Summary
Background
Pneumococcal conjugate vaccines (PCVs) are used in many low-income countries but their impact on the incidence of pneumonia is unclear. The Gambia introduced PCV7 in August, 2009, and PCV13 in May, 2011. We aimed to measure the impact of the introduction of these vaccines on pneumonia incidence.
Methods
We did population-based surveillance and case-control studies. The primary endpoint was WHO-defined radiological pneumonia with pulmonary consolidation. Population-based surveillance was for suspected pneumonia in children aged 2–59 months (minimum age 3 months in the case-control study) between May 12, 2008, and Dec 31, 2015. Surveillance for the impact study was limited to the Basse Health and Demographic Surveillance System (BHDSS), whereas surveillance for the case-control study included both the BHDSS and Fuladu West Health and Demographic Surveillance System. Nurses screened all outpatients and inpatients at all health facilities in the surveillance area using standardised criteria for referral to clinicians in Basse and Bansang. These clinicians recorded clinical findings and applied standardised criteria to identify patients with suspected pneumonia. We compared the incidence of pneumonia during the baseline period (May 12, 2008, to May 11, 2010) and the PCV13 period (Jan 1, 2014, to Dec 31, 2015). We also investigated the effectiveness of PCV13 using case-control methods between Sept 12, 2011, and Sept 31, 2014. Controls were aged 90 days or older, and were eligible to have received at least one dose of PCV13; cases had the same eligibility criteria with the addition of having WHO-defined radiological pneumonia.
Findings
We investigated 18 833 children with clinical pneumonia and identified 2156 cases of radiological pneumonia. Among children aged 2–11 months, the incidence of radiological pneumonia fell from 21·0 cases per 1000 person-years in the baseline period to 16·2 cases per 1000 person-years (23% decline, 95% CI 7–36) in 2014–15. In the 12–23 month age group, radiological pneumonia decreased from 15·3 to 10·9 cases per 1000 person-years (29% decline, 12–42). In children aged 2–4 years, incidence fell from 5·2 to 4·1 cases per 1000 person-years (22% decline, 1–39). Incidence of all clinical pneumonia increased by 4% (–1 to 8), but hospitalised cases declined by 8% (3–13). Pneumococcal pneumonia declined from 2·9 to 1·2 cases per 1000 person-years (58% decline, 22–77) in children aged 2–11 months and from 2·6 to 0·7 cases per 1000 person-years (75% decline, 47–88) in children aged 12–23 months. Hypoxic pneumonia fell from 13·1 to 5·7 cases per 1000 person-years (57% decline, 42–67) in children aged 2–11 months and from 6·8 to 1·9 cases per 1000 person-years (72% decline, 58–82) in children aged 12–23 months. In the case-control study, the best estimate of the effectiveness of three doses of PCV13 against radiological pneumonia was an adjusted odds ratio of 0·57 (0·30–1·08) in children aged 3–11 months and vaccine effectiveness increased with greater numbers of doses (p=0·026). The analysis in children aged 12 months and older was underpowered because there were few unvaccinated cases and controls.
Interpretation
The introduction of PCV in The Gambia was associated with a moderate impact on the incidence of radiological pneumonia, a small reduction in cases of hospitalised pneumonia, and substantial reductions of pneumococcal and hypoxic pneumonia in young children. Low-income countries that introduce PCV13 with reasonable coverage can expect modest reductions in hospitalised cases of pneumonia and a marked impact on the incidence of severe childhood pneumonia.
Funding
GAVI's Pneumococcal vaccines Accelerated Development and Introduction Plan, Bill & Melinda Gates Foundation, and UK Medical Research Council.
Research in context.
Evidence before this study
We did a systematic literature search on PubMed, Embase, and Web of Science for observational studies of the impact of pneumococcal conjugate vaccine (PCV) published between Jan 1, 2008, and Jan 15, 2017. We searched PubMed using the MeSH terms “pneumococcal vaccines”, “vaccines, conjugate”, “pneumonia”, and the (All Fields) terms “pneumococcal”, “conjugate vaccines”, “pneumonia”, “impact”, and “effectiveness”, restricting the search to childhood populations. For other data sources, we used the key search terms as above. We searched for prospective case-control and population-based longitudinal studies of childhood pneumonia that included at least 2 years of data following the introduction of PCV. After reviewing 225 articles, 27 met inclusion criteria; PCV7 impact was measured in eight, PCV10/13 impact in 19, and three were case-control studies. We found no eligible reports from low-income countries. Reports varied in quality and most reported administrative data on hospital admissions without ensuring consistency of procedures for the investigation of patients. The reports showed consistent evidence of an overall reduction of variable magnitude in childhood hospital admissions coded as pneumonia after the introduction of PCV7 (13–43%) and PCV10/13 (13–72%). Studies of the impact of PCV10/13 showed consistent evidence of an overall reduction in hospital admissions with radiological pneumonia (16–72%) and WHO-defined radiological pneumonia with consolidation (27–47%). Case-control studies reported 21–39% effectiveness against presumed bacterial pneumonia, 77% effectiveness against pneumococcal pneumonia, and 72% effectiveness against death from pneumonia.
Added value of this study
This study showed substantial relative and absolute reductions in severe pneumonia in young children 4 years after the introduction of PCV13. We report reductions in the standard endpoints of hospital admission for pneumonia and WHO-defined radiological pneumonia with consolidation, as well as large reductions in proven pneumococcal and hypoxic pneumonia. This study used standardised procedures for prospective case ascertainment over 8 years and thus provides new and robust evidence of the impact of PCV introduction on pneumonia in a low-income country. Our findings are relevant to many low-income countries because the study was based in a typical African population, where the PCV programme used a standard schedule without a catch-up campaign, as is the case in almost all low-income countries. These data will be valuable to countries that need data from a low-income country to support their policy and financing for pneumococcal vaccination.
Implications of all available evidence
The routine use of PCV13, with a standard schedule and reasonable coverage, will reduce the burden of hospital admissions for pneumonia and substantially reduce severe pneumonia in low-income and middle-income countries, where pneumococcal disease burden and deaths are greatest. The ongoing burden of pneumonia emphasises the importance of developing new vaccines and interventions. Robust data to assess the impact of PCV in Africa and Asia are scarce and ongoing high-quality impact studies provide crucial data to inform global decision making about vaccination with PCV.
Introduction
In 2013, pneumonia caused an estimated 935 000 childhood deaths worldwide.1 The most common cause of severe pneumonia is Streptococcus pneumoniae2, 3, 4 and pneumonia is estimated to cause 90% of pneumococcal deaths.5 In high-income countries, pneumococcal conjugate vaccines (PCVs) have reduced the incidence of childhood pneumonia.6, 7, 8 54 low-income countries have introduced PCV, yet data from these settings on the effect of the vaccine against pneumonia are scarce.
The Gambia has a high burden of pneumonia.9 Results from a trial of a nonavalent PCV (PCV9) in The Gambia from 2000 to 2004, showed an efficacy of 37% against WHO-defined radiological pneumonia and that 58% of radiological pneumonia was caused by the PCV9 serotypes.9 The Government of The Gambia introduced PCV7 into the Expanded Programme on Immunisation (EPI) on Aug 19, 2009. The schedule consisted of three doses given at ages 2, 3, and 4 months. Children younger than 6 months were eligible to receive all three doses, whereas older children were eligible for one dose. There was no catch-up campaign. PCV13 was introduced at EPI clinics in the study area during May, 2011, using the same three-dose schedule. In this study, we aimed to measure the impact and effectiveness of routine infant vaccination with PCV on pneumonia in children in The Gambia.
Methods
Surveillance study
We did population-based surveillance for suspected pneumonia, septicaemia, and meningitis in the Basse Health and Demographic Surveillance System (BHDSS; appendix p 8; population of 171 269 in 2012) between May 12, 2008, and Dec 31, 2015. The surveillance population included all residents aged 2 months or older and was enumerated every 4 months. This surveillance analysis was restricted to children aged 2–59 months.
Case-control study
We did a case-control study to estimate the effectiveness of PCV13 against radiological pneumonia with consolidation between Sept 12, 2011, and Sept 31, 2014. During this period, surveillance was extended to all residents younger than 5 years in the Fuladu West Health and Demographic Surveillance System (FWHDSS; appendix p 8), as well as in the BHDSS. The FWHDSS population was enumerated annually. Cases had WHO-defined radiological pneumonia and were aged 90 days or older (ie, aged ≥3 months) and eligible to have received at least one dose of PCV13; matched community controls had to meet the same eligibility criteria, with the exception of the radiological pneumonia. All cases in both the surveillance and case-control studies were confirmed residents in the study area. Children who had received three doses of PCV7 were ineligible for the case-control study and those who had received one or more dose of PCV7 were excluded from the case-control analysis. Cases and controls were excluded if they had a major congenital abnormality or suspected or confirmed immune deficiency. Individuals were eligible to be selected as controls more than once but were ineligible if they had previously been enrolled as a case (appendix p 5). For each case, three community controls were randomly selected from the population register matched on the date of birth plus or minus 15 days. Controls were enrolled at home visits within 3 months of case enrolment.
Procedures
The surveillance methods have been described previously.11, 12 In brief, nurses screened all outpatients and inpatients at all health facilities in the BHDSS and FWHDSS using standardised criteria for referral to clinicians in Basse and Bansang (appendix p 18). Screening included measurement of O2 saturation with a pulse oximeter (Nellcor N-65, Covidien, Boulder, CO, USA). Clinicians recorded clinical findings and applied standardised criteria to identify patients with suspected pneumonia, septicaemia, or meningitis, and requested blood culture, lumbar puncture, or chest radiography in accordance with a standardised protocol (appendix, pp 19, 20). Aspiration of pleural fluid or lung aspiration was done for selected patients with pleural effusions or large, dense, peripheral consolidation. All enrolled patients underwent rapid malaria tests (ICT Diagnostics, Cape Town, South Africa) from August to December (the malaria transmission season) every year. Surveillance was interrupted between Oct 5 and Nov 3, 2010, when the field station flooded.
Radiographs were obtained with consistent methods to produce digital images in accordance with WHO recommendations.10 Radiographs were read by two independent readers and readings discordant for end-point consolidation were resolved by a paediatric radiologist. All readers were calibrated to the WHO standard, achieving κ scores of 0·8 or higher before reading radiographs.
For the case-control study, radiographs were read in real time by two independent readers with discordant readings resolved by a third reader. All readers were recalibrated to the WHO standard every 6 months achieving κ scores of 0·7 or higher before continuing to read radiographs. Vaccination dates were recorded from hand-held cards and in real time at maternal-child-health clinics in the BHDSS. Standardised questionnaires were used to collect data on risk factors and potential confounders (appendix p 24).
Laboratory samples for the surveillance study were processed in Basse with consistent standardised methods.13 S pneumoniae was identified by morphology and optochin sensitivity. Pneumococcal isolates were serotyped at the Medical Research Council (MRC) Fajara laboratory, by use of latex agglutination.12
The Gambia Government/MRC Joint Institutional Ethics Committee (number 1087) and the London School of Hygiene & Tropical Medicine ethics committee approved the study. Parents or guardians of study participants gave written informed consent.
Outcomes
The primary endpoint was radiological pneumonia with consolidation, as defined by WHO.10 Secondary endpoints were radiological pneumonia with consolidation plus isolation of S pneumoniae from a sterile site (blood, cerebrospinal fluid, lung aspirate, or pleural fluid); clinical pneumonia, defined as cough or difficulty breathing for less than 14 days accompanied by one or more of raised respiratory rate for age, lower chest wall indrawing, nasal flaring, grunting, O2 saturation less than 92%, altered consciousness, inability to sit or feed, convulsions, dull chest percussion note, coarse crackles, or bronchial breathing; clinical pneumococcal pneumonia (defined as for clinical pneumonia with the addition of isolation of S pneumoniae from a sterile site; and hypoxic pneumonia, defined as clinical pneumonia with peripheral O2 saturation less than 90%.
We further categorised pneumococcal pneumonia outcomes according to PCV13 serotype (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) or non-PCV13 serotype groups. We defined the exploratory endpoint of bronchiolitis as clinical pneumonia with wheeze detectable on auscultation without dullness to percussion, bronchial breathing, or radiological pneumonia.
Statistical analysis
We calculated annual incidence of each type of pneumonia by dividing number of cases by the mid-year population estimates from the BHDSS. To calculate annual incidence in 2008 and 2010, we used the number of observed cases to extrapolate the unobserved cases from Jan 1 to May 11, 2008, and during the flood in 2010 (appendix p 3). Repeated episodes in an individual were included if they were separated by more than 30 days. We adjusted for observed increases in the number of children referred to clinicians per unit population over time by multiplying annual event counts by a correction factor that assumed the rate of referral in the absence of bias was constant (appendix p 4).12
We calculated the ratio of the incidence in the last 2 years of surveillance (2014–15) to the incidence in the baseline first 2 years (May 12, 2008, to May 11, 2010; ie, extrapolated cases were not included). We assumed a Poisson distribution to calculate incidence rate ratios (IRRs) and 95% CIs. CIs for the 2–4 year age group were inflated to allow for over dispersion, which was estimated from a subject-level Poisson regression analysis of radiological pneumonia data from 2008 to 2009. Categorical analyses used Fisher's exact test. Statistical significance was set at a p value of less than 0·05. We used STATA version 12.1 and MATLAB version R2015a for the analyses.
To investigate potential bias due to temporal changes in health-care seeking, patient investigation, or confounding by secular trends in epidemic serotypes, we did three a-priori stratified analyses, which excluded outpatients, cases identified by lung aspiration alone, and cases caused by serotype 1 or 5. To assess the effect of temporal trends related to bacterial pneumonia, we evaluated the incidence of clinical pneumonia due to bacteria other than pneumococcus as a control condition. We also evaluated the prevalence of malnutrition and malaria over time in patients with suspected pneumonia.12
The sample size for the case-control study assumed three-dose coverage of 90% in controls. Enrolment of 881 cases with three controls each would have 80% power to detect vaccine effectiveness of 35% at a significance level of 5%. We used conditional logistic regression to estimate odds ratios of radiological pneumonia for three compared with zero doses of PCV13 and to test for trend by number of doses. Vaccine effectiveness was defined as 1 minus the odds ratio. Effectiveness estimates were adjusted for age and all potential confounding variables: gender, maternal age, mother's education, number of children younger than 5 years in the household, number of children sleeping in the same room, illness in previous 3 months, previous hospital admission, distance to clinic or hospital, malnutrition, and socioeconomic status based on asset score.14 We based vaccination status on doses received at least 14 days before presentation of the case. Since a high proportion of Gambian children are fully vaccinated by the age of 12 months, we stratified vaccine effectiveness by age 3–11 months and 12 months or older.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
3837 of 4036 children born in the last 6 months of 2014 had received two or more doses of PCV13 before age 12 months, giving a coverage of 95%. Coverage of at least two doses of PCV13 in the 2–23 month age group plateaued at about 70% in early 2014 (appendix p 9).
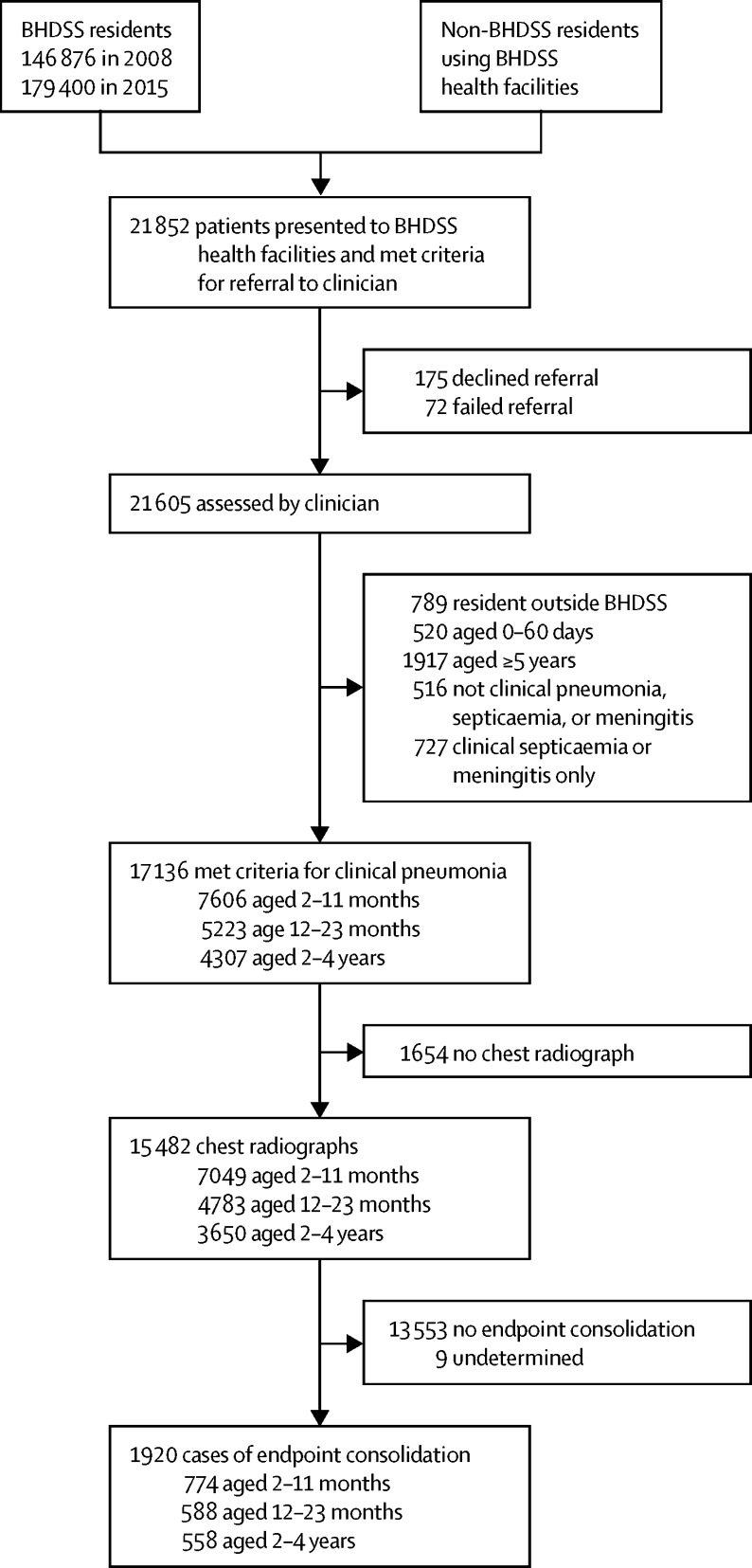
21 852 patients were screened in the BHDSS for referral to a clinician (figure 1). Surveillance was maintained at a consistently high level (appendix pp 10–12). Overall, 17 136 children aged 2–59 months met the criteria for clinical pneumonia (figure 1) and 1920 children had radiological pneumonia (table 1). Mortality from clinical and radiological pneumonia was similar. However, mortality was significantly higher in pneumococcal versus other pneumonia cases (p<0·0001) and hypoxic pneumonia versus other pneumonia cases (p<0·0001).
Figure 1.
Study profile
The profile covers the longitudinal observation period in the BHDSS from May 12, 2008, to Dec 31, 2015. 1920 cases had radiological pneumonia with consolidation, 17 136 had clinical pneumonia, 265 had pneumococcal pneumonia, and 672 had hypoxic pneumonia. All values are crude. The adjustment corrects for trends in the rate of patients presenting who met referral criteria. BHDSS=Basse Health and Demographic Surveillance System.
Table 1.
Characteristics of children with pneumonia by category of pneumonia
| Clinical pneumonia*(n=17 136) | Radiological pneumonia†(n=1920) | Pneumococcal pneumonia‡(n=265) | Hypoxic clinical pneumonia§(n=672) | ||
|---|---|---|---|---|---|
| Age | |||||
| 2–11 months | 7606 (44%) | 774 (40%) | 90 (34%) | 362 (54%) | |
| 12–23 months | 5223 (30%) | 588 (31%) | 74 (28%) | 189 (28%) | |
| 2–4 years | 4307 (25%) | 558 (29%) | 101 (38%) | 121 (18%) | |
| Male | 9676 (56%) | 1045 (54%) | 149 (56%) | 345 (51%) | |
| Weight-for-height Z score less than −3, age 2–59 months¶ | 2121 (12%) | 279 (15%) | 45 (17%) | 116 (17%) | |
| Treated as inpatient | 10 309 (60%) | 1599 (83%) | 243 (92%) | 643 (96%) | |
| Length of hospital stay, days | 3·5 (2·4) | 3·8 (2·3) | 4·8 (3·4) | 3·9 (2·9) | |
| Mortality | 379 (2%) | 47 (2%) | 27 (10%) | 81 (12%) | |
Data are n (%) or mean (SD). Patients were identified in Basse Health and Demographic Surveillance System between May 12, 2008, and Dec 31, 2015.
Defined as acute cough or shortness of breath with raised respiratory rate for age, inability to feed or sit, reduced conscious state, convulsions, lower chest wall indrawing, peripheral arterial oxygen saturation less than 93%, dull chest percussion note, or bronchial breathing on auscultation.
Defined in accordance with the WHO standard for childhood radiological pneumonia with consolidation.10
Defined as clinical pneumonia with isolation of Streptococcus pneumoniae from a normally sterile site.
Defined as clinical pneumonia with peripheral arterial O2 saturation less than 90% at presentation.
Defined with the 2006 WHO anthropometry standards.
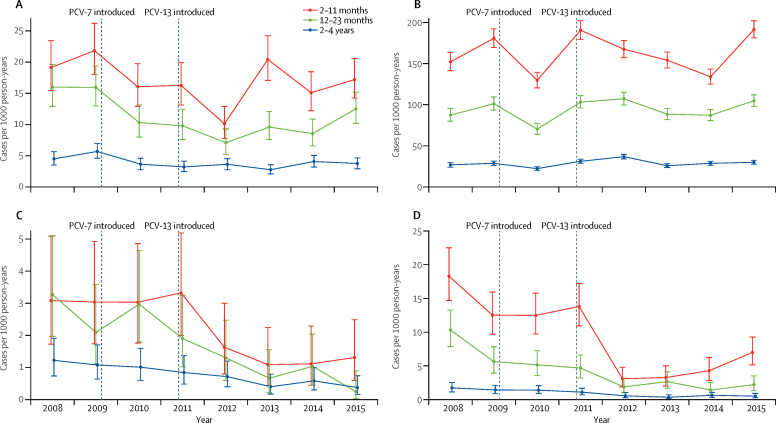
The incidence of radiological pneumonia in the 2–11 month age group fell from 21·0 cases per 1000 person-years before PCV introduction, to 16·3 cases per 1000 person-years in 2011, declined further to 10·1 cases per 1000 person-years in 2012 after the introduction of PCV13, increased to 20·2 cases per 1000 person-years in 2013, and then fell to 16·2 in 2014–15 (figure 2). The pattern was similar in the older age groups although without an increase in 2013.
Figure 2.
Adjusted annual pneumonia incidence
Incidence of radiological pneumonia with consolidation (A), clinical pneumonia (B), pneumococcal pneumonia (C), and hypoxic pneumonia (D) in the Basse Health and Demographic Surveillance System from 2008 to 2015, by age group. Bars show 95% CI. PCV=pneumococcal conjugate vaccine.
In the baseline period from May 12, 2008, to May 11, 2010, 477 cases (565 adjusted) of radiological pneumonia were recorded (table 2). In the 2014–15 period, there were 687 cases (544 adjusted). Crude and adjusted numbers of cases differed substantially, showing that the crude analyses were affected by the increased rate of referrals to clinicians over time. Between the baseline period and 2014–15, the adjusted incidence of radiological pneumonia declined from 21·0 to 16·2 cases per 1000 person-years (23% decline, 95% CI 7–36) in children aged 2–11 months, from 15·3 to 10·9 cases per 1000 person-years (29% decline, 12–42) in children aged 12–23 months, and from 5·2 to 4·1 cases per 1000 person-years (22% decline, 1–39) in children aged 2–4 years.
Table 2.
Crude and adjusted numbers of cases, incidence of pneumonia endpoints, and incidence rate ratios by age group
|
May 12, 2008, to May 11, 2010 |
2014–15 |
Incidence rate ratio for 2014–15 vs May 12, 2008, to May 11 (95% CI) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases |
Incidence per 1000 person-years |
Cases |
Incidence per 1000 person-years |
Crude | Adjusted | ||||||
| Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | ||||
| Age 2–11 months | |||||||||||
| Radiological pneumonia with consolidation | 180 | 213 | 17·8 | 21·0 | 288 | 213 | 21·9 | 16·2 | 1·23 (1·02–1·48) | 0·77 (0·64–0·93) | |
| Admitted to hospital | 154 | 182 | 15·2 | 18·0 | 214 | 181 | 16·3 | 13·8 | 1·07 (0·87–1·32) | 0·77 (0·62–0·94) | |
| Pneumococcal | 13 | 15 | 1·3 | 1·5 | 7 | 6 | 0·5 | 0·4 | 0·41 (0·17–1·04) | 0·31 (0·12–0·79) | |
| PCV13 vaccine-type | 7 | 8 | 0·7 | 0·8 | 1 | 1 | 0·08 | 0·08 | 0·11 (0·01–0·89) | 0·10 (0·01–0·77) | |
| Non-vaccine-type | 6 | 7 | 0·6 | 0·7 | 6 | 5 | 0·5 | 0·4 | 0·77 (0·25–2·39) | 0·55 (0·17–1·73) | |
| Clinical pneumonia | 1421 | 1693 | 140·2 | 167·1 | 2561 | 2156 | 194·6 | 163·8 | 1·39 (1·30–1·48) | 0·98 (0·92–1·04) | |
| Admitted to hospital | 912 | 1088 | 90·0 | 107·4 | 1534 | 1291 | 116·6 | 98·1 | 1·30 (1·19–1·41) | 0·91 (0·84–0·99) | |
| Hypoxic | 111 | 133 | 11·0 | 13·1 | 89 | 75 | 6·8 | 5·7 | 0·62 (0·47–0·82) | 0·43 (0·33–0·58) | |
| Pneumococcal | 25 | 29 | 2·5 | 2·9 | 19 | 16 | 1·4 | 1·2 | 0·59 (0·32–1·06) | 0·42 (0·23–0·78) | |
| PCV13 vaccine-type | 15 | 17 | 1·5 | 1·7 | 3 | 3 | 0·2 | 0·2 | 0·15 (0·04–0·53) | 0·14 (0·04–0·46) | |
| Non-vaccine-type | 10 | 12 | 1·0 | 1·2 | 16 | 13 | 1·2 | 1·0 | 1·23 (0·56–2·71) | 0·83 (0·38–1·83) | |
| Age 12–23 months | |||||||||||
| Radiological pneumonia with consolidation | 149 | 184 | 12·4 | 15·3 | 205 | 167 | 13·4 | 10·9 | 1·08 (0·88–1·34) | 0·71 (0·58–0·88) | |
| Admitted to hospital | 123 | 154 | 10·2 | 12·8 | 165 | 135 | 10·8 | 8·8 | 1·05 (0·83–1·33) | 0·69 (0·55–0·87) | |
| Pneumococcal | 23 | 29 | 1·9 | 2·4 | 6 | 5 | 0·4 | 0·3 | 0·21 (0·08–0·50) | 0·14 (0·05–0·35) | |
| PCV13 vaccine-type | 21 | 26 | 1·7 | 2·2 | 2 | 2 | 0·1 | 0·1 | 0·07 (0·02–0·32) | 0·06 (0·01–0·25) | |
| Non-vaccine-type | 2 | 2 | 0·2 | 0·2 | 4 | 4 | 0·3 | 0·3 | 1·57 (0·29–8·58) | 1·57 (0·29–8·58) | |
| Clinical pneumonia | 901 | 1123 | 74·9 | 93·3 | 1857 | 1519 | 121·3 | 99·2 | 1·62 (1·50–1·75) | 1·06 (0·98–1·15) | |
| Admitted to hospital | 539 | 674 | 44·8 | 56·0 | 995 | 815 | 65·0 | 53·3 | 1·45 (1·30–1·61) | 0·95 (0·86–1·05) | |
| Hypoxic | 65 | 82 | 5·4 | 6·8 | 35 | 29 | 2·3 | 1·9 | 0·42 (0·28–0·64) | 0·28 (0·18–0·42) | |
| Pneumococcal | 25 | 31 | 2·1 | 2·6 | 12 | 10 | 0·8 | 0·7 | 0·38 (0·19–0·75) | 0·25 (0·12–0·53) | |
| PCV13 vaccine-type | 23 | 28 | .. | 2·3 | 4 | 4 | 0·3 | 0·3 | 0·14 (0·05–0·40) | 0·11 (0·04–0·32) | |
| Non-vaccine-type | 2 | 2 | .. | 0·2 | 8 | 7 | 0·5 | 0·5 | 3·14 (0·67–14·8) | 2·75 (0·57–13·2) | |
| Age 2–4 years | |||||||||||
| Radiological pneumonia with consolidation | 148 | 168 | 4·6 | 5·2 | 194 | 164 | 4·8 | 4·1 | 1·04 (0·82–1·32) | 0·78 (0·61–0·99) | |
| Admitted to hospital | 119 | 135 | 3·7 | 4·2 | 147 | 129 | 3·6 | 3·2 | 0·98 (0·75–1·28) | 0·76 (0·58–0·99) | |
| Pneumococcal | 24 | 28 | 0·7 | 0·9 | 14 | 12 | 0·3 | 0·3 | 0·46 (0·22–0·96) | 0·34 (0·16–0·72) | |
| PCV13 vaccine-type | 21 | 23 | 0·7 | 0·7 | 5 | 5 | 0·1 | 0·1 | 0·19 (0·06–0·57) | 0·17 (0·06–0·50) | |
| Non-vaccine type | 3 | 3 | 0·1 | 0·1 | 9 | 8 | 0·2 | 0·2 | 1·86 (0·42–8·27) | 2·12 (0·49–9·19) | |
| Clinical pneumonia | 800 | 910 | 24·8 | 28·2 | 1391 | 1225 | 34·4 | 30·3 | 1·38 (1·26–1·52) | 1·07 (0·98–1·18) | |
| Admitted to hospital | 499 | 569 | 15·5 | 17·7 | 692 | 609 | 17·1 | 15·0 | 1·10 (0·97–1·25) | 0·85 (0·75–0·97) | |
| Hypoxic | 38 | 43 | 1·2 | 1·3 | 27 | 24 | 0·7 | 0·6 | 0·57 (0·32–0·98) | 0·44 (0·26–0·77) | |
| Pneumococcal | 33 | 37 | 1·0 | 1·1 | 22 | 20 | 0·5 | 0·5 | 0·53 (0·29–0·96) | 0·43 (0·24–0·78) | |
| PCV13 vaccine-type | 29 | 33 | 0·9 | 1·0 | 7 | 6 | 0·2 | 0·1 | 0·19 (0·08–0·48) | 0·14 (0·06–0·38) | |
| Non-vaccine-type | 4 | 5 | 0·1 | 0·2 | 15 | 13 | 0·4 | 0·3 | 2·99 (0·88–10·09) | 2·07 (0·66–6·47) | |
Data are for the baseline period from May 12, 2008, to May 11, 2010, and the 2014–15 period after the introduction of PCV13. Case counts are adjusted for temporal trends in referral of patients meeting screen criteria to surveillance clinicians, and rounded to the nearest integer. 95% CIs were calculated with an inflated variance because of overdispersion in the 2–4 years age group. PCV=pneumococcal conjugate vaccine.
Across all age groups, incidence of pneumococcal pneumonia declined by 63% (47–74), from 180 to 65 cases per 1000 person-years (declined from 2·9 to 1·2 cases per 1000 person-years [58% decline, 22–77] in children aged 2–11 months and from 2·6 to 0·7 cases per 1000 person-years [75% decline, 47–88] in children aged 12–23 months). Pneumonia caused by PCV13 serotypes declined by 86% (75–92), from 145 to 17 cases per 1000 person-years. The incidence of pneumonia due to non-PCV13 serotypes did not increase significantly (IRR 1·50, 95% CI 0·86–2·61).
The incidence of clinical pneumonia was not significantly different between the baseline and 2014–15 periods (table 2). Cases of clinical pneumonia admitted to hospital declined by 8% (3–13), from 4250 to 3969 cases per 1000 person-years, across all age groups, with the greatest absolute reduction occurring in the 2–11 month age group, from 107·4 cases per 1000 person-years at baseline to 98·1 cases per 1000 person-years in 2014–15.
There was a 61% (95% CI 52–68) fall in the incidence of hypoxic pneumonia in all age groups; in the 2–11 month age group, incidence fell from 13·1 cases per 1000 person-years at baseline to 5·7 cases per 1000 person-years in 2014–15 (57% decline, 42–67), and in the 12–23 month age group, incidence fell by from 6·8 to 1·9 cases per 1000 person-years (72% decline, 58–82; table 2). Bronchiolitis incidence fell by 39% (31–47) in the 2–11 month age group, from 51·7 to 31·4 cases per 1000 person-years, and by 29% (15-41) in the 12–23 month age group, from 21·4 to 15·2 cases per 1000 person-years (appendix pp 13, 20).
The incidence of the control condition, non-pneumococcal bacterial pneumonia, was not significantly different between the baseline and 2014–15 periods (appendix pp 14, 23). The prevalence of malaria-positive rapid tests was also not significantly different between the baseline and 2014–15 periods (appendix p 15). The prevalence of malnutrition (weight-for-height Z score <–3) was stable throughout the study at about 10% (appendix p 16). Estimates of vaccine impact were unchanged in stratified analyses excluding outpatients (table 2), cases detected only by lung aspiration (appendix p 21), and cases of serotype 1 or 5 pneumonia (appendix p 22).
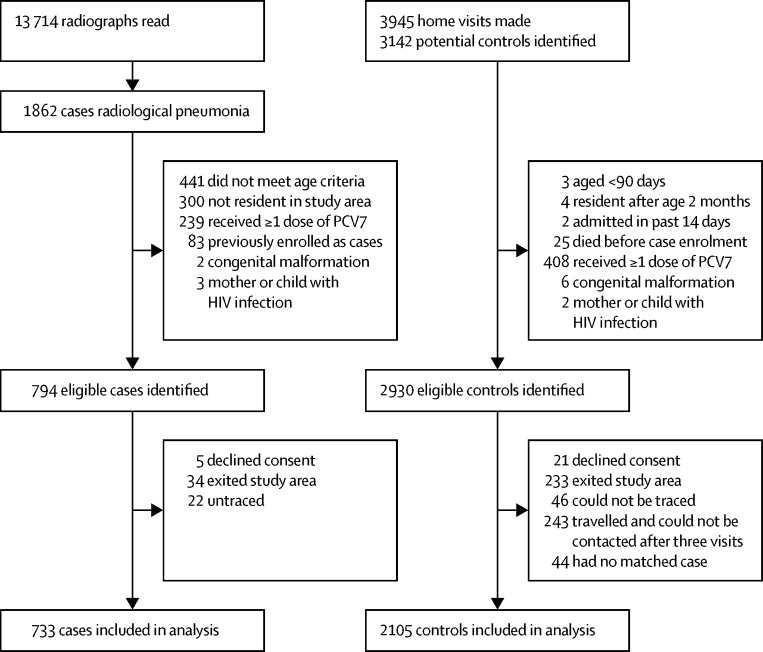
In the case-control study, we identified 794 eligible cases of radiological pneumonia with consolidation, of whom 733 were included in the analysis, and 2930 eligible controls, of whom 2105 were included in the analysis (figure 3). Cases were more likely than controls to have had low birthweight, a recent illness, previous hospital admission, malnutrition, a greater number of children younger than 5 years in the household, and to live closer to a hospital (appendix p 24). Vaccine effectiveness was similar in the crude (odds ratio 0·71, 95% CI 0·43–1·18) and adjusted (adjusted odds ratio 0·72, 0·42–1·23) analyses (table 3). The adjusted odds ratio to assess vaccine effectiveness in the 3–11 month age group was 0·59 (0·33–1·06) in the crude analysis and 0·57 (0·30–1·08) in the adjusted analysis. An increasing number of doses of PCV13 was associated with increased effectiveness in children aged 3–11 months (p=0·026). The analysis in children aged 12 months or older had insufficient power (ptrend=0·884) because there were only four unvaccinated cases and ten unvaccinated controls.
Figure 3.
Case-control study profile
The case-control study investigated the effectiveness of PCV13 against radiological pneumonia with consolidation. PCV=pneumococcal conjugate vaccine.
Table 3.
Case-control analysis of PCV13 against radiological pneumonia with consolidation
| Cases (%) | Controls (%) | Crude odds ratio (95% CI) | Adjusted odds ratio (95% CI) | p value for trend | ||
|---|---|---|---|---|---|---|
| Age 3–11 months | .. | .. | .. | .. | 0·026 | |
| Total | 336 | 969 | .. | .. | .. | |
| No doses | 38 (11%) | 103 (11%) | Reference | Reference | .. | |
| One dose | 83 (25%) | 198 (20%) | 1·09 (0·67–1·79) | 1·08 (0·63–1·83) | .. | |
| Two doses | 87 (26%) | 247 (25%) | 0·81 (0·47–1·39) | 0·83 (0·46–1·50) | .. | |
| Three doses | 128 (38%) | 421 (43%) | 0·59 (0·33–1·06) | 0·57 (0·30–1·08) | .. | |
| Two or three doses | 215 (64%) | 668 (69%) | 0·73 (0·43–1·24) | 0·74 (0·42–1·32) | .. | |
| Age ≥12 months | .. | .. | .. | .. | 0·884 | |
| Total | 397 | 1136 | .. | .. | .. | |
| No doses | 4 (1%) | 10 (1%) | Reference | Reference | .. | |
| One dose | 9 (2%) | 16 (1%) | 1·64 (0·35–7·74) | 1·29 (0·26–6·36) | .. | |
| Two doses | 18 (5%) | 49 (4%) | 1·28 (0·32–5·10) | 0·74 (0·17–3·16) | .. | |
| Three doses | 366 (92%) | 1061 (93%) | 1·21 (0·33–4·48) | 0·93 (0·24–3·64) | .. | |
| Two or three doses | 384 (97%) | 1110 (98%) | 1·22 (0·33–4·52) | 0·73 (0·23–3·49) | .. | |
| Overall | .. | .. | .. | .. | 0·067 | |
| Total | 733 | 2105 | .. | .. | .. | |
| No doses | 42 (6%) | 113 (5%) | Reference | Reference | .. | |
| One dose | 92 (13%) | 214 (10%) | 1·15 (0·72–1·83) | 1·12 (0·68–1·82) | .. | |
| Two doses | 105 (14%) | 296 (14%) | 0·87 (0·53–1·44) | 0·84 (0·50–1·43) | .. | |
| Three doses | 494 (67%) | 1482 (70%) | 0·71 (0·43–1·18) | 0·72 (0·42–1·23) | .. | |
| Two or three doses | 599 (82%) | 1778 (84%) | 0·79 (0·49–1·28) | 0·78 (0·47–1·30) | .. | |
Radiological pneumonia with consolidation was defined in accordance with WHO criteria. Adjusted odds ratios were calculated from a conditional logistic regression model adjusted for age, gender, maternal age, mother's education, number of children younger than 5 years in the household, number of children sleeping in the same room, illness in previous 3 months, previous hospital admission, distance to clinic or hospital, malnutrition, and socioeconomic status based on asset score. PCV13=13-valent pneumococcal conjugate vaccine.
Discussion
The results from our population-based surveillance in The Gambia showed an estimated 24% reduction in the incidence of radiological pneumonia with consolidation in children aged 2–59 months following the introduction of PCV. We found substantial reductions in the incidence of culture-proven pneumococcal pneumonia caused by PCV13 serotypes (86% reduction). Despite a small, non-significant increase in pneumonia due to non-PCV13 serotypes, there was a 63% reduction in all pneumococcal pneumonia. Although we detected no effect of PCV on all clinical pneumonia, which included outpatients, there was an 8% reduction in hospital admissions for clinical pneumonia, and the incidence of hypoxic pneumonia fell by 61%.
Our findings for radiological pneumonia are similar to those reported in Israel, where the introduction of PCV was associated with a 47% reduction in childhood hospital admissions for WHO-defined radiological pneumonia.8 Our estimated individual-level effectiveness of PCV13 against radiological pneumonia (table 3) is similar to that in the randomised trial of PCV9 in The Gambia.9 Sampling for invasive pneumococcal disease and routine measurement of O2 saturation enabled us to show a large effect of PCV against proven pneumococcal pneumonia and hypoxic pneumonia. The substantial impact of PCV on these two disorders underscores the vaccines capability to prevent the most severe forms of pneumonia.
Population-based studies in high-income countries have recorded PCV impact and detected 13–72% reductions in hospital admissions coded as pneumonia.6, 15, 16, 17, 18, 19, 20, 21, 22 We found only an 8% reduction in hospital admissions for clinical pneumonia across all ages. However, in absolute terms there was a reduction of 9·3 cases per 1000 person-years in the 2–11 month age group which contrasted with the reduction of 4·8 cases of radiological pneumonia per 1000 person-years in the same age group. Our estimate of vaccine impact against all clinical pneumonia might have been affected by our case definition and setting. Our definition included non-specific clinical signs, such as convulsions and raised respiratory rate, and when applied in a tropical malaria-endemic setting, this could lead to misclassification of other illnesses as pneumonia,23, 24 biasing effect estimates towards the null. A lack of impact of PCV against clinical pneumonia has been noted in other settings25, 26 and is consistent with the 7% efficacy reported in the Gambian PCV9 trial.9
The reduction in bronchiolitis that we detected is consistent with the reported 16% reduction in bronchiolitis admissions in infants in the USA following the introduction of PCV7.27 The US investigators also showed that, following PCV7 introduction, hospital admissions for respiratory syncytial virus infection declined by 18% in infants and 9% in children aged 12–23 months. Evidence of synergistic relationships between pneumococcus and respiratory viruses was provided by a trial in South Africa, in which PCV9 prevented 31% of hospital admissions for virus-associated lower respiratory disease, probably those caused by co-infections with virus and pneumococcus.28
All screening, investigations, and laboratory practices were standardised and consistently applied throughout this study. The reading of radiographs was done in accordance with the WHO recommendations11 and all readers were regularly recalibrated to the WHO standard. Measurement of O2 saturation and clinical and radiographic findings enabled us to show significant impact of PCV against the novel endpoints of hypoxic pneumonia and bronchiolitis. The limitations of this study include the relatively short baseline period and the need for adjustment of annual case counts to account for increased referrals of patients over time. Our adjustment of case counts resulted in less precise estimates of vaccine impact and an interpretation that assumes referrals over time were constant. The adjustment accounts for a systematic bias leading to increasing case detection during the course of the observation period. We, and others, have used this approach in the past.12, 29
The estimates of direct vaccine effectiveness in the case-control study support the longitudinal results of a significant impact of PCV, while suggesting that the longitudinal study might have underestimated the impact of the vaccine slightly. The case-control estimate might have been affected by biases in either direction; a falling proportion of radiological pneumonia caused by vaccine serotypes over time would tend to underestimate vaccine effectiveness, whereas overestimation is possible because of unvaccinated children being at an inherently higher risk than vaccinated children in ways unrelated to vaccination. In the longitudinal study, the baseline period was partly affected by the first few months of vaccine implementation, although the effect was limited by the absence of a catch-up campaign. Coverage of PCV13 in the 2–4 year age group peaked later in the PCV13 period, so impact could not be fully estimated in this age group. Our study has several features that help to alleviate some concerns of bias and confounding resulting from changes in factors that affect the detection or risk of pneumonia: the incidence of the control condition, pneumonia due to non-pneumococcal bacteria, was stable over time, estimates of the vaccine impact were unchanged in stratified analyses, and there was no difference in the prevalence of malaria or malnutrition between the baseline and 2014–15 periods.
Extended surveillance will be needed to establish the maximum impact of PCV13 in the 2–4 year age group, as vaccine coverage increases in that age group, and to identify the serotypes causing disease in the PCV13 era to guide the development of the next generation of vaccines. The vaccine impact that we saw resulted from a standard EPI schedule without catch-up—ie, the programmatic circumstances found in most low-income countries. Therefore, other low-income countries that introduce PCV with reasonable coverage can expect to have significant reductions in hospital admissions for pneumonia and substantial reductions in cases of severe pneumonia.
Acknowledgments
Acknowledgements
This study was funded by GAVI's Pneumococcal vaccines Accelerated Development and Introduction Plan (PneumoADIP; Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, USA), the Bill & Melinda Gates Foundation (OPP 1020372); and Medical Research Council (UK). The Gambia Government, Upper River Region and Central River Region, Regional Health Teams delivered the PCV; surveillance was done at Basse Major Health Centre, Bansang Hospital and government health facilities in Gambissara, Demba Kunda, Fatoto, Garawol, Koina, Brikama Ba, and Jakhaly. We thank all staff of the MRC Basse Field Station and the residents of the BHDSS and FWHDSS for supporting the study.
Contributors
RAA, PCH, OSL, TC, and GAM proposed the study idea. GAM, IP, DSa, SRH, MJ, MK, PCH, RAA, and TC established the surveillance system and GAM oversaw it throughout the study. GAM, AA, and DSa trained and supervised clinicians and staff on study procedures. SMS, IH, UU, DA, MN, CDO, OA, JP, YO, BA, EA, BSM, AEF, RM, BE, RCI, BK, PG, EO, OO, EU, and EG clinically evaluated and investigated the patients and maintained quality assurance during clinical procedures. SMS, IH, RM, and AA read the radiographs in the longitudinal study. SMS, IH, MN, CDO, RM, BSM, and GAM read the radiographs in the case-control study. IH, MJ, DSa, and GAM supervised the collection of demographic and vaccination data. HB, UNAI, AM, and RS supervised the microbiology in Basse. EDN, SJ, and MA supervised serotyping in Fajara. SS, YL-J, and DSo provided central level logistic support and supervision to the EPI and Disease Control department in the Ministry of Health. LC supervised the EPI in the Upper River Region. TC provided institutional support and central level liaison with the Ministry of Health. MK and OSL provided administrative support and technical feedback. GAM, DJJ, PCH, SMS, CB, and BMG developed the analysis plan and did the analysis. GAM, PCH, DJJ, KM, CB, and BMG interpreted the findings. GAM, DJJ, BMG, and PCH drafted the paper. All authors contributed to the writing of the final manuscript.
Declaration of interests
RAA and DSa are currently employees of GlaxoSmithKline Vaccines. The opinion presented in this paper is that of the authors and does not reflect GlaxoSmithKline's position. RAA received grant awards whilst employed at the MRC Unit, The Gambia. MK, SRH, and BMG received grants from the Bill & Melinda Gates Foundation. MK received grants from the Gavi Alliance, Merck, and Pfizer, and personal fees from Pfizer. EU has a current consultancy with the GlaxoSmithKline malaria vaccine group. All other authors declare no competing interests.
Supplementary Material
References
- 1.Liu L, Oza S, Hogan D. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Silverman M, Stratton D, Diallo A, Egler LJ. Diagnosis of acute bacterial pneumonia in Nigerian children. Value of needle aspiration of lung of countercurrent immunoelectrophoresis. Arch Dis Child. 1977;52:925–931. doi: 10.1136/adc.52.12.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shann F, Gratten M, Germer S, Linnemann V, Hazlett D, Payne R. Aetiology of pneumonia in children in Goroka Hospital, Papua New Guinea. Lancet. 1984;2:537–541. doi: 10.1016/s0140-6736(84)90764-5. [DOI] [PubMed] [Google Scholar]
- 4.Falade AG, Mulholland EK, Adegbola RA, Greenwood BM. Bacterial isolates from blood and lung aspirate cultures in Gambian children with lobar pneumonia. Ann Trop Paediatr. 1997;17:315–319. doi: 10.1080/02724936.1997.11747904. [DOI] [PubMed] [Google Scholar]
- 5.O'Brien KL, Wolfson LJ, Watt JP. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 6.Griffin MR, Grijalva CG. Hospitalizations for pneumonia after a decade of pneumococcal vaccination. N Engl J Med. 2013;369:1662–1663. doi: 10.1056/NEJMc1310477. [DOI] [PubMed] [Google Scholar]
- 7.Koshy E, Murray J, Bottle A, Sharland M, Saxena S. Impact of the seven-valent pneumococcal conjugate vaccination (PCV7) programme on childhood hospital admissions for bacterial pneumonia and empyema in England: national time-trends study, 1997–2008. Thorax. 2010;65:770–774. doi: 10.1136/thx.2010.137802. [DOI] [PubMed] [Google Scholar]
- 8.Greenberg D, Givon-Lavi N, Ben-Shimol S, Ziv JB, Dagan R. Impact of PCV7/PCV13 introduction on community-acquired alveolar pneumonia in children <5 years. Vaccine. 2015;33:4623–4629. doi: 10.1016/j.vaccine.2015.06.062. [DOI] [PubMed] [Google Scholar]
- 9.Cutts FT, Zaman SM, Enwere G. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 10.WHO Pneumonia Vaccine Trial Investigators' Group . Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. World Health Organization Department of Vaccines and Biologicals; Geneva: 2001. http://apps.who.int/iris/handle/10665/66956 (accessed Oct 3, 2016). [Google Scholar]
- 11.Mackenzie GA, Plumb ID, Sambou S. Monitoring the introduction of pneumococcal conjugate vaccines into West Africa: design and implementation of a population-based surveillance system. PLoS Med. 2012;9:e1001161. doi: 10.1371/journal.pmed.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackenzie GA, Hill PC, Jeffries DJ. Effect of the introduction of pneumococcal conjugate vaccination on invasive pneumococcal disease in The Gambia: a population-based surveillance study. Lancet Infect Dis. 2016;16:703–711. doi: 10.1016/S1473-3099(16)00054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adegbola RA, Falade AG, Sam BE. The etiology of pneumonia in malnourished and well-nourished Gambian children. Pediatr Infect Dis J. 1994;13:975–982. doi: 10.1097/00006454-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Greenland S. Invited commentary: variable selection versus shrinkage in the control of multiple confounders. Am J Epidemiol. 2008;167:523–529. doi: 10.1093/aje/kwm355. [DOI] [PubMed] [Google Scholar]
- 15.Griffin MR, Mitchel E, Moore MR, Whitney CG, Grijalva CG. Declines in pneumonia hospitalizations of children aged <2 years associated with the use of pneumococcal conjugate vaccines—Tennessee, 1998–2012. MMWR Morb Mortal Wkly Rep. 2014;63:995–998. [PMC free article] [PubMed] [Google Scholar]
- 16.Scotta MC, Veras TN, Klein PC. Impact of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on childhood pneumonia hospitalizations in Brazil two years after introduction. Vaccine. 2014;32:4495–4499. doi: 10.1016/j.vaccine.2014.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014;2:387–394. doi: 10.1016/S2213-2600(14)70032-3. [DOI] [PubMed] [Google Scholar]
- 18.Becker-Dreps S, Amaya E, Liu L. Changes in childhood pneumonia and infant mortality rates following introduction of the 13-valent pneumococcal conjugate vaccine in Nicaragua. Pediatr Infect Dis J. 2014;33:637–642. doi: 10.1097/INF.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 19.Saxena S, Atchison C, Cecil E, Sharland M, Koshy E, Bottle A. Additive impact of pneumococcal conjugate vaccines on pneumonia and empyema hospital admissions in England. J Infect. 2015;71:428–436. doi: 10.1016/j.jinf.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Lindstrand A, Bennet R, Galanis I. Sinusitis and pneumonia hospitalization after introduction of pneumococcal conjugate vaccine. Pediatrics. 2014;134:e1528–e1536. doi: 10.1542/peds.2013-4177. [DOI] [PubMed] [Google Scholar]
- 21.Berglund A, Ekelund M, Fletcher MA, Nyman L. All-cause pneumonia hospitalizations in children <2 years old in Sweden, 1998 to 2012: impact of pneumococcal conjugate vaccine introduction. PLoS One. 2014;9:e112211. doi: 10.1371/journal.pone.0112211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jardine A, Menzies RI, McIntyre PB. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr Infect Dis J. 2010;29:607–612. doi: 10.1097/inf.0b013e3181d7d09c. [DOI] [PubMed] [Google Scholar]
- 23.O'Dempsey TJ, Laurence BE, McArdle TF, Todd JE, Lamont AC, Greenwood BM. The effect of temperature reduction on respiratory rate in febrile illnesses. Arch Dis Child. 1993;68:492–495. doi: 10.1136/adc.68.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–665. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 25.Angoulvant F, Levy C, Grimprel E. Early impact of 13-valent pneumococcal conjugate vaccine on community-acquired pneumonia in children. Clin Infect Dis. 2014;58:918–924. doi: 10.1093/cid/ciu006. [DOI] [PubMed] [Google Scholar]
- 26.Nelson JC, Jackson M, Yu O. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine. 2008;26:4947–4954. doi: 10.1016/j.vaccine.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger DM, Klugman KP, Steiner CA, Simonsen L, Viboud C. Association between respiratory syncytial virus activity and pneumococcal disease in infants: a time series analysis of US hospitalization data. PLoS Med. 2015;12:e1001776. doi: 10.1371/journal.pmed.1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madhi SA, Klugman KP. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.