Abstract
Objectives
Menthol, a flavoring agent, is found in approximately 90% of cigarettes, but at much higher levels in menthol than non-menthol cigarettes. Menthol is reportedly included in cigarettes for its cooling and soothing effects, but also additional actions that affect smokers’ receipt and processing of nicotine. In this study we investigated the response to short-term abstinence and acute nicotine delivery in menthol-preferring and non-menthol-preferring smokers.
Methods
Nicotine dependent participants (N = 134) participated in an intravenous nicotine delivery session following overnight smoking abstinence. Participants were intravenously administered a placebo and 2 escalating nicotine doses. We compare subjective and physiological responses to nicotine and smoking urges, withdrawal, and cognitive performance following overnight abstinence and post-nicotine between regular ‘menthol’ smokers and ‘non-menthol’ cigarette smokers.
Results
Relative to non-menthol-preferring smokers, menthol-preferring smokers re a smaller reduction in smoking urges from overnight abstinence baseline to post-nicotine end-of-session and rated less subjective differences between nicotine doses.
Conclusions
Differences between menthol-preferring and non-menthol-preferring smokers’ responses to abstinence or acute nicotine could reflect pre-existing individual differences that may have in initial development of menthol preferences, or could have arisen secondarily to pro use of menthol versus non-menthol cigarettes.
Keywords: menthol, intravenous nicotine, craving, smoking urges, subjective drug effects
Menthol is present in approximately 90% of all cigarettes and approximately 28%–35% of smokers smoke menthol cigarettes that contain sufficient levels for menthol to be considered a characterizing flavor.1,2 The Food and Drug Administration (FDA) has requested information on menthol that could inform their future regulatory decisions including whether menthol, when present at levels considered ‘char, affects the appeal and addiction potential of cigarettes. The Tobacco Control Act (2009)3 charged the Tobacco Products Scientific Advisory Committee (TPSAC) to generate a report on the impact on public health of the menthol contained in cigarettes. After a comprehensive review of peer-reviewed literature, FDA reports, tobacco industry documents and public comments, the report con there was sufficient evidence to support a role for menthol in the appeal and addiction potential of cigarettes. However, it noted substan gaps in the menthol literature including the consequences of menthol smoking on the rates of smoking cessation and sustained quitting, and longitudinal data on the effects of menthol cigarette availability on experimentation, initiation and development of nicotine dependence within adolescents and young adults.4
Because personal cigarette preferences strongly impact participants’ responses to cigarettes, assessing the impact of menthol on nicotine’s effects using standard cigarette smoking laboratory paradigms is problematic. An alternative method is to group individuals as menthol or non-menthol cigarette users and then assess differences in the effects of short-term nicotine abstinence and in acute subjective and physiological responses to nicotine delivered in the absence of associated smoking cues by using intravenous (IV) nicotine infusions. Intravenous nicotine is comparable to smoking in terms of the rapid rate of nicotine delivery and its positive and negative subjective effects, and enables precise dose delivery and assessment of acute effects without associated cigarette cues that carry their own associative learning history.5–9
Nicotine is an addictive component of cigarettes.10 Nicotine contributes to positive subjective effects of smoking that promote continued use and also promotes dependence, in part, by contributing to short-term abstinence-induced aversive effects of nicotine withdrawal. If menthol-preferring and non-menthol-preferring smokers differ in responses to acute nicotine delivery, or aversive short-term abstinence-related symptoms, this could offer insight into how menthol smokers may respond to nicotine-containing products if menthol were to be eliminated from cigarettes.
There are several mechanisms through which menthol preference may have relevance to abstinence-related symptoms or acute responses to nicotine, in the absence of acute menthol co-administration.4,11,12 First, pre-existing differences, including biological differences, between menthol-preferring and non-preferring individuals may affect their response to short-term abstinence or perception of nicotine in a manner that impacted their initial choice preference.13,14 Second, menthol provides a robust sensory experience that influences the experience of nicotine-related sensory cues, thereby producing differential associative-learning to smoking- and nicotine-related cues in long-term menthol and non-menthol smokers. Menthol provides a reinforcing cooling sensation,15 inhibits nicotine-related pain,16 and provides its own ‘impact’ (eg, throat hit) which is qualitatively different (eg, cooler, longer-lasting) than nicotine’s impact, but can partially substitute for the sensory effects of nicotine at low nicotine doses and diminish the aversive impact of high dose nicotine.17–19 Third, menthol also may increase nicotine exposure from cigarettes, as indicated by higher cotinine or CO levels,20–23 by increasing reflexive breath hold times,24 and may slow nicotine clearance.25–28 Finally, emerging evidence indicates that menthol has effects on the same neural receptor systems to which nicotine binds. Preclinical26,29 and human neuroimaging30 studies show functional up-regulation of nicotinic acetylcholine receptors (nAChR) in the brain associated with menthol plus nicotine, relative to nicotine alone, and menthol also may affect desensitization of these receptors.31
Collectively, the existing scientific evidence indicates that menthol- and non-menthol-preferring smokers may differ in ways that affect the initial perception of nicotine. Furthermore, throughout their smoking history, these groups of smokers are exposed to different smoking-related cues and tobacco constituents, including nicotine, and therefore, may undergo differential changes in the neural systems that underlie nicotine’s behavioral and psychological effects. Therefore, these groups may differ in their aversive experience of nicotine abstinence or in acute responses to nicotine, even in the absence of menthol.
The current study addresses these possibilities by comparing menthol- and non-menthol-preferring smokers in their responses to IV nicotine on a range of outcomes including withdrawal severity, cognitive performance, and physiological and self-report drug effects following overnight abstinence. We hypothesized that the menthol-preferring group would show diminished sensitivity to nicotine effects in the absence of menthol-related cues.
METHODS
Participants
Non-treatment-seeking cigarette smokers (N = 134) were recruited from the New Haven, Connecticut area. Preference for menthol (N = 110) or non-menthol (N = 24) was assessed based on self-reported preferred usual brand/type of cigarette. Smoking status was defined as 10–25 cigarettes/day for the past year, Fagerström Test of Nicotine Dependence (FTND32) score ≥ 5, and expired carbon monoxide (CO) >10 parts-per-million (ppm) at initial screening. Participants were aged 18–50, medically healthy as determined by a physician and blood laboratory test battery, did not meet criteria for current Axis I psychiatric disorders according to the Structured Clinical Interview for DSM-IV33 and clinician assessment, including dependence on alcohol or drugs other than nicotine, were not using psychotropic medication, and were not pregnant or breast-feeding. Screening and experimental sessions were held on separate days.
Procedures
We asked participants to abstain from smoking and eating overnight from midnight prior to the 8AM experimental session; therefore, they arrived in a state of short-term abstinence. Participants continued their usual caffeine intake. Drug and pregnancy urine screens confirmed continued eligibility. Expired CO (≤ 8ppm) helped verify overnight abstinence.34
An indwelling catheter with multiple ports was inserted into an antecubital vein for blood sampling saline and nicotine administration. Baseline blood samples were collected for determining plasma nicotine, and cotinine and 3′-hydroxycotinine (3HC) levels, to calculate nicotine metabolite ratio (NMR = 3HC/cotinine).
Withdrawal symptoms (Minnesota Nicotine Withdrawal Scale (MNWS)),35 cigarette/nicotine craving (Brief Questionnaire of Smoking Urges (BQSU)),36 affect (Positive and Negative Affect Schedule (PANAS))37 and a brief computerized cognitive battery were collected at overnight-abstinence baseline (prior to any IV saline or nicotine administration) and end-of-session (following IV delivery of saline and both nicotine doses). The battery consisted of tasks from ANAM (Automated Neuropsychological Assessment Metrics):38,39 Stroop, a measure of cognitive control, including response inhibition; Running Memory - Continuous Performance Task (CPT), a one-back task that assesses sustained attention and working memory; and Mathematical Processing Task (MPT) that taps basic computational skills, attention and working memory. The outcome measure for each task was ‘throughput’, an overall performance indicator accounting for speed and accuracy.
After we collected overnight abstinence-baseline measures, participants received IV saline and 2 weight-adjusted nicotine doses in an escalating fashion (saline, 0.5 then 1.0 mg nicotine/70kg bodyweight) to avoid nicotine carryover into the saline dose and as a safety precaution, consistent with prior studies.39–41 We administered infusions 30 minutes apart to enable recovery of subjective and physiological measures towards baseline.
We assessed subjective drug effects with the Drug Effects Questionnaire (DEQ), a 10-item visual analogue scale, immediately prior to then 1, 3, 5, 8 and 10 minutes following each saline and nicotine delivery. The primary DEQ analyses were focused on 3 factors: ‘Feel Good’ factor comprised of the average of ‘feel good’, ‘want more’ and ‘like’ scales; ‘Stimulatory’ factor comprised of ‘feel stimulated’, ‘feel high’ and ‘feel drug strength’ scales; and ‘Negative’ factor comprised of ‘feel anxious’, ‘feel down’ and ‘feel bad effects’. Heart rate, systolic and diastolic blood pressure were collected immediately prior to and 1, 2, 3, 5, 8, 10 and 15 minutes following saline and each nicotine delivery.
Data Analyses
Analyses were carried out in JMP 11 (SAS Institute Inc.). Values of p ≤ .05 in 2-tailed tests were considered statistically significant.
Baseline differences by menthol versus non-menthol preference
Menthol-preferring (N = 110) and non-menthol-preferring participants (N = 24) were compared on demographic and baseline self-report and biochemical smoking indicators using analysis of variance (ANOVA) for continuous, or chi-square (χ2) or logistic regression for categorical, variables.
Overnight-abstinence baseline versus end-of-session measures by menthol-preferring status
Measures of withdrawal (MNWS Total Score), craving (BQSU Factors 1 and Factor 2), affect (PANAS Positive and Negative), and cognitive function (Stroop, CPT and MPT Throughput) were analyzed separately with repeated-measures mixed-models including time-point (overnight-abstinence baseline, end-of-session), menthol-preference status, and menthol-preference status by time-point contrasts. Sex and a dichotomized race variable (African-American/black vs non-African-American/black) were included in the model, and then removed if they were not statistically significant (p ≤ .05) or demonstrating trend (p < .10).
Subjective and physiological responses to IV nicotine by menthol-preferring status
Subjective drug effects (‘Feel good’, ‘Stimulatory’ and ‘Negative’ DEQ factors) and physiological responses (heart rate, diastolic and systolic blood pressure (BP)) were analyzed in separate repeated-measures mixed-models models that included dose, menthol-preference-status, minute (ie, number of minutes post-dose), dose-by-minute, menthol-preference status-by-dose, menthol-preference status-by-minute, and menthol-preference status-by-dose-by-minute contrasts. Sex and dichotomous race were included in the model, and then removed if they were not statistically significant or trend contributors.
RESULTS
Baseline Differences by Menthol versus Non-menthol Preference
Table 1 shows baseline and demographic data. Race and ethnicity significantly differed by menthol-preferring status, with more African Americans (relative to non-African Americans) and more Hispanics (relative to non-Hispanics) reporting that their usual brand of cigarettes was mentholated, generally consistent with population studies.1,42 A higher nicotine metabolite ratio (3HC/cotinine) in the non-menthol-preferring group (relative to the menthol-preferring group) indicated faster nicotine metabolism rates in the non-menthol-preferring group. This difference was driven by higher plasma 3HC in the non-menthol-preferring group. Menthol-preference groups did not differ significantly on other demographic, self-reported smoking or biochemical indicators of recent smoking at baseline.
Table 1.
Baseline Measures for the Study Sample by Menthol Preference
| Measures | Menthol Preferring Status | Statisticsa | ||
|---|---|---|---|---|
|
| ||||
| Non-menthol Preferring (N = 24)d |
Menthol Preferring (N = 110)d |
Menthol Preference |
||
| Demographics | N (%) | N (%) | Wald | p |
|
| ||||
| Raceb | 65.53 | <.001 | ||
| African-American/Black | 1 (4.17) | 70 (63.64) | ||
| Not of Hispanic Origin | 1 (4.17) | 68 (61.82) | ||
| Hispanic Origin | 0 (0.00) | 2 (1.82) | ||
| Non-African-American | 23 (95.83) | 40 (36.36) | ||
| European American | 23 (95.83) | 38 (34.55) | ||
| Not of Hispanic Origin | 23 (95.83) | 23 (20.91) | ||
| Hispanic Origin | 0 (0.00) | 15 (13.64) | ||
| Native American/American Indian | 0 (0.00) | 1 (0.91) | ||
| Other | 0 (0.00) | 1 (0.91) | ||
| Sex | 1.85 | .174 | ||
| Men | 19 (79.17) | 71 (64.55) | ||
| Women | 5 (20.83) | 39 (35.45) | ||
| Mean (SD) | Mean (SD) | F | p | |
| Age, years | 37.00 (9.95) | 34.56 (9.01) | 1.39 | .241 |
| Body mass index (BMI) | 27.26 (4.70) | 29.59 (6.12) | 3.06 | .082 |
| Weight (lbs) | 181.67 (40.52) | 193.20 (41.70) | 1.52 | .220 |
|
| ||||
| Self-reported Smoking History and Severity | ||||
|
| ||||
| Fagerström Test of Nicotine Dependence (FTND) | 5.04 (2.33) | 5.52 (1.90) | 1.14 | .287 |
| Average cigarette consumption/day | 18.08 (7.34) | 18.15 (10.39) | 0.00 | .975 |
| Age onset regular smoking | 16.33 (2.85) | 16.15 (3.71) | 0.05 | .816 |
| Estimated years of smokingc | 20.67 (9.73) | 18.42 (9.20) | 1.15 | .285 |
|
| ||||
| Biochemical Smoking Indices at Baseline | ||||
|
| ||||
| Expired carbon monoxide (CO) | 5.33 (2.73) | 4.89 (2.51) | 0.60 | .442 |
| Plasma cotinine, ng/ml | 192.30 (90.72) | 200.60 (130.80) | 0.08 | .771 |
| Plasma 3′-hydroxycotinine (3HC) | 83.46 (37.53) | 58.90 (40.95) | 7.29 | .008 |
| Nicotine metabolite ratio (NMR; 3HC/cotinine) | 0.52 (0.31) | 0.32 (0.17) | 18.61 | <.001 |
| Plasma nicotine, ng/ml | 3.27 (4.37) | 2.87 (2.83) | 0.32 | .574 |
Note.
Statistics (F(p) or χ2 or Wald (p) as appropriate, are reported. Results reaching the statistical significance at p ≤ .05 level are considered statistically significant (bold) and reaching p < .10 are considered trend (italics).
The race variable included in analyses was dichotomous (African-American vs non-African-American). Further breakdown of the non-African-American sample by race and both samples by ethnicity (Hispanic or non-Hispanic) are for clarification of the sample but not represented in the statistics.
Estimated years of smoking was derived from age at testing and age of onset of regular smoking and does not account for periods of nicotine abstinence.
Data were available on the full sample with the exception of the following missing data points within the mentholpreferring sample (N = 1 missing bodyweight and BMI; N = 1 missing CO; N = 5 missing NMR).
Overnight-abstinence Baseline versus End-of-session Measures by Menthol-preferring Status
Raw data (means, SD, N) and full statistics are presented in Supplemental Table 1. Expected main effects of time-point were observed with diminished craving (BQSU) and withdrawal (MNWS) and improved cognitive function (ANAM tasks) at end-of-session relative to overnight-abstinence baseline. Sex and race contributed significantly to MPT Throughput and sex contributed significantly to BQSU Factor 1, so those variables were kept in the respective models. However, removal of sex and race from each model did not change the pattern of significance of menthol or menthol-by-time point results. Neither sex nor race contributed to remaining models; therefore, they were removed from the remaining final models.
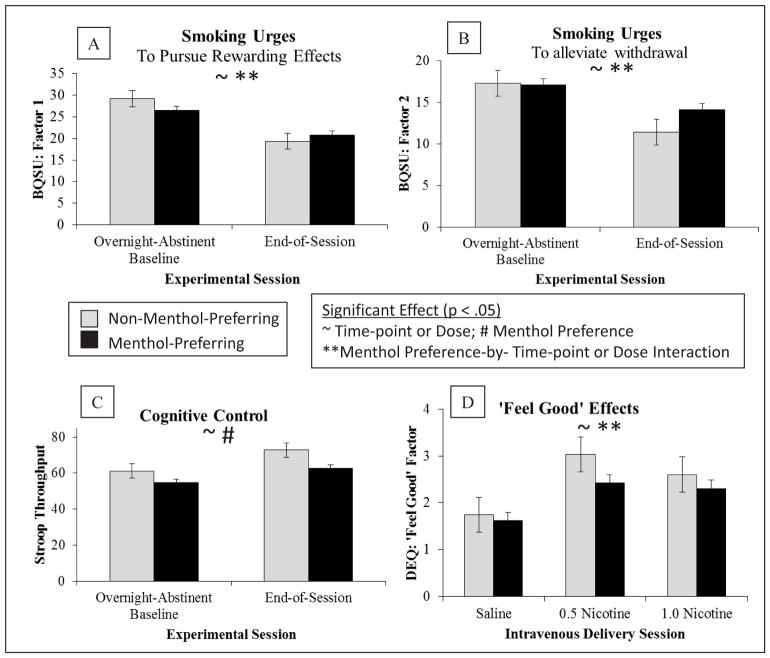
The menthol-preferring, relative to non-menthol-preferring, group reported less reduction in smoking urges at post-IV-nicotine-administration relative to the overnight abstinence baseline (Figure 1). Significant menthol-preferring status by time-point interactions were observed for both BQSU factors (menthol-preferring-status-by-time-point interaction: BQSU Factor 1: F = 5.34, p = .022; Factor 2: 4.49, p = .036).
Figure 1. Menthol Preference Status Effects on Smoking Urges, Cognition and Subjective Responses to Nicotine.
Note.
Means (standard error of the means) are presented by menthol-preferring status (black bars = menthol; grey bars = non-menthol) and either time-point or dose, as appropriate. Statistically significant effects (p < .05) are indicated for Time-point (~) or Dose (~); Menthol Preference (#); and Menthol Preference-by-Time-point Interaction (**) or Menthol Preference-by-Dose Interaction (**). For full statistical reporting of results, see Supplemental Tables 1 and 2. The menthol-preferring, relative to non-menthol-preferring, group reported less reduction in smoking urges to pursue positive smoking effects (A) and to alleviate aversive withdrawal symptoms (B) at post-IV-nicotine-administration relative to the overnight abstinence baseline (menthol-preferring-status-by-time-point interactions: BQSU Factor 1: F = 5.34, p = .0224; Factor 2: 4.49, p = .036). The menthol-preferring, relative to non-menthol-preferring, group performed worse on the Stroop task (C) across time-points (menthol-preferring-status: Stroop F = 4.16, p = .044). Both groups reported significantly higher ‘feel good’ ratings (D) for both nicotine doses relative to saline, within menthol-preferring group ‘feel good’ ratings did not significantly differ by dose, while within non-menthol-preferring group ‘feel good’ ratings were higher with the lower dose (0.5 nicotine) relative to the higher dose (1.0 nicotine) (menthol-preferring-status-by-dose interaction DEQ ‘Feel Good’ Factor: F = 4.34, p = .013)
The menthol-preferring, relative to non-menthol-preferring, group showed a trend towards lower withdrawal symptoms across both time-points (menthol-preference-status: MNWS Total Score F = 2.86, p = .093), but no menthol-by-time-point interactions on MNWS. There were no significant main or interactive effects of menthol-preferring status on affect (PANAS Positive or Negative).
The menthol-preferring, relative to non-menthol-preferring, group performed poorer on the Stroop and MPT across time-points (menthol-preferring-status: Stroop F = 4.16, p = .044 (Figure 1); MPT F = 6.99, p = .009). There were no significant menthol-by-time-point interactions for Stroop, MPT or CPT Throughput and no significant main effects of menthol-preferring status on CPT Throughput.
Subjective and Physiological Responses to IV Nicotine by Menthol-preferring Status
Raw data (means, SD, N) and full statistics are presented in Supplemental Table 2. As expected, main effects of dose were observed with subjective drug effects (DEQ) and physiological responses (heart rate, BP) increasing with nicotine relative to saline. Race and sex did not contribute significantly to any models; therefore, they were removed from final models. The inclusion or exclusion of race or sex from these models did not alter the pattern of statistical significance of menthol or menthol-by-dose results.
Both groups reported significantly higher ‘feel good’ ratings for both nicotine doses relative to saline; within menthol-preferring group ‘feel good’ ratings did not significantly differ by dose, and within non-menthol-preferring group ‘feel good’ ratings were higher with the lower dose (0.5 nicotine) relative to the higher dose (1.0 nicotine) (menthol-preferring-status-by-dose interaction DEQ ‘Feel Good’ Factor F = 4.34, p = .013; Figure 1). There were no significant main or interactive effects on menthol-preference status on DEQ ‘Stimulatory’ or ‘Negative’ factors.
The menthol-preferring, relative to non-menthol-preferring, group had a trend towards higher systolic BP (menthol-preferring-status: F = 2.97, p = .087) but there were no significant menthol interactions with dose or minute. There were no significant main or interactive effects of menthol-preferring status on heart rate or diastolic BP.
DISCUSSION
The primary findings were that menthol-preferring smokers had less alleviation of short-term abstinence-induced craving, both in terms of urges to pursue positive smoking effects (Factor 1) and to alleviate negative withdrawal effects (Factor 2), following IV nicotine administration and less subjective dissociation between the nicotine doses in terms of subjective positive (‘feel good’) effects compared to non-menthol-preferring smokers. In addition, the nicotine metabolite ratio in menthol-preferring smokers was consistent with the slower nicotine clearance demonstrated in prior research,25,26 suggesting greater overall exposure to nicotine per cigarette. Combined, these findings may reflect blunted sensitivity to some of nicotine’s effects and smoking urges being less driven by nicotine in menthol-preferring smokers. Several non-mutually exclusive mechanisms are feasible, although future studies will be necessary to test for a direct impact of each mechanism on these effects. Blunted nicotine sensitivity could arise due to a combination of prolonged nicotine exposure per cigarette (eg, via slowed nicotine clearance), menthol’s effects on neuroadaptation of nAChRs,26,29–31 and an unpairing of smoking behavior from potent peripheral nicotine sensory cues (eg, throat hit) and alternative pairing with menthol-related peripheral sensory cues.
There are several limitations with this study that may be addressed in future research. First, the imbalance of race across the menthol groups, although consistent with population studies showing higher proportions of African-American smokers preferring menthol relative to non-menthol cigarettes,1,42 could present a confounding influence. Race was accounted for in the analyses and did not significantly impact the menthol findings; however, it was not possible to account statistically for potential race-by-menthol group interactions in the current sample due to insufficient sample sizes per cell. Second, these findings should be considered preliminary due to the limited sample size in the non-menthol-preferring group relative to the menthol-preferring group, which may have resulted in imbalanced sensitivity to detect effects of abstinence or nicotine responses in each group. Third, menthol versus non-menthol-preferring status was based on current preferred brand and information was not collected on whether participants ever switched between menthol or non-menthol brands in the past. Therefore, although the menthol and non-menthol-preferring groups were well matched for duration, age of onset and frequency of use, nicotine dependence severity, and biochemical indicators of recent use, lifetime cumulative exposure to menthol versus non-menthol cigarettes is unknown. Finally, future studies combining the intravenous nicotine paradigm with acute menthol could provide valuable insight into the role of menthol-related sensory cues or central or peripheral effects of acute menthol administration on response to abstinence or nicotine administration.
IMPLICATIONS FOR TOBACCO REGULATION
Industry documents recognize that nicotine level determines perceived ‘impact’ of peripheral sensory cues (eg, throat hit), but with sufficient menthol doses, this impact is primarily driven by menthol rather than nicotine, indicating that these characteristics of menthol could be used to substitute, in part, for the sensory effects of nicotine.18 Whereas this effect was discussed in industry documents as a means of using menthol to enhance the impact of low nicotine cigarettes, an additional implication is that regular menthol smokers’ associative cues driving continued smoking may be sustained more by menthol-related cues rather than nicotine-related cues, despite physiological dependence upon nicotine. This would be consistent with this study’s findings of menthol smokers’ blunted response to IV nicotine, and less alleviation of smoking urges in response to IV nicotine, in the absence of menthol and its associated cues. If menthol smokers are less sensitive to the primary reinforcing effects of nicotine, then lowering nicotine levels as a means of reducing addiction potential, without concurrent regulation of menthol levels, may have less effect on menthol-preferring smokers’ behavior if their sensitivity to nicotine is blunted and smoking behavior and alleviation of craving is substantially driven by peripheral menthol-related cues. Concurrent regulation of nicotine levels and menthol levels, in contrast, could be more effective in reducing the addiction potential of cigarettes for menthol-preferring smokers.
Human Subjects Statement
The VA Connecticut Healthcare System Human Subjects Subcommittee and Yale Human Research Protections Program approved the study.
Supplementary Material
Acknowledgments
We thank Stacy Minnix, Lance Barnes, Christopher Cryan, Ellen Mitchell for their valuable contributions to study, including but not limited to subject recruitment, data collection, or data management and Noah Konkus for assistance with manuscript preparation. We also thank the participants for their time.
Footnotes
Conflict of Interest Statement
This research was supported by the National Institute on Drug Abuse (NIDA), a VA Career Award, and the Veterans Administration Mental Illness Research, Education and Clinical Center (MIRECC). EE DeVito, G Valentine and M Sofuoglu are affiliated with and partially supported by the Yale Tobacco Center of Regulatory Science (Yale TCORS; P50DA036151) from the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products (CTP). AIH was supported by K12 DA00167 from NIDA. EE DeVito and KP Jensen have no conflicts of interest to disclose. M Sofuoglu previously served as an expert witness on behalf of Pfizer in lawsuits related to varenicline. The funding bodies had no role in the collection, analysis or the decision to publish these data.
Contributor Information
Elise E. DeVito, Associate Research Scientist in Psychiatry, Yale School of Medicine, New Haven, CT.
Gerald W. Valentine, Clinician in Psychiatry, Yale School of Medicine, New Haven, CT.
Aryeh I. Herman, Associate Research Scientist in Psychiatry, Yale School of Medicine, New Haven, CT.
Kevin P. Jensen, Associate Research Scientist in Psychiatry, Yale School of Medicine, New Haven, CT.
Mehmet Sofuoglu, Professor of Psychiatry, Yale School of Medicine, New Haven, CT.
References
- 1.Lawrence D, Rose A, Fagan P, et al. National patterns and correlates of mentholated cigarette use in the United States. Addiction. 2010;105(Suppl 1):S13–S31. doi: 10.1111/j.1360-0443.2010.03203.x. [DOI] [PubMed] [Google Scholar]
- 2.Substance Abuse Mental Health Services Administration (SAMHSA) The NSDUH Report: Use of Menthol Cigarettes. Washington, DC: SAMHSA Office of Applied Studies Substance Abuse Mental Health Services; 2009. pp. 1135–1140. [Google Scholar]
- 3.Food and Drug Administration. [Accessed July 26, 2016];Family Smoking Prevention and Tobacco Control Act. Available at: http://www.fda.gov/TobaccoProducts/GuidanceComplianceRegulatoryInformation/ucm237092.htm.
- 4.Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations. Rockville, MD: Food and Drug Administration; 2011. [Google Scholar]
- 5.Harvey DM, Yasar S, Heishman SJ, et al. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology (Berl) 2004;175(2):134–142. doi: 10.1007/s00213-004-1818-6. [DOI] [PubMed] [Google Scholar]
- 6.Henningfield JE, Miyasato K, Jasinski DR. Abuse liability and pharmacodynamic characteristics of intravenous and inhaled nicotine. J Pharmacol Exp Ther. 1985;234(1):1–12. [PubMed] [Google Scholar]
- 7.Mello NK, Peltier MR, Duncanson H. Nicotine levels after iv nicotine and cigarette smoking in men. Exp Clin Psychopharmacol. 2013;21(3):188–195. doi: 10.1037/a0031799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose JE, Behm FM, Westman EC, Coleman RE. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction. Drug Alcohol Depend. 1999;56(2):99–107. doi: 10.1016/s0376-8716(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 9.Sofuoglu M, Yoo S, Hill KP, Mooney M. Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology. 2008;33(4):715–720. doi: 10.1038/sj.npp.1301460. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Drug therapy. Pharmacologic aspects of cigarette smoking and nicotine addition. N Engl J Med. 1988;319(20):1318–1330. doi: 10.1056/NEJM198811173192005. [DOI] [PubMed] [Google Scholar]
- 11.Ahijevych K, Garrett BE. Menthol pharmacology and its potential impact on cigarette smoking behavior. Nicotine Tob Res. 2004;6(Suppl 1):S17–S28. doi: 10.1080/14622200310001649469. [DOI] [PubMed] [Google Scholar]
- 12.Wickham RJ. How menthol alters tobacco-smoking behavior: a biological perspective. Yale J Biol Med. 2015;88(3):279–287. [PMC free article] [PubMed] [Google Scholar]
- 13.Oncken C, Feinn R, Covault J, et al. Genetic vulnerability to menthol cigarette preference in women. Nicotine Tob Res. 2015;17(12):1416–1420. doi: 10.1093/ntr/ntv042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uhl GR, Walther D, Behm FM, Rose JE. Menthol preference among smokers: association with TRPA1 variants. Nicotine Tob Res. 2011;13(12):1311–1315. doi: 10.1093/ntr/ntr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Front Behav Neurosci. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendt Nielsen T, Nielsen BP, Wang K, et al. Psychophysical and vasomotor responses of the oral tissues: a nicotine dose-response and menthol interaction study. Nicotine Tob Res. 2016;18(5):596–603. doi: 10.1093/ntr/ntv163. [DOI] [PubMed] [Google Scholar]
- 17.Rosbrook K, Green BG. Sensory effects of menthol and nicotine in an e-cigarette. Nicotine Tob Res. 2016 Jan 17; doi: 10.1093/ntr/ntw019. pii: ntw019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferris WG, Connolly GN. Application, function, and effects of menthol in cigarettes: a survey of tobacco industry documents. Nicotine Tob Res. 2004;6(Suppl 1):S43–S54. doi: 10.1080/14622203310001649513. [DOI] [PubMed] [Google Scholar]
- 19.Kreslake JM, Yerger VB. Tobacco industry knowledge of the role of menthol in chemosensory perception of tobacco smoke. Nicotine Tob Res. 2010;12(Suppl 2):S98–S101. doi: 10.1093/ntr/ntq208. [DOI] [PubMed] [Google Scholar]
- 20.Clark PI, Gautam S, Gerson LW. Effect of menthol cigarettes on biochemical markers of smoke exposure among black and white smokers. Chest. 1996;110(5):1194–1198. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 21.Ha MA, Smith GJ, Cichocki JA, et al. Menthol attenuates respiratory irritation and elevates blood cotinine in cigarette smoke exposed mice. PLoS One. 2015;10(2):e0117128. doi: 10.1371/journal.pone.0117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarvik ME, Tashkin DP, Caskey NH, et al. Mentholated cigarettes decrease puff volume of smoke and increase carbon monoxide absorption. Physiol Behav. 1994;56(3):563–570. doi: 10.1016/0031-9384(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 23.Ahijevych K, Parsley LA. Smoke constituent exposure and stage of change in black and white women cigarette smokers. Addict Behav. 1999;24(1):115–120. doi: 10.1016/s0306-4603(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 24.Garten S, Falkner RV. Role of mentholated cigarettes in increased nicotine dependence and greater risk of tobacco-attributable disease. Prev Med. 2004;38(6):793–798. doi: 10.1016/j.ypmed.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Fagan P, Pokhrel P, Herzog TA, et al. Nicotine metabolism in young adult daily menthol and nonmenthol smokers. Nicotine Tob Res. 2016;18(4):437–446. doi: 10.1093/ntr/ntv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alsharari SD, King JR, Nordman JC, et al. Effects of menthol on nicotine pharmacokinetic, pharmacology and dependence in mice. PLoS One. 2015;10(9):e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abobo CV, Ma J, Liang D. Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res. 2012;14(7):801–808. doi: 10.1093/ntr/ntr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramlinger VM, von Weymarn LB, Murphy SE. Inhibition and inactivation of cytochrome P450 2A6 and cytochrome P450 2A13 by menthofuran, beta-nicotyrine and menthol. Chem Biol Interact. 2012;197(2–3):87–92. doi: 10.1016/j.cbi.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson BJ, Wall TR, Henley BM, et al. Menthol alone upregulates midbrain nAChRs, alters nAChR subtype stoichiometry, alters dopamine neuron firing frequency, and prevents nicotine reward. J Neurosci. 2016;36(10):2957–2974. doi: 10.1523/JNEUROSCI.4194-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brody AL, Mukhin AG, La Charite J, et al. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16(5):957–966. doi: 10.1017/S1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ton HT, Smart AE, Aguilar BL, et al. Menthol enhances the desensitization of human alpha3beta4 nicotinic acetylcholine receptors. Mol Pharmacol. 2015;88(2):256–264. doi: 10.1124/mol.115.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pomerleau CS, Carton SM, Lutzke ML, et al. Reliability of the Fagerstrom Tolerance Questionnaire and the Fagerstrom Test for Nicotine Dependence. Addict Behav. 1994;19(1):33–39. doi: 10.1016/0306-4603(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders – Patient Edition. New York, NY: Biometrics Research Department, New York State Psychiatric Institution; 1996. [Google Scholar]
- 34.Benowitz N, Ahijevych K, Jarvis MJ, et al. SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 35.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43(3):289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 36.Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief ) in laboratory and clinical settings. Nicotine Tob Res. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- 37.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 38.Reeves D, Winter K, Kane R, et al. ANAM 2001 User’s Manual. San Diego, CA: National Cognitive Recovery Foundation; 2002. [Google Scholar]
- 39.DeVito EE, Herman AI, Waters AJ, et al. Subjective, physiological, and cognitive responses to intravenous nicotine: effects of sex and menstrual cycle phase. Neuropsychopharmacology. 2014;39(6):1431–1440. doi: 10.1038/npp.2013.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sofuoglu M, Mooney M. Subjective responses to intravenous nicotine: greater sensitivity in women than in men. Exp Clin Psychopharmacol. 2009;17(2):63–69. doi: 10.1037/a0015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology. 2012;37(6):1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giovino GA, Sidney S, Gfroerer JC, et al. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(Suppl 1):S67–S81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.