Abstract
Overexpression of multidrug-resistant efflux transporters is one of the major causes of chemotherapy failure. MRP1, a 190 kDa efflux transporter, confers resistance to a wide of range of chemotherapeutic drugs. Here we study the cellular effects of GSK1904529A in reversing MRP1-mediated drug resistance. Cytotoxicity of GSK1904529A was determined by MTT assay. Reversal effects of GSK1904529A in combination with MRP1 substrates were determined. The intracellular accumulation and efflux of MRP1 substrate was measured by scintillation counter and protein expression was determined by Western blotting analysis. Cell cycle effects of GSK1904529A in combination with MRP1 substrates were determined by flow cytometric analysis. GSK1904529A, at non-toxic concentrations, enhanced the cytotoxicity of MRP1 substrates in HEK293/MRP1 cells. Furthermore, GSK1904529A increased the intracellular accumulation of [3H]-vinblastine by inhibiting the efflux function of MRP1. GSK1904529A did not alter the expression level of MRP1, induced a G0/G1 phase cell cycle arrest. Our results indicated that GSK1904529A significantly increased the sensitivity of MRP1 overexpressing cells to chemotherapeutic agents. Furthermore, GSK1904529A enhanced the efficacy of chemotherapeutic drugs that are substrates of MRP1.
Keywords: GSK1904529A;, ABC transporters;, MRP1;, Multidrug-resistance
1. Introduction
Multidrug resistance (MDR) is the resistance to drugs that are different in their structures and mechanism of action [Gupta et al., 2016a; Kathawala et al., 2015a; Li et al., 2016; Zhang et al., 2015]. A number of cellular MDR mechanisms have been identified, and overexpression of ATP-binding cassette (ABC) transporters is considered to be the major factor causing MDR[Danks et al., 1988; Giaccone et al., 1992]. The ABC transporters have an efflux function, pumping out the substrate anti-cancer drugs such as vincristine, vinblastine, doxorubicin, and paclitaxel from the cancer cells[Shukla et al., 2012; Sodani et al., 2012].
Till date, 48 ABC transporters have been identified and classified into seven subfamilies from ABC-A through –G. Among them, ABCB1 (MDR1/P-glycoprotein), ABCG2/MXR/BCRP (breast cancer resistance protein), and ABCC1/MRP1 (multidrug resistance protein 1) are the primary determinants of MDR in cancer cells[Anreddy et al., 2014; Anreddy et al., 2015]. MRP1, a 190 kDa transporter, is the first discovered member of the C family of ABC transporters [Chen and Tiwari, 2011; Sodani et al., 2012]. MRPI was identified in the anthracycline resistant small-cell lung cancer cells, H69AR and was named as multidrug resistance protein 1 (MRP1)[Cole et al., 1992]. Studies conducted in 1994 first reported that MRP1 has a basolateral location and is responsible for the efflux of GSH and other xenobiotics[Müller et al., 1994]. Since MRP1 pumps out toxins from normal cells, its actions are considered protective and homeostatic. However, overexpression of MRP1 in cancer cells causes the efflux of anti-cancer drugs from tumors and thus its function is deleterious. Clinically, the overexpression of MRP1 has been known to cause chemotherapeutic drug resistance in childhood neuroblastomas, lung and oesophageal cancers[Burger et al., 1994; Norris et al., 1996].
Over the years a number of modulators have been developed and investigated for their blockade of MRP1-mediated drug transport. Drugs such as indomethacin, probenecid, MK571, and ONO-1078 have been successfully shown to inhibit MRP1, but their clinical significance is still questionable. Furthermore, small molecule tyrosine kinase inhibitors (TKIs) such as imatinib, ibrutinib, and AG1393 have been reported to inhibit MRP1 thus indicating the possible inhibitory action of TKIs [Hegedus et al., 2002; Zhang et al., 2014a; Zheng et al., 2009]. GSK1904529A is an inhibitor of the insulin-like growth factor-I receptor (IGF-IR) tyrosine kinase[Sabbatini et al., 2009]. IGF-IR regulates cellular proliferation, differentiation, and motility and has been known to cause breast, colon, prostate, ovarian, and pancreatic cancer [Pollak et al., 2004]. Recently, GSK1904529A was also reported to inhibit the glioma tumor growth both in vitro and in vivo[Zhou et al., 2015].
In the present study, we investigated whether GSK1904529A reverses MRP1-mediated MDR in cells overexpressing MRP1. We looked at the anti-proliferative effects of GSK1904529A in combination with known substrates of MRP1. In addition, the effects of GSK1904529A on MRP1 expression, efflux function and cell cycle arrest were elucidated.
2. Materials and Methods
2.1 Chemicals and reagents
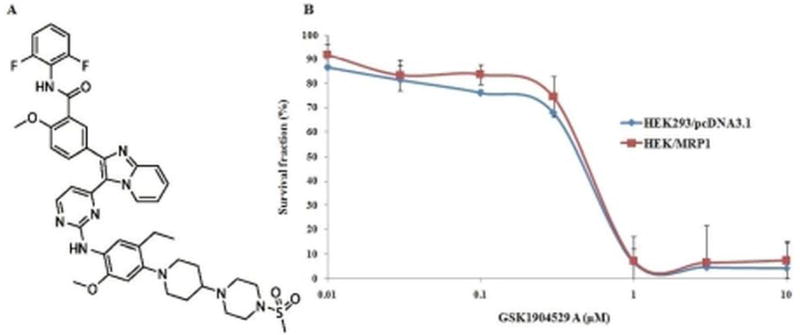
GSK1904529A (figure 1A) was obtained from ChemieTek (Indianapolis, IN). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), phosphate buffer saline (PBS), 10,000 IU/ml penicillin, 10,000 µg/ml streptomycin, and trypsin 0.25% were purchased from Hyclone (Waltham, MA). [3H]-vinblastine (25 Ci/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO). The monoclonal antibodies against MRP1 (D5C1X) and β-actin were purchased from Cell Signaling Technology Inc (Beverly, MA). Vinblastine, vincristine, cisplatin, MK571, Triton X-100, paraformaldehyde, 3-(4,5-dimethylthiazol-yl)-2,5-diphenyl-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) and other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). Propidium iodide (PI) was purchased from BD biosciences (San Jose, CA).
Figure 1. Cytotoxicity of GSK1904529A.
(A) The chemical structure of GSK1904529A (N-(2,6-difluorophenyl)-5-[3-[2-[5-ethyl-2-methoxy-4-[4-(4-methylsulfonylpiperazin-1-yl)piperidin-1-yl]anilino]pyrimidin-4-yl]imidazo[1,2-a]pyridin-2-yl]-2-methoxybenzamide). (B) Cytotoxicity of GSK1904529A was determined by the MTT assay in HEK293/pcDNA3.1 and HEK/MRP1 cells. Error bars indicate SD.
2.2 Cell lines and cell culture
HEK293/pcDNA3.1 and MRP1-transfected HEK293/MRP1 cell lines were kindly provided by Dr Suresh V. Ambudkar (NCI, NIH, Bethesda, MD)[Müller et al., 2002]. The MRP1 expressing KB/MRP1 cells were made by transfecting the parental human epidermoid carcinoma cell line KB-3-1 with the MRP1 plasmid. The cells were cultured in DMEM supplemented with 10% FBS and 1% PS at 37°C in a humidified atmosphere of 5% CO2. The cells were grown in monolayer in drug-free medium for more than 2 weeks before assay.
2.3 Cell viability assay
The cytotoxicity and reversal effects of GSK1904529A were determined by MTT colorimetric assay as described previously [Anreddy et al., 2015; Kathawala et al., 2015b]. Briefly, HEK293/pcDNA3.1 and HEK293/MRP1 cells were harvested and resuspended in a final concentration of 5 × 103 cells/well and seeded into 96 well plates. After 24h of incubation 20 µl of GSK1904529A at the indicated concentrations (0–10 µM) was added to determine the cytotoxicity. To determine the reversal effects of GSK1904529A, different concentrations chemotherapeutic drugs (20 µl/ well) was added after pre-incubation with GSK1904529A or MK571. After 72 h of incubation, MTT reagent (4 mg/ml) was added to each well and further incubated for 4 h. Subsequently, the MTT/medium was removed and 100 µl DMSO was added to dissolve the formazan crystals formed by the viable cells. Absorbance was the determined at 570 nm by Opsys microplate reader (Dynex Technologies, Chantilly, VA). IC50 (concentration required to inhibit the growth by 50%) was calculated from cell viability curves. Resistance folds were calculated by dividing the IC50 for the resistant cells with or without an inhibitor by that of the parental cells without an inhibitor. The concentrations of GSK1904529A as a potential reversal agent used in this study were 0.01 µM and 0.1 µM. MK571 at 25 µM was used as a positive control inhibitor of MRP1
2.4 [3H]-vinblastine accumulation assay
The accumulation of [3H]-vinblastine in HEK293/pcDNA3.1 and HEK293/MRP1 cells was measured in the presence or absence of GSK1904529A and MK571. Briefly, the cells were trypsinized and incubated in DMEM containing GSK1904529A (0.1 and 1 µM) and MK571 (50 µM) at 37°C for 2 h. Cells were further incubated in DMEM containing 0.01 µM [3H]-vinblastine with or without tan inhibitor at 37°C for 2 h. Subsequently, the cells were washed twice in ice cold PBS and lysed by 10 mM lysis buffer (pH 7.4, containing 1% Triton X-100 and 0.2% SDS). The lysed cells were placed in scintillation vials with 5 ml scintillation fluid and radioactivity was measured in a Packard TRI-CARB 1900CA liquid scintillation analyzer from Packard Instrument Company, Inc (Downers Grove, IL).
2.5 [3H]-vinblastine efflux assay
To measure the efflux of [3H]-vinblastine from HEK293/pcDNA3.1 and HEK293/MRP1 cells, the cells were incubated with 0.01 µM [3H]-vinblastine as described in the accumulation experiment. After washing two times with ice cold PBS, the cells were incubated in fresh DMEM at 37°C with or without an inhibitor. Aliquots of cell suspension were taken at 0, 30, 60, and 120 min and washed twice with ice cold PBS. Subsequently, the cells were lysed by 10 mM lysis buffer (pH 7.4, containing 1% Triton X-100 and 0.2% SDS) and placed in scintillation vials with 5 ml scintillation fluid. Radioactivity was measured in a Packard TRI-CARB 1900CA liquid scintillation analyzer from Packard Instrument Company, Inc (Downers Grove, IL).
2.6 Preparation of total cell lysates
The cells were treated with or without inhibitors at the indicated time periods (0, 24, 48, and 72h) and concentrations (0, 0.01, 0.05, and 0.1 µM). The treated and control cells were harvested and washed with ice cold PBS three times. Cell lysates were prepared with lysis buffer (10 mM Tris HCl, pH 7.5, 1 mM EDTA, 0.1% SDS, 150 mM NaCl, 1% Triton X-100 and 0.01% leupeptin) for 30 min on ice, followed by centrifugation at 12,000 rpm at 4°C for 20 min. The supernatant was collected and stored at −80°C for the Western blot experiment. Protein concentration was determined by bicinchoninic acid (BCA™)-based protein assay (Thermo Scientific, Rockford, IL).
2.7 Western blotting
Equal amounts of total cell lysates (60 µg protein) were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto polyvinylidene fluoride (PVDF) membranes. The PVDF membranes were blocked with 5% skim milk dissolved in TBST buffer (10 mmol·L−1 Tris-HCL, 150 mmol·L−1 NaCl and 0.1% Tween20 pH 8.0) to block non specific binding for 2 h at room temperature. The membranes were incubated overnight with primary monoclonal antibodies against MRP1 (1:500 dilution) and β-actin (at 1:1000 dilution) at 4°C. The membranes were further incubated with horseradish peroxide (HRP)-conjugated secondary antibody (1:1000 dilution) for 2 h at room temperature. The enhanced chemiluminescence detection system (Amersham, NJ) was used to detect the protein-antibody complex. The expression of β-actin was used as a loading control and the protein expression was quantified by ImageJ 1.47v Software (NIH, USA)
2.8 Cell cycle analysis
The cells were incubated with 0.1 nM vincristine alone or in combination with GSK1904529A (0.01 µM and 0.1 µM) for 72h. The cells were harvested and washed twice with PBS supplemented with 0.5% bovine serum albumin (BSA) at 4°C. The cell pellets were fixed overnight in ice cold 70% ethanol at 4°C. After washing with PBS the cells were stained with 50 µg/ml PI and 100 µg/ml RNase for 1 h at room temperature in the dark. The percentage of cells in specific phases of the cell cycle (G0/G1, S, and G2/M) was determined by flow cytometric analysis using a BD Accuri™ C6 flow cytometer (San Jose, CA)[Gupta et al., 2016b].
2.9 Statistical analysis
All experiments were repeated at least three times and the differences were determined by using the Student's t-test. The statistical significance was determined as P < 0.05.
3. Results
3.1 Cytotoxic effects of GSK1904529A on parental-sensitive and MRP1-overexpressing cells
Prior to investigating the reversal effects of GSK1904529A, the cytotoxicity was evaluated in MRP1 overexpressing and parental cells by MTT assay. As shown in figure 1B, although GSK1904529A is toxic to cells at the concentration than 1 µM, more than 85% of cells survived at the concentrations of 0.1 µM GSK1904529A, indicating that GSK1904529A is safe to be use at a concentration upto 0.1 µM. Based on these results, GSK1904529A at 0.01 µM and 0.1 µM was tested in combination with chemotherapeutic drugs for its ability to reverse MRP1-mediated MDR.
3.2 GSK1904529A increases the sensitivity of MRP1 overexpressing cells to the substrates of MRP1
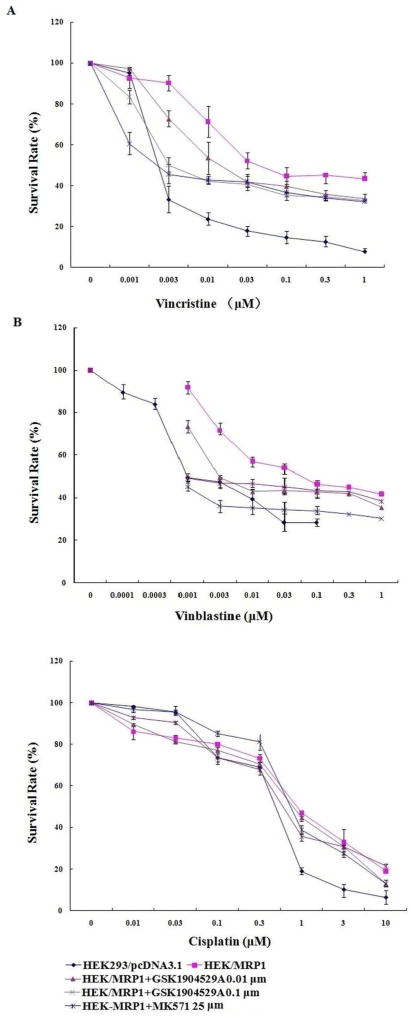
To investigate the reversal effects of GSK1904529A on MRP1 overexpresing cells, cell survival assays were performed in the presence and absence of GSK1904529A. Vincristine (figure 2A) and vinblastine (figure 2 B) were used as MRP1 substrates and cisplatin (figure 2C) was used as a non-MRP1 substrate. As shown in Table 1, the MRP1-overexpressing HEK293/MRP1 cells exhibited resistance to MRP1 substrates such as vincristine and vinblastine compared with HEK293/pcDNA3.1 cells. GSK1904529Aat 0.01 or 0.1 µM significantly sensitized HEK293/MRP1 cells to the MRP1 substrates but not to cisplatin. The sensitizing effect of GSK1904529A was more potent than that of MK571 (25 µM), a known inhibitor of MRP1.
Figure 2. GSK1904529A increases the sensitivity of MRP1 overexpressing cells to the substrates of MRP1.
HEK293/pcDNA3.1 and HEK/MRP1 cells were treated with vincristine (A), vinblastine (B), and cisplatin (C) alone or in combination with GSK1904529A at 0.01 and 0.1 µM. MTT assay was carried out to determine the change in resistance fold. MK571 at 25 µM was used as a positive control inhibitor of MRP1 and cisplatin was used as negative control substrate drug. Points with error bars represent the mean ± SD. Each of the above figures is a representative of three independent experiments, each done in triplicate.
Table 1.
The effect of GSK1904529A on reversal of MRP1-mediated MDR in HEK293/pcDNA3.1 and HEK293/MRP1 cells
| IC50 ±SDa (nM) (FRb) | ||
|---|---|---|
|
|
||
| Compounds | HEK293/pcDNA3.1 | HEK293/MRP1 |
| Vincristine | 2.42 ± 0.52 (1.00) | 31.16 ± 9.86 (12.85) |
| +0.01 µM GSK1904529A | 2.40 ± 0.66 (0.99) | 16.75 ± 0.02 (6.91)c |
| +0.1 µM GSK1904529A | 0.99 ± 0.08 (0.41) | 4.40 ± 2.90 (1.82)d |
| +25 µM MK571 | 1.69 ± 0.82 (0.70) | 2.55 ± 0.49 (1.05)d |
|
| ||
| Vinblastine | 0.99 ± 0.01 (1.00) | 60.31 ± 0.38 (60.92) |
| +0.01 µM GSK1904529A | 0.87 ± 0.01 (0.88) | 3.07 ± 0.15 (3.10)d |
| +0.1 µM GSK1904529A | 0.73 ± 0.03 (0.74) | 0.90 ± 0.01 (0.91)d |
| +25 µM MK571 | 0.46 ± 0.04 (0.46) | 1.05 ± 0.01 (1.02)d |
|
| ||
| Cisplatin | 566.56 ± 6.52 (1.00) | 955.50 ±39.29 (1.68) |
| +0.01 µM GSK1904529A | 600.83 ± 2.20 (1.06) | 846.63 ± 5.47 (1.49) |
| +0.1 µM GSK1904529A | 553.56 ± 25.05 (0.98) | 811.86 ± 29.40 (1.43) |
| +25 µM MK571 | 678.00 ± 45.86 (1.20) | 708.29 ± 2.88 (1.25) |
IC50 values are represented as mean ± SD of at least three independent experiments performed in triplicate
FR: Resistance fold was calculated by dividing the IC50 values of substrates in the presence or absence of inhibitor by the IC50 of parental cells without inhibitor
P < 0.05,
P<0.01 versus the control group without reversal agent
3.3 GSK1904529A increases the intracellular accumulation of [3H]-vinblastine in MRP1 overexpressing cells
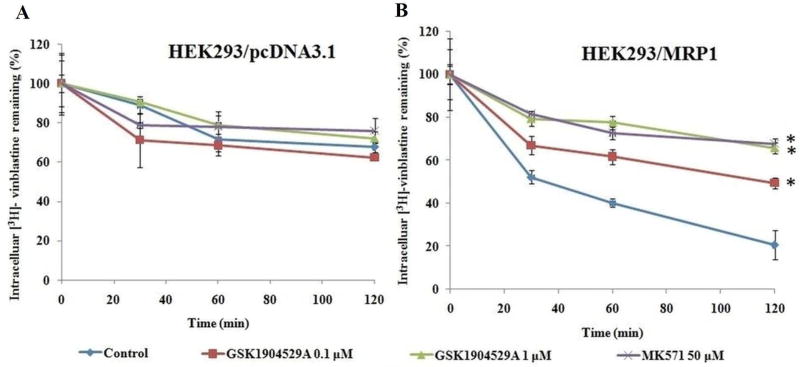
In order to determine the mechanism of action responsible for GSK1904529A-mediated reversal of MRP1 activity, we investigated the effect of GSK1904529A on the intracellular accumulation of [3H]-vinblastine in HEK293/pcDNA3.1 and HEK293/MRP1 cells. The intracellular accumulation of [3H]-vinblastine were measured in the presence or absence of GSK1904529A at 0.1 µM and 1 µM. MK571 (50 µM) was used as a positive control. The intracellular accumulation of [3H]-vinblastine in HEK293/MRP1 cells were significantly lower than those in HEK293/pcDNA3.1 cells, after incubation with [3H]-vinblastine for 2 h. (figures 3A and 3B). Treatment with GSK1904529A significantly increased the intracellular accumulation of [3H]-vinblastine in HEK293/MRP1, in a concentration-dependent manner. Moreover, GSK1904529A at 0.1 µM and 1 µM had no effect on the intracellular accumulation levels of [3H]-vinblastine in HEK293/pcDNA3.1 cells, indicating that its action is specific to MRP1 efflux function. In addition, the effects obtained from GSK1904529A treatment were comparable to the intracellular accumulation of [3H]-vinblastine achieved by the treatment with MK571 at 50 µM.
Figure 3. GSK1904529A increased the intracellular accumulation of [3H]-vinblastine.
The accumulation of [3H]-vinblastine was measured after HEK293/pcDNA3.1 (A) and HEK293/MRP1 (B) cells were preincubated with or without GSK1904529A or MK571 for 2 h at 37°C and then incubated with 0.01 µM [3H]-vinblastine for another 2 h at 37°C. Error bars indicate SD. **: p < 0.01 versus the control group.
3.4 GSK1904529A decreases the efflux of [3H]-vinblastine in MRP1 overexpressing cells
To ascertain that the increase in intracellular accumulation of [3H]-vinblastine was due to inhibition of MRP1 efflux function, we determined the efflux of [3H]-vinblastine from HEK293/pcDNA3.1 and HEK293/MRP1 cells. The cells were treated with GSK1904529A at 0.1 µM and 1 µM and MK571 at 50 µM for different time periods (0, 30, 60, and 120 min). Our results indicated that in the absence of GSK1904529A, the remaining intracellular amount of [3H]-vinblastine in HEK293/MRP1 cells was significantly lower than that of HEK293/pcDNA3.1 cells, due to the efflux of [3H]-vinblastine by MRP1 transporter (figures 4A and 4B. However, in the presence of GSK1904529A, the efflux of [3H]-vinblastine from HEK293/MRP1 cells significantly decreased in a time-dependent manner. Moreover, these results were comparable to the decrease in efflux of [3H]-vinblastine by MK571 at 50µM.
Figure 4. GSK1904529A decreased the efflux of [3H]-vinblastine.
The effect of GSK1904529A on the efflux of [3H]-vinblastine from HEK293/pcDNA3.1 (A) and HEK293/MRP1 (B) cells was measured. A time-dependent decrease of efflux of [3H]-vinblastine was found (0, 30, 60 and 120 min). Data shown are means ± SDs from three independent determinations, in triplicate. * P < 0.05, significantly different from control.
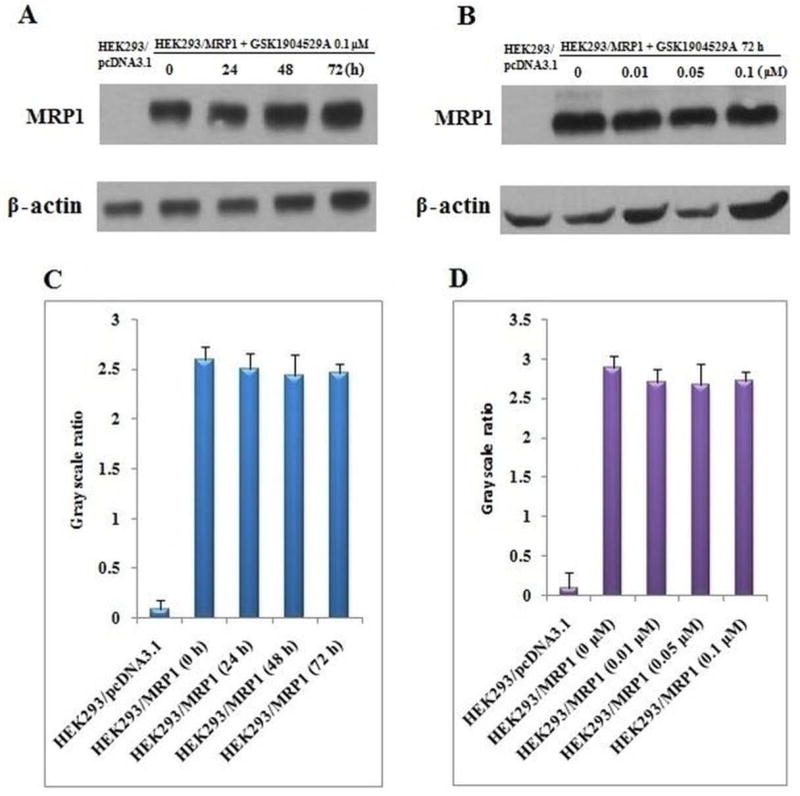
3.5 GSK1904529A does not alter the expression of MRP1
The reversal effects of GSK1904529A could be either due to the blockade of the efflux function or a decrease in the expression of MRP1 transporter. We conducted Western blot analysis to determine the effect of GSK1904529A on the expression of MRP1. As shown in figure 5, a protein band with a molecular weight of approximately 190-kDa appeared in the HEK293/MRP1 cell lysates but not in the HEK293/pcDNA3.1 cell lysates, suggesting the presence of MRP1 protein in HEK293/MRP1 cells. On treatment with GSK1904529A, the MRP1 protein expression of HEK293/MRP1 cell lysates remained unaltered at different time and concentration (figures 5 A–D). These results indicated that the sensitization effect of GSK1904529A did not result from the alteration of MRP1 expression.
Figure 5. GSK1904529A did not alter the expression levels of MRP1.
(A) The effect of GSK1904529A on the protein levels of MRP1 was tested after HEK293/MRP1 cells were treated with 0.1 µM GSK1904529A for 0, 24, 48 and 72 h. (B) The effect of GSK1904529A on the protein levels of MRP1 was tested after HEK293/MRP1 cells were treated with 0.01, 0.05, 0.1 µM GSK1904529A for 72 h. (C and D) ImageJ was used to analyze the grayscale ratios of MRP1/β-actin. The greyscale ratios were proportional to the MRP1 protein levels.
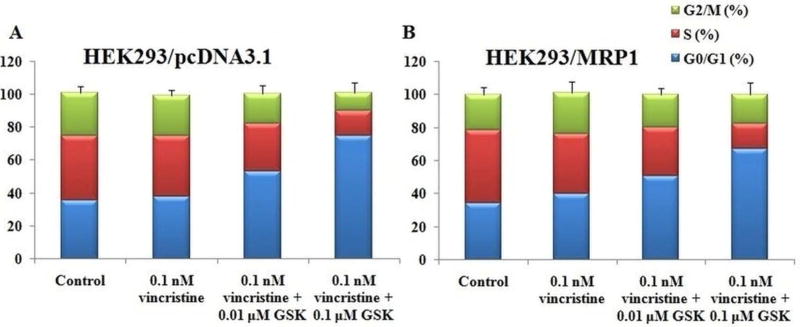
3.6 GSK1904529A potentiates the cell cycle effects of vincristine
To further elucidate the cellular mechanism of action of GSK1904529A, we determined the cell cycle effects of GSK1904529A in combination with vincristine. The cells were treated with vincristine in the presence or absence of GSK1904529A for 72 h and stained with PI to analyze the distribution of cells in specific phase of cell cycle (G0/G1, S, and G2/M). As shown in figure 6, treatment with 0.1 nM vincristine arrested the cells in G0/G1 phase of cell cycle as compared to the control, in both HEK293/pcDNA3.1 and HEK293/MRP1 cells. On combination with GSK1904529A, a dose-dependent increase in the arrest of cells in G0/G1 phase was observed (Table 2). These results suggested that GSK1904529A augments the cell cycle effects of vincristine in HEK293/pcDNA3.1 and HEK293/MRP1 cells.
Figure 6. Effect of GSK1904529A on the cell cycle of HEK293/pcDNA3.1 and HEK293/MRP1cells.
HEK293/pcDNA3.1 (A) and HEK293/MRP1 (B) cells were treated with vincristine alone or in combination with GSK1904529A. Cells were harvested and stained with propidium iodide (PI) and analyzed by flow cytometer for cell cycle. Quantification of cell population in G0/G1, S, and G2/M phase of the cell cycle was done.
Table 2.
The effect of GSK1904529A on the cell cycle of HEK293/pcDNA3.1 and HEK293/MRP1 cells
| Compounds | HEK293/pcDNA3.1 | ||
| G0/G1 (%) ± SD | S (%) ± SD | G2/M (%) ± SD | |
| Control | 35.3 ± 3.3 | 39.3 ± 2.1 | 26.14 ± 2.1 |
| 0.1 nM vincristine | 38 ± 1.3 | 36.59 ± 1.4 | 24.8 ± 2.4 |
| 0.1 nM vincristine + 0.01 µM GSK1904529A | 53 ± 4.2 | 29.34 ± 1.5 | 17.8 ± 0.8 |
| 0.1 nM vincristine + 0.1 µM GSK1904529A | 75 ± 4.1 | 15.1 ± 0.7 | 11 ± 0.4 |
| Compounds | HEK293/MRP1 | ||
| G0/G1 (%) ± SD | S (%) ± SD | G2/M (%) ± SD | |
| Control | 34.23 ± 2.8 | 44.3 ± 3.1 | 21.14 ± 1.8 |
| 0.1 nM vincristine | 39.8 ± 4.3 | 36.59 ± 4.1 | 24.8 ± 1.3 |
| 0.1 nM vincristine + 0.01 µM GSK1904529A | 50.7 ± 3.1 | 29.34 ± 1.4 | 19.8 ± 0.7 |
| 0.1 nM vincristine + 0.1 µM GSK1904529A | 67 ± 6.1 | 15.1 ± 0.5 | 18 ± 1.2 |
4. Discussion
A number of small molecule TKIs has been reported as potent inhibitors of multi-drug efflux transporters at non-toxic concentrations [Kathawala et al., 2014; Kathawala et al., 2015b; Wang et al., 2014a; Wang et al., 2014b; Zhang et al., 2014b]. In this study we investigated if GSK1904529A, a potent IGF-IR inhibitor, could be an inhibitor of ABC transporters. The cytotoxicity of GSK1904529A in parental HEK293/pcDNA3.1 and MRP1 overexpressing HEK293/MRP1 cells was evaluated and it was found that GSK1904529A had little cytotoxicity at the concentration up to 0.1 µM. We used 0.1 µM to study the reversal effects of GSK1904529A on HEK293/pcDNA3.1 and MRP1 overexpressing HEK293/MRP1 cells. One of the major findings of this study was that GSK1904529A, at 0.1 µM, significantly enhanced the sensitivity of HEK293/MRP1 cells to the substrates of MRP1. This effect was more potent than that of MK571[Burns et al., 1995], a well-known inhibitor of MRP1 efflux function. Moreover, GSK1904529A did not enhance the toxic effects of cisplatin, a drug that is not a substrate for the MRP1 transporter. This suggested that GSK1904529A is specific in its action on the substrates of the MRP1 transporter. In addition, GSK1904529A at 0.01 and 0.1 µM reversed MRP1 mediated MDR to vincristine and vinblastine in MRP1 expressing human epidermoid carcinoma cell line KB/MRP1. (Supplement figure 1).
Our findings also indicated that GSK1904529A produces a significant concentration-dependent increase in the intracellular accumulation of [3H]-vinblastine in HEK293 cells expressing MRP1. Furthermore, GSK1904529A produces a significant concentration-dependent decrease in the efflux of [3H]-vinblastine from cells overexpressing the MRP1 transporter. This effect was significantly better than MK571 at 50 µM, thus, suggesting that GSK1904529A increases the sensitivity of MRP1-overexpressing cells to vinblastine by inhibiting its efflux from the cells. Our results were consistent with Zhang et al. who reported a potent inhibitor of MRP1 as compared to 50 µM MK571 [Zhang et al., 2014a]. To understand the effects of GSK1904529A on the expression levels of the MRP1 protein, concentration and time-dependent immunoblot assays were conducted. GSK1904529A at 0.1 µM and upto 72 h did not significantly alter the expression of the MRP1 protein. This finding suggests that GSK1904529A re-sensitizes HEK293/MRP1 cells to its substrates without significantly altering its expression. Furthermore, our mechanistic study showed that a combination of 0.1 nM vincristine and 0.1 µM GSK1904529A induces a G0/G1-mediated cell cycle arrest in HEK293/MRP1 cells. Interestingly, this effect was significantly more than that of vincristine alone group. This finding suggested that GSK1904529A potentiates the cell cycle effects of vincristine in HEK293/pcDNA3.1 and HEK293/MRP1 cells.
In conclusion, this is the first study to report the reversal effects of GSK1904529A in cells overexpressing the MRP1 transporter. The inhibitory activity is due to the blockade of efflux function of MRP1 transporter and not due to the alteration of its expression. However, the exact mechanism of interaction of GSK1904529A with the MRP1 transporter is yet to be explored. Combination of GSK1904529A with the substrates of MRP1 could be of great interest in clinical applications and thus further clinical studies are warranted.
Supplementary Material
Acknowledgments
CONTRACT GRANT SPONSOR: St. John's University Research Seed Grant.
CONTRACT GRANT NUMBER: 579-1110-7002.
The authors thank Dr. Suresh Ambudkar for providing HEK293/pcDNA3.1 and HEK293/MRP1 cells (NCI, NIH, Bethesda, MD) and late Dr. Kruh GD (UIC Cancer Center, University of Illinois at Chicago College of Medicine, Chicago, IL) for proving us the MRP1 plasmid.
Footnotes
CONFLICTS OF INTEREST: No potential conflicts of interest were disclosed.
References
- Anreddy N, Gupta P, Kathawala RJ, Patel A, Wurpel JN, Chen ZS. Tyrosine kinase inhibitors as reversal agents for ABC transporter mediated drug resistance. Molecules. 2014;19:13848–77. doi: 10.3390/molecules190913848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anreddy N, Patel A, Zhang YK, Wang YJ, Shukla S, Kathawala RJ, Kumar P, Gupta P, Ambudkar SV, Wurpel JN, Chen ZS, Guo H. A-803467, a tetrodotoxin-resistant sodium channel blocker, modulates ABCG2-mediated MDR in vitro and in vivo. Oncotarget. 2015;6:39276–91. doi: 10.18632/oncotarget.5747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger H, Nooter K, Zaman GJ, Sonneveld P, van Wingerden KE, Oostrum RG, Stoter G. Expression of the multidrug resistance-associated protein (MRP) in acute and chronic leukemias. Leukemia. 1994;8:990–7. [PubMed] [Google Scholar]
- Burns BM, Taylor JF, Herring KL, Herring AD, Holder MT, Holder DA, Collins JS, Sanders JO, Davis SK. Bovine microsatellite dinucleotide repeat polymorphisms at the TEXAN16, TEXAN17, TEXAN18, TEXAN19 and TEXAN20 loci. Anim Genet. 1995;26:208–9. doi: 10.1111/j.1365-2052.1995.tb03174.x. [DOI] [PubMed] [Google Scholar]
- Chen ZS, Tiwari AK. Multidrug resistance proteins (MRPs/ABCCs) in cancer chemotherapy and genetic diseases. FEBS J. 2011;278:3226–45. doi: 10.1111/j.1742-4658.2011.08235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–4. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Danks MK, Schmidt CA, Cirtain MC, Suttle DP, Beck WT. Altered catalytic activity of and DNA cleavage by DNA topoisomerase II from human leukemic cells selected for resistance to VM-26. Biochemistry. 1988;27:8861–9. doi: 10.1021/bi00424a026. [DOI] [PubMed] [Google Scholar]
- Giaccone G, Gazdar AF, Beck H, Zunino F, Capranico G. Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res. 1992;52:1666–74. [PubMed] [Google Scholar]
- Gupta P, Jani KA, Yang DH, Sadoqi M, Squillante E, Chen ZS. Revisiting the role of nanoparticles as modulators of drug resistance and metabolism in cancer. Expert Opin Drug Metab Toxicol. 2016a;12:281–9. doi: 10.1517/17425255.2016.1145655. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kathawala RJ, Wei L, Wang F, Wang X, Druker BJ, Fu LW, Chen ZS. PBA2, a novel inhibitor of imatinib-resistant BCR-ABL T315I mutation in chronic myeloid leukemia. Cancer Lett. 2016b doi: 10.1016/j.canlet.2016.09.025. [DOI] [PubMed] [Google Scholar]
- Hegedus T, Orfi L, Seprodi A, Váradi A, Sarkadi B, Kéri G. Interaction of tyrosine kinase inhibitors with the human multidrug transporter proteins, MDR1 and MRP1. Biochim Biophys Acta. 2002;1587:318–25. doi: 10.1016/s0925-4439(02)00095-9. [DOI] [PubMed] [Google Scholar]
- Kathawala RJ, Chen JJ, Zhang YK, Wang YJ, Patel A, Wang DS, Talele TT, Ashby CR, Chen ZS. Masitinib antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance. Int J Oncol. 2014;44:1634–42. doi: 10.3892/ijo.2014.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathawala RJ, Gupta P, Ashby CR, Chen ZS. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist Updat. 2015a;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Kathawala RJ, Wei L, Anreddy N, Chen K, Patel A, Alqahtani S, Zhang YK, Wang YJ, Sodani K, Kaddoumi A, Ashby CR, Chen ZS. The small molecule tyrosine kinase inhibitor NVP-BHG712 antagonizes ABCC10-mediated paclitaxel resistance: a preclinical and pharmacokinetic study. Oncotarget. 2015b;6:510–21. doi: 10.18632/oncotarget.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhang H, Assaraf YG, Zhao K, Xu X, Xie J, Yang DH, Chen ZS. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist Updat. 2016;27:14–29. doi: 10.1016/j.drup.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Müller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994;91:13033–7. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Yong M, Peng XH, Petre B, Arora S, Ambudkar SV. Evidence for the role of glycosylation in accessibility of the extracellular domains of human MRP1 (ABCC1) Biochemistry. 2002;41:10123–32. doi: 10.1021/bi026075s. [DOI] [PubMed] [Google Scholar]
- Norris MD, Bordow SB, Marshall GM, Haber PS, Cohn SL, Haber M. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med. 1996;334:231–8. doi: 10.1056/NEJM199601253340405. [DOI] [PubMed] [Google Scholar]
- Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–18. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- Sabbatini P, Rowand JL, Groy A, Korenchuk S, Liu Q, Atkins C, Dumble M, Yang J, Anderson K, Wilson BJ, Emmitte KA, Rabindran SK, Kumar R. Antitumor activity of GSK1904529A, a small-molecule inhibitor of the insulin-like growth factor-I receptor tyrosine kinase. Clin Cancer Res. 2009;15:3058–67. doi: 10.1158/1078-0432.CCR-08-2530. [DOI] [PubMed] [Google Scholar]
- Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012;15:70–80. doi: 10.1016/j.drup.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodani K, Patel A, Kathawala RJ, Chen ZS. Multidrug resistance associated proteins in multidrug resistance. Chin J Cancer. 2012;31:58–72. doi: 10.5732/cjc.011.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DS, Patel A, Shukla S, Zhang YK, Wang YJ, Kathawala RJ, Robey RW, Zhang L, Yang DH, Talele TT, Bates SE, Ambudkar SV, Xu RH, Chen ZS. Icotinib antagonizes ABCG2-mediated multidrug resistance, but not the pemetrexed resistance mediated by thymidylate synthase and ABCG2. Oncotarget. 2014a;5:4529–42. doi: 10.18632/oncotarget.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Kathawala RJ, Zhang YK, Patel A, Kumar P, Shukla S, Fung KL, Ambudkar SV, Talele TT, Chen ZS. Motesanib (AMG706), a potent multikinase inhibitor, antagonizes multidrug resistance by inhibiting the efflux activity of the ABCB1. Biochem Pharmacol. 2014b;90:367–78. doi: 10.1016/j.bcp.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Patel A, Ma SL, Li XJ, Zhang YK, Yang PQ, Kathawala RJ, Wang YJ, Anreddy N, Fu LW, Chen ZS. In vitro, in vivo and ex vivo characterization of ibrutinib: a potent inhibitor of the efflux function of the transporter MRP1. Br J Pharmacol. 2014a;171:5845–57. doi: 10.1111/bph.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhang YK, Wang YJ, Kathawala RJ, Patel A, Zhu H, Sodani K, Talele TT, Ambudkar SV, Chen ZS, Fu LW. WHI-P154 enhances the chemotherapeutic effect of anticancer agents in ABCG2-overexpressing cells. Cancer Sci. 2014b;105:1071–8. doi: 10.1111/cas.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YK, Wang YJ, Gupta P, Chen ZS. Multidrug Resistance Proteins (MRPs) and Cancer Therapy. AAPS J. 2015;17:802–12. doi: 10.1208/s12248-015-9757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng LS, Wang F, Li YH, Zhang X, Chen LM, Liang YJ, Dai CL, Yan YY, Tao LY, Mi YJ, Yang AK, To KK, Fu LW. Vandetanib (Zactima, ZD6474) antagonizes ABCC1- and ABCG2-mediated multidrug resistance by inhibition of their transport function. PLoS One. 2009;4:e5172. doi: 10.1371/journal.pone.0005172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Zhang J, Cui Q, Li X, Gao G, Wang Y, Xu Y, Gao X. GSK1904529A, an insulin-like growth factor-1 receptor inhibitor, inhibits glioma tumor growth, induces apoptosis and inhibits migration. Mol Med Rep. 2015;12:3381–5. doi: 10.3892/mmr.2015.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.