Abstract
Dysregulation of MST1/STK4, a key kinase component of the Hippo-YAP pathway, is linked to the etiology of many cancers with poor prognosis. However, how STK4 restricts the emergence of aggressive cancer remains elusive. Here, we investigated the effects of STK4, primarily localized in the cytoplasm, lipid raft, and nucleus, on cell growth and gene expression in aggressive prostate cancer. We demonstrated that lipid raft and nuclear STK4 had superior suppressive effects on cell growth in vitro and in vivo compared with cytoplasmic STK4. Using RNA sequencing and bioinformatics analysis, we identified several differentially expressed (DE) genes that responded to ectopic STK4 in all three subcellular compartments. We noted that the number of DE genes observed in lipid raft and nuclear STK4 cells were much greater than cytoplasmic STK4. Our functional annotation clustering showed that these DE genes were commonly associated with oncogenic pathways such as AR, PI3K/AKT, BMP/SMAD, GPCR, WNT, and RAS as well as unique pathways such as JAK/STAT, which emerged only in nuclear STK4 cells. These findings indicate that MST1/STK4/Hippo signaling restricts aggressive tumor cell growth by intersecting with multiple molecular pathways, suggesting that targeting of the STK4/Hippo pathway may have important therapeutic implications for cancer.
Introduction
Mammalian STE20-like serine-threonine kinase MST1, encoded by the STK4 gene, is a multifunctional protein [1, 2]. MST1 and its closest paralogs MST2 (encoded by the STK3 gene), MST3, and MST4 are members of the Class II Germinal Center Family of Protein Kinases [3]. Here, we use STK4, an official gene name for MST1, to avoid confusion with the MST1 official gene name that encodes macrophage stimulating 1 or hepatocyte growth factor-like protein. STK3/4 and LATS1/2 (large tumor suppressor 1 and 2) are core kinase components of the Hippo tumor suppressor pathway in mammalians [4]. In the conventional Hippo pathway, the STK3/4 and LATS1/2 signaling cascade phosphorylates and inactivates the transcriptional coactivator YAP1 (yes associated protein 1) and its close paralog WWTR1 [5]. YAP1 and WWTR1 do not have DNA binding domains and they exert their biological outputs, such as cell proliferation and survival, by interacting with the TEAD1-4 transcription factors.
Lines of evidence have indicated that dysregulation or loss of STK4/Hippo signaling is linked to developmental disorders and carcinogenesis with poor prognosis [6–12]. For example, a genetic deletion of hippo (hpo) in Drosophila results in a tumor-like phenotype due to the loss of apoptosis [13]. Similarly, mice with the conditional STK3/4 gene knockouts show stem cell expansion, and tumorigenesis [11, 14, 15]. STK4 is a stress-induced kinase and it can be activated in response to cell-death inducers. Autophosphorylation of STK4 at Thr183 (Thr180 in STK3) in the activation loop is a key activation mechanism for STK4/3 because phosphorylation of Thr183/180 causes the cleavage of STK4 by caspases under apoptotic conditions [3, 16, 17]. The caspase-cleavage results in a more active STK4 protein (STK4-N, an amino-terminally truncated STK4), which localizes into the nucleus and induces apoptosis through histone modifications and chromatin condensations [18, 19].
Previously, we identified STK4 as a binding partner of AKT protein complexes that were isolated from lipid raft of the androgen-sensitive LNCaP prostate cancer (PC) cell line [7]. Lipid raft is the specialized cholesterol-rich membrane microdomain and plays a critical role in signal transductions and cell survival [20–23]. In that study, we demonstrated that levels of STK4 protein progressively declined during PC progression to the metastatic castration-resistant state, which coincided with the activation of AKT1 [7, 24]. In addition, we and others reported that DNA hypermethylation [17, 25] and post-translational modification [17, 25] meadiated the loss of STK4 activity. Interestingly, a recent study suggested that the dimerization of STK3 and STK4 that was mediated by H-ras signaling caused the loss of STK4 activity [26]. Moreover, we reported that the full-length STK4 (STK4-FL) enriched in cell nuclei, even in the presence of cell-death inducer, was devoid of Thr183 phosphorylation [25]. Nevertheless, how STK4 in a defined cell location regulates PC cell growth remains elusive.
In the present study, we developed and utilized the cytoplasm-, lipid raft- and nuclear-localized STK4 expressing PC cell models to gain more insights into the role of STK4 in aggressive PC. We found that STK4 enriched in the defined subcellular compartment differentially regulated cell growth in vitro and tumor growth in vivo. We identified several differentially expressed (DE) genes that responded to the enrichment of ectopic STK4 in all three cell compartments. Our functional annotation clustering showed that these DE genes were associated with a wide range of molecular pathways including tumor suppressor and oncogenesis as well as cellular metabolisms. Our findings suggest that STK4 signaling controls aggressive prostate tumor cell growth by modulating with multiple signaling mechanisms.
Materials and methods
Plasmids
Construction of the tetracycline (Tet) or doxycycline (Dox)-inducible STK4 plasmid (pRX-HA-STK4) was described previously [27]. Dox is a tetracycline analog. To express Hemagglutinin (HA)-tagged STK4 protein in the lipid raft membrane domain, we constructed lipid raft (LR)-targeted pRX-LR-HA-STK4 mammalian expression vector. To generate pRX-LR-HA-STK4 vector, we took a series of approaches. First, we generated a pRX-LR-HA vector, for which double-stranded 5’-phosphorylated DNA consisting of palmitoylation and myristoylation (PM) signal from the Lck gene and the HA tag sequences were ligated into the BamH1 and NotI restriction enzyme (RE) sites in the pRetro-X-Pur (Pur: puromycin) retroviral vector (Clontech Laboratories, Inc.). The resulting vector was designated as pRX-PM-HA. Second, the PCR-amplified full-length STK4 cDNA was inserted into the NotI and MluI enzyme sites in the pRX-PM-HA vector. To express HA-tagged STK4 protein in the nucleus, we constructed nuclear (NL)-targeted pRX-NL-HA-STK4 mammalian expression vector. To construct pRX-NL-HA-STK4, first, we generated pRX-3NLS-HA vector, for which double-stranded 5’-phosphorylated DNA containing three consecutive copies of nuclear localization signal (3NLS) from SV40 large T-antigen separated with three base-pair spacers and HA-tag sequences were ligated into the BamH1 and NotI RE sites in the pRetro-X-Pur retroviral vector. The resulting vector was designated as pRX-NLS-HA. Second, the PCR-amplified full length STK4 cDNA was inserted into the NotI and MluI RE sites in the pRX-3NLS-HA vector. AccuPrime™ Pfx SuperMix (Invitrogen; Grand Island, NY) was used in PCR reactions. Standard molecular biology techniques in cloning and DH5-α competent cells in plasmid amplification were utilized [27]. In-frame and fidelity of all constructs were confirmed by DNA sequencing.
Cell models
Establishment of Tet or Dox-responsive C4-2/Vector and C4-2/HA-STK4 cell models was previously described [27]. Here, we renamed the C4-2/HA-STK4 cell as C4-2/CL-STK4 because we noted that ectopic expression of HA-STK4 was naturally accumulated in the cytoplasm ([27] and Fig 1A and 1D). To establish the C4-2/LR-STK4 cell model, first retrovirus carrying pRX-LR-HA-STK4 and pRX-NL-HA-STK4 vector were produced in HEK-293T cells as previously described [27]. Then, C4-2/TetON cells were infected with retrovirus encoding pRX-LR-HA-STK4 or pRX-NL-HA-STK4 vector, followed by Puromycin (Pur) selection (3 μg/mL) to generate Tet-inducible C4-2/LR-HA-STK4 or C4-2/NS-HA-STK4 expressing cells. We designated these cells as C4-2/LR-STK4 and C4-2/NL-STK4, respectively. All protocols and procedures were performed according to the manufacturer's instructions (Clontech Laboratories, Inc.). Growth conditions for C4-2 and HEK 292T cells were previously described [27].
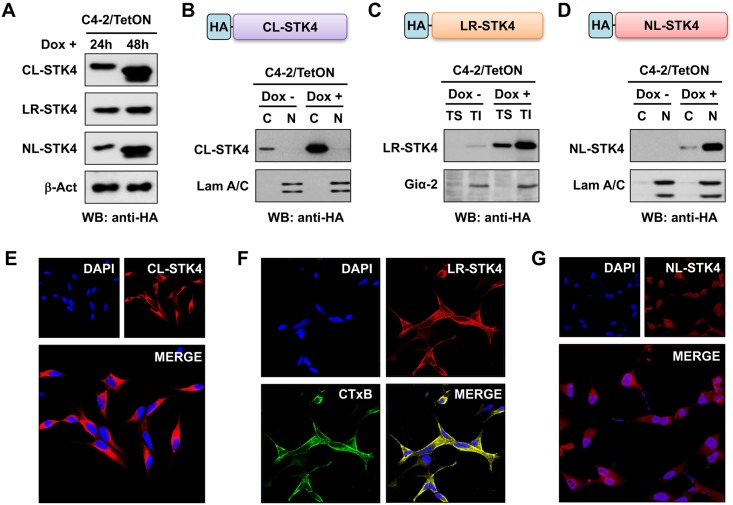
Fig 1. Expression of CL-STK4, LR-STK4, and NL-STK4 protein in Tet-inducible C4-2 prostate cancer cells.
(A) Time-dependent expression of CL-STK4, LR-STK4, and NL-STK4 protein in the engineered cells that were exposed to doxycycline (Dox) for 24 and 48h. Levels of ectopic STK4 proteins were assessed by Western blotting (WB) using the HA-tagged antibody. (B-D) Analysis of CL-STK4, LR-STK4, and NL-STK4 protein in cytoplasmic, lipid raft, and nuclear fractions, respectively. Expression of HA-STK4 protein was evaluated by WB with the HA-tag antibody at 48h after treatment with and without doxycycline (Dox, 4 μg/ml). Lam (lamin) A/C was used as a nuclear marker. Giα2 was used as a lipid raft marker. (E-G) Immunofluorescence (IF) analysis of CL-STK4, LR-STK4, and NL-STK4 protein in the C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells, respectively. IF was performed at 24h post Dox (4 μg/ml) treatment. Micrographs are the representation of two independent experiments. CTxB-FITC labeled lipid raft in C4-2/LR-STK4 cells. For both experiments, cells were grown in Tet-approved serum conditions. CL: Cytoplasmic localization, LR: Lipid raft; NL: nuclear localization, HA: Hemagglutinin.
Protein analysis
Cytoplasmic and nuclear fractions were prepared using our established method [28]. TS (Triton-X-100-soluble) fraction and TI (Triton-X-100 insoluble, but n-octyl-β-D-glucoside soluble) fraction—TI fraction by definition, represents lipid raft—were isolated according to the established protocol [7, 29]. Presence of ectopic HA-tagged STK4 protein in cytoplasmic, lipid raft and nuclear fractions was determined by Western blotting using the HA antibody (Covance). Briefly, proteins were resolved by SDS-PAGE. PBST (0.1% Tween-20) containing 5% (w/v) skim milk powder or PBST containing 5% immunoglobulin G (IgG)-free bovine serum albumin (Sigma-Aldrich) was used in membrane blocking and antibody dilutions. Giα2 protein was included as a negative and positive control for TS and TI fractionations, respectively. Lamin A/C (Cell Signaling Technology) was used as a negative and positive control for cytoplasmic and nuclear fractionations, respectively [27]. Signals were visualized by chemiluminescence method (GE HealthCare).
Growth assays
C4-2/Vector, C4-2/CL-STK4, C4-2/LR-STK4 and C4-2/NL-STK4 cells were seeded in tissue culture medium supplemented with 10% Tet-approved serum in 96-well cell culture plate. Cells were then treated with Dox (3 μ/ml) for 72h to induce STK4 expression in the cell. Cell growth was assessed by imaging and MTS assay. For imaging, cells were washed with PBS and bright-field images were captured using microscopy (Nikon Eclipse Ti model; USA) at 20x magnification. CellTiter 96 AQueous system was used to assess cell growth according to manufacturer’s protocol (Promega) and as described [27]. This system uses MTS and it has been widely used to evaluate cell growth in cultures [27].
Immunofluorescence
Immunofluorescence analysis of HA-STK4 protein in C4-2 cells was performed with modifications [27]. Briefly, cells were fixed with freshly prepared 4% PFA (paraformaldehyde that was prepared in PBS) for 30 min and permeabilized with 0.2% Triton-X-100 and incubated with anti-HA (Covance, 1:50) antibody overnight at 4°C. Cells were washed with PBS after each step. In addition, lipid rafts were labeled with CTxB-FITC conjugated (Sigma-Aldrich) as described [21]. Briefly, live cells were washed with cold PBS and incubated with CTxB-FITC (20 ng/ml, which was prepared in cold serum-free media) on ice for 30 min prior to fixation with 4% PFA. Alexa Fluor 532 conjugated anti-mouse (1:1000 dilution) was used to detect HA-STK4 signals in the cell. Slides were mounted with VectaShield containing DAPI (Vector Labs, H-1200). Immunofluorescence images were captured by microscopy (Zeiss 700) at 40x magnification with oil immersion.
Xenograft assays
C4-2/Vector, C4-2/LR-STK4, and C4-2/NL-STK4 cells mixed with Matrigel (1:1 ratio) were implanted subcutaneously at the right and left flanks of the nude and immunocompromised male mice (n = 10 per condition). 1×106 cells/100 μL were used per injection per site under anesthesia by isoflurane. Starting 24h post cell inoculation, mice were treated with Dox (0.5 mg/mL) in drinking water for 6 weeks to induce STK4 expression. A weekly tumor size measurement was assessed by caliper manually [27, 29]. Upon completion of the experiment, mice were sacrificed by humane way (by CO2 inhalation, followed by cervical dislocation) and tumor tissues extracted from mice were fixed in 5% formaldehyde or “snap” frozen at −80°C for future analysis. Animal study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) at Cedars-Sinai Medical Center. All experimental procedures in live animals were performed under isoflurane anesthesia and all efforts were made to minimize suffering. Mice were euthanized by CO2 inhalation, followed by cervical dislocation.” Student's t-test (two-tailed) was used to determine the significance between the two groups. P-value ≤ 0.05 was considered significant.
RNA isolation and sequencing
Total RNA in biological replicates was isolated from C4-2/Vector, C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells using RNA isolation kit according to manufacturer’s instruction (Life Technologies). Cells were grown in 10% Tet-approved serum-fed conditions at 80% confluence prior to RNA isolation. Quality of total RNA was assessed prior to library construction. RNA-sequencing (RNAseq) libraries were prepared using the standard protocol and sequenced by Illumina Genome Analyzer IIx in Genomic Core at Cedar-Sinai Medical Center.
RNAseq data analysis
The quality of 72 base-pair reads was assessed with FastQC 0.10.1 software [30]. FastQC identifies lingering TruSeq adapter sequences present on reads. We used cutadapt tool to trim low quality base pairs and the TruSeq adapter sequences from the end of reads [31]. The reads were then aligned against the Illumina iGenomes Homo Sapiens NCBI build 37.2 reference sequence using Tophat 2.2.08b [32]. To compute DE genes, gene counts (i.e., number of sequence reads assigned to each gene) were calculated using htseq-count tool [33]. The gene count data were fed into DESeq2 Bioconductor package in R to identify DE genes using the false discovery rate (FDR) and fold change cutoffs [34]. To demonstrate the consistency of transcript abundance between replicates, scatterplot of the DESeq2 normalized gene counts were plotted in R.
Gene ontology and pathway enrichment analysis
To compute the functional annotation of the DE genes, we performed gene ontology (GO) [35] and KEGG pathway [36] enrichment analysis using STRINGDb Bioconductor package (version 1.14.0) in R [37]. All transcripts in Homo Sapiens NCBI build 37.2 were used as background and Benjamini-Hochberg based FDR threshold of 0.05 was used to select significant GO biological process terms and KEGG pathways for each DE gene list [38]. We visualized the top 20 enriched KEGG pathways and GO terms in a bar chart using the lattice library (version 0.20–34) in R. We computed the union of top 20 enriched KEGG pathways/GO terms and displayed the significant FDR values (i.e., ≤ 0.05) of these pathways/terms in the three conditions.
Network enrichment analysis of DE genes
Reactome Functional Interaction (FI) plugin in Cytoscape was used to study the known functional interactions among the DE genes [39]. First, we imported the DE genes to build a network of known functional interactions among the DE genes using the most recent Reactome FI Network annotation (version 2015). In this network, each node is a DE gene and edges represent the known functional interactions. We filtered the network by removing computationally predicted interactions and genes with zero degree (i.e., did not have any functional interaction to any other gene in the network). We clustered nodes into modules using “Cluster FI Network” function in Reactome FI plugin. We performed GO biological process enrichment on modules of size ≥ 10 and determined a representative GO term for each module by examining the GO enrichment results manually. Finally, we edited the network by setting the layout to “grouping by module ID”, labeling each module by its GO term, coloring nodes based on their upregulation/downregulation status, and adjusting the size of nodes and the font size of node labels proportional to their degree (i.e., number of interactions that they have).
Availability of RNAseq data
RNAseq data are available for download from the National Center for Biotechnology Information Sequence Read Archive database (Accession Number: SRP102205).
Results
Establishment of lipid raft and nuclear localized STK4 prostate cancer cell models
To better understand the effects of STK4 enriched in the cytoplasm, lipid raft and nucleus on cell growth and gene expression in PC, first we established the Tet-inducible lipid raft (LR)- and nuclear (NL)-localized STK4 expressing C4-2 cell models in addition to the cytoplasm (CL)-localized STK4 expressing C4-2 cell model. Previously, we described the establishment of CL-localized STK4 C4-2 cell, which was included as a control in this study [27]. The Tet-inducible system allowed us to control STK4 expression in the cell. We designated these cell models as C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells (Fig 1A–1C). In this study, we utilized the C4-2 cell line because it is the castration-resistant subline of LNCaP cells and expresses significantly lower levels of STK4 transcript and protein than parental LNCaP [24]. Ectopic expression of STK4 protein in the engineered C4-2 cells was analyzed by Western blotting (Fig 1A–1D). First, we showed that doxycycline (Dox, a tetracycline analog) exposure increased the expression of ectopic CL-STK4, LR-STK4, and NL-STK4 in a time-dependent manner relative to the no Dox treatment (Fig 1A). Second, we demonstrated that ectopic STK4 protein was primarily enriched in the intended subcellular locations: cytoplasm in C4-2/CL-STK4 cell (Fig 1B), lipid raft in C4-2/LR-STK4 (Fig 1C), and nucleus in C4-2/NL-STK4 (Fig 1D) cells. Here, it is worth mentioning that the cleavage of ectopic STK4 protein (i.e. STK4-N) due to the overexpression was not detectible by Western blotting under these experimental conditions (not shown). In addition, we performed immunofluorescence imaging to verify the subcellular localization of ectopic CL-STK4, LR-STK4, and NL-STK4 protein (Fig 1E, 1F and 1G, respectively).
STK4 enriched in cytoplasm, lipid raft, and nucleus differentially regulates cell growth
To determine whether cytoplasmic-, lipid raft-, and nuclear STK4 distinctly regulate cell growth in vitro, C4-2/CL-STK4, C4-2/LR-STK4, C4-2/NL-STK4 and C4-2/Vector (mock) cells were exposed to Dox to induce ectopic STK4 expression in the cell. C4-2/CL-STK4 and C4-2/Vector were included as a positive and negative control, respectively, to accurately evaluate the effects of LR-STK4 and NL-STK4 on C4-2 cell growth (Fig 2A and 2B). The results showed that the growth suppressive effects of LR-STK4 were significantly greater than NL-STK4 and CL-STK4 (P < 0.01). CL-STK4 showed the least inhibitory effects on cell growth, which is consistent with our earlier observation [27]. Therefore, the degree of growth suppression by STK4 is LR-STK4 > NL-STK4 > CL-STK4.
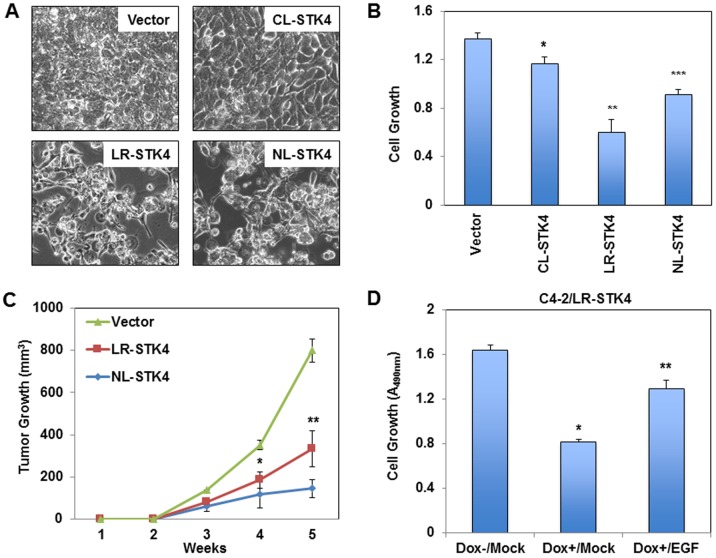
Fig 2. Regulation of C4-2 cell growth by STK4 signaling in all three subcellular locations.
(A) Representative bright field images of C4-2/Vector, C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells. Cell images were captured at 72h post to Dox treatment (4 μg/ml). (B) Growth of C4-2/Vector, C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells in vitro. Cell growth was determined by MTS assay at 72h post Dox exposure. Data (±SD) are the representation of two independent experiments in triplicates, *, **, ***P < 0.007. (C) Prostate tumor xenografts in mice (n = 10 per conditions). C4-2/Vector, C4-2/LR-STK4, and C4-2/NL-STK4 cells were subcutaneously inoculated into the intact nude, immunocompromised male mice. Animals were treated with Dox (0.5 mg/ml) for 6 weeks in drinking water. Tumor sizes were measured weekly for 5 weeks. Tumor growth (volumes) was presented as a function of time, *, **P < 0.01. (D) Growth of C4-2/LR-STK4 cells treated with and without Dox and epidermal growth factor (EGF). Cell growth was determined by MTS assay at 72h post Dox and/or EGF treatment, *, **P < 0.001. Data (±SD) are the representation of two independent experiments in triplicates.
To determine the biological significance of the above findings, we conducted xenograft experiments in mice, according to a protocol approved by the IACUC. C4-2/LR-STK4, C4-2/NL-STK4, and C4-2/Vector cells were implanted under the skin of the hormonally intact and immunocompromised male mice (n = 10 per condition). Mice were fed with Dox in drinking water to induce STK4 expression in tumor cells. The results demonstrated that C4-2/LR-STK4 and C4-2/NL-STK4 cells formed much smaller tumors in number and size than C4-2/Vector (Fig 2C).
We noted that in vitro and in vivo growth suppression caused by the induction of LR-STK4 did not correlate. One possible explanation for it was that the growth suppressive effects of LR-STK4 might be attenuated. Evidence suggested that growth factors or cytokines could negatively regulate STK4 signaling [17, 40–42]. Indeed, treatment of LR-STK4 cells with epidermal growth factor (EGF) significantly reversed the growth inhibitory effects of LR-STK4 relative to the mock control (Fig 2D), indicating that our observations were internally consistent.
STK4 enriched in cytoplasm, lipid raft, and nucleus differentially regulates gene expression
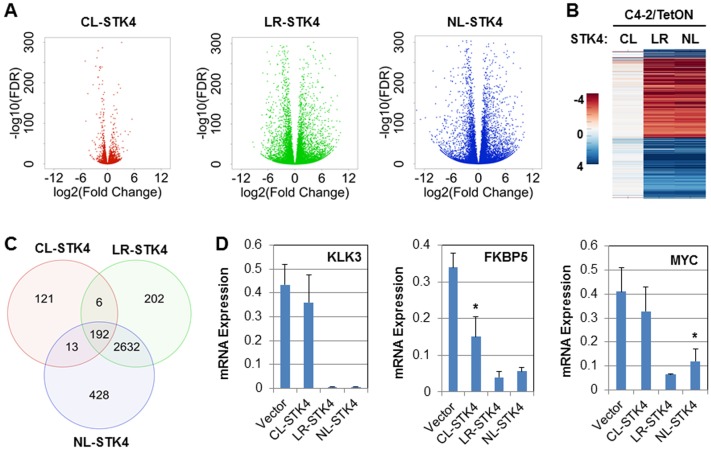
To determine whether the enrichment of STK4 signaling in the cytoplasm, lipid raft, and nucleus changes the gene expression patterns of C4-2 cells, we performed mRNA expression profiling of mock, CL-STK4, LR-STK4, and NL-STK4 cell using RNAseq (see Materials & methods). First, our statistical and bioinformatics analysis of the RNAseq data showed that the normalized gene counts between replicates were highly correlated (S1 Fig), indicating that technical variability of the sequencing between replicates were minimal. Second, we identified a list of DE genes with respect to the vector control using DESeq2. The FDR and fold change values computed by DESeq2 for all genes in all three STK4 conditions are shown in the S1 Table. To determine DE genes, we used absolute log2 fold change values ≥ 2, False Discovery Rate (FDR) ≤ 0.01 cutoffs for LR-STK4, and NL-STK4 cells. To increase the number of DE genes for CL-STK4 cells, however, we used a slightly less stringent fold change cutoff (absolute log2 fold change ≥ 1.5; FDR ≤ 0.01). The results, as illustrated in volcano plots (Fig 3A), heatmap (Fig 3B) and Venn diagram (Fig 3C) demonstrated that the number of DE genes in NL-STK4 and LR-STK4 cells were much greater than CL-STK4, and about 90% DE genes in NL-STK4 and LR-STK4 cells overlap. As detailed in the Venn diagram (Fig 3C), CL-STK4 cells resulted in 332 DE genes (226 upregulated and 106 downregulated), LR-STK4 cells resulted in 3032 DE genes (1780 upregulated and 1252 downregulated), and NL-STK4 cells resulted in 3265 DE genes (1938 upregulated and 1327 downregulated). The complete list of DE genes identified from CL-STK4, LR-STK4, and NL-STK4 cells are shown in S2–S4 Tables, respectively. The top 100 DE genes in each condition are shown in Table 1. There were 192 DE genes intersected with STK4 expression in all three subcellular compartments (Table 2). Among the intersected DE genes, seven of them were downregulated in CL-STK4, but upregulated in NL-STK4 and LR-STK4 cells, and 28 of them were upregulated in CL-STK4, but downregulated in NL-STK4 and LR-STK4 cells (Table 2). There were 2830 DE genes intersected in NL-STK4 and LR-STK4 cells (Fig 3C).
Fig 3. Overview of the RNAseq data and the validation.
(A) Volcano plots of DE genes from the DESeq2 analysis. (B) Heatmap of DE genes using fold-change values in all three STK4 conditions (C) Venn diagram of DE genes in C4-2/CL-STK4, C4-2/LR-STK4, and C4-2/NL-STK4 cells. (D) Quantitative RT-PCR analysis of select DE genes, *P < 0.01. Data are representation of the two independent experiments in duplicates.
Table 1. Top 100 DE genes regulated by the enrichment of STK4 in three subcellular locations (FDR ≤ 0.01).
Numbers are in log2 fold change. Minus signs indicate downregulation with respect to the vector control.
| Entrez ID | Gene Symbol | CL-STK4 | Entrez ID | Gene Symbol | LR-STK4 | Entrez ID | Gene Symbol | NL-STK4 |
|---|---|---|---|---|---|---|---|---|
| 2042 | EPHA3 | -4.00 | 10417 | SPON2 | -8.14 | 158471 | PRUNE2 | -8.36 |
| 54898 | ELOVL2 | -3.41 | 3817 | KLK2 | -7.88 | 4311 | MME | -7.19 |
| 6860 | SYT4 | -2.92 | 354 | KLK3 | -7.28 | 367 | AR | -7.05 |
| 1644 | DDC | 2.84 | 26298 | EHF | -6.42 | 8611 | PPAP2A | -6.78 |
| 1836 | SLC26A2 | -2.78 | 7345 | UCHL1 | 6.41 | 7345 | UCHL1 | 6.74 |
| 55504 | TNFRSF19 | -2.70 | 158471 | PRUNE2 | -6.22 | 4128 | MAOA | -6.71 |
| 7982 | ST7 | 2.66 | 85414 | SLC45A3 | -6.16 | 4824 | NKX3-1 | -6.52 |
| 2181 | ACSL3 | -2.39 | 9415 | FADS2 | -5.83 | 999 | CDH1 | -6.39 |
| 2887 | GRB10 | 2.39 | 2042 | EPHA3 | -5.75 | 85414 | SLC45A3 | -6.00 |
| 2289 | FKBP5 | -2.12 | 4824 | NKX3-1 | -5.72 | 2042 | EPHA3 | -5.94 |
| 64853 | AIDA | 1.99 | 999 | CDH1 | -5.37 | 9415 | FADS2 | -5.91 |
| 444 | ASPH | 1.65 | 8611 | PPAP2A | -5.29 | 56937 | PMEPA1 | -5.57 |
| 8287 | USP9Y | 3.16 | 2346 | FOLH1 | -5.27 | 200916 | RPL22L1 | -5.47 |
| 58480 | RHOU | -2.10 | 4128 | MAOA | -5.17 | 6720 | SREBF1 | -5.41 |
| 79884 | MAP9 | -2.36 | 4311 | MME | -5.14 | 2182 | ACSL4 | 5.41 |
| 23671 | TMEFF2 | -4.99 | 154796 | AMOT | 4.97 | 261729 | STEAP2 | -5.31 |
| 1846 | DUSP4 | -2.64 | 2182 | ACSL4 | 4.95 | 4784 | NFIX | -5.14 |
| 60481 | ELOVL5 | -1.64 | 1021 | CDK6 | 4.76 | 3169 | FOXA1 | -5.13 |
| 2982 | GUCY1A3 | -1.75 | 3169 | FOXA1 | -4.71 | 4685 | NCAM2 | -5.09 |
| 79993 | ELOVL7 | -1.58 | 367 | AR | -4.67 | 154796 | AMOT | 5.06 |
| 22936 | ELL2 | -1.84 | 200916 | RPL22L1 | -4.66 | 6482 | ST3GAL1 | -4.97 |
| 1948 | EFNB2 | -2.07 | 1803 | DPP4 | -4.66 | 5567 | PRKACB | -4.96 |
| 54491 | FAM105A | -2.15 | 55504 | TNFRSF19 | -4.63 | 1803 | DPP4 | -4.95 |
| 563 | AZGP1 | -1.83 | 283349 | RASSF3 | -4.60 | 1021 | CDK6 | 4.92 |
| 3158 | HMGCS2 | 2.49 | 5567 | PRKACB | -4.56 | 283349 | RASSF3 | -4.74 |
| 3479 | IGF1 | -3.24 | 9590 | AKAP12 | 4.55 | 94241 | TP53INP1 | -4.74 |
| 57007 | ACKR3 | 2.87 | 23052 | ENDOD1 | -4.38 | 10982 | MAPRE2 | 4.71 |
| 4430 | MYO1B | -1.96 | 6652 | SORD | -4.37 | 26872 | STEAP1 | -4.65 |
| 6192 | RPS4Y1 | 2.20 | 3315 | HSPB1 | -4.34 | 9590 | AKAP12 | 4.62 |
| 4094 | MAF | -4.42 | 94241 | TP53INP1 | -4.27 | 55504 | TNFRSF19 | -4.61 |
| 9518 | GDF15 | 2.03 | 4784 | NFIX | -4.27 | 4324 | MMP15 | -4.61 |
| 1026 | CDKN1A | 1.70 | 80031 | SEMA6D | 4.25 | 26084 | ARHGEF26 | -4.55 |
| 79822 | ARHGAP28 | -1.51 | 7782 | SLC30A4 | -4.23 | 7782 | SLC30A4 | -4.49 |
| 2550 | GABBR1 | 2.15 | 261729 | STEAP2 | -4.20 | 3315 | HSPB1 | -4.48 |
| 10602 | CDC42EP3 | -2.44 | 79993 | ELOVL7 | -4.17 | 7431 | VIM | 4.43 |
| 4285 | MIPEP | -1.52 | 4685 | NCAM2 | -4.15 | 439921 | MXRA7 | 4.39 |
| 85414 | SLC45A3 | -1.65 | 26084 | ARHGEF26 | -4.15 | 6652 | SORD | -4.37 |
| 56937 | PMEPA1 | -1.60 | 114569 | MAL2 | -4.08 | 10129 | FRY | -4.35 |
| 9687 | GREB1 | -1.86 | 6482 | ST3GAL1 | -4.03 | 79993 | ELOVL7 | -4.35 |
| 120 | ADD3 | 1.71 | 4436 | MSH2 | 3.87 | 80031 | SEMA6D | 4.29 |
| 4316 | MMP7 | 5.48 | 3909 | LAMA3 | -3.82 | 23052 | ENDOD1 | -4.29 |
| 8756 | ADAM7 | -4.84 | 5358 | PLS3 | 3.82 | 3909 | LAMA3 | -4.27 |
| 445 | ASS1 | 1.58 | 27347 | STK39 | -3.76 | 6678 | SPARC | 4.26 |
| 4100 | MAGEA1 | 1.84 | 23705 | CADM1 | 3.65 | 286077 | FAM83H | -4.22 |
| 84898 | PLXDC2 | 3.94 | 1534 | CYB561 | -3.65 | 9515 | STXBP5L | -4.18 |
| 147381 | CBLN2 | 5.02 | 25874 | MPC2 | -3.63 | 114569 | MAL2 | -4.11 |
| 8653 | DDX3Y | 2.06 | 10481 | HOXB13 | -3.62 | 3716 | JAK1 | 4.06 |
| 3899 | AFF3 | -1.56 | 22998 | LIMCH1 | -3.59 | 4436 | MSH2 | 4.00 |
| 23266 | LPHN2 | -2.08 | 11057 | ABHD2 | -3.58 | 22998 | LIMCH1 | -3.95 |
| 1292 | COL6A2 | 1.53 | 667 | DST | 3.56 | 25874 | MPC2 | -3.91 |
| 1001 | CDH3 | 2.92 | 22894 | DIS3 | 3.53 | 11057 | ABHD2 | -3.82 |
| 4109 | MAGEA10 | 4.63 | 2982 | GUCY1A3 | -3.47 | 22894 | DIS3 | 3.79 |
| 4857 | NOVA1 | 2.36 | 5580 | PRKCD | -3.41 | 27347 | STK39 | -3.75 |
| 1135 | CHRNA2 | -2.40 | 55748 | CNDP2 | -3.37 | 25923 | ATL3 | -3.74 |
| 57633 | LRRN1 | 1.73 | 25923 | ATL3 | -3.37 | 5358 | PLS3 | 3.72 |
| 3397 | ID1 | -1.86 | 10257 | ABCC4 | -3.32 | 10481 | HOXB13 | -3.67 |
| 10551 | AGR2 | -3.40 | 57221 | KIAA1244 | -3.25 | 57683 | ZDBF2 | 3.65 |
| 8612 | PPAP2C | 1.89 | 60481 | ELOVL5 | -3.23 | 1534 | CYB561 | -3.56 |
| 4233 | MET | 3.42 | 2181 | ACSL3 | -3.23 | 23705 | CADM1 | 3.55 |
| 138046 | RALYL | -2.96 | 2335 | FN1 | -3.05 | 667 | DST | 3.54 |
| 162394 | SLFN5 | 1.58 | 23195 | MDN1 | 3.02 | 2335 | FN1 | -3.44 |
| 285025 | CCDC141 | -2.76 | 11167 | FSTL1 | 2.99 | 57221 | KIAA1244 | -3.41 |
| 54842 | MFSD6 | 1.86 | 23327 | NEDD4L | -2.93 | 55748 | CNDP2 | -3.39 |
| 116443 | GRIN3A | 1.87 | 6675 | UAP1 | -2.89 | 22873 | DZIP1 | 3.35 |
| 1740 | DLG2 | -2.69 | 5836 | PYGL | 2.86 | 64780 | MICAL1 | -3.31 |
| 80150 | ASRGL1 | -1.83 | 55884 | WSB2 | -2.84 | 2982 | GUCY1A3 | -3.28 |
| 3934 | LCN2 | 2.35 | 3417 | IDH1 | -2.81 | 5580 | PRKCD | -3.28 |
| 4137 | MAPT | 1.72 | 288 | ANK3 | -2.75 | 7105 | TSPAN6 | 3.25 |
| 23467 | NPTXR | 1.79 | 56994 | CHPT1 | -2.74 | 9882 | TBC1D4 | 3.22 |
| 6091 | ROBO1 | -1.63 | 7764 | ZNF217 | -2.71 | 56204 | FAM214A | -3.17 |
| 90362 | FAM110B | -1.55 | 5530 | PPP3CA | -2.70 | 64839 | FBXL17 | -3.14 |
| 4664 | NAB1 | 2.07 | 55704 | CCDC88A | 2.69 | 60481 | ELOVL5 | -3.13 |
| 9783 | RIMS3 | 2.37 | 2289 | FKBP5 | -2.50 | 5836 | PYGL | 3.11 |
| 389206 | BEND4 | -1.71 | 3992 | FADS1 | -2.43 | 2181 | ACSL3 | -3.11 |
| 6286 | S100P | -2.03 | 79718 | TBL1XR1 | -2.42 | 5530 | PPP3CA | -3.04 |
| 330 | BIRC3 | 1.91 | 57222 | ERGIC1 | -2.39 | 1528 | CYB5A | -3.02 |
| 5796 | PTPRK | 1.97 | 55827 | DCAF6 | -2.38 | 11167 | FSTL1 | 3.01 |
| 5801 | PTPRR | 3.23 | 2195 | FAT1 | 2.30 | 57211 | GPR126 | -2.96 |
| 57628 | DPP10 | 3.56 | 7163 | TPD52 | -2.28 | 51347 | TAOK3 | -2.95 |
| 51351 | ZNF117 | 2.05 | 8826 | IQGAP1 | 2.25 | 928 | CD9 | -2.88 |
| 79674 | VEPH1 | -2.65 | 64062 | RBM26 | 2.24 | 288 | ANK3 | -2.85 |
| 64881 | PCDH20 | 2.63 | 2194 | FASN | -2.22 | 22936 | ELL2 | -2.85 |
| 2026 | ENO2 | 1.95 | 23013 | SPEN | 2.19 | 6091 | ROBO1 | 2.84 |
| 10133 | OPTN | 2.20 | 29968 | PSAT1 | -2.10 | 23327 | NEDD4L | -2.83 |
| 5066 | PAM | 1.87 | 6747 | SSR3 | -2.03 | 4942 | OAT | 2.82 |
| 7102 | TSPAN7 | 4.40 | 6678 | SPARC | 4.19 | 7764 | ZNF217 | -2.81 |
| 80031 | SEMA6D | -4.13 | 51347 | TAOK3 | -3.23 | 56994 | CHPT1 | -2.80 |
| 26053 | AUTS2 | 1.78 | 9882 | TBC1D4 | 3.10 | 55884 | WSB2 | -2.79 |
| 284119 | PTRF | 2.13 | 56937 | PMEPA1 | -5.13 | 57619 | SHROOM3 | -2.74 |
| 162514 | TRPV3 | -2.41 | 6091 | ROBO1 | 2.79 | 6675 | UAP1 | -2.70 |
| 5268 | SERPINB5 | 1.65 | 159195 | USP54 | -2.51 | 55704 | CCDC88A | 2.70 |
| 9644 | SH3PXD2A | 1.59 | 2235 | FECH | -2.55 | 3417 | IDH1 | -2.69 |
| 55243 | KIRREL | 3.62 | 57211 | GPR126 | -2.86 | 1174 | AP1S1 | -2.69 |
| 4117 | MAK | -1.67 | 57683 | ZDBF2 | 3.45 | 79718 | TBL1XR1 | -2.63 |
| 3371 | TNC | 1.71 | 2131 | EXT1 | 3.50 | 1946 | EFNA5 | -2.63 |
| 57562 | KIAA1377 | 2.32 | 7431 | VIM | 4.54 | 5768 | QSOX1 | -2.62 |
| 10158 | PDZK1IP1 | 1.51 | 440 | ASNS | -2.34 | 159195 | USP54 | -2.61 |
| 9086 | EIF1AY | 1.85 | 23657 | SLC7A11 | -3.54 | 51166 | AADAT | -2.60 |
| 8284 | KDM5D | 1.64 | 51635 | DHRS7 | -2.74 | 57222 | ERGIC1 | -2.54 |
| 5352 | PLOD2 | 1.57 | 595 | CCND1 | -2.25 | 3304 | HSPA1B | 2.52 |
Table 2. Common DE genes regulated by the enrichment of STK4 in three subcellular locations (FDR ≤ 0.01).
Numbers are in log2 fold change. Minus signs indicate downregulation with respect to the vector control.
| Entrez ID | Gene Symbol | CL-STK4 | LR-STK4 | NL-STK4 | Entrez ID | Gene Symbol | CL-STK4 | LR-STK4 | NL-STK4 |
|---|---|---|---|---|---|---|---|---|---|
| 23316 | CUX2 | -1.61 | 2.64 | 2.8 | 3113 | HLA-DPA1 | 2.03 | 4.98 | 4.8 |
| 3087 | HHEX | -2.09 | 2.33 | 2.4 | 3239 | HOXD13 | 1.70 | 7.53 | 7.6 |
| 3400 | ID4 | -2.00 | 3.57 | 3.5 | 11255 | HRH3 | -1.53 | -3.61 | -3.8 |
| 5064 | PALM | -2.19 | 2.76 | 2.8 | 9951 | HS3ST4 | -3.12 | -4.71 | -5.3 |
| 6091 | ROBO1 | -1.63 | 2.79 | 2.8 | 3434 | IFIT1 | 1.86 | 2.86 | 2.7 |
| 80031 | SEMA6D | -4.13 | 4.25 | 4.3 | 3479 | IGF1 | -3.24 | -6.94 | -8.7 |
| 57713 | SFMBT2 | -1.60 | 4.41 | 4.4 | 3751 | KCND2 | -1.58 | -3.06 | -3.7 |
| 57007 | ACKR3 | 2.87 | -2.45 | -5.7 | 57562 | KIAA1377 | 2.32 | 3.17 | 2.9 |
| 6364 | CCL20 | 1.74 | -3.12 | -4.5 | 3798 | KIF5A | 2.68 | 6.71 | 6.6 |
| 51816 | CECR1 | 2.08 | -2.60 | -2.6 | 55243 | KIRREL | 3.62 | 6.78 | 6.8 |
| 170712 | COX7B2 | 2.12 | -2.71 | -3.9 | 84894 | LINGO1 | 1.84 | 3.70 | 3.9 |
| 1644 | DDC | 2.84 | -3.41 | -7.1 | 54947 | LPCAT2 | 1.92 | 4.68 | 5.0 |
| 8653 | DDX3Y | 2.06 | -4.39 | -7.1 | 84230 | LRRC8C | 1.99 | 5.70 | 5.6 |
| 9086 | EIF1AY | 1.85 | -3.23 | -5.2 | 130576 | LYPD6B | 2.36 | 2.40 | 3.1 |
| 9518 | GDF15 | 2.03 | -6.69 | -7.5 | 4117 | MAK | -1.67 | -2.40 | -2.3 |
| 219970 | GLYATL2 | 1.55 | -3.86 | -6.0 | 22983 | MAST1 | 1.50 | 2.29 | 2.3 |
| 3158 | HMGCS2 | 2.49 | -3.53 | -7.1 | 154141 | MBOAT1 | 1.71 | 4.01 | 4.1 |
| 153572 | IRX2 | 1.76 | -3.08 | -3.6 | 2122 | MECOM | 2.76 | 5.13 | 5.0 |
| 8284 | KDM5D | 1.64 | -3.18 | -5.7 | 4233 | MET | 3.42 | 6.10 | 6.2 |
| 3934 | LCN2 | 2.35 | -3.90 | -4.9 | 11320 | MGAT4A | 2.14 | 3.91 | 3.9 |
| 57633 | LRRN1 | 1.73 | -4.01 | -6.5 | 4281 | MID1 | 2.09 | 7.94 | 7.8 |
| 4100 | MAGEA1 | 1.84 | -3.60 | -7.4 | 4285 | MIPEP | -1.52 | -2.76 | -2.4 |
| 4316 | MMP7 | 5.48 | -3.03 | -3.6 | 26002 | MOXD1 | 2.85 | 7.08 | 7.6 |
| 22829 | NLGN4Y | 1.53 | -3.68 | -4.3 | 4664 | NAB1 | 2.07 | 3.12 | 3.3 |
| 10158 | PDZK1IP1 | 1.51 | -4.07 | -6.6 | 57701 | NCKAP5L | 1.62 | 2.41 | 2.6 |
| 5284 | PIGR | 1.82 | -2.72 | -2.8 | 23114 | NFASC | 1.57 | 2.58 | 2.3 |
| 8612 | PPAP2C | 1.89 | -3.94 | -5.9 | 7025 | NR2F1 | 1.61 | 3.62 | 3.3 |
| 64063 | PRSS22 | 1.73 | -3.55 | -4.1 | 2908 | NR3C1 | 2.58 | 8.50 | 8.3 |
| 5696 | PSMB8 | 2.53 | -2.10 | -2.6 | 4922 | NTS | 2.08 | 4.71 | 4.7 |
| 6192 | RPS4Y1 | 2.20 | -3.63 | -7.8 | 93145 | OLFM2 | 2.42 | 2.62 | 2.5 |
| 5268 | SERPINB5 | 1.65 | -4.31 | -5.5 | 10133 | OPTN | 2.20 | 2.02 | 2.1 |
| 8764 | TNFRSF14 | 1.62 | -3.95 | -4.2 | 390190 | OR5B2 | -2.17 | -3.93 | -4.5 |
| 7367 | UGT2B17 | 1.63 | -2.16 | -4.7 | 5066 | PAM | 1.87 | 3.88 | 4.0 |
| 8287 | USP9Y | 3.16 | -2.47 | -6.6 | 64881 | PCDH20 | 2.63 | 2.51 | 2.9 |
| 7404 | UTY | 2.08 | -2.79 | -4.9 | 56034 | PDGFC | 2.02 | 6.40 | 6.4 |
| 728763 | AC104809.3 | -1.89 | -4.27 | -4.9 | 114770 | PGLYRP2 | -2.23 | -5.75 | -6.8 |
| 2181 | ACSL3 | -2.39 | -3.23 | -3.1 | 5241 | PGR | 2.90 | 6.04 | 6.1 |
| 23305 | ACSL6 | 1.75 | 3.86 | 3.6 | 5569 | PKIA | 2.53 | 5.84 | 5.9 |
| 10863 | ADAM28 | -2.48 | -4.17 | -3.6 | 51365 | PLA1A | -2.18 | -5.94 | -6.6 |
| 8756 | ADAM7 | -4.84 | -7.51 | -8.2 | 440107 | PLEKHG7 | -1.51 | -3.55 | -4.1 |
| 150 | ADRA2A | -1.72 | -4.08 | -5.9 | 5352 | PLOD2 | 1.57 | 3.94 | 4.3 |
| 100130776 | AGAP2-AS1 | 1.58 | 3.05 | 3.4 | 84898 | PLXDC2 | 3.94 | 3.66 | 3.4 |
| 10551 | AGR2 | -3.40 | -7.05 | -7.1 | 56937 | PMEPA1 | -1.60 | -5.13 | -5.6 |
| 183 | AGT | -1.96 | -4.95 | -5.1 | 57718 | PPP4R4 | 1.93 | 2.56 | 2.8 |
| 57491 | AHRR | 1.52 | 5.95 | 6.2 | 5743 | PTGS2 | 1.53 | 2.96 | 3.8 |
| 115701 | ALPK2 | -2.23 | -3.52 | -3.7 | 5796 | PTPRK | 1.97 | 3.36 | 3.3 |
| 347902 | AMIGO2 | 1.83 | 3.16 | 3.3 | 284119 | PTRF | 2.13 | 2.65 | 2.9 |
| 319 | APOF | -1.82 | -4.53 | -4.7 | 138046 | RALYL | -2.96 | -6.93 | -7.6 |
| 79822 | ARHGAP28 | -1.51 | -2.60 | -2.6 | 11069 | RAPGEF4 | 1.51 | 4.05 | 4.2 |
| 115557 | ARHGEF25 | 1.81 | 2.30 | 2.4 | 85004 | RERG | 2.02 | 3.99 | 4.3 |
| 26053 | AUTS2 | 1.78 | 3.23 | 3.1 | 10287 | RGS19 | 1.70 | 3.28 | 3.3 |
| 563 | AZGP1 | -1.83 | -7.37 | -10.2 | 58480 | RHOU | -2.10 | -2.27 | -2.4 |
| 25825 | BACE2 | 1.98 | 3.56 | 3.5 | 9783 | RIMS3 | 2.37 | 2.45 | 2.4 |
| 54796 | BNC2 | 2.07 | 4.92 | 4.9 | 140730 | RIMS4 | 1.51 | 6.12 | 6.2 |
| 89927 | C16orf45 | 1.92 | 2.32 | 2.2 | 57484 | RNF150 | 1.54 | 4.52 | 4.6 |
| 767 | CA8 | 3.00 | 6.92 | 7.3 | 221687 | RNF182 | 2.10 | 3.82 | 3.8 |
| 57118 | CAMK1D | 1.91 | 3.68 | 3.9 | 6286 | S100P | -2.03 | -6.00 | -5.5 |
| 858 | CAV2 | 3.15 | 6.26 | 6.5 | 55511 | SAGE1 | -2.23 | -4.94 | -5.6 |
| 285025 | CCDC141 | -2.76 | -4.30 | -4.8 | 389432 | SAMD5 | 2.55 | 3.04 | 3.0 |
| 159989 | CCDC67 | -2.41 | -4.89 | -5.5 | 54809 | SAMD9 | 2.68 | 3.25 | 3.8 |
| 151887 | CCDC80 | 2.66 | 5.68 | 5.3 | 6326 | SCN2A | 2.46 | 4.71 | 4.6 |
| 9308 | CD83 | 1.68 | 2.84 | 2.7 | 56256 | SERTAD4 | 2.46 | 6.28 | 6.1 |
| 64781 | CERK | 2.54 | 5.64 | 5.7 | 9644 | SH3PXD2A | 1.59 | 3.65 | 3.8 |
| 1135 | CHRNA2 | -2.40 | -7.59 | -8.3 | 6565 | SLC15A2 | -1.65 | -2.46 | -2.1 |
| 9435 | CHST2 | 2.14 | 4.65 | 4.9 | 7781 | SLC30A3 | 2.20 | 5.05 | 5.0 |
| 7123 | CLEC3B | -1.51 | -3.95 | -4.1 | 55089 | SLC38A4 | -3.73 | -2.73 | -2.1 |
| 80034 | CSRNP3 | 2.12 | 3.16 | 3.2 | 85414 | SLC45A3 | -1.65 | -6.16 | -6.0 |
| 1519 | CTSO | -1.57 | -2.58 | -2.2 | 146857 | SLFN13 | 2.50 | 3.17 | 3.0 |
| 10563 | CXCL13 | -2.96 | -4.32 | -4.4 | 84189 | SLITRK6 | -1.68 | -3.89 | -3.5 |
| 80319 | CXXC4 | 2.35 | 5.90 | 5.8 | 8406 | SRPX | 2.25 | 2.73 | 2.8 |
| 260293 | CYP4X1 | 2.04 | 2.66 | 3.1 | 7903 | ST8SIA4 | 2.61 | 2.73 | 3.4 |
| 1740 | DLG2 | -2.69 | -3.44 | -3.8 | 6769 | STAC | 2.32 | 4.58 | 4.6 |
| 93099 | DMKN | 1.60 | 5.54 | 5.8 | 112755 | STX1B | 1.83 | 4.45 | 4.9 |
| 10655 | DMRT2 | -1.69 | -3.91 | -4.0 | 55061 | SUSD4 | 1.54 | 2.47 | 3.0 |
| 1846 | DUSP4 | -2.64 | -3.71 | -3.5 | 221711 | SYCP2L | 1.75 | 4.82 | 4.8 |
| 80303 | EFHD1 | 1.50 | 4.55 | 4.8 | 23345 | SYNE1 | 1.90 | 4.46 | 4.4 |
| 22936 | ELL2 | -1.84 | -3.10 | -2.8 | 11346 | SYNPO | 1.52 | 2.86 | 2.7 |
| 54898 | ELOVL2 | -3.41 | -2.33 | -2.1 | 6857 | SYT1 | 1.72 | 4.63 | 5.0 |
| 60481 | ELOVL5 | -1.64 | -3.23 | -3.1 | 6860 | SYT4 | -2.92 | -6.38 | -8.5 |
| 79993 | ELOVL7 | -1.58 | -4.17 | -4.3 | 80731 | THSD7B | -1.91 | -3.73 | -4.0 |
| 2026 | ENO2 | 1.95 | 3.18 | 3.4 | 23671 | TMEFF2 | -4.99 | -6.34 | -6.3 |
| 2042 | EPHA3 | -4.00 | -5.75 | -5.9 | 55321 | TMEM74B | 2.55 | 3.90 | 3.7 |
| 2119 | ETV5 | 1.88 | 3.40 | 4.3 | 3371 | TNC | 1.71 | 4.63 | 3.9 |
| 90362 | FAM110B | -1.55 | -2.47 | -2.4 | 55504 | TNFRSF19 | -2.70 | -4.63 | -4.6 |
| 9715 | FAM131B | 1.85 | 3.45 | 4.1 | 23043 | TNIK | 1.64 | 3.97 | 3.8 |
| 23359 | FAM189A1 | 2.02 | 2.02 | 2.0 | 84951 | TNS4 | -1.94 | -5.00 | -5.3 |
| 2217 | FCGRT | 2.29 | 4.06 | 4.2 | 10345 | TRDN | -1.76 | -5.87 | -6.3 |
| 9638 | FEZ1 | 1.90 | 2.14 | 2.8 | 117854 | TRIM6 | 1.74 | 4.92 | 5.0 |
| 2289 | FKBP5 | -2.12 | -2.50 | -2.3 | 7220 | TRPC1 | 1.89 | 6.36 | 6.6 |
| 121643 | FOXN4 | 2.14 | 2.19 | 2.2 | 162514 | TRPV3 | -2.41 | -3.49 | -3.7 |
| 53827 | FXYD5 | 2.27 | 6.68 | 7.0 | 10100 | TSPAN2 | 1.78 | 5.64 | 6.1 |
| 2550 | GABBR1 | 2.15 | 2.39 | 2.2 | 7102 | TSPAN7 | 4.40 | 5.84 | 5.8 |
| 2706 | GJB2 | 1.72 | 2.16 | 3.1 | 23508 | TTC9 | 1.55 | 2.72 | 2.7 |
| 647309 | GMNC | -1.95 | -3.46 | -5.3 | 10382 | TUBB4A | 1.52 | 2.80 | 2.8 |
| 387509 | GPR153 | 1.50 | 3.25 | 3.1 | 54490 | UGT2B28 | -2.08 | -4.54 | -5.2 |
| 2982 | GUCY1A3 | -1.75 | -3.47 | -3.3 | 79674 | VEPH1 | -2.65 | -5.42 | -6.2 |
To validate our RNAseq data, we analyzed the levels of KLK3, FKBP5, and MYC mRNA expression in the engineered C4-2 cells. We selected these genes because (i) their expressions were differentially regulated by STK4 in all three conditions (S1 Table), (ii) KLK3 and FKBP5 are well-known targets of AR that is also negatively regulated by STK4 [43] and (iii) MYC is a YAP/TEAD target and it intersected with STK4 signaling in PC cells [24]. Our RNAseq data revealed that expression of KLK3 was inhibited 7-fold in LR-STK4 and 13-fold in NL-STK4 cells. Similarly, expression of FKBP5 was inhibited 2-fold in CL-STK4, 2.5-fold in LR-STK4, and 2.3-fold in NL-STK4 cells. In addition, expression of MYC was inhibited 1.6-fold in LR-STK4 and NL-STK4 cells. These fold change values are in log2. Induction of CL-STK4 inhibited the expression of KLK3 and MYC less than half fold. Our quantitative PCR analysis verified the RNAseq data that, indeed, STK4 expression in all three cell compartments differentially regulates the expression of KLK3, FKBP5, and MYC (Fig 3D). We also examined the impact of STK4 on the AR pathway genes (Table 3) obtained from Wikipathways [44]. The results showed that several AR targets were upregulated or downregulated by STK4 expression in these subcellular compartments, further validating our RNAseq data.
Table 3. Fold change values of AR targets in response to STK4 enrichment in all three subcellular compartments (FDR ≤ 0.01).
Fold change values are in log2. Minus signs indicate downregulation with respect to the vector control.
| Entrez ID | Gene Symbol | CL-STK4 | LR-STK4 | NL-STK4 | Entrez ID | Gene Symbol | CL-STK4 | LR-STK4 | NL-STK4 |
|---|---|---|---|---|---|---|---|---|---|
| 367 | AR | 0.28 | -4.67 | -7.05 | 9612 | NCOR2 | 0.09 | -0.97 | -0.99 |
| 595 | CCND1 | -0.05 | -2.25 | -2.04 | 1499 | CTNNB1 | 0.15 | 0.41 | 0.43 |
| 8611 | PPAP2A | -0.18 | -5.29 | -6.78 | 860 | RUNX2 | -0.71 | -1.48 | -1.74 |
| 57178 | ZMIZ1 | 0.24 | -1.71 | -1.81 | 5925 | RB1 | 0.44 | 0.61 | 0.75 |
| 23028 | KDM1A | -0.34 | 1.06 | 1.28 | 8648 | NCOA1 | -0.02 | -0.55 | -0.63 |
| 3725 | JUN | 0.25 | 2.52 | 2.54 | 10401 | PIAS3 | 0.43 | -0.72 | -0.74 |
| 1956 | EGFR | 0.77 | -2.41 | -2.36 | 1387 | CREBBP | -0.29 | 0.53 | 0.53 |
| 4088 | SMAD3 | 0.93 | 2.94 | 2.95 | 2119 | ETV5 | 1.88 | 3.40 | 4.32 |
| 998 | CDC42 | -0.14 | 1.64 | 1.75 | 898 | CCNE1 | -0.14 | 0.47 | 0.80 |
| 354 | KLK3 | -0.71 | -7.28 | -13.71 | 2033 | EP300 | -0.12 | 0.35 | 0.35 |
| 24149 | ZNF318 | 0.34 | 1.44 | 1.48 | 29893 | PSMC3IP | -0.31 | 0.61 | 0.85 |
| 811 | CALR | -0.06 | -1.49 | -1.11 | 10499 | NCOA2 | 0.13 | -0.46 | -0.47 |
| 1026 | CDKN1A | 1.70 | -2.11 | -1.95 | 6714 | SRC | 0.05 | -0.66 | -0.61 |
| 573 | BAG1 | 0.22 | -2.03 | -1.78 | 5728 | PTEN | 0.20 | 0.54 | 0.44 |
| 6093 | ROCK1 | -0.01 | 0.98 | 1.12 | 11034 | DSTN | -0.10 | 0.61 | 0.54 |
| 5901 | RAN | -0.39 | 0.64 | 0.72 | 90427 | BMF | -0.35 | -0.54 | -0.90 |
| 11143 | KAT7 | 0.05 | 0.91 | 1.21 | 2288 | FKBP4 | -0.10 | -0.31 | -0.23 |
| 9475 | ROCK2 | 0.20 | 0.91 | 1.05 | 23598 | PATZ1 | -0.03 | 0.45 | 0.53 |
| 207 | AKT1 | 0.16 | -1.03 | -1.11 | 64800 | EFCAB6 | 0.27 | 2.63 | 2.62 |
| 9611 | NCOR1 | 0.33 | 0.69 | 0.86 | 7041 | TGFB1I1 | 0.40 | 1.79 | 3.00 |
| 2308 | FOXO1 | -0.26 | 1.58 | 1.85 | 11315 | PARK7 | -0.22 | -0.21 | -0.21 |
| 2932 | GSK3B | 0.03 | -0.79 | -0.92 | 1385 | CREB1 | -0.10 | -0.37 | -0.43 |
| 6774 | STAT3 | 0.24 | -1.06 | -0.90 | 25942 | SIN3A | 0.19 | 0.08 | 0.19 |
| 2274 | FHL2 | -0.34 | 3.70 | 3.66 | 8554 | PIAS1 | -0.53 | -0.38 | -0.26 |
| 672 | BRCA1 | -0.26 | 0.83 | 0.97 | 3065 | HDAC1 | -0.11 | 0.05 | 0.15 |
| 387 | RHOA | 0.06 | 0.54 | 0.57 | 7329 | UBE2I | -0.04 | -0.19 | -0.19 |
| 7341 | SUMO1 | -0.13 | 0.80 | 0.87 | 6667 | SP1 | 0.17 | 0.33 | 0.17 |
| 8850 | KAT2B | 0.21 | 1.20 | 1.28 | 6013 | RLN1 | 0.36 | -2.68 | -2.21 |
| 10273 | STUB1 | 0.11 | -1.77 | -1.77 | 4193 | MDM2 | 0.42 | -0.10 | -0.16 |
| 5052 | PRDX1 | 0.31 | 0.56 | 0.65 | 5970 | RELA | 0.26 | 0.01 | 0.16 |
| 5295 | PIK3R1 | 0.12 | -1.06 | -1.39 | 5879 | RAC1 | -0.10 | 0.03 | 0.10 |
| 6049 | RNF6 | -0.15 | -1.17 | -0.86 | 388 | RHOB | 0.42 | 0.22 | 0.17 |
| 7337 | UBE3A | -0.13 | -0.82 | -0.68 | 5296 | PIK3R2 | -0.22 | -0.78 | -1.21 |
| 5747 | PTK2 | -0.10 | 0.66 | 0.61 | 9604 | RNF14 | -0.17 | 0.00 | -0.07 |
| 166 | AES | 0.24 | -0.84 | -0.77 | 8202 | NCOA3 | 0.30 | -0.08 | 0.06 |
| 23411 | SIRT1 | -0.33 | 0.97 | 1.00 | 5883 | RAD9A | 0.24 | 0.12 | -0.10 |
| 10399 | GNB2L1 | 0.17 | 0.37 | 0.36 | 56924 | PAK6 | 0.36 | -1.07 | 0.55 |
| 857 | CAV1 | 1.09 | 6.33 | 6.42 | 8431 | NR0B2 | 0.14 | 0.84 | 0.69 |
| 3985 | LIMK2 | -0.39 | -0.73 | -0.85 | 10524 | KAT5 | 0.11 | -0.24 | -0.09 |
| 6605 | SMARCE1 | -0.05 | 0.71 | 0.93 | 6047 | RNF4 | -0.05 | -0.19 | -0.04 |
| 1616 | DAXX | 0.12 | 0.67 | 0.66 | 4089 | SMAD4 | 0.06 | 0.04 | -0.03 |
| 7050 | TGIF1 | 0.24 | -1.23 | -1.24 | 7182 | NR2C2 | 0.15 | -0.01 | -0.03 |
| 9063 | PIAS2 | 0.24 | -0.98 | -0.84 | 51588 | PIAS4 | 0.01 | 0.29 | -0.03 |
| 10498 | CARM1 | 0.09 | 0.93 | 0.83 | 2316 | FLNA | -0.19 | -0.03 | 0.01 |
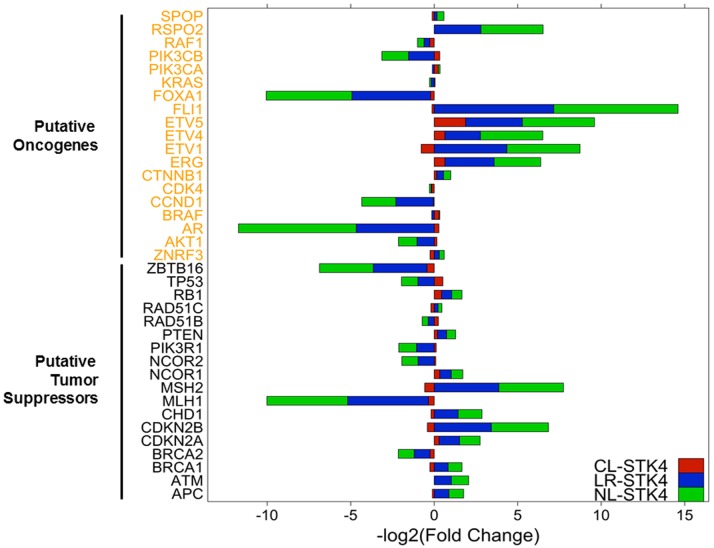
In addition, we compared our DE genes to the known putative oncogenes and tumor suppressors [45, 46]. Fig 4 shows that enrichment of STK4 in all three subcellular compartments selectively regulated the expression of putative oncogenes and tumor suppressors. Many of these genes are directly linked to the AR pathway (e.g. FOXA1, SPOP, NCOR1/2, and ZBTB16), the DNA repair mechanism (e.g. MLH1 and MSH2), a member of the EST factors (e.g. ERG, ETV1/4/5, and FLH1), and cycle regulators (e.g. CDKN1A and CDKN2B). These genes and pathways are suggested to play a critical role in PC biology including metastatic CRPC [45, 46].
Fig 4. STK4 selectively regulates the expression of putative oncogenes and tumor suppressors.
Log2 fold change values for a list of putative oncogenes (black) and tumor suppressors (orange) in CL-STK4, LR-STK4, and NL-STK4 cells.
DE genes regulated by STK4 signaling are associated with multiple biological pathways
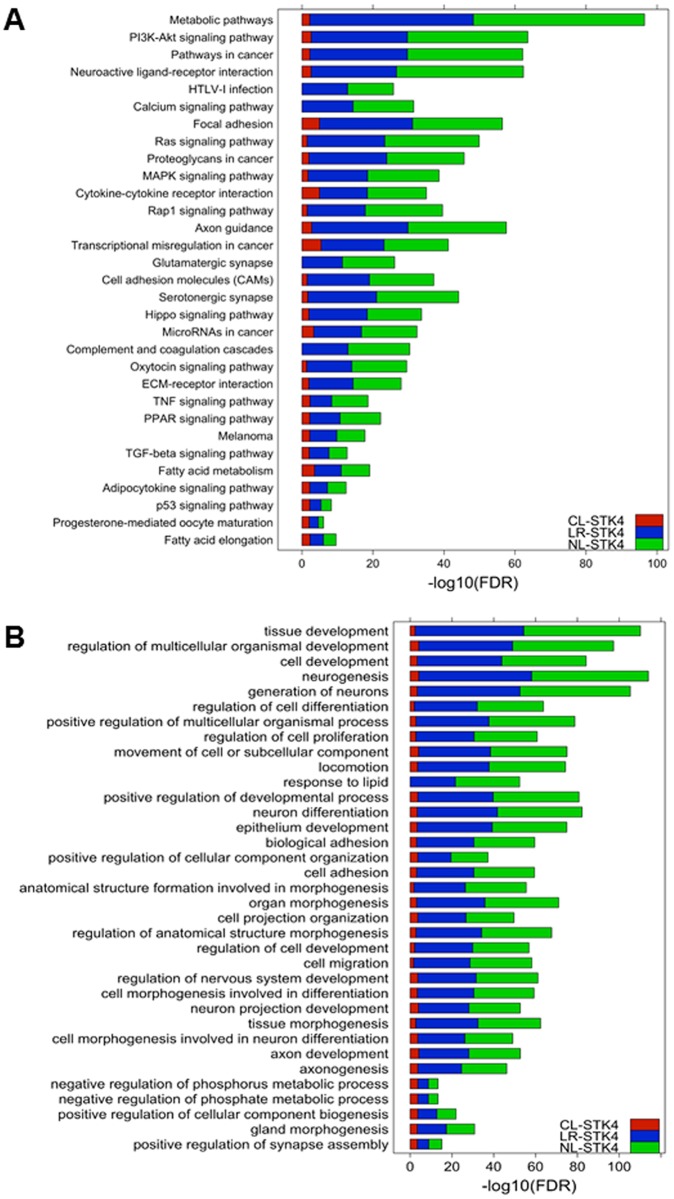
To gain insights into the biological processes and pathways associated with DE genes, we performed KEGG pathway and Gene Ontology (GO) biological processes enrichment analysis using STRINGdb package in R [37]. We found 45, 214, and 216 KEGG pathways enriched by DE genes in CL-STK4, LR-STK4, and NL-STK4 cells, respectively (S5–S7 Tables). There were eight and five KEGG pathways uniquely enriched in LR-STK4 and NL-STK4 cells, respectively. On the other hand, there were no KEGG pathways uniquely enriched in CL-STK4 cells. Top 20 enriched KEGG pathways are shown in Fig 5A and the complete results are shown in S5–S7 Tables. The KEGG pathway enrichment results show that majority of the top enriched pathways (e.g., Metabolic pathways, PI3K-AKT signaling, Pathways in cancer, Focal adhesion, Axon guidance, MAPK signaling, and Fatty acid metabolism) are common in all three conditions. Due to the large number of DE genes in NL-STK4 and LR-STK4 conditions, GO enrichment analysis returned over 2,000 terms. Top 20 enriched GO biological process terms are shown in Fig 5B and complete results are shown in S8–S10 Tables. Top GO terms included terms such as development, proliferation, differentiation, axonogenesis, metabolic process, and cell adhesion.
Fig 5. Top 20 KEGG pathways and GO biological process terms enriched by DE genes in all three STK4 cells.
Each term in the bar chart appears in the top 20 (A) KEGG pathways (B) GO biological process terms enriched by DE genes in at least one condition. Red, blue, and green bars refer to the log2 enrichment FDR values in CL-STK4, LR-STK4, and NL-STK4, respectively. The terms with missing FDR values for CL-STK4 indicate that the DE genes in CL-STK4 were not significantly enriched for those terms.
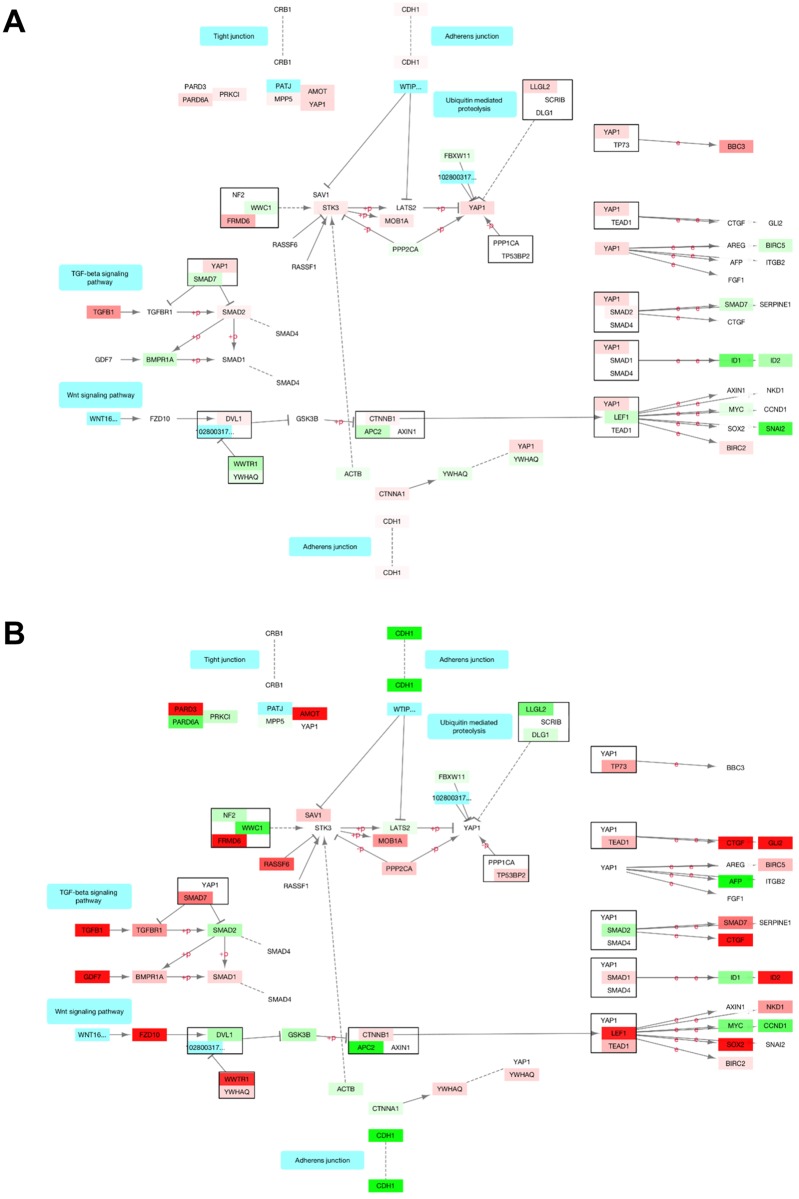
In addition, because STK4 is a potent inhibitor of the YAP1/WWTR1-dependent transcriptions, we evaluated the status of YAP1/WWTR1 targets with respect to the subcellular localization of STK4 (S11 Table). We obtained 48 YAP1/WWTR1 targets from published studies [47–50]. We also illustrated the expression status of the Hippo pathway components in CL-STK4 (Fig 6A), NL-STK4 (Fig 6B), and LR-STK4 (S2 Fig) cells. The results of these investigations demonstrated that CL-STK4 modestly and distinctly altered the expression of YAP1/WWTR1 targets and the Hippo pathway members compared with LR-STK4 and NL-STK4. For example, CL-STK4 slightly increased STK3 and LLGL2 transcripts while LR-STK4 and NL-STK4 downregulated LLGL2 without affecting STK3 transcripts. In addition, LR-STK4 and NL-STK4 differentially regulated YAP1/WWTR1 targets (Fig 6B). For instance, LR-STK4 downregulated YAP1 target ITGB2, whereas NL-STK4 had no effect. Likewise, CL-STK4 and LR-STK4 slightly increased YAP1 expression, but NL-STK4 did not.
Fig 6. KEGG Hippo signaling pathway.
Each gene is colored by gene expression fold change in (A) CL-STK4 (B) NL-STK4 conditions using Cytoscape. Red: Upregulated, green: downregulated, white: not differentially expressed. Edge labels “e”: expression interaction, “+p”: phosphorylation, “-p”: dephosphorylation.
DE genes in NL-STK4 and LR-STK4 cells associate with overlapping and unique modules
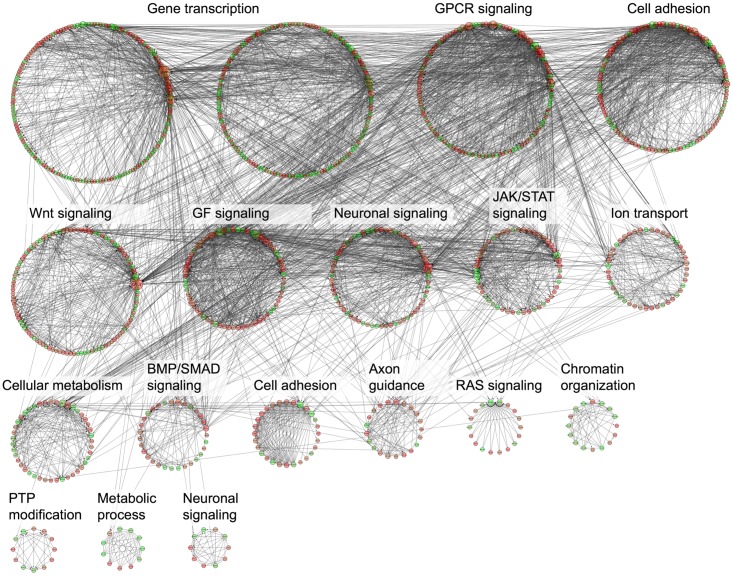
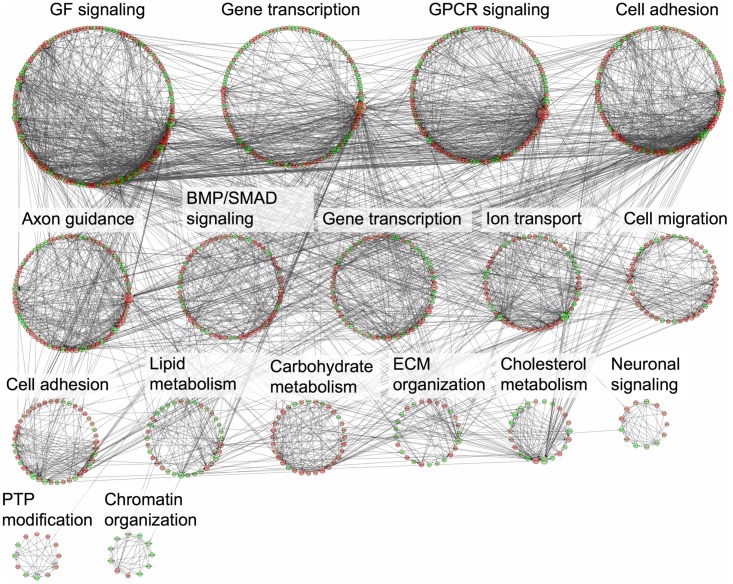
We investigated known functional interactions between DE genes in each condition using Reactome FI plugin in Cytoscape [51]. We built an interaction network of DE genes based on known functional interactions and clustered the network into modules. We annotated each module with a significantly enriched GO term (see Materials & methods). The annotated networks of DE genes for LR-STK4 and NL-STK4 cells were shown in Figs 7 and 8, respectively. Because a few number of DE genes existed in CL-STK4 cells, the network annotation for those DE genes were not performed. The interaction network of NL-STK4 had 1086 genes grouped into 18 modules of size ≥ 10 genes. The interaction network of LR-STK4 had 974 genes grouped into 17 modules of size ≥ 10 genes. Both networks had eight common module annotations such as gene transcription, cell adhesion, and axon guidance, which also appeared in the KEGG pathway enrichment results. The NL-STK4 network had unique GO terms such as WNT signaling, RAS signaling, JAK/STAT signaling, and metabolic processes whereas the LR-STK4 network had unique GO terms such as cell migration, lipid metabolism, and ECM organization. Since clustering of the nodes were not based on GO annotation of genes, but solely based on the known functional interactions between the genes, some modules were enriched in the same GO term, namely gene transcription, cell adhesion, and axon guidance. These findings indicate that STK4/Hippo signaling initiating from or transiting through lipid raft or cell nuclei not only has overlapping functions but it also confers unique functions in regulating cell growth.
Fig 7. Network enrichment annotation of DE genes in NL-STK4 cells.
DE genes in NL-STK4 cells that have known functional interactions were clustered based on their connectivity in the network using Reactome FI plugin in Cytoscape. Each clustered module was annotated to a representative significantly-enriched GO term. Each edge represents a known interaction between two genes (i.e., nodes) in the network.
Fig 8. Network enrichment annotation of DE genes in LR-STK4 cells.
DE genes in LR-STK4 cells that have known functional interactions were clustered based on their connectivity in the network using Reactome FI plugin in Cytoscape. Each clustered module was annotated to a representative significantly-enriched GO term. Each edge represents a known interaction between two genes (i.e., nodes) in the network.
Discussion
In this study, we have demonstrated that controlled expression of STK4, primarily enriched in the cytoplasm, lipid raft, and nucleus, differentially regulates PC cell growth and gene expression. We have identified several DE genes whose expression is downregulated or upregulated by CL-STK4, LR-STK4, and NL-STK4 signaling in all three subcellular locations. Surprisingly, about 90% of DE genes that were regulated by LR-STK4 and NL-STK4 overlapped and the number of DE genes identified from LR-STK4 and NL-STK4 cells were much greater than CL-STK4 cells. Our functional annotation clustering showed that these DE genes were associated with a broad cellular biology including cell proliferation, differentiation, motility, adhesion, survival, apoptosis, axon guidance, and metabolisms. Overall, our data suggest that STK4 signaling transiting through or initiating from a different subcellular compartment may result in distinct gene expression patterns and cellular biology.
PC is the most commonly diagnosed malignancy and the second leading cause of cancer death among men in the US [52]. Because androgen hormone signaling plays a critical role in PC development, progression, and metastasis, androgen deprivation is the first line of therapy for patients with locally advanced disease [45, 46]. However, almost all patients who receive primary androgen deprivation therapy (ADT) develop metastatic CRPC [45, 46]. Evidence indicates that AR is still a key driver of metastatic CRPC cell growth and survival, even in the absence of sub-physiological levels of androgens and in the presence of the second generation of ADT such as abiraterone acetate (ABI), a direct inhibitor of CYP17A that is a key enzyme in the androgen biosynthesis pathway [53, 54] and enzalutamide (ENZ), a direct AR inhibitor [55–57]. However, nearly all men with metastatic CRPC who are treated with ABI and ENZ also develop resistance to these agents, albeit with unknown mechanisms.
Here, we showed that enrichment of STK4 in the cytoplasm, lipid raft, and nucleus selectively regulates AR transcript. We also showed that STK4 signaling depending on its subcellular locations suppressed AR transcript with varying degrees. We noted that although CL-STK4 slightly increased AR transcript, LR-STK4 and NL-STK4 reduced it 4.6- and 7-fold, respectively, which correlated with the inhibition of AR target genes such as FKBP5, KLK3, and TMPRSS2 [43, 58]. However, many AR targets were minimally affected or unaltered by the targeted STK4 signaling (Table 3), suggesting that the regulation of AR targets by STK4 is selective or context-dependent. Previously, we reported that crosstalk between YAP1 and AR signaling could contribute to CRPC [29]. In that study, we showed that STK4 depletion increased YAP1/AR interaction, which coincided with CRPC cell growth. Therefore, it is possible that YAP1 may function as a key intermediate for the selective regulation of AR targets by STK4, but this warrants further investigation, which is not the subject of the current study.
Alterations of the DNA methylation, DNA repair, PI3K/AKT, RAS/RAF, WNT, and cell-cycle pathways are commonly observed both in primary prostate tumors [45] and metastatic CRPC [46]. Aberrant expression of the DNA repair genes such as MLH1 and MSH2 is implicated in advanced PC [59]. Here, we showed that LR-STK4 and NL-STK4, but not CL-STK4, reduced the expression of MLH1 about 5-fold while increasing MSH2 expression about 4-fold under the same growth conditions. Similarly, altered ZBTB16 (also known as PLZF1) signaling is also implicated in metastatic CRPC [46]. Induction of LR-STK4 and NL-STK4, but not of CL-STK4, downregulated the expression of ZBTB16 3.2-fold. ZBTB16 is an AR target gene [46]. Furthermore, CDKN2A and CDKN2B are potent suppressors of cell-cycle progression [46, 60]. LR-STK4 and NL-STK4 increased the expression of CDKN2B 3.4-fold. These findings suggest that STK4 restricts aggressive cancer cell growth by modulating key oncogenic pathways including DNA repair and cell cycle regulators.
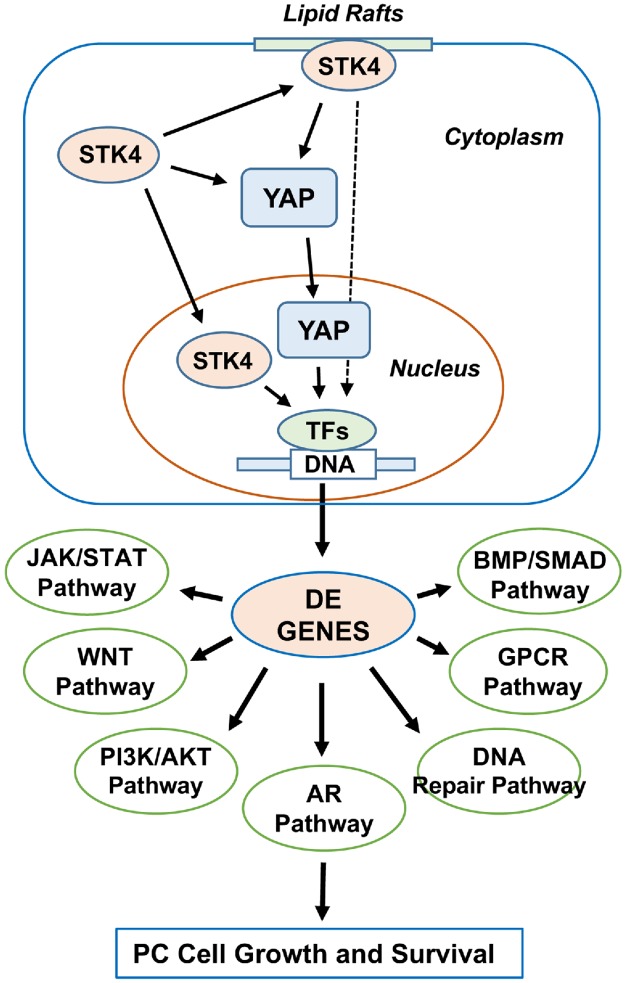
STK4 is a key negative regulator of YAP1/WWTR1-mediated gene transcription and oncogenesis. Herein, our data demonstrated that ectopic STK4 protein enriched in the cytoplasm, lipid raft, and nucleus had differential effects on YAP1/WWTR1 and YAP1/WWTR1-dependent gene expression. We noted that unlike NL-STK4, CL-STK4 and LR-STK4 showed similar trends in regulating YAP1 expression, although about 90% of genes regulated by LR-STK4 and NL-STK4 overlapped. These observations suggest the possibility that (a) CL-STK4 regulates gene expression by signaling through YAP1/WWTR1, (b) LR-STK4 regulates gene expression by YAP1/WWTR1-dependent and YAP1/WWTR1-independent mechanisms, and (c) NL-STK4 most likely regulates gene expression independently of YAP1/WWTR1. Nevertheless, future studies are necessary to test these hypotheses. In summary, we identified several DE genes and molecular pathways that are responded to the targeted STK4 expression and these pathways are known to be biologically and clinically relevant to human cancer including PC. The model in Fig 9 summarizes our main findings.
Fig 9. Schematic representation of the current study.
TFs: Transcription factors. DE: Differentially expressed.
Supporting information
Scatterplot of gene counts from the technical replicates show a high degree of correlation in all three conditions.
(TIF)
Each gene is colored by gene expression fold change in LR-STK4 condition. Red: Upregulated, green: downregulated, white: not differentially expressed. Edge labels “e”: expression interaction, “+p”: phosphorylation, “-p”: dephosphorylation.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Acknowledgments
The authors thank Drs. Jaideep Chaudhary and Shafiq Khan for scientific support, critics, and discussion about the manuscript.
Data Availability
RNA-seq data are available for download from the National Center for Biotechnology Information Sequence Read Archive database (accession number: SRP102205).
Funding Statement
This study was supported in part by the NIH/National Center for Advancing Translational Science (grant #UL1TR000124), and the NIH/National Institute on Minority Health and Health Disparities and Research Centers in Minority Institution Program (grant #5G12MD007590). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Barry ER, Camargo FD. The Hippo superhighway: signaling crossroads converging on the Hippo/Yap pathway in stem cells and development. Curr Opin Cell Biol. 2013;25(2):247–53. doi: 10.1016/j.ceb.2012.12.006 . [DOI] [PubMed] [Google Scholar]
- 2.Plouffe SW, Hong AW, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med. 2015;21(4):212–22. doi: 10.1016/j.molmed.2015.01.003 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381(Pt 2):453–62. doi: 10.1042/BJ20040025 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26(19):2138–43. doi: 10.1101/gad.197582.112 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang K, Qi HX, Hu ZM, Chang YN, Shi ZM, Han XH, et al. YAP and TAZ Take Center Stage in Cancer. Biochemistry. 2015;54(43):6555–66. doi: 10.1021/acs.biochem.5b01014 . [DOI] [PubMed] [Google Scholar]
- 6.Avruch J, Zhou D, Fitamant J, Bardeesy N. Mst1/2 signalling to Yap: gatekeeper for liver size and tumour development. Br J Cancer. 2011;104(1):24–32. doi: 10.1038/sj.bjc.6606011 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinar B, Fang PK, Lutchman M, Di Vizio D, Adam RM, Pavlova N, et al. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. EMBO J. 2007;26(21):4523–34. doi: 10.1038/sj.emboj.7601872 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13(3):188–92. doi: 10.1016/j.ccr.2008.02.011 . [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Lei Q, Guan KL. Mst out and HCC in. Cancer Cell. 2009;16(5):363–4. doi: 10.1016/j.ccr.2009.10.008 . [DOI] [PubMed] [Google Scholar]
- 10.Gomez M, Gomez V, Hergovich A. The Hippo pathway in disease and therapy: cancer and beyond. Clin Transl Med. 2014;3:22 doi: 10.1186/2001-1326-3-22 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107(4):1437–42. doi: 10.1073/pnas.0911427107 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976 doi: 10.1038/ncomms3976 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–9. doi: 10.1101/gad.1134003 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–38. doi: 10.1016/j.ccr.2009.09.026 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17(1):7–19. doi: 10.1038/ncb3084 . [DOI] [PubMed] [Google Scholar]
- 16.Graves JD, Draves KE, Gotoh Y, Krebs EG, Clark EA. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem. 2001;276(18):14909–15. doi: 10.1074/jbc.M010905200 . [DOI] [PubMed] [Google Scholar]
- 17.Yuan Z, Kim D, Shu S, Wu J, Guo J, Xiao L, et al. Phosphoinositide 3-kinase/Akt inhibits MST1-mediated pro-apoptotic signaling through phosphorylation of threonine 120. J Biol Chem. 2010;285(6):3815–24. doi: 10.1074/jbc.M109.059675 ; [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Ahn SH, Cheung WL, Hsu JY, Diaz RL, Smith MM, Allis CD. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell. 2005;120(1):25–36. doi: 10.1016/j.cell.2004.11.016 . [DOI] [PubMed] [Google Scholar]
- 19.Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113(4):507–17. . [DOI] [PubMed] [Google Scholar]
- 20.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, et al. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67(13):6238–46. doi: 10.1158/0008-5472.CAN-07-0288 . [DOI] [PubMed] [Google Scholar]
- 21.Cinar B, Mukhopadhyay NK, Meng G, Freeman MR. Phosphoinositide 3-kinase-independent non-genomic signals transit from the androgen receptor to Akt1 in membrane raft microdomains. J Biol Chem. 2007;282(40):29584–93. doi: 10.1074/jbc.M703310200 . [DOI] [PubMed] [Google Scholar]
- 22.Freeman MR, Cinar B, Lu ML. Membrane rafts as potential sites of nongenomic hormonal signaling in prostate cancer. Trends Endocrinol Metab. 2005;16(6):273–9. doi: 10.1016/j.tem.2005.06.002 . [DOI] [PubMed] [Google Scholar]
- 23.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–31. . [PubMed] [Google Scholar]
- 24.Kuser-Abali G, Alptekin A, Cinar B. Overexpression of MYC and EZH2 cooperates to epigenetically silence MST1 expression. Epigenetics. 2014;9(4):634–43. doi: 10.4161/epi.27957 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collak FK, Yagiz K, Luthringer DJ, Erkaya B, Cinar B. Threonine-120 phosphorylation regulated by phosphoinositide-3-kinase/Akt and mammalian target of rapamycin pathway signaling limits the antitumor activity of mammalian sterile 20-like kinase 1. J Biol Chem. 2012;287(28):23698–709. doi: 10.1074/jbc.M112.358713 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rawat SJ, Araiza-Olivera D, Arias-Romero LE, Villamar-Cruz O, Prudnikova TY, Roder H, et al. H-ras Inhibits the Hippo Pathway by Promoting Mst1/Mst2 Heterodimerization. Curr Biol. 2016;26(12):1556–63. doi: 10.1016/j.cub.2016.04.027 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinar B, Collak FK, Lopez D, Akgul S, Mukhopadhyay NK, Kilicarslan M, et al. MST1 is a multifunctional caspase-independent inhibitor of androgenic signaling. Cancer Res. 2011;71(12):4303–13. doi: 10.1158/0008-5472.CAN-10-4532 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cinar B, Yeung F, Konaka H, Mayo MW, Freeman MR, Zhau HE, et al. Identification of a negative regulatory cis-element in the enhancer core region of the prostate-specific antigen promoter: implications for intersection of androgen receptor and nuclear factor-kappaB signalling in prostate cancer cells. Biochem J. 2004;379(Pt 2):421–31. doi: 10.1042/BJ20031661 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuser-Abali G, Alptekin A, Lewis M, Garraway IP, Cinar B. YAP1 and AR interactions contribute to the switch from androgen-dependent to castration-resistant growth in prostate cancer. Nat Commun. 2015;6:8126 doi: 10.1038/ncomms9126 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andrews S. FastQC: a quality control tool for high throughput sequence data. http://wwwbioinformaticsbabrahamacuk/projects/fastqc. 2010.
- 31.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetJournal. 2011;17(1):10–2. http://dx.doi.org/10.14806/ej.17.1.200. [Google Scholar]
- 32.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36 doi: 10.1186/gb-2013-14-4-r36 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31(2):166–9. doi: 10.1093/bioinformatics/btu638 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550 doi: 10.1186/s13059-014-0550-8 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gene Ontology C. Gene Ontology Consortium: going forward. Nucleic Acids Res. 2015;43(Database issue):D1049–56. doi: 10.1093/nar/gku1179 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017;45(D1):D353–D61. doi: 10.1093/nar/gkw1092 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franceschini A, Szklarczyk D, Frankild S, Kuhn M, Simonovic M, Roth A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41(Database issue):D808–15. doi: 10.1093/nar/gks1094 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benjamini Y, Hochberg Y. Controlling the false fiscovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 39.Wu G, Feng X, Stein L. A human functional protein interaction network and its application to cancer data analysis. Genome Biol. 2010;11(5):R53 doi: 10.1186/gb-2010-11-5-r53 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim W, Khan SK, Yang Y. Interacting network of Hippo, Wnt/beta-catenin and Notch signaling represses liver tumor formation. BMB Rep. 2016. doi: 10.5483/BMBRep.2017.50.1.196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357 doi: 10.1038/ncomms9357 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito A, Nagase T. Hippo and TGF-beta interplay in the lung field. Am J Physiol Lung Cell Mol Physiol. 2015;309(8):L756–67. doi: 10.1152/ajplung.00238.2015 . [DOI] [PubMed] [Google Scholar]
- 43.Magee JA, Chang LW, Stormo GD, Milbrandt J. Direct, androgen receptor-mediated regulation of the FKBP5 gene via a distal enhancer element. Endocrinology. 2006;147(1):590–8. doi: 10.1210/en.2005-1001 . [DOI] [PubMed] [Google Scholar]
- 44.Kutmon M, Riutta A, Nunes N, Hanspers K, Willighagen EL, Bohler A, et al. WikiPathways: capturing the full diversity of pathway knowledge. Nucleic Acids Res. 2016;44(D1):D488–94. doi: 10.1093/nar/gkv1024 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cancer Genome Atlas Research N. The Molecular Taxonomy of Primary Prostate Cancer. Cell. 2015;163(4):1011–25. doi: 10.1016/j.cell.2015.10.025 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–28. doi: 10.1016/j.cell.2015.05.001 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen LT, Tretiakova MS, Silvis MR, Lucas J, Klezovitch O, Coleman I, et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell. 2015;27(6):797–808. doi: 10.1016/j.ccell.2015.05.005 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. beta-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–73. doi: 10.1016/j.cell.2012.11.026 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11(8):e1005465 doi: 10.1371/journal.pgen.1005465 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17(9):1218–27. doi: 10.1038/ncb3216 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387 . [DOI] [PubMed] [Google Scholar]
- 53.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. doi: 10.1056/NEJMoa1014618 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48. doi: 10.1056/NEJMoa1209096 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–38. doi: 10.1056/NEJMoa1315815 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(18):1755–6. doi: 10.1056/NEJMc1410239 . [DOI] [PubMed] [Google Scholar]
- 57.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97. doi: 10.1056/NEJMoa1207506 . [DOI] [PubMed] [Google Scholar]
- 58.Yu J, Yu J, Mani RS, Cao Q, Brenner CJ, Cao X, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17(5):443–54. doi: 10.1016/j.ccr.2010.03.018 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988 doi: 10.1038/ncomms5988 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McNeal AS, Liu K, Nakhate V, Natale CA, Duperret EK, Capell BC, et al. CDKN2B Loss Promotes Progression from Benign Melanocytic Nevus to Melanoma. Cancer Discov. 2015;5(10):1072–85. doi: 10.1158/2159-8290.CD-15-0196 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplot of gene counts from the technical replicates show a high degree of correlation in all three conditions.
(TIF)
Each gene is colored by gene expression fold change in LR-STK4 condition. Red: Upregulated, green: downregulated, white: not differentially expressed. Edge labels “e”: expression interaction, “+p”: phosphorylation, “-p”: dephosphorylation.
(TIF)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
(XLSX)
Data Availability Statement
RNA-seq data are available for download from the National Center for Biotechnology Information Sequence Read Archive database (accession number: SRP102205).