Abstract
The metastatic activity of breast carcinomas results from complex genetic changes in epithelial tumor cells and accounts for 90% of deaths in affected patients. Although the invasion of the local lymphatic vessels and veins by malignant breast tumor cells and their subsequent metastasis to the lung, has been recognized, the mechanisms behind the metastatic activity of breast tumor cells to other distal organs and the pathogenesis of metastatic cancer are not well understood. In this study, we utilized derivatives of the well-established and highly metastatic triple negative breast cancer (TNBC) cell line MDA-MB-231 (MDA-231) to study breast tumor metastasis in a mouse model. These MDA-231 derivatives had depleted expression of Kaiso, a POZ-ZF transcription factor that is highly expressed in malignant, triple negative breast cancers. We previously reported that Kaiso depletion attenuates the metastasis of xenografted MDA-231 cells. Herein, we describe the pathological features of the metastatic activity of parental (Kaisopositive) versus Kaisodepleted MDA-231 cells. Both Kaisopositive and Kaisodepleted MDA-231 cells metastasized from the original tumor in the mammary fat pad to the lung. However, while Kaisopositive cells formed large masses in the lung parenchyma, invaded large pulmonary blood vessels and formed secondary metastases and large tumors in the distal organs, Kaisodepleted cells metastasized only to the lung where they formed small metastatic lesions. Importantly, intravascular invasion and secondary metastases in distal organs were not observed in mice xenografted with Kaisodepleted cells. It thus appears that the lung may constitute a barrier for less invasive breast tumors such as the Kaisodepleted TNBC cells; this barrier may limit tumor growth and prevents Kaisodepleted TNBC cells from invading the pulmonary blood vessels and forming secondary metastases in distal organs.
Introduction
An estimated 90% of medical fatalities in cancer patients are due to metastases [1]. Carcinomas of the mammary gland [2,3], prostate gland [4], liver [5–9], pancreas [10], endometrium [11], thyroid gland [12,13] and Merkel cell [14] have been shown to invade lymphoid vessels and blood vessels [2,6,8,9,11–14] resulting in metastases to distant organs, particularly the lung [7,9]. According to the systemic and pulmonary circulatory patterns, cancer cells that escape the primary tumor site and enter the bloodstream would first disseminate to the lung via the right ventricle before dispersing from the lung through the left heart to distal organs. Characterization of the mechanisms of malignant mammary carcinoma indicates that in the primary tumor, a small population of cells travel towards the blood vessels, and invade them in a complex process involving enhanced activity of genes regulating the dynamics of the actin cytoskeleton, e.g. Mena [2,15–17] and LIM kinase1 [18]. In a series of in vivo experiments in mouse and rat models utilising multiphoton microscopy, migrating tumor cells were seen to be assisted by perivascular macrophages in the process of vascular invasion and metastases to distant organs, which involved chemotaxis regulated by EGF and CSF-1 [1,15–17,19–23].
The progression of breast tumors towards an invasive behaviour and metastasis are postulated to involve several molecular factors associated with the complex epithelial-to-mesenchymal transformation (EMT) process that endows tumor cells with the ability to modulate their cell-cell adhesion and the extracellular matrix [24–26,47,48,50–52], apparently involved in the first line of defense against metastatic tumors. EMT is frequently accompanied by loss of the epithelial marker E-cadherin, concurrent with elevated expression of vimentin [41–45], an intermediate filament that participates in cell motility [46], as well as increased expression of matrix metalloproteases-2 and -9 (MMP-2, MMP-9) [27–32] that are often assessed in the determination of poor prognosis in breast cancer patients [33–36]. Tissue plasminogen activator (tPA) and urokinase plasminogen activator (uPA) are known to activate pro-enzyme forms of MMP-2 and MMP-9 to active forms [37,38] and tPA and uPA have been proposed as markers for breast cancer progression [39,40].
Despite remarkable advancements in our understanding of cellular and molecular mechanisms involved in tumor growth and intravascular invasion at primary tumor sites, relatively little is known about how malignant tumors travel to distant organs. Since all lymph and venous blood from the body flows via the right heart ventricle to the lung, it is expected that most if not all primary metastases of carcinomatous tumors are trapped in small pulmonary blood vessels. However, the relevant questions regarding the pathogenesis of metastatic cancer remain; how do secondary metastases travel to other distant organs, and what are the mechanisms involved in the generation of these secondary metastases?
To gain insight into the mechanisms involved in breast tumor metastases to distant organs, we studied the pathogenesis of secondary metastases of parental, Kaisopositive and experimental Kaisodepleted MDA-231 TNBC cells in immunocompromised mice [53]. Kaiso is a dual-specificity transcription factor that is highly expressed in, and linked with the aggressive features of breast, prostate, colon and pancreatic carcinomas [54–57]. We previously reported that Kaiso depletion strongly inhibited the metastasis of TNBC cells to distal organs [53]. Herein, we describe histological analyses of the metastases generated by parental Kaisopositive and Kaisodepleted MDA-231 cells.
We found that Kaisopositive MDA-231 breast cancer cells implanted into the mammary fad pad of immunocompromised mice [53] formed large tumor masses in the lung parenchyma, invaded large blood vessels and metastasised to other distant organs where it also formed large masses. In contrast, Kaisodepleted tumor cells formed small clusters only in the lung parenchyma and did not invade blood vessels and did not metastasize to distant organs. While the role of high Kaiso expression in the metastatic activity of human breast cancer cells was reported in our previous paper [53], we describe here the critical importance of high Kaiso expression in the propagation of breast tumors to distal organs beyond the lung, which we call “secondary metastases”. We propose that the lung serves as the second line of defense against carcinomas with metastatic potential; tumors with less malignant characteristics such as Kaisodepleted MDA-231 cells are trapped, do not progress and perhaps are eliminated. In contrast, malignant tumors such as Kaisopositive MDA-231 cells thrive in the lung to form large masses that then, invade the large pulmonary blood vessels, travel via the left heart ventricle and lodge in small blood vessels of a variety of peripheral organs where they form secondary metastases.
Materials and methods
Generation of stable Kaiso-depleted MDA-231 cells
The MDA-231 TNBC cell line was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA), and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Lonza BioWhittaker®, Walkersville, MD, USA) supplemented with 10% Fetal Bovine Serum (FBS; Hyclone, Logan, Utah, USA), 0.1 mg/mL penicillin/streptomycin and 0.25 g/mL Fungizone (Invitrogen, Grand Island, NY, USA). Cells were passaged every 2 or 3 days and grown in a 5% CO2 humidified incubator at 37°C. Stable Kaiso-depletion was achieved by using multiple Kaiso-specific short hairpin RNAs (shRNA) that target the Kaiso mRNA specifically as previously described [49]. A scrambled shRNA that does not target the Kaiso mRNA was used as a control. Stable control (Kaisopositive) and Kaisodepleted (sh-K) MDA-231 cells were maintained in DMEM-supplemented media treated with Puromycin (Invitrogen) at 0.8 μg/mL.
Western blot analysis
Stable control Kaisopositive and Kaisodepleted (sh-K1 and sh-K2) MDA-231 cells were cultured until ~80–90% confluent, washed twice with cold PBS, and then harvested by cell scraping into cold microfuge tubes. Control, sh-K1 and sh-K2 MDA-231 cells were then pelleted by centrifugation, lysed, and protein isolated as previously described [59]. Rabbit anti-Kaiso polyclonal (1:5,000 dilution; a generous gift from Dr. A. Reynolds), and mouse anti-β-actin monoclonal (1:50,000 dilution, Sigma Aldrich, Oakville, ON, Canada) primary antibody incubations were performed overnight at 4°C. Secondary antibody incubations were performed with goat anti-rabbit- or donkey anti-mouse-horseradish peroxidase-conjugated secondary antibodies. (1;10,000 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Signals were then amplified with Clarity Western Enhanced Chemiluminescence substrate as previously described [53]. The sh-K2 MDA-231 cells, hereafter referred as sh-K or Kaisodepleted MDA-231 cells, where chosen for all subsequent studies as these cells displayed the most efficient Kaiso knockdown.
Animal studies
All animal studies were approved and performed at McMaster University, Ontario, Canada according to the guidelines by the Canadian Council for Animal Care. Extensive description of animal studies has been outlined previously [53]. Briefly, 4.5 x 106 Kaisopositive or Kaisodepleted MDA-231 cells were injected subcutaneously into the mammary fat pad of 6–8 week old female NOD SCID Gamma (NSG, Jackson Laboratories) mice (n = 5 each per condition), and allowed to form prominent subcutaneous masses up to 3,300 mm3 in volume. Non-invasive monitoring of mice was performed once a week, and increased to 2–3 times per week upon tumor appearance. Tumor growth was monitored externally with vernier calipers and tumor volume (in mm3) measured using the following formula; length/2 × width2, 2–3 times per week [53]. This tumor mass volume was achieved before the onset of serious clinical signs such as body weight loss, dehydration and lethargy that would require the application of the endpoint and euthanasia. The endpoint tumor volume of 3,300 mm3 was determined in pilot experiments prior to the study. At endpoint, the mice were euthanized by overdosing with intraperitoneal injection of sodium pentobarbital (100 mg/kg body weight), perfused when deeply anaesthetized, and fixed in 10% formalin and all routine tissues collected for histological examination.
Histology and Immunohistochemistry
Harvested and formalin-fixed tissues were processed, embedded in paraffin wax, and 5 μm thick tissue sections were then mounted on glass slides and stained with either hematoxylin & eosin (H&E) or Masson’s trichrome. Immunohistochemical (IHC) analyses of tissue sections were performed as described previously [53]. Briefly, tissues were rehydrated in decreasing concentrations of alcohol, and deparaffinized in xylene before antigen retrieval by heating tissues in a sodium citrate solution (pH 6.0) in a microwave. Primary antibody incubations were performed overnight at 4°C with the following antibodies: anti-Kaiso 6F mouse monoclonal (1:500), anti-Kaiso 12H mouse monoclonal (1:800) [58], anti-Vimentin rabbit monoclonal antibody (1:500; Cell Signaling Technology (CST), Danvers, MA, USA #5741), anti-E-cadherin mouse monoclonal antibody (1:50; BD Biosciences, Mississauga, ON, Canada 610182), anti-MMP-2 rabbit polyclonal antibody (1:1000; CST #4022BC), and anti-MMP-9 rabbit polyclonal antibody (1:1000; CST # 3852BC). Secondary antibody incubations were performed for 2 hours at room temperature with either biotinylated goat anti-mouse or donkey anti-rabbit antibody at a dilution of 1:1000. Negative controls were obtained by excluding primary antibody. Histological analysis of H&E, Masson’s trichome and IHC-stained tissue sections were performed using a Nikon Eclipse 50 light microscope and representative phenotypes photographed.
Results and discussion
Protein expression
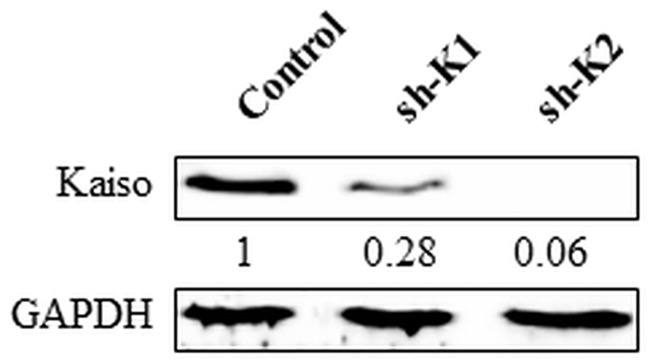
Expression of Kaiso in MDA-231 cells was abundant but remarkably reduced in sh-K1 (to 28%) and sh-K2 (to 6%) (see Fig 1) as the result of the stable transfection of the Kaiso-specific shRNA in these cells. Sh-K2 cells were utilized as Kaisodepleted in the xenograft studies.
Fig 1. Kaisodepleted MDA- 231 cells express negligible Kaiso compared to parental Kaisopositive cells.
Kaiso expression levels were determined using western blot. Both Kaisodepleted clones (sh-K1 & sh-K2) expressed little Kaiso compared to the Kaisopositive MDA-231 cells.
Clinical observations and histological analyses
The subcutaneous masses in the mammary gland fat pad reached the endpoint volume of 3,300 mm3 within 8 weeks in Kaisopositive tumors and 12 weeks in Kaisodepleted tumors [60]. Further characterization of the Kaisopositive and Kaisodepleted MDA-231 phenotypes revealed that the difference in tumor growth was due to Kaiso-depletion effects on cell proliferation, anchorage-independence and apoptosis [60].
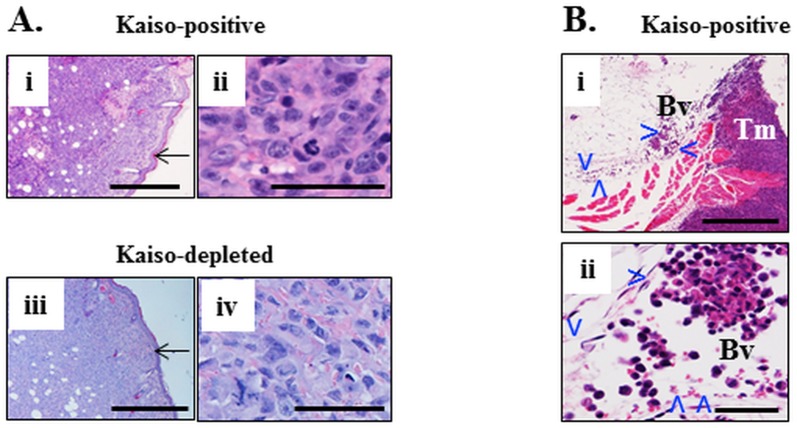
The injection of the Kaisopositive and Kaisodepleted MDA-231 cells into the mammary fat pad of immunocompromised mice resulted in the formation of large subcutaneous masses (Fig 2Ai and 2Aiii) formed by large, pleomorphic cells with high mitotic index (Fig 2Aii and 2Aiv). Primary tumor masses formed by both types of mammary carcinoma cells were morphologically indistinguishable from each other (Fig 2A). Veins and lymphatic vessels in vicinity to some subcutaneous masses were distended with clusters or single tumor cells scattered throughout the lumen (Fig 2B).
Fig 2. Primary subcutaneous tumors formed by Kaisopositive and kaisodepleted cells with invasion of the lumen of surrounding veins.
Subcutaneous tumor mass of Kaisopositive MDA-231 human mammary carcinoma cells (Ai, ii) and Kaisodepleted tumor cells (Aiii, iv)) implanted into the fat pad of the mammary gland of female NRG mice. Tumor cells abut against the epidermis (arrow in Ai, iii) but do not invade it. Tumor cells are large, markedly pleomorphic, there is high mitotic index. (Bi) A vein (Bv, delineated by arrowheads) is adjacent to the subcutaneous tumor mass (Tm). It is distended by clumps and individual large pleomorphic cells (Bii) and also has scattered red blood cells. H&E. Size bars Ai, ii, Bi– 500 microns; Aii, iv, Bii– 50 microns.
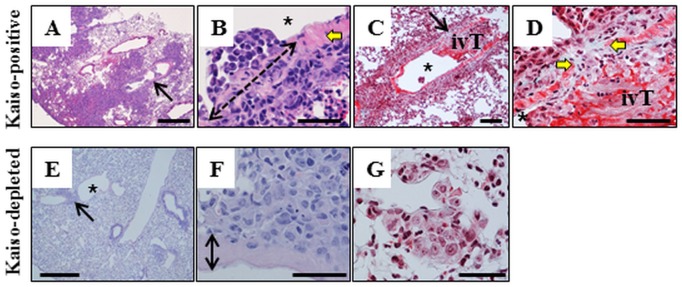
In the lung, Kaisopositive cells formed large, often coalescing, non-circumscribed tumor masses with obliteration of the alveolar architecture (Fig 3A) formed by large, pleomorphic cells with a high mitotic index. Scattered neutrophils infiltrated the periphery of the tumor masses and the surrounding alveolar tissue. A proportion of large blood vessels encompassed by or adjacent to tumors (Fig 3A) had masses of tumor cells protruding into the lumen, with the segmental concurrent obliteration of the vascular wall by tumor cells forming a continuity of perivascular and intravascular tumors (Fig 3B–3D). The apparent vascular invasion of Kaisopositive tumors was often associated by formation of thrombi infiltrated by tumor cells (Fig 3D). In contrast, Kaisodepleted tumor cells formed small aggregations scattered in the alveolar parenchyma (Fig 3E and 3G), sometimes adjacent to large blood vessels but with no invasion of the vascular wall or the lumen (Fig 3F). Kaisodepleted tumor aggregations were often infiltrated by scattered neutrophils (Fig 3G).
Fig 3. Metastasis of Kaisopositive and Kaisodepleted cells to the lung results in dramatically different tumor behavior.
(A-D) Kaisopositive tumors are numerous, large, obliterate the architecture of the pulmonary tissue and invade the lumen of large blood vessels (arrow in A). A segment of the vascular wall indicated by yellow arrows (B, D) is obliterated by tumor cells (double-headed interrupted arrow in B) that provide continuity between a perivascular mass and intravascular tumors (ivT) in the lumen (asterix in B, C). Apparent vascular invasion is associated with formation of intravascular thrombus (C, D). Intravascular surface of tumors or tumor thrombi is typically lined by endothelial cells (B). Tumor cells in intravascular masses or thrombi are large and pleomorphic (B, D). Kaisodepleted cells form small interstitial aggregations (arrow in E) of large pleomorphic cells (F, G) that do not invade the wall or the lumen (F) of adjacent blood vessels. H&E–A, B, E, F; Masson’s trichrome (C, D, G). Size bars; A, E– 500 microns, B-D, F, G– 50 microns.
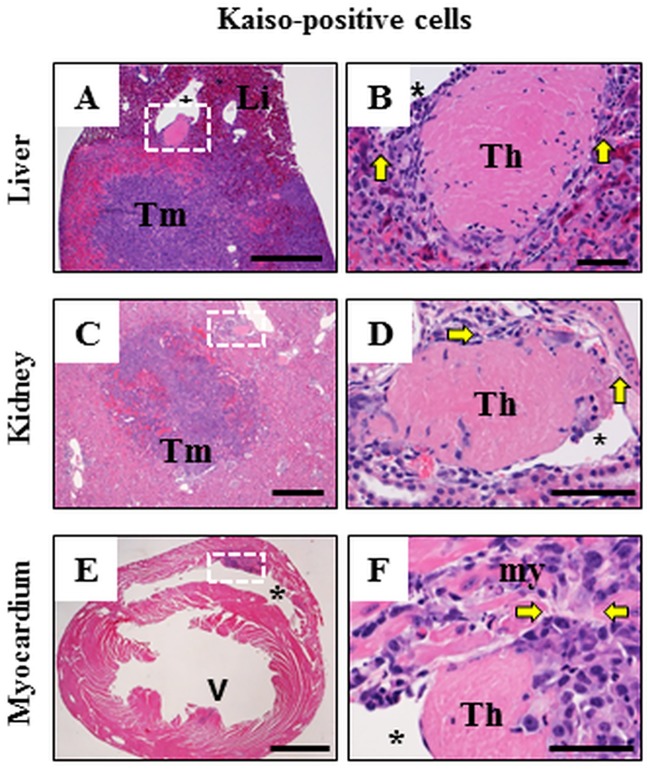
Although Kaisopositive and Kaisodepleted cancer cells both formed subcutaneous masses and invaded nearby veins and lymphatics resulting in pulmonary metastases, Kaiso-depletion markedly supressed the ability of cancer masses to grow expansively in the lung, and eliminated their ability to invade blood vessels thereby restricting their spread to other organs. Indeed, tumors were not observed in any other organ except in the lung of mice injected with Kaisodepleted cells. In contrast, in mice injected with Kaisopositive cells, large tumors were observed in the liver (Fig 4A and 4B), kidney (Fig 4C and 4D), myocardium (Fig 4E and 4F), and infrequently in the adrenal gland and leptomeninges of the brain (not shown). Thus, Kaiso appear to play a regulatory role to in the; (i) expansive growth of metastatic tumors in the lung and (ii) invasion of the pulmonary blood vessels to spread to other organs supports the notion of Kaiso as a crucial factor in highly aggressive subtypes of breast cancer [53,54,60]. Our findings suggest that Kaiso could be a target for therapeutic strategies in the treatment of aggressive breast cancers. Some large blood vessels within a tumor mass or adjacent to it, such as in the liver (Fig 4A), or in kidney (Fig 4C) had intraluminal invasion of tumor cells with formation of thrombus and obliteration of the adjacent segment of the wall of the blood vessel (Fig 4B and 4D). In the myocardium, protrusions of the tumor cells into the lumen of ventricles (Fig 4E) resulted in the formation of fibrinous thrombus (Fig 4F) infiltrated by tumor cells. Large, sometimes coalescing tumor masses obliterated the organ architecture and were composed of large pleomorphic cells similar morphologically and immunohistochemically to those described in the subcutaneous mass and in the lung of mice injected with Kaisopositive cells.
Fig 4. Intravascular invasion of secondary metastatic Kaisopositive tumors.
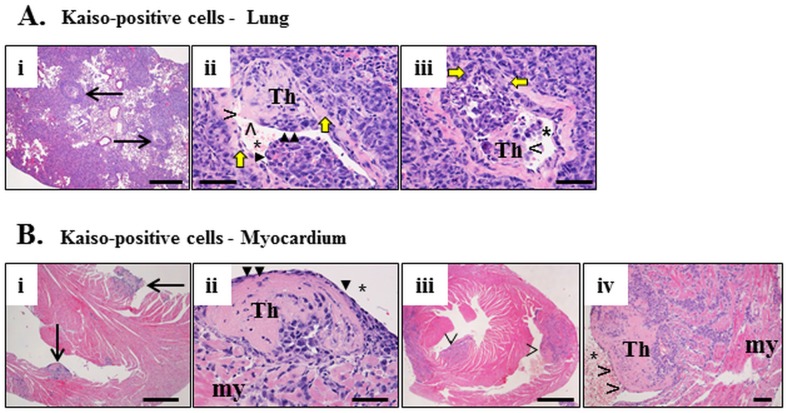
Low magnification images (A, C, E) and high magnification images (B, D, F) of tissue regions outlined by white dotted lines in A, C and E. Kaisopositive cells metastatic to the liver (A, B) and kidney (C, D) formed large tumors and invaded adjacent blood vessels with formation of thrombi (Th in B, D) delineated from the surrounding tissue by yellow arrows. Tumor masses in the myocardium (white box and arrowhead in E) often resulted in invasion of the ventricle (asterix) with formation of a mass (white box in E) and thrombus (Th in F). Thrombus is delineated from myocardium (my) by yellow arrows in F. There is continuity between the masses of tumor cells in the myocardium and in the intraventricular thrombus (F). H&E. Size bars; A, C, E– 1,000 microns, B, D, F– 50 microns.
Intravascular (Fig 5A) or cardiac intraventricular (Fig 5B) invasion by the Kaisopositive tumor cells often resulted in formation of a thrombus whose surface in some cases was apparently covered by endothelium (Fig 5Aii and 5Bii), delineating the remaining lumen of the blood vessel or the ventricle. Other thrombi however, where not delineated by endothelium but often by a layer of neutrophils (Fig 5Aiii and 5Biv). In some blood vessels adjacent areas of thrombi were or were not endothelialized while in others apparently endothelium-free small clusters or individual cancer cells were present in the lumen (Fig 5Aiii).
Fig 5. Thrombosis caused by Kaisopositive tumors invading the blood vessels and heart ventricles.
In the lung (A), a number of large blood vessels (two indicated by arrows) have intravascular thrombi delineated from the vascular wall by yellow arrows and protruding in the vascular lumen (Th in Aii, iii). The thrombi are infiltrated by neoplastic cells and are lined by endothelium (solid arrowheads in Aii) or not (open arrowhead in Aiii). In the myocardium (my, B) thrombi protruding into the ventricular lumen (Bi, iii) are also infiltrated by neoplastic cells (Th in Bii, iv) and either lined by endothelium (solid arrowheads in Bii) or not (open arrowheads in Biv). H&E. Size bars; Biii– 1,000 microns, Ai, Bi– 500 microns, Aii, iii, Bii, iv B– 50 microns.
The above data indicate that the subcutaneously implanted Kaisopositive MDA-231 cells efficiently penetrated the vascular wall and invaded the lumen of large blood vessels in the lung and other organs (Figs 4 and 5). This is in line with other studies that have also demonstrated vascular taxis and intravascular invasion of breast tumors implanted subcutaneously into mice [1,15–17,19–23]. The active penetration of the vascular wall by Kaisopositive cells lead to their accessing of the lumen with frequent formation of thrombus. This pathogenesis implies two potential mechanisms; (1) tumor cells breached the endothelium of the tumor–invaded blood vessel, which may have led to thrombosis; (2) the tumor cells then invaded the thrombus as the convenient substrate, which lead to the increase of the intravascular load of cancer cells destined to metastasize to other organs. We also observed endothelium lining of the intravascular tumor masses with or without thrombosis. We consider that neo-endothelialization of the intravascular tumor masses and tumor thrombi may serve as a defense mechanism preserving the patency of the blood flow. We thus postulate that this putative defense mechanism may actually be subverted by the invading tumor cells allowing for the increase of their intravascular load and presumably leading to a greater chance of success of secondary metastases.
Immunohistochemical analyses
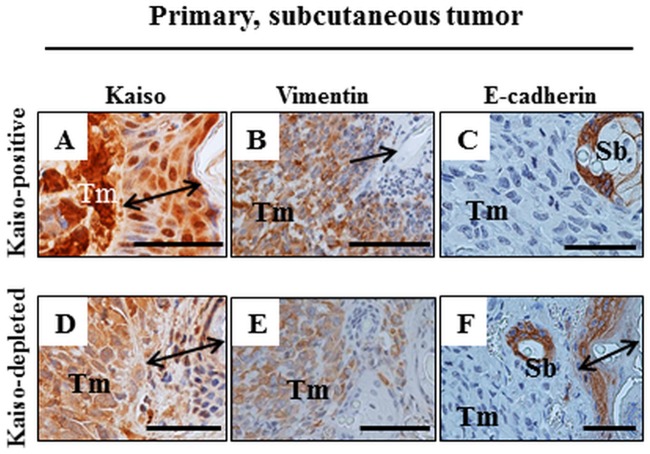
Kaisopositive primary tumor tissues, as expected, stained positive for Kaiso (Fig 6A), which localized to both the nucleus and cytoplasm. Further analysis of the Kaisopositive tumor tissues for other molecular markers implicated in tumor metastasis revealed that similar to our previous observations in vitro [53], Kaisopositive MDA-231 primary tumor tissues stained moderately for Vimentin (Fig 6B) and negative for E-cadherin (Fig 6C). We also examined the Kaisodepleted primary tumor tissues for Kaiso, Vimentin and E-cadherin expression. While we expected little to no Kaiso staining in the Kaisodepleted MDA-231 tumors as per our western blot results (Fig 1), we were surprised to observe weak Kaiso staining in the primary tumors which was predominantly cytoplasmic (Fig 6D). The weak Kaiso staining could be due to deselection of the Kaisodepleted MDA-231 cells as the mice were not subjected to constant treatment with Puromycin that would ensure selection and maintenance of shRNA plasmid in the stable cells in vitro. Nonetheless, the Kaiso staining observed in Kaisodepleted tumor tissues was still remarkably reduced compared to the staining observed in the Kaisopositive tumor tissues (Fig 6A and 6D). Kaisodepleted MDA-231 tumor tissues also stained weakly for Vimentin (Fig 6E) as expected from in vitro findings in our previous report [53]. In contrast, while we had observed increased E-cadherin expression in the Kaisodepleted MDA-231 cells in vitro [53], the Kaisodepleted MDA-231 tumor tissues stained negative for E-cadherin (Fig 6F). This lack of E-cadherin staining could be due to the weak levels of Kaiso expression observed in the Kaisodepleted MDA-231 primary tumors (Fig 6D) or due to other in vivo factors in the tumor microenvironment that are independent of Kaiso’s expression or Kaiso’s effect on E-cadherin expression.
Fig 6. IHC of primary subcutaneous Kaisopositive and Kaisodepleted MDA-231 tumors.
Tumor cells (Tm) of Kaisopositive (A-C) and Kaisodepleted (D-F) masses do not invade the epidermis (double-headed arrow in A, D, F, arrow in B). Kaisopositive tumor cells are labeled strongly positive for Kaiso (A) and vimentin (B) while the Kaisodepleted cells are labelled considerably less (D, E). The labeling with anti-E-cadherin antibody is negative for both types of tumor cells in contrast to the positive labelling of the mouse epithelium in sebaceous glands (Sb in C, F) and in epidermis (F). Size bars A-F– 50 microns.
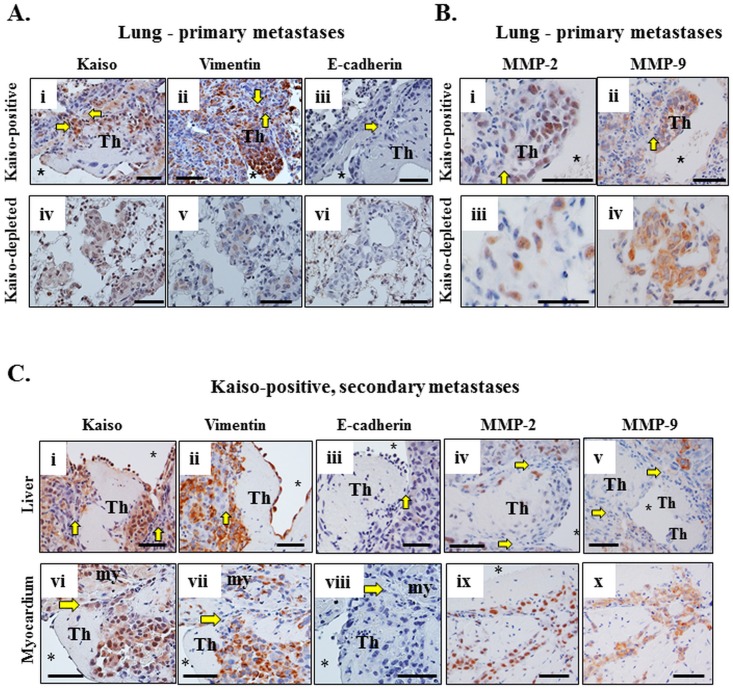
Analysis of the tumor masses observed in the lung (Fig 7A) of mice injected with the Kaisopositive and Kaisodepleted MDA-231 cells also revealed that the Kaisopositive tumor cells that metastasized to the lung exhibited strong Kaiso (Fig 7Ai) and Vimentin (Fig 7Aii) staining but no E-cadherin staining (Fig 7Aiii), while Kaisodepleted tumor metastases in the lung displayed weak Kaiso (Fig 7Aiv) and Vimentin (Fig 7Av) staining, but no E-cadherin staining (Fig 7vi). These findings suggest a persistence of the molecular phenotype of both Kaisopositive and Kaisodepleted MDA-231 cells as they metastasized to the lung. We further analyzed the Kaisopositive and Kaisonegative MDA-231 lung metastases for the expression of MMP-2 and MMP-9. Although, MMP-2 and MMP-9 had not previously been examined in the Kaisopositive and Kaisonegative MDA-231 cells in vitro, they have been implicated in EMT and breast cancer progression [61–63]. Consistent with the EMT phenotype in the Kaisopositive tumor cells, Kaisopositive lung metastases displayed strong MMP-2 and MMP-9 expression (Fig 7Bi and 7Bii). In contrast, Kaisodepleted lung metastases displayed reduced MMP-2 (Fig 7Biii) but not MMP-9 (Fig 7Biv) expression.
Fig 7. The molecular phenotype of the Kaisopositive MDA-231 cells persist as they metastasize to other distal organs (liver and myocardium).
(A) Neoplastic Kaisopositive cells in lung metastases or thrombi are large, pleomorphic, and stain positive for Kaiso (i) and vimentin (ii), but negative for E-cadherin (iii). In contrast, Kaisodepleted tumor cells are weakly stained for Kaiso (iv) and Vimentin (v) and negative for E-cadherin (vi). (B) Kaisopositive tumor cells in lung metastases also stain strongly for MMP-2 (i), and MMP-9 (ii), while the Kaisodepleted tumor cells stain weakly for MMP-2 (iii), but positive for MMP-9 (iv). The asterisk indicates the lumen of the blood vessel with the thrombus (Th) and the yellow arrows indicate the vascular wall and its obliteration by tumor cells in (A, B). (C) The thrombus (Th) formed in the large blood vessel of the liver (i-v) and in the ventricle of the heart (vi-viii) in mice injected with Kaisopositive cells partially obliterated the vascular wall or endocardium indicated by the yellow arrows (i-viii). Neoplastic cells are stained positive for Kaiso (i, vi), vimentin (ii, vii), MMP-2 (iv, ix), and MMP-9 (v, x), and negative for E-cadherin (iii, viii). Scale bars; A-C– 50 microns.
Examination of the Kaisopositive tumor cells in secondary metastases (Fig 7C) also revealed strong Kaiso (Fig 7Ci) and Vimentin (Fig 7Cii) staining but no E-cadherin staining (Fig 7Ciii) in liver metastases. Similar to the lung metastases, the Kaisopositive liver metastases also displayed strong MMP-2 (Fig 7Civ) and MMP-9 (Fig 7Cv) staining. Likewise, the Kaisopositive tumor masses in the myocardium displayed strong Kaiso (Fig 7Cvi) and Vimentin (Fig 7Cvii) staining but no E-cadherin staining (Fig 7Cviii), as well as strong MMP-2 (Fig 7Cix) and MMP-9 (Fig 7Cx) staining. These findings also imply a persistence of the molecular phenotype of the Kaisopositive MDA-231 cells as they metastasized to other distal organs.
In this study we analyzed the metastatic progression of Kaisopositive and Kaisonegative malignant mammary carcinomas using in vivo transplantation experiments in a mouse model. We found that Kaisopositive and Kaisonegative MDA-231 cancer cells both formed subcutaneous masses and invaded nearby veins and lymphatics apparently leading to pulmonary metastases. However, Kaiso-depletion was associated with remarkable suppression of the growth of cancer cells in the lung. In contrast, high Kaiso-expressing tumor cells thrived in the lung, invaded large pulmonary blood vessels and metastasized to other organs. These findings suggest that Kaiso plays a key role in metastatic activity of MDA-231 cancer cells. The penetration of the vascular wall and invasion of the lumen of large blood vessels abundant in the lung and other organs appears to be the fundamental factor of malignancy of the Kaisopositive tumor cells. This is in line with other studies that have also demonstrated vascular taxis and intravascular invasion of breast tumors implanted subcutaneously into mice [1,15–17,19–23]. Immunohistochemical characterization of Kaisopositive and Kaisodepleted tumors in this study revealed that Kaisopositive tumor cells exhibit more features associated with malignancy (increased Kaiso, Vimentin, MMP-2 and MMP-9 expression) than the Kaisodepleted tumor cells, which only displayed increased MMP-9 expression. Moreover, Kaiso expression seem to correlate positively with Vimentin and MMP-2 but not MMP-9 expression. Indeed Kaiso-depletion resulted in decreased Vimentin and MMP-2 but not MMP-9 expression. The similar staining of MMP-9 and lack of E-cadherin staining in both Kaisopositive and Kaisodepleted tumor cells, which is a marker of EMT, may explain the ability of both cell types to metastasize to the lungs. However, considering that only the Kaisopositive tumor cells were capable of surviving in the lungs, invading blood vessels and forming macrometastases in other distal organs, the higher Kaiso expression in concert with the increased Vimentin and MMP-2 expression could be considered as the critical determinants that allowed the Kaisopositive tumors to thrive after metastasis to the lung, and other distant organs.
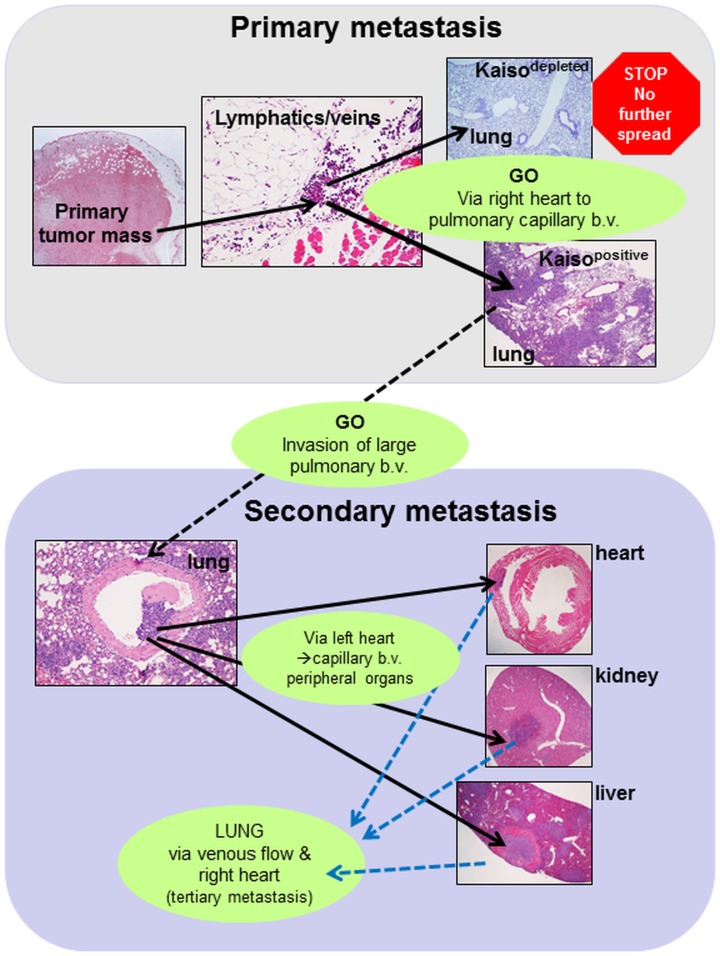
We thus propose that the lung serves as the second line of defense against carcinomas with metastatic potential where tumors with less malignant characteristics, e.g. with reduced expression of Kaiso, are trapped, do not progress and perhaps are eliminated. Consequently, secondary metastases to the distant organs are prevented. We further postulate, that malignant tumors such as human breast tumors with high Kaiso expression can overcome this defensive mechanism, thrive in the lung and form large masses whose cells invade the blood vessels, travel via the left heart ventricle to lodge in small blood vessels of a variety of peripheral organs, and initiate multiple secondary metastatic tumors leading to accelerated demise (Fig 8). If this hypothesis is proven to be correct, potential cancer-suppressive tissue mechanisms in the lung should be considered in the pathogenesis of cancer metastasis. Also, models used to evaluate the effectiveness of anti-cancer therapies should specifically include the analysis of the primary metastases in the lung and secondary metastases from the lung to other organs. The possibility of the lung as the second line of defence, with potential anti-cancer mechanisms sufficient to stall Kaisodepleted but not Kaisopositive breast cancer cells should be addressed in further studies.
Fig 8. Conceptual pathogenesis of cancer metastasis.
Primary metastasis: Invasion of Kaisopositive and Kaisodepleted mammary carcinoma cells of the local veins and lymphatics allows the cells to migrate via the right heart ventricle to the lung where they are trapped in the capillary blood vessels (b.v.) and form pulmonary metastases. While in the lung, Kaisopositive cells proliferate successfully and form large, coalescing masses that send the cells to actively cross the wall of adjacent blood vessels and invade their lumen. Kaisodepleted tumor cells form small aggregations that do not invade blood vessels therefore the secondary metastases do not occur. Secondary metastasis: The intravascular invasion by the Kaisopositive tumor cells in the lung presumably leads to its migration in the blood via the left heart to a variety of organs notably heart, liver and kidney, where they form metastases and tumors with the invasion of local blood vessels or heart ventricles in a fashion similar to that observed in the lung. This may lead to tertiary metastases; via the venous flow to the right heart and ultimately to the lung.
Conclusions
In this report, we analyzed the metastatic progression of Kaisopositive and Kaisonegative malignant mammary carcinomas using in vivo transplantation experiments in a mouse model. Although this study utilizes the end point metastasis analysis of disseminated breast tumor cells, it highlights potential novel mechanisms involved in secondary metastases and provides detailed histological evidence of different behaviour of MDA-231 malignant breast cancer cells depending on the expression level of Kaiso. Both Kaisopositive and Kaisodepleted tumor types; (1) formed subcutaneous masses of cells with morphological features of malignancy; (2) invaded adjacent veins and lymphatic vessels; and (3) metastasized to the lung. However, while Kaisopositive cells; (i) formed large pulmonary tumors; (ii) actively invaded pulmonary blood vessels apparently leading to (iii) secondary metastases and tumors in a variety of distal organs, Kaisodepleted tumors formed only small aggregates in the lungs, did not invade pulmonary blood vessels and did not form secondary metastases. Thus, Kaiso may be a potent factor enabling breast cancer cells to overcome apparent inhibitory mechanisms in the lung and to send secondary metastases throughout distant organs.
Supporting information
(PDF)
Acknowledgments
We wish to thank Dr. Albert Reynolds for the rabbit anti-Kaiso polyclonal antibody used in this study. We also thank Alyssa Giannotti and Omotunde Babatope for their technical assistance in the optimization of the E-cadherin antibody used for immunohistochemistry.
Data Availability
All relevant data are available from Harvard Dataverse (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/C8S8SN).
Funding Statement
This work was supported NSERC Discovery Grant # RGPIN6442-15 - JMD; Canadian Breast Cancer Foundation - JMD and the Schlumberger Faculty for the Future Fellowship - BIB-A. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011;331:1559–1564. doi: 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 2.Di Modugno F, Mottolese M, Di Benedetto A, Conidi A, Novelli F, Perracchio L, et al. The cytoskeleton regulatory protein hMena (ENAH) is overexpressed in human benign breast lesions with high risk of transformation and human epidermal growth factor receptor-2-positive/hormonal receptor-negative tumors. Clin Cancer Res 2006;12:1470–1478. doi: 10.1158/1078-0432.CCR-05-2027 [DOI] [PubMed] [Google Scholar]
- 3.Robinson BD, Sica GL, Liu YF, Rohan TE, Gertler FB, Condeelis JS, et al. Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clin Cancer Res 2009;15:2433–2441. doi: 10.1158/1078-0432.CCR-08-2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dasgupta S, Wasson LM, Raunyar N, Prokai L, Borejdo J, Vishvanatha JK. Novel gene C17orf37 in 17q12 amplicon promotes migration and invasion of prostate cancer cells. Oncogene 2009;28:2860–2872. doi: 10.1038/onc.2009.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung JW, Park JH, Han JK, Choi BI, Han MC. Hepatocellular carcinoma and portal vein invasion: results of treatment with transcatheter oily chemoembolization. Am J Roentgenol 1995;165:315–321. [DOI] [PubMed] [Google Scholar]
- 6.Lee IJ, Chung JW, Kim H-C, Kim MU, Kim JD, Kim GM, et al. Extrahepatic collateral artery supply to the tumor thrombi of hepatocellular carcinoma invading inferior vena cava: the prevalence and determinant factors. J Vasc Interv Radiol 2009;20:22–29. doi: 10.1016/j.jvir.2008.09.030 [DOI] [PubMed] [Google Scholar]
- 7.Nakashima T, Okuda K, Kojiro M, Jimi A, Yamaguchi R, Sakamoto K, et al. Pathology of hepatocellular carcinoma in Japan. 232 Consecutive cases autopsied in ten years. Cancer 1983;51:863–877. [DOI] [PubMed] [Google Scholar]
- 8.Quaglia A, Etessami N, Sim R, Difford J, Dhillon AP. Vascular invasion and herniation by hepatocellular carcinoma in cirrhosis: A wolf in sheep's clothing? Arch Pathol Lab Med 2005;129:639–644. doi: 10.1043/1543-2165(2005)129<0639:VIAHBH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 9.Sugino T, Yamaguchi T, Hoshi N, Kusakabe T, Oguza G, Goodison S, et al. Sinusoidal tumor angiogenesis is a key component in hepatocellular carcinoma metastasis. Clin Exp Metastasis 2008;25:835–841. doi: 10.1007/s10585-008-9199-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaneko T, Nakao A, Inoue S, Harada A, Nonami T, Itoh s, et al. , Intraportal endovascular ultrasonography in the diagnosis of portal vein invasion by pancreatobiliary carcinoma. Ann Surg 1995;222:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkins AK, Nevadunsky NS, Sleemudin A, Jarboe EA, Muto MG, Feltmate CM, et al. Evaluation of vascular space involvement in endometrial adenocarcinomas: laparoscopic vs abdominal hysterectomies. Mod Pathol 2010;23:1073–1079. doi: 10.1038/modpathol.2010.91 [DOI] [PubMed] [Google Scholar]
- 12.Al-Jarrah Q, Abou-Foul Ak, Heis H. Intravascular extension of papillary thyroid carcinoma to the internal jugular vein: A case report. Intl J Surg Case Rep 2014;5:551–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mete O, Asa SL. Pathological definition and clinical significance of vascular invasion in thyroid carcinomas of follicular epithelial derivation. Mod Pathol 2011;24:1545–1552. doi: 10.1038/modpathol.2011.119 [DOI] [PubMed] [Google Scholar]
- 14.Kukko HM, Koljonen VSK, Tukiainen EJ, Haglund CH, Bohling TO. Vascular invasion is an early event in pathogenesis of Merkel cell carcinoma. Mod Pathol 2010;23:1151–1156. doi: 10.1038/modpathol.2010.100 [DOI] [PubMed] [Google Scholar]
- 15.Philippar U, Roussos ET, Oser M, Yamaguchi H, Kim HD, Giampieri S, et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev Cell 2008;15:813–828. doi: 10.1016/j.devcel.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gertler F, Condeelis J. Metastasis: tumor cells becoming MENAcing. Trends Cell Biol 2011;21:81–90. doi: 10.1016/j.tcb.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roussos ET, Goswami S, Balsamo M, Wang Y, Stobezki R, Adler E, et al. Mena invasive (Mena(INV)) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin Exp Metastasis 2011;28:515–527. doi: 10.1007/s10585-011-9388-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang W, Mouneinme G, Sidani M, Wyckoff J, Chen X, Makris A, et al. The activity status of cofilin is directly related to invasion, intravasation, and metastasis of mammary tumors. J Cell Biol 2006;173:395–404. doi: 10.1083/jcb.200510115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol 2005;21:695–718. doi: 10.1146/annurev.cellbio.21.122303.120306 [DOI] [PubMed] [Google Scholar]
- 20.Pignatelli J, Goswami S, Jones JG, Rohan TE, Pieri E, Chen X, et al. Invasive breast carcinoma cells from patients exhibit MenaINV—and macrophage-dependent transendothelial migration. Sci Signal 2014;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patsialou A, Bravo-Cordero JJ, Wang Y, Entenberg D, Liu H, Clarke M, et al. Intravital multiphoton imaging reveals multicellular streaming as a crucial component of in vivo cell migration in human breast tumors. IntraVital 2013;2:e25295 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Wyckoff JB, Frohlich VC, Oleynikov Y, Huttelmaier S, Zavadil J, et al. Single cell behavior in metastatic primary mammary tumors correlated with gene expression patterns revealed by molecular profiling. Cancer Res 2002;62:6278–6288. [PubMed] [Google Scholar]
- 23.Wyckoff JB, Wang Y, Lin EY, Goswami S, Syanley ER, Segall JE, et al. Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res 2007;67:2649–2656. doi: 10.1158/0008-5472.CAN-06-1823 [DOI] [PubMed] [Google Scholar]
- 24.Baker EA, Stephenson TJ, Reed MW, Brown NJ. Expression of proteinases and inhibitors in human breast cancer progression and survival. Mol Pathol 2002;55:300–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshikawa N, Gianelli G, Cirulli V, Miyazaki K, Quaranta V. Role of cell surface metalloprotease MT1-MMP in epithelial cell migration over laminin-5. J Cell Biol 2000;148:615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 27.Asrani K, Keri RA, Galisteo R, Brown SA, Morgan SJ, Ghosh A, et al. The HER2- and heregulin β1 (HRG)-inducible TNFR superfamily member Fn14 promotes HRG-driven breast cancer cell migration, invasion, and MMP9 expression. Mol Cancer Res 2013;11:393–404. doi: 10.1158/1541-7786.MCR-12-0542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza m, et al. Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis. Oncogene 2014;33:2065–2074. doi: 10.1038/onc.2013.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin H, Eun SY, Lee JS, Park SW, Lee JH, Chang KC, et al. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res 2014;16:R77 doi: 10.1186/bcr3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung HW, Wang Z, Yue GG, Zhao SM, Lee JK, Fung KP, et al. Cyclopeptide RA-V inhibits cell adhesion and invasion in both estrogen receptor positive and negative breast cancer cells via PI3K/AKT and NF-κB signaling pathways. Biochim Biophys Acta 2015;1853:1827–1840. doi: 10.1016/j.bbamcr.2015.04.020 [DOI] [PubMed] [Google Scholar]
- 31.Perentes JY, Kirkpatrick ND, Nagano S, Smith EY, Shaver CM, Sgroi D, et al. Cancer cell-associated MT1-MMP promotes blood vessel invasion and distant metastasis in triple-negative mammary tumors. Cancer Res 2011;71:4527–4538. doi: 10.1158/0008-5472.CAN-10-4376 [DOI] [PubMed] [Google Scholar]
- 32.Rizwani W, Schaal C, Kunigal S, Coppola D, Chellappan S. Mammalian lysine histone demethylase KDM2A regulates E2F1-mediated gene transcription in breast cancer cells. PLoS ONE 2014;9:e100888 doi: 10.1371/journal.pone.0100888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puzovic V, Brcic I, Ranogaiec I, Rakic-Razumovic J. Prognostic values of ETS-1, MMP-2 and MMP-9 expression and co-expression in breast cancer patients. Neoplasma 2014;61:439–446. doi: 10.4149/neo_2014_054 [DOI] [PubMed] [Google Scholar]
- 34.Rabi ZA, Todorovic-Rakovicz N, Vujasinovic T, Milovanovic J, Nikolic-Vukosavljevic D. Markers of progression and invasion in short term follow up of untreated breast cancer patients. Cancer Biomark 2015;15:45–54. [DOI] [PubMed] [Google Scholar]
- 35.Van’t Veer LJ, He YD, van de Viiver MJ, Dai H, Hart AA, Voskuil DW, et al. Gene expression profiling predicts clinical outcome of breast cancer. N Engl J Med 2002;347:1999–2009. [DOI] [PubMed] [Google Scholar]
- 36.Vucemilo T, Skoko M, Sarcevic B, Puljiz M, Alvir I, Turudic TP, et al. The level of serum pro-matrix metalloproteinase-2 as a prognostic factor in patients with invasive ductal breast cancer. Coll Antropol 2014;38:135–140. [PubMed] [Google Scholar]
- 37.Hsu HH, Hu WS, Lin YM, Kuo WW, Chen LM, Chen WK, et al. JNK suppression is essential for 17β-Estradiol inhibits prostaglandin E2-Induced uPA and MMP-9 expressions and cell migration in human LoVo colon cancer cells. J Biomed Sci 2011;18:61 doi: 10.1186/1423-0127-18-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu HH, Liu CJ, Shen CY, Chen YJ, Chen LM, Kuo WH, et al. p38α MAPK mediates 17β-estradiol inhibition of MMP-2 and -9 expression and cell migration in human lovo colon cancer cells. J Cell Physiol 2012;227:3648–3660. doi: 10.1002/jcp.24072 [DOI] [PubMed] [Google Scholar]
- 39.Bouris P, Skandalis SS, Piperigkou Z, Afratis N, Karamanou K, Aletras AJ, et al. Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol 2015;43:42–60. doi: 10.1016/j.matbio.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 40.Maynadier M, Farnoud R, Lamy PJ, Laurent-Matha V, Garcia M, Rochefort H. Cathepsin D stimulates the activities of secreted plasminogen activators in the breast cancer acidic environment. Int J Oncol 2013;43:1683–1690. doi: 10.3892/ijo.2013.2095 [DOI] [PubMed] [Google Scholar]
- 41.Han M, Liu M, Wang Y, Chen X, Xu J, Sun Y, et al. Antagonism of miR-21 reverses epithelial-mesenchymal transition and cancer stem cell phenotype through AKT/ERK1/2 inactivation by targeting PTEN. PLoS ONE 2012;7:e39520 doi: 10.1371/journal.pone.0039520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhodes LV, Tate CR, Hoang VT, Burks HE, Gilliam D, Martin EC, et al. Regulation of triple-negative breast cancer cell metastasis by the tumor-suppressor liver kinase B1. Oncogenesis 2015;4:e168 doi: 10.1038/oncsis.2015.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Saleh S, Sharaf LH, Lugmani YA. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition (Review). Int J Oncol 2011;38:1197–1217. doi: 10.3892/ijo.2011.942 [DOI] [PubMed] [Google Scholar]
- 44.Xu Y, Qin L, Sun T, Wu H, He T, Yang Z, et al. Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene 2016; doi: 10.1038/onc.2016.286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang E, Cisowski J, Nguyen N, O’Callaghan K, Xu J, Agarwal A, et al. Dysregulated protease activated receptor 1 (PAR1) promotes metastatic phenotype in breast cancer through HMGA2. Oncogene 2016;35:1529–1540. doi: 10.1038/onc.2015.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thaiparambil JT, Bender L, Ganesh T, Kline E, Patel P, Liu Y, et al. Withaferin A inhibits breast cancer invasion and metastasis at sub-cytotoxic doses by inducing vimentin disassembly and serine 56 phosphorylation. Int J Cancer 2011;129:2744–2755. doi: 10.1002/ijc.25938 [DOI] [PubMed] [Google Scholar]
- 47.Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong G, et al. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Med Oncol 2014;31:180 doi: 10.1007/s12032-014-0180-8 [DOI] [PubMed] [Google Scholar]
- 48.Su P, Hu J, Zhang H, Li W, Jia M, Zhang X, et al. IMP3 expression is associated with epithelial-mesenchymal transition in breast cancer. Int J Clin Exp Pathol 2014;7:3008–3017. [PMC free article] [PubMed] [Google Scholar]
- 49.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol Cancer Res 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao YW, Wan GX, Sun JP, Cui XB, Hu JM, Liang WH, et al. Implications of the Notch1-Snail/Slug-epithelial to mesenchymal transition axis for lymph node metastasis in infiltrating ductal carcinoma. Kaohsiung J Med Sci 2015;31:70–76. doi: 10.1016/j.kjms.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 51.Fei F, Zhang D, Yang Z, Wang S, Wang X, Wu Z, et al. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J Exp Clin Cancer Res 2015;34:158 doi: 10.1186/s13046-015-0277-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murakami E, Nakanishi Y, Hirotani Y, Ohni S, Tang X, Masuda S, et al. Roles of Ras homolog A in invasive ductal breast carcinoma. Acta Histochem Cytochem 2016;49:131–140. doi: 10.1267/ahc.16020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassey-Archibong BI, Kwiecien JM, Milosavljevic SB, Hallett RM, Rayner LG, Erb MJ, et al. Kaiso depletion attenuates transforming growth factor-β signaling and metastatic activity of triple-negative breast cancer cells. Oncogenesis 2016;21: e208 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vermeulen JF, van de Ven RA, Ercan C, van der Groep P, van der Wall E, Bult P, et al. Nuclear Kaiso expression is associated with high grade and triple-negative invasive breast cancer. PLoS ONE 2012;7:e37864 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones J, Wang H, Karanam B, Theodore S, Dean-Colomb W, Welch DR, et al. Nuclear localization of Kaiso promotes the poorly differentiated phenotype and EMT in infiltrating ductal carcinomas. Clin Exp Metastasis 2014;31:497–510. doi: 10.1007/s10585-014-9644-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pierre CC, Longo J, Mavor M, Milosavljevic SB, Chaudhary R, Gilbreath E, et al. Kaiso overexpression promotes intestinal inflammation and potentiates intestinal tumorigenesis in Apc(Min/+) mice. Biochim Biophys Acta 2015;1852:1846–1855. doi: 10.1016/j.bbadis.2015.06.011 [DOI] [PubMed] [Google Scholar]
- 57.Jones J, Mukherjee A, Karanam B, Davis M, Jaynes J, Reams RR, et al. African Americans with pancreatic ductal adenocarcinoma exhibit gender differences in Kaiso expression. Cancer Lett 2016;380:513–522. doi: 10.1016/j.canlet.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Daniel JM, Ireton RC, Reynolds AB. Monoclonal antibodies to Kaiso: a novel transcription factor and p120ctn-binding protein. Hybridoma 2001;20:159–166. doi: 10.1089/027245701750293484 [DOI] [PubMed] [Google Scholar]
- 59.Donaldson NS, Norgaard CL, Pierre CC, Kelly KF, Robinson SC, Swystun L, et al. Kaiso regulates Znf131-mediated transcriptional activation. Exp Cell Res 2010;316:1692–1705. doi: 10.1016/j.yexcr.2010.03.011 [DOI] [PubMed] [Google Scholar]
- 60.Bassey-Archibong BI, Rayner LGA, Hercules SM, Aarts CW, Dvorkin-Gheva A, Bramson JL, et al. Kaiso depletion attenuates the growth and survival of triple negative breast cancer cells. Cell Death Dis 2017;8:e2689 doi: 10.1038/cddis.2017.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- 62.Jezierska A, Motyl T. Matrix metalloproteinase-2 involvement in breast cancer progression: a mini-review. Med Sci Monit 2009;15:RA32–40. [PubMed] [Google Scholar]
- 63.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014;5:2736–2749. doi: 10.18632/oncotarget.1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are available from Harvard Dataverse (https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/C8S8SN).