.
Pulmonary edema is a hallmark of VILI and the severity and susceptibility to pulmonary edema increases with age (25)(5)(21)(41). Some of the driving forces behind development and progression of pulmonary edema both in general and in VILI are increases in pulmonary intravascular pressure, decreased epithelial barrier integrity/increased permeability, and increased local and systemic inflammatory cytokine presence. Since conservative fluid strategies decease intravascular pressure, it is not surprising that a body of data has emerged linking them to decreased pulmonary edema and increased survival rates both in animal and human studies of ARDS (22)(25)(36). However, liberal fluid management strategies are not wholly without merit. Liberal fluid resuscitation is known to have positive effects on blood pressure and other hemodynamic factors in human studies (25).
The role of aquaporin channels in pulmonary fluid clearance and how they my specifically interact with VILI, aging, and edema is complicated at the very least. Zhongguo et al showed that aquaporin channel 1 and 5 (AQP1& AQP5) expression decreases in rats with hyperoxia induced lung injury and correlates with increases in pulmonary edema. And although low tidal volume mechanical ventilation was not found to increase alveolar barrier permeability or pulmonary edema in rats, it was shown to increase AQP1 and AQP5 expression. Age is known to be a predictive factor in the incidence and severity of pulmonary edema (40)(24). Zhang et al showed that aging lungs exhibit decreased aquaporin channel expression (42). Additionally it is known that age associated changes in lung physiology increase susceptibility to and severity of both VILI and pulmonary edema independently during mechanical ventilation. In the present study, we hypothesized that the negative synergy of the age associated increases allows them to be powerful regulators of the age-associated increases in ventilator mortality rates observed both in animal models and in older patients who are mechanically ventilated.
In our 1hr ventilations we found significant increases in lung wet to dry ratios associated with both advanced age and liberal fluid support. These results support the general finding that age increases the incidence and severity of edema (5)(33). They also demonstrate our experimental model’s ability to induce ventilator-associated pulmonary edema with HVT mechanical ventilation and subsequently attenuate it through conservative fluid administration alone.
Increased lavage protein concentration is a general marker of lung injury and of enhanced pulmonary permeability and edema (35). In our 4hr subjects, lavage protein concentration was significantly increased by HVT ventilation. Importantly, old subjects on low fluid protocol exhibited near complete attenuation of the high protein containing BAL fluid produced by HVT ventilation. As with our 1hr ventilation results, these data establish our ability to use HVT mechanical ventilation and conservative fluid management both induce and ablate pulmonary edema respectively.
Pulmonary disease changes the physiology of the lungs, which manifests as changes in respiratory mechanics. Therefore, measurement of respiratory mechanics allows a clinician to monitor closely the course of pulmonary disease(13). The area of the pressure volume loop (i.e., the hysteresis) is the geometric representation of the energy lost during each breath cycle. Increased hysteresis is indicative of compromised lung mechanics function and is a hallmark of aging and various lung pathologies (16)(11). The one hour PV-Loop data demonstrated that conservative fluid support in the old HVT subjects attenuated age-related increase in MV induced pulmonary mechanical disruption. The success of these conservative fluid strategies in decreasing the severity of pulmonary edema and improving pulmonary mechanics in our 1Hr HVT subjects precipitated us to confidently apply our conservative fluid protocols to a group of old subjects being mechanically ventilated for 4hrs in hopes of increasing their survival rate. Predictably the four hour PV-Loop data showed an even more significant pattern of disruption and resolution of pulmonary mechanics with the administration of liberal and conservative fluid protocols respectively in our old murine subjects. In young subjects we saw a similar pattern but none of hysteresis differences reached statistical significance.
In addition to their hysteresis values, the PV loops also contain information in the shape of their ascending limb. In figure 3, we see that while the shapes of the PV loops of young LVT and HVT and old LVT subjects vary only minimally over time, the old HVT loops splay strongly to the left. This represents an increasing rise in pulmonary compliance over time. Increased lung compliance is a hallmark of the age-associated decline in lung mechanics and of many pathological conditions (19)(31)(3). TLC is not considered to significantly change with age (34). It is generally known that this effect results from the almost perfect balance between the age-associated increase in lung compliance, the decrease in respiratory muscle tone, and the increase in chest wall stiffness (7). However in our experiments, the Flexivent system calculates TLC based upon the volume of air required to inflate the lung to a specified pressure. Therefore, the age-associated loss of muscle tone does not affect the TLC measurements. Consequently, we expect the loss of lung compliance to dominate these measurements and expect to see TLC measurements taken with the Flexivent system to increase with age. Furthermore, certain types of lung injury (e.g., emphysema) increase lung compliance, and thus, they further increase total lung capacity as measured by our Flexivent system (27).
Both age and injury related increases in these pulmonary mechanical measures were observed in our study. The significantly increased 0hr lung compliance and total lung capacity in old mice demonstrates the predicted age-associated changes in pulmonary physiology and mechanics. The strong trend toward increased mean values of both lung compliance and TLC observed in HVT vs LVT ventilator protocols seen in our older subjects therefore notable as well. Even more striking is that both lung compliance and TLC values are significantly decreased in older HVT subjects when treated with conservative fluid protocols. These findings demonstrate that HVT ventilation promotes greater injury compared to LVT ventilation in old animal subjects, and that this injury can be attenuated with a low fluid protocol alone.
Collectively these mechanics data paint a picture of an aging lung whose mechanical dysfunction is a downstream effect of the loss of elasticity and subsequent increase in compliance. This is in agreement with the known age-associated decrease in elastin fibers of the lung parenchyma(17). However, it must be noted that in general having increased pulmonary edema classically decreases lung compliance. This would seem to be in opposition to the pattern we observed of increasing lung compliance with injury. However, VILI induced changes in the lung parenchyma do operate in the direction of increasing lung compliances. In our histological analysis, we found a qualitative decrease in the presence and quality of elastin in older mice (Supplemental Figure).
We further investigated our model’s effect on lung parenchyma destruction through measurement of ventilation-induced alveolar airspace enlargement. Increased airspace enlargement values are associated with both increased age and pathophysiological conditions such as emphysema (23) and VILI (20). In our case, the differences we observed in the airspace enlargement values for the experimental groups represent the extent of the alveolar barrier injury/destruction resulting from mechanical ventilation. Just as with our mechanics, edema, and cytology data, we expected to observe a group-wise difference in the airspace enlargement values across age, with older mice exhibiting greater airspace enlargement values than younger mice. Further, we expected to see significant increases in airspace enlargement values of HVT subjects over non-ventilated controls and LVT subjects. Our data not only showed both of these patterns, but additionally there was a significant interaction effect of increased airspace enlargement when advanced age combined with HVT ventilation. These pro-pulmonary compliance changes in the lung parenchyma work in opposition to the anti-compliant mechanics classically observed in an edematous lung. The fact we seen an increasing compliance in our most injured subjects despite their increasingly edematous lungs suggests that the mechanical effect of the injury/destruction of the parenchyma has a more profound impact on the overall lung mechanics than the edema.
Our cytological analysis of BAL fluid demonstrates a pattern of inflammatory cell recruitment that further validates our experimental model of VILI in aging mice. Recruitment of lymphocytes, neutrophils, basophils, and eosinophils to the site of an injury is part of the normal immuno-inflammatory and wound healing response (15)(44). Literature, experimental data, and our research lead us to conclude that age-related increases in the severity of VILI should amplify this damage-associated pattern both systemically and in the microenvironment of the lung (33)(28)(9). However, several studies have found little or no differences in BAL fluid cell count ratios between young and old healthy mice (10)(36)(26). For this reason, we expected that we would only see significant differences in differential cell counts between ventilation control and HVT subjects and that furthermore; this pattern would be more exaggerated in the old vs. the young subjects.
As expected, our cytological analysis failed to yield significant differences between young and old control groups. The only significant differences we observed were between old subjects ventilated with HVT and young and old control groups. These cytology data support our hypothesis by demonstrating the synergistic effect of advanced age and HVT ventilation on lung inflammation and injury. This age-associated increase in ventilator –induced pulmonary “biotrauma” is decidedly “mid-stream” in the VILI process. Alveolar epithelial cell barrier function is known to be disrupted by inflammatory cytokines. Increased pro-inflammatory cytokine levels in the lung promote disruption of the lung’s barrier function thus resulting in enhanced permeability and development of pulmonary edema.
Thus, the work reported here demonstrates that a low fluid support strategy alone can reverse the age-associated increases in the 4hr hour mortality rate of older subjects ventilated with HVT. The significantly important finding arising from this work is that development of pulmonary edema has both deleterious up and down stream impacts on the development of VILI. Further, we have identified the crucial role conservative fluid administration has in diminishing lung edema and the severity of VILI.
In conclusion, our findings suggest that the age-associated increase in VILI-induced pulmonary edema is not simply a downstream marker of injury but also a mechanism for further injury and increased mortality. And while already known that conservative fluid support strategies give favorable outcomes to patients with ARDS, this is the first evidence that these strategies also attenuate VILI and its age-associated increase in severity. Considering the relative lack of preventative treatments for VILI and the age-associated increases in injury and death, the development of age-dependent fluid support strategies may be of critical importance in increasing the survival rate of elderly ventilator patients.
Limitations
The volume parameter of mechanical ventilations is generally determined by the application of a ratio between tidal volume and patient weight. For this purpose it is often the preferred clinical practice to use a calculation of the patient’s ideal body weight instead of their actual weight as to not over or under ventilate under or overweight patients. This is of particular importance in our study as 20 month old mice are reliably ~30% more massive than their younger counterparts. If not accounted for, this would result in the lungs of the older subjects being systematically inflated to larger volumes based on their size than those of their younger counterparts. However it has also been noted that while the lungs of the older mice may not be larger than those of the young adult mice, the increased mass of the older mice does correlate with an increased circulatory volume. Therefore while the weight-adjusted tidal volume may provide a lung volume normalized tidal volume, the older mice are receiving a lower tidal volume relative to their circulatory volume. This is of course not different from how overweight human patients may be ventilated but is still a potentially important limitation to consider.
Supplementary Material
4Hr mechanical ventilation causes lung injury and death in elderly mice.
The effect is strongly blunted in young subjects or by using a low tidal volume.
Pulmonary edema was hypothesized as an upstream mechanism of this mortality.
A novel conservative fluid protocol was proposed to attenuate these effects.
Conservative fluid support significantly decreased edema and mortality in old mice.
Acknowledgments
The authors would like to thank Dr. Ramesh Natarajan for critically reading this manuscript. The authors would also like to thank Dr. David Edwards, Associate Professor of Statistics, at Virginia Commonwealth University, for his consultation on statistical analysis of this study.
Grants
Financial Support for this study: Research reported in this publication was supported by the National Institute On Aging of the National Institutes of Health under Award Number R01AG041823. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures
No conflicts of interest, financial or otherwise are declared by the authors.
Authors’ Contributions
JH designed the experimental set-up, performed experiments, analyzed the results and drafted the manuscript. MV performed experiments and analyzed results. NS helped perform experiments. MS helped perform analysis. RP helped design the experimental set-up. AF helped design study and revised the manuscript. AR helped design study and revise manuscript. RH designed the study, helped analyze the data, and revised the manuscript. All authors read and approved of the final manuscript.
References
- 1.Andrade PV, dos Santos JM, Silva HCA, Wilbert DD, Cavassani SS, Oliveira-Júnior IS. Influence of Hyperoxia and Mechanical Ventilation in Lung Inflammation and Diaphragm Function in Aged Versus Adult Rats. Inflammation. 2013;37:486–494. doi: 10.1007/s10753-013-9762-4. [DOI] [PubMed] [Google Scholar]
- 2.Baudouin S. Ventilator induced lung injury and infection in the critically ill. Thorax. 2001;56:ii50–ii57. [PMC free article] [PubMed] [Google Scholar]
- 3.Biehl M, Kashiouris MG, Gajic O. Ventilator-induced lung injury: minimizing its impact in patients with or at risk for ARDS. Respir Care. 2013;58:927–937. doi: 10.4187/respcare.02347. [DOI] [PubMed] [Google Scholar]
- 4.Caironi P, Langer T, Carlesso E, Protti A, Gattinoni L. Time to generate ventilator-induced lung injury among mammals with healthy lungs: a unifying hypothesis. Intensive Care Med. 2011;37:1913–1920. doi: 10.1007/s00134-011-2388-9. [DOI] [PubMed] [Google Scholar]
- 5.Chamorro-Marín V, García-Delgado M, Touma-Fernández A, Aguilar-Alonso E, Fernández-Mondejar E. Intratracheal dopamine attenuates pulmonary edema and improves survival after ventilator-induced lung injury in rats. Crit Care Lond Engl. 2008;12:R39. doi: 10.1186/cc6829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chioncel O, Ambrosy AP, Bubenek S, Filipescu D, Vinereanu D, Petris A, Christodorescu R, Macarie C, Gheorghiade M, Collins SP the Romanian Acute Heart Failure Syndromes study investigators. Epidemiology, pathophysiology, and in-hospital management of pulmonary edema: data from the Romanian Acute Heart Failure Syndromes registry. J Cardiovasc Med Hagerstown Md. 2014 Sep 23; doi: 10.2459/JCM.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 7.Cotes JE, Cotes JE. Lung function: physiology, measurement and application in medicine. Malden, Mass.; Oxford: Blackwell Pub; 2006. [Google Scholar]
- 8.Crane SD. Epidemiology, treatment and outcome of acidotic, acute, cardiogenic pulmonary oedema presenting to an emergency department. Eur J Emerg Med Off J Eur Soc Emerg Med. 2002;9:320–324. doi: 10.1097/00063110-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elder AC, Finkelstein J, Johnston C, Gelein R, Oberdörster G. Induction of adaptation to inhaled lipopolysaccharide in young and old rats and mice. Inhal Toxicol. 2000;12:225–243. doi: 10.1080/08958370050165085. [DOI] [PubMed] [Google Scholar]
- 11.Fahlman A, Loring SH, Johnson SP, Haulena M, Trites AW, Fravel VA, Van Bonn WG. Inflation and deflation pressure-volume loops in anesthetized pinnipeds confirms compliant chest and lungs. Front Physiol. 2014;5 doi: 10.3389/fphys.2014.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gattinoni L, Protti A, Caironi P, Carlesso E. Ventilator-induced lung injury: the anatomical and physiological framework. Crit Care Med. 2010;38:S539–548. doi: 10.1097/CCM.0b013e3181f1fcf7. [DOI] [PubMed] [Google Scholar]
- 13.Grinnan DC, Truwit JD. Clinical review: Respiratory mechanics in spontaneous and assisted ventilation. Crit Care. 2005;9:472–484. doi: 10.1186/cc3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halbertsma FJJ, Vaneker M, Scheffer GJ, van der Hoeven JG. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med. 2005;63:382–392. [PubMed] [Google Scholar]
- 15.Hammerschmidt S, Kuhn H, Sack U, Schlenska A, Gessner C, Gillissen A, Wirtz H. Mechanical stretch alters alveolar type II cell mediator release toward a proinflammatory pattern. Am J Respir Cell Mol Biol. 2005;33:203–210. doi: 10.1165/rcmb.2005-0067OC. [DOI] [PubMed] [Google Scholar]
- 16.Harris RS. Pressure-volume curves of the respiratory system. Respir Care. 2005;50:78–98. discussion 98–99. [PubMed] [Google Scholar]
- 17.Huang K, Rabold R, Schofield B, Mitzner W, Tankersley CG. Age-dependent changes of airway and lung parenchyma in C57BL/6J mice. J Appl Physiol. 2007;102:200–206. doi: 10.1152/japplphysiol.00400.2006. [DOI] [PubMed] [Google Scholar]
- 18.Jacob RE, Carson JP, Gideon KM, Amidan BG, Smith CL, Lee KM. Comparison of two quantitative methods of discerning airspace enlargement in smoke-exposed mice. PloS One. 2009;4:e6670. doi: 10.1371/journal.pone.0006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 13:197. doi: 10.1034/j.1399-3003.1999.13a36.x. 9901. [DOI] [PubMed] [Google Scholar]
- 20.Loring SH, Pecchiari M, Della Valle P, Monaco A, Gentile G, D’Angelo E. Maintaining end-expiratory transpulmonary pressure prevents worsening of ventilator-induced lung injury caused by chest wall constriction in surfactant-depleted rats. Crit Care Med. 2010;38:2358–2364. doi: 10.1097/CCM.0b013e3181fa02b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthay MA. Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med. 2014;189:1301–1308. doi: 10.1164/rccm.201403-0535OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semler Matthew W, Wheeler Art P, Bernard Gordon R, Thompson BT, Rice Todd W. C95. WHAT’S NEW INACUTE RESPIRATORY DISTRESS SYNDROME. American Thoracic Society; Conservative Fluid Management Decreases Mortality In Acute Respiratory Distress Syndrome Patients With Low Central Venous Pressure [Online] pp. A5094–A5094. http://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2014.189.1_MeetingAbstracts.A5094 [1 Jul. 2015] [Google Scholar]
- 23.Muñoz-Barrutia A, Ceresa M, Artaechevarria X, Montuenga LM, Ortiz-de-Solorzano C. Quantification of lung damage in an elastase-induced mouse model of emphysema. Int J Biomed Imaging. 2012;2012:734734. doi: 10.1155/2012/734734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray JF. Pulmonary edema: pathophysiology and diagnosis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2011;15:155–160. i. [PubMed] [Google Scholar]
- 25.National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, de Boisblanc B, Connors AF, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 26.Olsen HH, Grunewald J, Tornling G, Sköld CM, Eklund A. Bronchoalveolar lavage results are independent of season, age, gender and collection site. PloS One. 2012;7:e43644. doi: 10.1371/journal.pone.0043644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papandrinopoulou D, Tzouda V, Tsoukalas G. Lung compliance and chronic obstructive pulmonary disease. Pulm Med. 2012;2012:542769. doi: 10.1155/2012/542769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxson JA, Gruntman A, Parkin CD, Mazan MR, Davis A, Ingenito EP, Hoffman AM. Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PloS One. 2011;6:e23232. doi: 10.1371/journal.pone.0023232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pedreira PR, García-Prieto E, Parra D, Astudillo A, Diaz E, Taboada F, Albaiceta GM. Effects of melatonin in an experimental model of ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2008;295:L820–827. doi: 10.1152/ajplung.90211.2008. [DOI] [PubMed] [Google Scholar]
- 30.Plötz FB, Slutsky AS, van Vught AJ, Heijnen CJ. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med. 2004;30:1865–1872. doi: 10.1007/s00134-004-2363-9. [DOI] [PubMed] [Google Scholar]
- 31.De Prost N, Ricard J-D, Saumon G, Dreyfuss D. Ventilator-induced lung injury: historical perspectives and clinical implications. Ann Intensive Care. 2011;1:1–15. doi: 10.1186/2110-5820-1-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 33.Setzer F, Oschatz K, Hueter L, Schmidt B, Schwarzkopf K, Schreiber T. Susceptibility to ventilator induced lung injury is increased in senescent rats. Crit Care. 2013;17:R99. doi: 10.1186/cc12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma G, Goodwin J. Effect of aging on respiratory system physiology and immunology. Clin Interv Aging. 2006;1:253–260. doi: 10.2147/ciia.2006.1.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsukimoto K, Yoshimura N, Ichioka M, Tojo N, Miyazato I, Marumo F, Mathieu-Costello O, West JB. Protein, cell, and LTB4 concentrations of lung edema fluid produced by high capillary pressures in rabbit. J Appl Physiol Bethesda Md 1985. 1994;76:321–327. doi: 10.1152/jappl.1994.76.1.321. [DOI] [PubMed] [Google Scholar]
- 36.Valentine SL, Sapru A, Higgerson RA, Spinella PC, Flori HR, Graham DA, Brett M, Convery M, Christie LM, Karamessinis L, Randolph AG Pediatric Acute Lung Injury, Sepsis Investigator’s (PALISI) Network, Acute Respiratory Distress Syndrome Clinical Research Network (ARDSNet) Fluid balance in critically ill children with acute lung injury. Crit Care Med. 2012;40:2883–2889. doi: 10.1097/CCM.0b013e31825bc54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clarke WPC, Coull B, Koutrakis P, Krishna Murthy GG, Rice T, Godleski JJR. Age-related responses in rats to concentrated urban air particles. Inhal Toxicol. 2000;12:73–84. [Google Scholar]
- 38.Wilson MR, Patel BV, Takata M. Ventilation with “clinically-relevant” high tidal volumes does not promote stretch-induced injury in the lungs of healthy mice. Crit Care Med. 2012;40:2850–2857. doi: 10.1097/CCM.0b013e31825b91ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolthuis EK, Vlaar APJ, Choi G, Roelofs JJTH, Juffermans NP, Schultz MJ. Mechanical ventilation using non-injurious ventilation settings causes lung injury in the absence of pre-existing lung injury in healthy mice. Crit Care Lond Engl. 2009;13:R1. doi: 10.1186/cc7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wunsch H, Linde-Zwirble WT, Angus DC, Hartman ME, Milbrandt EB, Kahn JM. The epidemiology of mechanical ventilation use in the United States. Crit Care Med. 2010;38:1947–1953. doi: 10.1097/CCM.0b013e3181ef4460. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Kharge AB, Perlman CE. Lung ventilation injures areas with discrete alveolar flooding, in a surface tension-dependent fashion. J Appl Physiol Bethesda Md 1985. 2014;117:788–796. doi: 10.1152/japplphysiol.00569.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y-W, Bi L-T, Hou S-P, Zhao X-L, Song Y-L, Ma T-H. Reduced lung water transport rate associated with downregulation of aquaporin-1 and aquaporin-5 in aged mice. Clin Exp Pharmacol Physiol. 2009;36:734–738. doi: 10.1111/j.1440-1681.2009.05156.x. [DOI] [PubMed] [Google Scholar]
- 43.Zuurbier CJ, Emons VM, Ince C. Hemodynamics of anesthetized ventilated mouse models: aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol. 2002;282:H2099–2105. doi: 10.1152/ajpheart.01002.2001. [DOI] [PubMed] [Google Scholar]
- 44.Interstitial lung disease. 5. Shelton, Conn: People’s Medical Pub. House; 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.