Abstract
Heparin/heparin sulfate (HS) interacts with a number of proteins thereby playing an essential role in the regulation of many physiological processes. The understanding of heparin/HS-protein interactions at the molecular level is of fundamental importance to biology and will aid in the development of highly specific glycan-based therapeutic agents. The heparin-binding proteins (HBPs) interact with sulfated domains of heparin/HS chains primarily through ionic attraction between negatively charged groups in HS/heparin chains and basic amino acid residues within the protein. Reports in literature have been shown that heparin molecules have a high affinity for a wide range of metal ions. In the present study, we used surface plasmon resonance (SPR) to study the effects of metal ions (under physiological and non-physiological concentrations) on heparin/HS-protein interactions. The results showed that under non-physiological of metal ion concentration, different metal ions showed different effects on heparin binding to fibroblast growth factor-1 (FGF1) and interleakin-7 (IL7). While the effects of individual metal ion at physiological concentrations had little impact on protein binding, the mixed metal ions reduced the FGF1/heparin or IL7/heparin binding affinity, changing its binding profile.
Keywords: Heparin, Potein, Metalion, Interaction, Surface Plasmon Resonance
4. Introduction
Heparin/heparan sulfate (HS) glycosaminoglycans (GAGs) are anionic and often highly sulfated, poly disperse linear polysaccharides. GAGs are ubiquitous molecules exhibiting a wide range of biological functions by interaction with various growth and differentiation factors and morphogens, extracellular matrix components, protease inhibitors, protease, lipoprotein lipase, and various pathogens [1–4]. Interactions between heparin/HS and proteins mediate diverse patho-physiological processes including: blood coagulation, cell growth and differentiation, host defense and viral infection, lipid transport and metabolism, cell-to-cell and cell-to-matrix signaling, inflammation, angiogenesis and cancer [4–6]. Thus, an understanding of heparin/HS-protein interactions at the molecular level is of fundamental importance to biology and should aid in the development of highly specific glycan-based therapeutic agents [3,5].
Metals play crucial roles in biological processes which are involved in cellular and sub cellular functions [7]. For instance, the divalent magnesium and calcium ions play important regulatory roles in cells. Lack of body iron is common in cancer patients and it is associated with complications in surgery and in animal experiments. Metal ions play essential roles in about one third of enzyme interactions [8]. These ions can modify electron flow in a substrate or enzyme, thus effectively controlling an enzyme-catalyzed reaction. They can serve to bind and orient substrate with respect to functional groups in the active site of the enzyme [9]. Copper is recognized as an essential metalloelement and is primarily associated with copper-dependent cellular enzymes. Metal ions function in numerous metalloenzymes, are incorporated into pharmaceuticals and used as inorganic drugs for many diseases [7,10].
The heparin-binding proteins (HBPs) interact with sulfated domains of HS/heparin chains by ionic attraction between negatively charged groups in HS/heparin chains and basic amino acid residues in the protein. Previous study has shown that heparin molecules have a high affinity for a wide range of metal ions [11–19], which suggests the presence of metals may play a significant role in heparin/HS-protein interactions. For example, divalent cations play an important role in regulating the anti-Factor Xa activity of heparin [20]. It has also reported that divalent cations and heparin/heparan sulfate cooperate to control assembly and activity of the fibroblast growth factor [21]. Unfortunately, the effects of metal ions on protein-heparin/HS complexes and their biological activities are largely unknown. Thus, we undertook this study to evaluate the impact of metal ions protein-heparin/HS interaction. The present study uses surface plasmon resonance (SPR) spectroscopy to evaluate the effect of common metal ions on heparin/HS interactions with fibroblast growth factor-1 (FGF1) and interleukin-7 (IL7).
5. Materials and Methods
5.1 Materials
Porcine intestinal heparin (16 kDa) and porcine intestinal heparan sulfate (12 kDa) were obtained from Celsus Laboratories (Cincinnati, OH). Sensor SA chips were from GE Healthcare (Uppsala, Sweden). Fibroblast growth factor 1 (FGF1) was a gift from Amgen (Thousands Oaks, CA). Human interleukin 7 (IL7) was provided by Dr. Walsh (Center for Advanced Research in Biotechnology, University of Maryland Biotechnology Institute). SPR measurements were performed on a BIAcore 3000 (GE Healthcare, Uppsala, Sweden) operated using BIAcore 3000 control and BIAevaluation software (version 4.0.1).
5.2 Preparation of heparin biochip
Biotinylated heparin or HS was prepared by reaction of sulfo-N-hydroxysuccinimide long-chain biotin (Piece, Rockford, IL) with free amino groups of unsubstituted glucosamine residues in the polysaccharide chain following a published procedure [22]. Biotinylated heparin was immobilized to streptavidin chip based on the manufacturer’s protocol. In brief, 20-μl solution of the heparin-biotin conjugate (0.1 mg/ml) in HBS-EP running buffer was injected over flow cell 2 of the streptavidin chip at a flow rate of 10μl/min. The successful immobilization of heparin was confirmed by the observation of a ~250 resonance unit (RU) increase in the sensor chip. The control flow cell was prepared by 1 min injection with saturated biotin.
5.3 Measurement of the effects of metal ions on the interaction between heparin/HS and protein (FGF1 or IL-7) Using SPR
The protein samples were diluted in HBS-P buffer (0.01 M HEPES, 0.15 M NaCl, 0.005% surfactant P20, pH 7.4). Different dilutions of protein samples with or without addition of metal ions were injected at a flow rate of 30 μl/min. At the end of the sample injection, the same buffer was flowed over the sensor surface to facilitate dissociation. After a 3 min dissociation time, the sensor surface was regenerated by injecting 30 μl of 2 M NaCl to obtain a regenerated surface. The response was monitored as a function of time (sensorgram) at 25 °C.
The additions of metal ions to the heparin/HS and protein binding measurement were in three categories: 1) addition of CaCl2, ZnCl2, FeCl3, MgCl2, and KCl in concentration of 0, 10, 100 and 1000 μM, respectively; 2) addition of metal ions in physiological lower/upper limit concentrations (Table 1); 3) the addition of mixed metals ions with Mg2+ (50μM), Zn2+(15μM), Fe3+ (20μM), K+(2000μM), Ca2+(1150μM ), and Cu2+ (15μM)in physiological concentrations.
Table 1.
Physiological metal ions concentration reference ranges for blood tests.
| Lower limit conc. | Upper limit conc. | |
|---|---|---|
| Mg2+ | 10 μM | 100 μM |
| Zn2+ | 10 μM | 20 μM |
| Fe3+ | 10 μM | 35 μM |
| K+ | 1000 μM | 3500 μM |
| Ca2+ | 1000 μM | 1300 μM |
| Cu2+ | 10 μM | 24 μM |
6. Results and Discussion
6.1 The effects of metal ions on heparin/HS-protein interactions in non-physiological concentrations
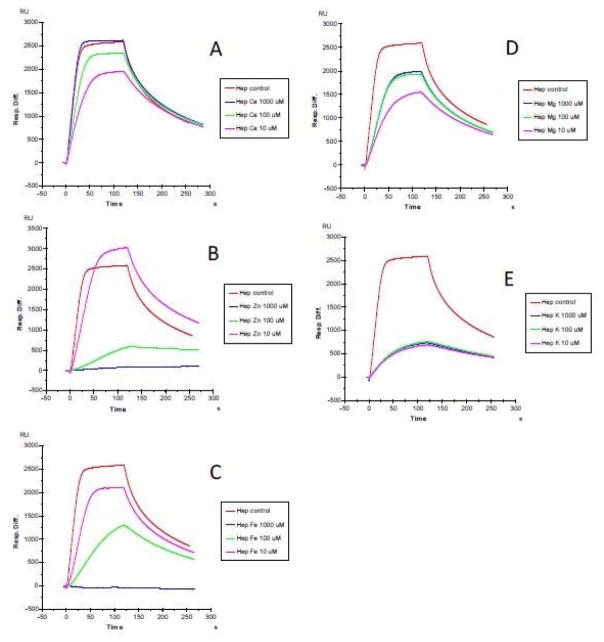
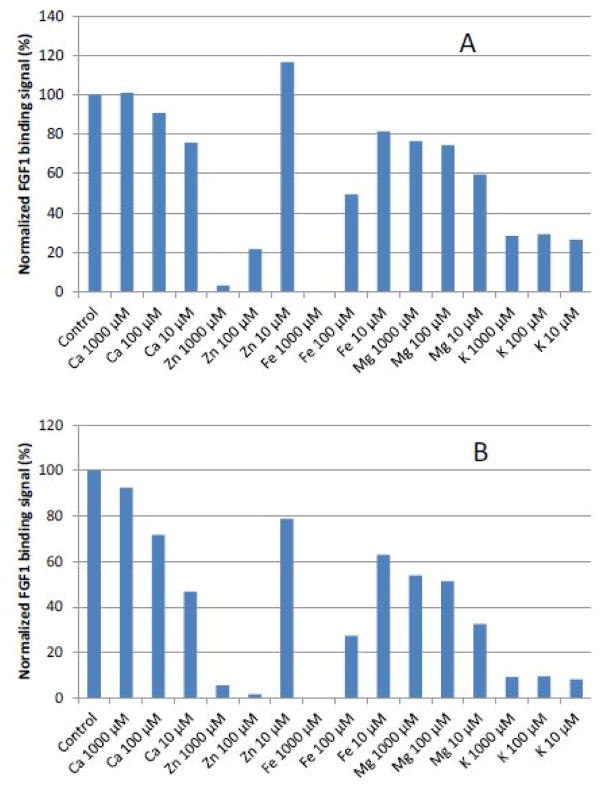
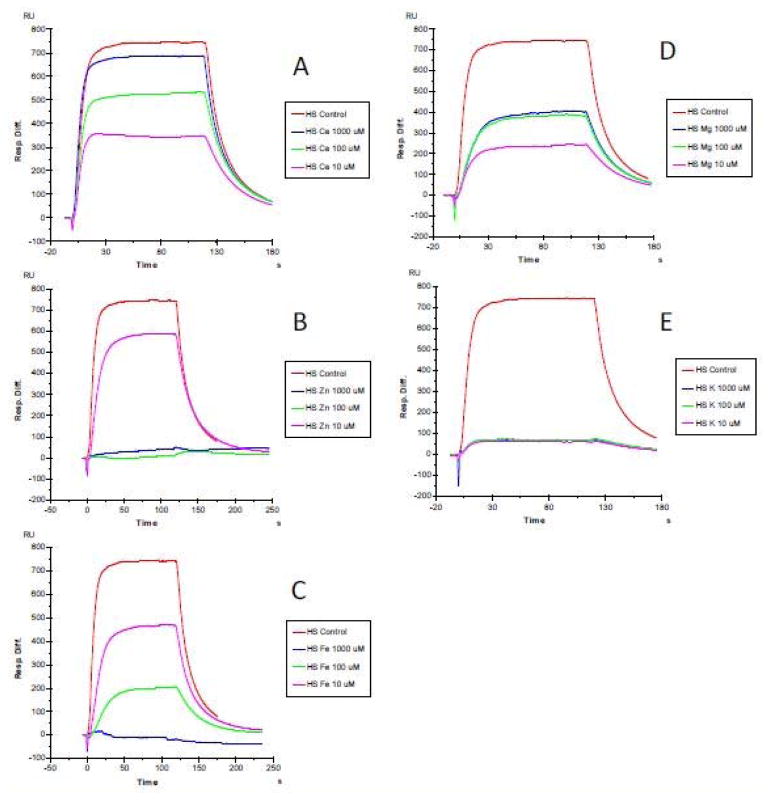
The first set of SPR measurements on heparin/HS-protein interaction were conducted through the addition of CaCl2, ZnCl2, FeCl3, MgCl2, and KCl at concentration of 0, 10, 100 and 1000 μM, respectively. FGF1 which is well known as a heparin-binding protein (HBP), was used in this initial experiment. The results (Figures 1 to 3) showed that at non-physiological concentrations, different metal ions showed different effects on the heparin/HS-protein binding. The metal ions showed a greater effect on the HS-FGF1 interaction than on the heparin-FGF1 interaction and most of the effects of most of the metal ions were concentration dependent. FGF1 binding to heparin/HS was reduced with addition of Ca2+ or Mg2+ at 10 μM and the effects were decreased at 100 and 1000μM concentrations of Ca2+ or Mg2+. FGF1 binding to heparin/HS was unaffected with addition of 10 μM Zn2+ but binding was dramatically reduced at Zn2+concentrations of 100 and 1000 μM. FGF1 binding to heparin/HS was reduced at 10 μM Fe3+ and further decreased at 100μM Fe3+ and no binding was detected at 1000 μM Fe3+. FGF1 to heparin/HS was greatly reduced at all concentrations of K+, ranging from 10 to 1000 μM. Some studies have reported that physiological metal ions such as sodium, calcium, and magnesium bind to heparin based on the polyelectrolyte theory [23–26].
Figure 1.
SPR sensorgrams of heparin-FGF1 interaction with the addition of metals ions. FGF1 concentration was 500 nM. A: heparin-FGF1 interaction with the addition of CaCl2 (0, 10, 100 and 1000 μM); B: heparin-FGF1 interaction with the addition of ZnCl2 (0, 10, 100 and 1000 μM); C: heparin-FGF1 interaction with the addition of FeCl3 (0, 10, 100 and 1000 μM); D: heparin-FGF1 interaction with the addition of MgCl2, (0, 10, 100 and 1000 μM); E: heparin-FGF1 interaction with the addition of KCl (0, 10, 100 and 1000 μM).
Figure 3.
A: Normalized FGF1 binding to heparin in the presence of different concentration of metal ions. B: Normalized FGF1 binding to HS in the presence of different concentration of metal ions.
Using atomic absorption and spectrophotometry, it was reported the overall trend for heparin–metal affinity to be Mn2+>Cu2+>Ca2+>Zn2+>Co2+>Na+>Mg2+>Fe3+>Ni2+>Al3+>Sr2+ [11]. There is evidence that divalent metal ions (Ca2+, Cu2+, and Zn2+) are necessary in many protein-heparin interactions thus influencing the affinity, specificity and stability of these complexes [27–28]. A previous study by our group showed the conformational changes induced by calcium ions are necessary for the interaction between heparin and annexin V [25].
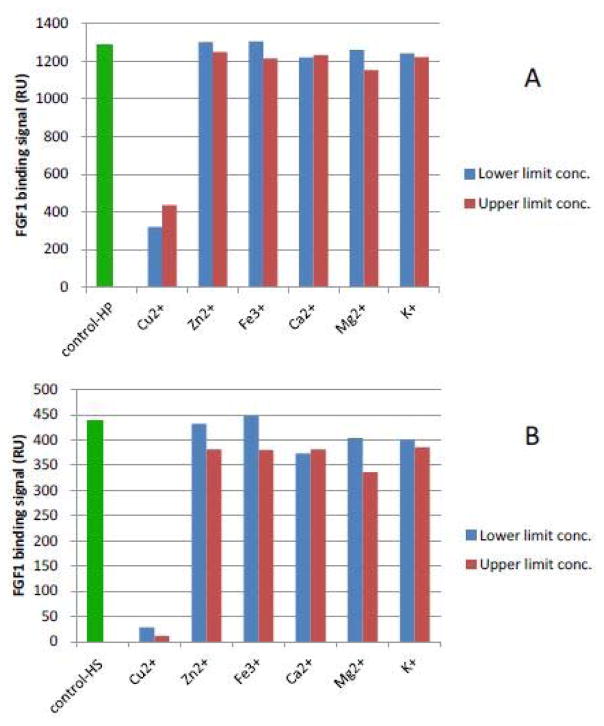
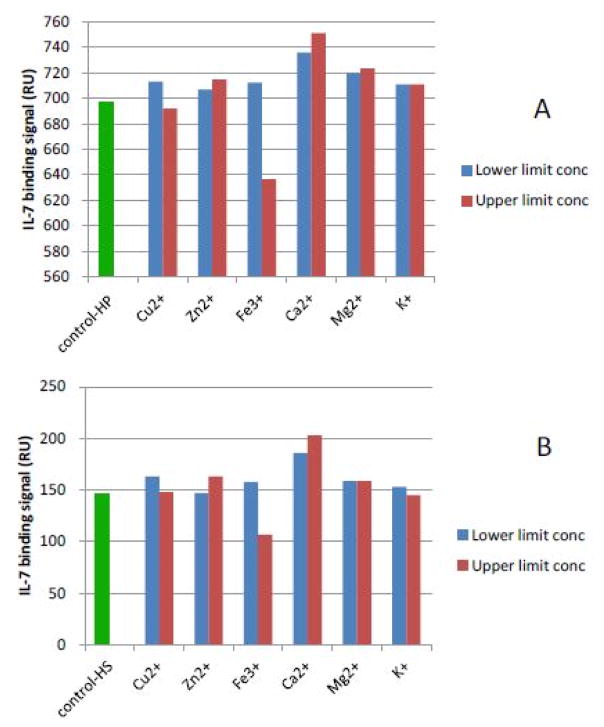
6.2 The effects of metal ions on heparin/HS-protein interactions in physiological concentrations
Next, heparin/HS FGF1 and heparin/HS-IL7 interactions were studied with the addition of metal ions at the physiological lower/upper limit concentrations (Table 1) [29]. The results (Figures 4 and 5) showed effects, of most individual metal ions at physiological lower/upper limit concentrations, on these interactions were minimal. One exception was the effect of Cu2+ on the interaction of heparin/HS with FGF1 (Figure 4). A second exception was the effect of Fe3+, at its upper limit concentration, on the interaction of heparin/HS with IL7 (Figure 5) obviously reduced with the addition of Fe3+ (Figure 5). We previously reported the formation of a Cu2+ - heparin complex gave extremely sensitive detection of heparin, permitting the analysis of as low as 10 ng with capillary electrophoresis [30]. Copper, along with FGF, [31] plays an important role in promoting physiological and malignant angiogenesis, the formation of new blood vessels by a tumor, enabling tumor growth, invasion, and metastasis [22]. It also has been reported that the heparin-copper complex is angiogenicin vivo and stimulates migration of capillary endothelium in vitro [32].
Figure 4.
A: FGF1 binding (RU) to heparin in the presence of physiological lower/upper limit concentrations of metal ions. FGF1 concentration was 500 nM; B: FGF1 binding (RU) to HS in the presence of physiological lower limit/upper limit concentrations of metal ions. FGF1 concentration was 500 nM.
Figure 5.
A: IL7 binding (RU) to heparin in the presence of physiological lower/upper limit concentrations of metal ions. IL7 concentration was 500 nM; B: IL7 binding (RU) to HS in the presence of physiological lower/upper limit concentrations of metal ions. IL7 concentration was 500 nM.
6.3 The effects of mixed metal ions on heparin-protein interactions
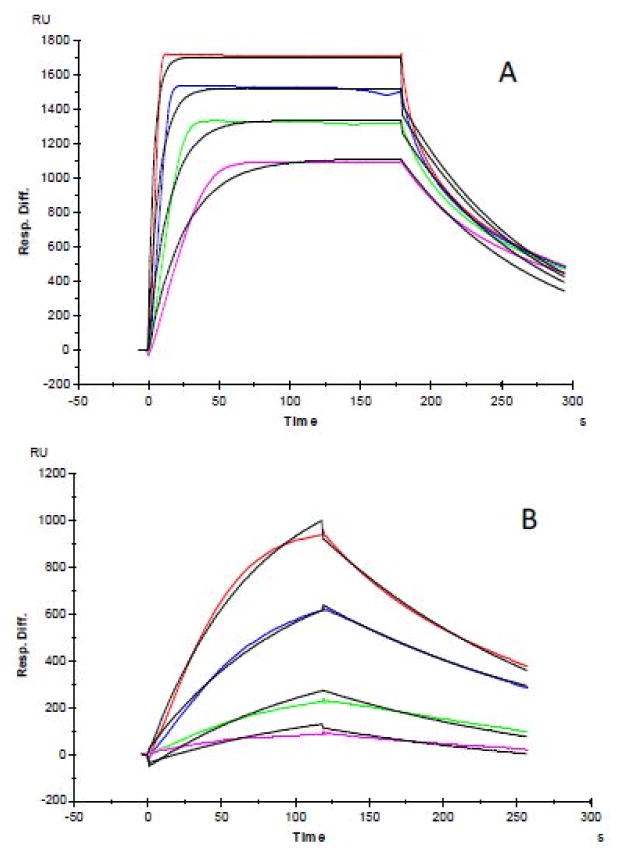
Finally, we measured the effect of mixed metals ions in physiological concentrations, i.e. Mg2+ (50μM), Zn2+(15μM), Fe3+ (20μM), K+(2000μM), Ca2+ (1150μM ), and Cu2+ (15μM), on the heparin-FGF1 interaction. Sensorgrams of heparin and FGF1 interactions are shown in Figure 6. The kinetic parameters (Table 2) of FGF1/heparin interactions were obtained by fitting the sensorgrams with a Langmuir 1:1 binding model. The SPR data showed different FGF1/heparin binding profiles (Figure 6A, and B) with and without the added mixed metals ions. Without the addition of mixed metals ions (control), the KD for FGF1/heparin interaction was 22 nM, with the addition of mixed metals ions, the KD for FGF1/heparin interaction was 350 nM. SPR analysis also showed different binding kinetics for FGF1/heparin interactions in the absence and presence of mixed metals ions. Without the added mixed metals ions, FGF1/heparin interaction exhibited aka = 4.5 × 105(1/Ms), and a kd = 0.01 (1/s), while FGF1/heparin interaction exhibited aka = 1.6×104 (1/Ms) and a kd = 5.7 ×10-3 (1/s) the presence of mixed metals ions.
Figure 6.
SPR sensorgrams of heparin-FGF1 interaction with the addition of mixed metals ions in physiological concentrations. A: SPR sensorgrams of heparin-FGF1 interaction without addition of mixed metals ions; Concentrations of FGF1 (from top to bottom): 500, 250, 125 and 63 nM, respectively. The black curves are the fitting curves using models from BIAevaluate 4.0.1.
Table 2.
Summary of kinetic data of FGF1-heparin interactions in present of mixed metal ions.
| Interaction | ka (1/MS) | kd (1/S) | KD(M) |
|---|---|---|---|
| FGF1/Heparin (control) | 4.5 × 105 | 0.01 | 2.2 × 10−8 |
| FGF1/Heparin in present of mixed metal ions | 1.6 × 104 | 5.7 × 10−3 | 3.5 × 10−7 |
In conclusion, the results of this study clearly show different metal ions can have different effects on the heparin/HS-protein binding at non-physiological concentrations. Metal ions in the range of physiological concentrations with few exceptions generally show little impact on heparin/HS-protein interactions. However, mixed metal ions can alter binding affinity in the case of FGF1/heparin or IL7/heparin binding. This study provides useful information for the formulation of heparin/HS-based agent to promote or block biological processes with heparin-protein interactions.
Figure 2.
SPR sensorgrams of HS-FGF1 interaction with the addition of metals ions. FGF1 concentration was 500 nM. A: HS-FGF1 interaction with the addition of CaCl2 (0, 10, 100 and 1000 μM); B: HS-FGF1 interaction with the addition of ZnCl2 (0, 10, 100 and 1000 μM); C: HS-FGF1 interaction with the addition of FeCl3 (0, 10, 100 and 1000 μM); D: HS-FGF1 interaction with the addition of MgCl2, (0, 10, 100 and 1000 μM); E: HS-FGF1 interaction with the addition of KCl (0, 10, 100 and 1000 μM).
Acknowledgments
Funding
This work was supported by grants from the National Institutes of Health in the form of GM-38060 to R.J.L
Abbreviations
- SPR
Surface Plasmon Resonance
- GAG
Glycosaminoglycan
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HS
Heparin Sulfate
- RU
Resonance Unit
- FGF1
Fibroblast Growth Factor 1
- IL7
Human Interleukin 7
References
- 1.Parish CR. The role of heparan sulphate in inflammation. Nat Rev Immunol. 2006;6(9):633–43. doi: 10.1038/nri1918. [DOI] [PubMed] [Google Scholar]
- 2.Powell AK, Yates EA, Fernig DG, Turnbull JE. Interactions of heparin/heparan sulfate with proteins: appraisal of structural factors and experimental approaches. Glycobiology. 2004;14(4):17R–30R. doi: 10.1093/glycob/cwh051. [DOI] [PubMed] [Google Scholar]
- 3.Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglycans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- 4.Qiu H1, Jiang JL, Liu M, Huang X, Ding SJ, Wang L. Quantitative phosphoproteomics analysis reveals broad regulatory role of heparan sulfate on endothelial signaling. Mol Cell Proteomics. 2013;12(8):2160–73. doi: 10.1074/mcp.M112.026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Capila I, Linhardt RJ. Heparin-protein interactions. Angew Chem Int Ed Engl. 2002;41(3):391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 6.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: the sweet side of development. Nat Rev Mol Cell Biol. 2005;6(7):530–41. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 7.Anastassopoulou J, Theophanides T. The Role of Metal Ions in Biological Systems and Medicine. Bioinorganic Chemistry. 459:209–218. [Google Scholar]
- 8.Frausto da, Williams RJP. The Biological Chemistry of the Elements. Clarendon Press; Oxford: 2001. [Google Scholar]
- 9.Hambley TW. Chemistry. Metal-based therapeutics. Science. 2007;318(5855):1392–3. doi: 10.1126/science.1150504. [DOI] [PubMed] [Google Scholar]
- 10.Lippard SJ. The inorganic side of chemical biology. Nat Chem Biol. 2006;2(10):504–7. doi: 10.1038/nchembio1006-504. [DOI] [PubMed] [Google Scholar]
- 11.Stevic I, Parmar N, Paredes N, Berry LR, Chan AK. Binding of heparin to metals. Cell Biochem Biophys. 2011;59(3):171–8. doi: 10.1007/s12013-010-9129-5. [DOI] [PubMed] [Google Scholar]
- 12.Whitfield DM, Choay J, Sarkar B. Heavy metal binding to heparin disaccharides. I: Iduronic acid is the main binding site. Biopolymers. 1992;32(6):585–96. doi: 10.1002/bip.360320603. [DOI] [PubMed] [Google Scholar]
- 13.Hunter GK, Wong KS, Kim JJ. Binding of calcium to glycosaminoglycans: An equilibrium dialysis study. Arch Biochem Biophys. 1988;260(1):161–7. doi: 10.1016/0003-9861(88)90437-7. [DOI] [PubMed] [Google Scholar]
- 14.Woodhead NE, Long WF, Williamson FB. Binding of zinc ions to heparin. Analysis by equilibrium dialysis suggests the occurrence of two, entropy-driven, processes. Biochem J. 1986;237(1):281–284. doi: 10.1042/bj2370281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grant D, Long WF, Moffat CF, Williamson FB. A study of Ca2+–heparin complex-formation by polarimetry. Biochem J. 1992;282( Pt 2):601–4. doi: 10.1042/bj2820601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu ZC, Perlin AS. A selective, copper-mediated reduction in the anti Xa activity of heparin. Thromb Haemost. 1991;66(6):742. [PubMed] [Google Scholar]
- 17.Mattai J, Kwak JC. Quantitative similarity of zinc and calcium binding to heparin in excess salt solution. Biophys Chem. 1988;31(3):295–9. doi: 10.1016/0301-4622(88)80035-8. [DOI] [PubMed] [Google Scholar]
- 18.Lages B, Stivala SS. Interaction of the polyelectrolyte heparin with copper(II) and calcium. Biopolymers. 1973;12(1):127–43. doi: 10.1002/bip.1973.360120112. [DOI] [PubMed] [Google Scholar]
- 19.Parrish RF, Fair WF. Selective binding of zinc ions to heparin rather than to other glycosaminoglycans. Biochem J. 1981;193(2):407–410. doi: 10.1042/bj1930407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenberg CS, Adams JP, Mullen PE, Koepke JA. The effect of calcium ions on the activated partial thromboplastin time of heparinized plasma. Am J Clin Pathol. 1986;86(4):484–9. doi: 10.1093/ajcp/86.4.484. [DOI] [PubMed] [Google Scholar]
- 21.Kan M, Wang F, To B, Gabriel JL, McKeehan WL. Divalent cations and heparin/heparan sulfate cooperate to control assembly and activity of the fibroblast growth factor receptor complex. J Biol Chem. 1996;271(42):26143–8. doi: 10.1074/jbc.271.42.26143. [DOI] [PubMed] [Google Scholar]
- 22.Goodman VL, Brewer GJ, Merajver SD. Control of copper status for cancer therapy. Curr Cancer Drug Targets. 2005;5(7):543–9. doi: 10.2174/156800905774574066. [DOI] [PubMed] [Google Scholar]
- 23.Rabenstein DL, Robert JM, Peng J. Multinuclear magnetic resonance studies of the interaction of inorganic cations with heparin. Carbohydr Res. 1995;278(2):239–56. doi: 10.1016/0008-6215(95)00263-4. [DOI] [PubMed] [Google Scholar]
- 24.Ricard-Blum S, Féraud O, Lortat-Jacob H, Rencurosi A, Fukai N, Dkhissi F, et al. Characterization of endostatin binding to heparin and heparan sulfate by surface plasmon resonance and molecular modeling: role of divalent cations. J Biol Chem. 2004;279(4):2927–36. doi: 10.1074/jbc.M309868200. [DOI] [PubMed] [Google Scholar]
- 25.Shao C, Zhang F, Kemp MM, Linhardt RJ, Waisman DM, Head JF, et al. Crystallographic analysis of calcium-dependent heparin binding to annexin A2. J Biol Chem. 2006;281(42):31689–95. doi: 10.1074/jbc.M604502200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lages B, Stivala SS. Interaction of Polyelectrolyte Heparin with Copper (II) and Calcium. Biopolymers. 1973;12(1):127–43. doi: 10.1002/bip.1973.360120112. [DOI] [PubMed] [Google Scholar]
- 27.Chevalier F, Lucas R, Angulo J, Martin-Lomas M, Nieto PM. The heparin-Ca(2+) interaction: the influence of the O-sulfation pattern on binding. Carbohydr Res. 2004;339(5):975–83. doi: 10.1016/j.carres.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Srinivasan SR, Radhakrishnamurthy B, Berenson GS. Studies on Interaction of Heparin with Serum-Lipoproteins in Presence of Ca2+, Mg2+, and Mn2+ Arch Biochem Biophys. 1975;170(1):334–40. doi: 10.1016/0003-9861(75)90125-3. [DOI] [PubMed] [Google Scholar]
- 29.Bishop ML, Fody EP, Schoeff LE. Clinical Chemistry: Techniques, principles, correlations. 6. Philadelphia: Lippincott Williams& Wilkin; 2010. [Google Scholar]
- 30.Toida T, Linhardt RJ. Detection of glycosaminoglycans as a copper (II) complex in capillary electrophoresis. Electrophoresis. 1996;17(2):341–6. doi: 10.1002/elps.1150170209. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221(4612):719–25. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 32.Alessandri G, Raju K, Gullino PM. Angiogenesis in vivo and selective mobilization of capillary endothelium in vitro by heparin-copper complex. Microcirc Endothelium Lymphatics. 1984;1(3):329–46. [PubMed] [Google Scholar]