Abstract
Background
There is need for better treatments of addictive behaviors, both substance and non-substance related, termed “Reward Deficiency Syndrome” (RDS). While the FDA has approved pharmaceuticals under the umbrella term Medication Assisted Treatment (MAT), these drugs are not optimal.
Objectives
It is our contention that these drugs work well in the short-term by blocking dopamine function leading to psychological extinction. However, use of buprenorphine/Naloxone over a long period of time results in unwanted addiction liability, reduced emotional affect, and mood changes including suicidal ideation.
Methods
We are thus proposing a paradigm shift in addiction treatment, with the long-term goal of achieving “Dopamine Homeostasis.” While this may be a laudable goal, it is very difficult to achieve. Nevertheless, this commentary briefly reviews past history of developing and subsequently, utilizing a glutaminergic-dopaminergic optimization complex [Kb220Z] shown to be beneficial in at least 20 human clinical trials and in a number of published and unpublished studies.
Results
It is our opinion that, while additional required studies could confirm these findings to date, the cited studies are indicative of achieving enhanced resting state functional connectivity, connectivity volume, and possibly, neuroplasticity.
Conclusions/Importance
We are proposing a Reward Deficiency Solution System (RDSS) that includes: Genetic Addiction Risk Score (GARS); Comprehensive Analysis of Reported Drugs (CARD); and a glutaminergic-dopaminergic optimization complex (Kb220Z). Continued investigation of this novel strategy may lead to a better-targeted approach in the long-term, causing dopamine regulation by balancing the glutaminergic-dopaminergic pathways. This may potentially change the landscape of treating all addictions leading us to the promised land.
Keywords: Dopamine Homeostasis, Neuropharmacological, Neuroimaging, Reward Deficiency Syndrome (RDS), KB220Z
Background
The nation and the world are facing an epidemic of opiate/opioid addiction never seen before (Kanate et al., 2015). When we think of the powerful addiction drug – heroin - glimpses of pictures of the “Man with the Golden Arm” or the sharing of dirty needles in smack houses seems to predominate. However, today with 127 people dying every day from narcotic overdose, as detailed by the CDC of Atlanta, suggests a very different picture (Dasgupta et al., 2014). Children from even well to do neighborhoods are dying.
According to SAMHSA, 75% of opiate/opioid dependent individuals, both young and old, are getting the required treatment. Unfortunately, the only treatment available that has been approved by the FDA are “Medication Assisted Treatment” (MAT), which favors maintenance with even more powerful addictive drugs like Methadone and Buprenorphine/Naloxone (Nosyk et al., 2013). In addition, MAT-approved drugs for alcohol, opiates, and nicotine tend to reduce the function of brain dopamine, and most agencies do not recommend long-term use (Blum et al., 2011; Badgaiyan et al., 2015; Mitchell et al., 2016).
This approach is based on the notion that the path to recovery involves blocking dopamine in the reward circuitry of the brain (Gorelick et al., 2004). This blocking of dopamine leads to psychological extinction and may be useful in the short–term, but harmful in the long-term. While many studies show the benefit of the combination of Buprenorphine and naloxone (Demetrovics et al., 2009), other studies raise the prospect of increased suicidal ideation, withdrawal symptoms upon termination of use, and fatalities by long-term use of Buprenorphine/Naloxone due to potential anti-reward properties and reduced affect (Kosten et al., 1990; Häkkinen et al., 2013; Blum et al., 2014; Hill et al., 2013). Now if we expand our thinking, all kinds of addictive behaviors, both drug and non-drug, represent an enormous global problem, comparable in a sense to the dangers posed by terrorism.
The following information, especially the past history, is presented to familiarize the newer clinician with the rationale for the development of KB220 variants as an adjunct to both prevention and treatment of Reward Deficiency Syndrome [RDS] (Blum et al., 1996).
Our proposition maintains that treatment of all addictive behaviors must address the root cause of the risk for these behaviors - a “Reward Deficiency” (Elman & Borsook, 2016). This notion is underscored by recent work showing that baseline or resting state levels in certain cohorts, like ADHD patients, show a “hypodopaminergic trait” (low dopamine tone) (Badgaiyan et al., 2015); while there are other studies showing that, for example, during alcohol acute abstinence there is an opposite “hyper-dopaminergic state” (Hirth et al., 2016). As a result, we are proposing that a common treatment modality incorporate the goal of inducing “dopamine homeostasis” (regulation of dopamine dysfunction across brain regions).
Have we developed a Glutaminergic-Dopaminergic Optimization Complex (KB220Z) to Regulate Brain Dopamine Function?
We propose that, based on many clinical and neuroimaging, peer-reviewed publications, KB220z may indeed induce dopamine homeostasis by an optimizing a balancing of glutaminergic-dopaminergic activity in the brain’s reward circuitry. While there may be other nutraceuticals, including N-Acetyl-Cysteine (NAC) which has important benefits in terms of balancing the glutaminergic system, we will describe some research directed to understanding KB220z (Blum et al., 2016). To understand this 40-year journey, our story begins in 1977, when Blum’s group reported that the D-amino-acid of phenylalanine (DPA) induced a pharmacogenetic shift of changing the genetically prone c57/bl mice to reduce their alcohol intake and actually mimic a mouse stain that genetically was aversive to alcohol (Blum, 1987). Specifically, administration of D-Phenylalanine for 18 days in alcohol-loving c57/blk mice showed that this substance raised endorphin levels in both the pituitary and the striatum. This is how it changed mice that were genetically prone to seek alcohol to reduce their alcohol intake so much that the treated DPA alcohol loving mice now had alcohol intake levels of non-preferring alcohol-hating DBA mice.
Subsequent to this seminal study, Blum’s laboratory also found that drinking alcohol was linked to low amounts of the endogenous neuropeptide enkephalins. Specifically, Blum et al. (1982) found that in Golden Syrian hamsters after 1 year, presented a markedly decreased concentration of leucine-enkephalin-like immunoreactive substance in the basal ganglia than did the control hamsters. This result suggested that the function of ethanol implicates endogenous peptidyl opiates. Simply put, DPA a known inhibitor of the enzyme called enkephalinase, which breaks down the enkephalins, raised brain levels of these neuropeptides (e.g., enkephalins, endorphins) with concomitant reduction of alcohol drinking (Blum, 1983).
Subsequent to this work, many years later following the actual commercialization of a formula developed to mimic the “Brain Reward Cascade” (Figure 1), whereby the net release of dopamine released at the Nucleus accumbens (NAc) can determine well- being, the untangling of a genetic predisposition for reward deficiency had occurred. Many reiterations have been developed over the years following these discoveries and have been supported most recently by the important work of the Morales’ group at NIDA (Morales & Root, 2014).
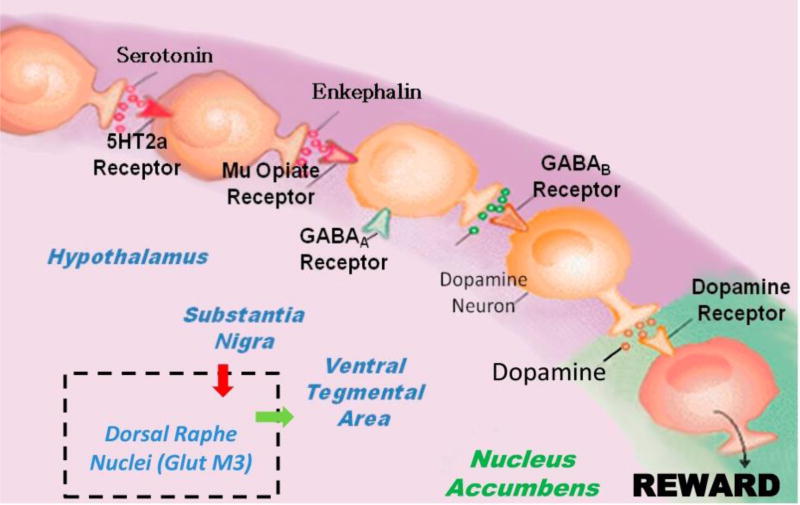
Figure 1. Brain Reward Cascade showing the inclusion of Dorsal Raphe Nuclei (GlutM3) interaction regulating VTA dopamine.
Figure 1 is an illustration of the Brain Reward Cascade, which involves the release of serotonin at the hypothalamus, which stimulates enkephalin. The enkephalin then inhibits GABA at the substantia nigra, which in turn, regulates the amount of dopamine released at the nucleus accumbens (or “reward site”). The dopamine originates in the VTA. Various receptors (including 5HT2a receptors, µ-opiate receptors, GABAA receptors, GABAB receptors, and dopamine receptors) are utilized in the reward cascade. Recent evidence demonstrates the role of the dorsal raphe nuclei in this cascade [Morales & Root 2014]. It is accepted that, under normal conditions, dopamine in the nucleus accumbens through a number of cascading events and neurotransmitter interaction works to maintain a person’s normal drives [Blum et al. 2015] (with permission).
Utilizing the “Brain Reward Cascade” led to the first genetic association of a polymorphism and severe alcoholism, where the DRD2 gene Taq A1 allele was found to be highly prevalent in deceased alcoholics, 80% of whom had cirrhosis. This finding paved the way for the field of “Psychiatric Genetics.” More importantly, it established a central role for dopamine as an important neurotransmitter for inducing substance-seeking as well as non-substance seeking behaviors, especially when a “hypo-dopaminergic trait” is present in the individual (Tournier et al., 2013).
The scientific framework countered the drug–reinstatement approach by its potential induction of dopamine regulation (homeostasis) (Gold et al., 2015). This theory has been supported by the now thousands of studies on DNA polymorphisms (variations) involving many of the known reward genes, whereby simple molecular shifts of single-nucleotide polymorphisms (SNPs) work in concert to potentially either up-regulate dopamine (hyper) or down-regulate dopamine (hypo). While these changes may influence subsequent risk for all addictive behaviors, carriers of these DNA variants are not doomed to addictive behavior. This proposition is supported by the fact that, over the last decade, scientists have become more aware of the importance of “epigenetics” (environmental influences), which can affect the expression of such DNA shortcomings.
Environmentally induced epigenetics appears to be either positive or negative in its effects, with a positive example being the fact that exercise can offset the FTO gene that increases the number of fat cells (Caruso et al., 2014); A negative example is the finding that the environment causes “methylation” (adding methyl groups on to chromatin histones), which blocks gene expression and has been found to occur on the DRD2 gene in individuals with a life-time addiction to gambling. In essence, methylation on the DRD2 gene reduces the number of D2 receptors, especially in the brain and, as such, increases the risk for addictive behavior like gaming (Hillemacher et al., 2015).
Recently, Wright et al. (2015) showed that chronic L-methionine (MET) treatment in rats blocked cocaine-primed reinstatement. Moreover, cocaine-induced c-Fos expression in the NAc was linked to decreased methylation at CpG dinucleotides in the c-Fos gene promoter, with outcomes reversed by MET treatment. These data indicate that drug-seeking behaviors partly contribute to a DNA methylation-dependent procedure, possibly occurring at specific gene loci (e.g., c-Fos) in the reward pathway. These and many other studies provide evidence that manipulation of the reward circuitry can have both positive and negative effects involving all RDS behaviors, which are both substance- and non-substance-related (Blum et al., 2015) (Table 1).
Table 1.
Reward Deficiency Syndrome (RDS)
| Addictive Behaviors |
Impulsive Behaviors | Compulsive Behaviors | Personality Disorders |
|---|---|---|---|
| severe alcoholism | attention-deficit disorder hyperactivity | aberrant sexual behavior | conduct disorder |
| polysubstance abuse | Tourette syndrome | Internet gaming | antisocial personality |
| smoking | autism | pathological gambling | aggressive behavior |
| obesity |
KB220 Variant Clinical Studies
To date, there are over 30 clinical trials on a number of KB220 variants (Blum et al., 2012; Blum et al., 2016). In accord with the latest definition of addiction, published by the American Society of Addiction Medicine (ASAM), it is now accepted that patients who present to a treatment facility associated with chemical dependency or other recognized reward dependence behaviors have impaired brain reward circuitry. They may have hypo-dopaminergic activity due to genetic and/or environmental negative effects upon the reward neuro-circuitry. This damage causes abnormal craving behavior and other behaviors, such as Substance Use Disorder (SUD). As discussed above, neuro-genetic research in both animal and humans indicates that there is a clear cascade in the reward system of the brain that point to normal dopamine release. Any damage due to either genetics or environmental effects upon this cascade will cause a decreased quantity of dopamine release in the brain’s reward site. Influence of the Brain Reward Cascade (BRC) has been productively attained with neuro-nutrient therapy employing nutrigenomic principles (T. J. Chen et al., 2011). After over four decades of improvement, neuro-nutrient therapy has produced significant clinical benefits, when properly applied.
Our basic tenet is that predisposition for opiate abuse has a heredity or possible genetic component, based on a reward deficiency (a hypo-dopaminergic trait) that may be impacted by the environment, through what has been termed epigenetics. We believe that in order to change the “revolving-door” for chronic pain and abusing opiates/opioids in epidemic numbers, a solution is desperately needed to break this cycle with a adjunctive solution that tackles the problem at its source: “The Brain.” In addition with, for example, holistic approaches, KB220z could assist in the defense against opiates and other addictive drugs like alcohol, psychostimulants, methadone, buprenorphine, and/or other addictive substances. While this may seem like a bold statement, it is backed by extensive scientific, peer-reviewed articles published in prestigious journals. We now know that addiction originating from RDS [now featured in SAGE Encyclopedia of Abnormal Psychology 2016 & APA DSM-5] is a brain disorder, and affects over one-third of the U.S. population carrying a well-researched dopaminergic genetic variant.
Most treating clinicians seem to embrace the concept that “opiate addiction” and subsequent substance-seeking and continued abuse is due primarily to a hypo-dopaminergic trait (genetic) or state (epigenetic) or a combination of both. Any major solution must address the low dopamine brain function early on in the recovery process, especally when an individual seeks help and clinicians should try to promote long-term balancing of dopamine function with the laudable goal of inducing “Dopamine Homeostasis” (regulation).
0 published human studies clearly showing major societal benefits, anti-craving effects, enhanced well-being (stress reduction); reduced AMA rates helping patients continue with treatment; increased focus; reduced relapse increased energy; and overall redemption of joy (Blum et al., 2012; Blum et al., 2016).
Examples of the Positive Effects of KB220 Variants in Human Studies
There is continuing excitement concerning the consistent positive effects of KB220 variants with at least 3 compared to controls, clinical subjects had lower building up to drink score, required no PRN benzodiazepines, ceased having tremors 24 hours earlier, and had less depression (Blum, Trachtenberg, & Ramsay, 1988).
Results reduced stress as measured by skin conductance, improved Physical and BESS (behavioral, emotional, social and spiritual) Scores, and had a six-fold decrease in leaving Against Medical Advice (AMA) rates (Blum, Trachtenberg, Elliott, et al., 1988).
KB220 variants decreased the AMA rate by significant reduction of drug hunger (Blum, Allison, et al., 1988).
Reduced relapse rates and enhanced recovery in 10-week outpatient setting (Brown et al., 1990).
The KB220 variants average weight loss was 26.96 lbs. vs. 10.2 lbs. in the control group. Relapse 18.2% in the KB220 variants group vs. 81.8% in the control group (Blum, Trachtenberg, & Cook, 1990).
Cocaine craving decreased significantly (Cold, 1996).
Cognitive processing speeds were enhanced by a statistically significant amplitude of the P300 component of the Event Related Potentials (ERPs). Focus improved as well (DeFrance et al., 1997).
The subject group, when compared to the control, lost twice as much weight, regained 14.7% of the weight while the control group regained 41.7%, decrease in food cravings for females 70% and males 63%, and decrease in binge eating for females 66% and males 41% (Blum et al., 1997).
98% of 100 patients similarly treated and evaluated reported significant improvement in both mood and reduced substance craving (Ross, 2001).
Results were dramatic in terms of significantly enhancing compliance to continue taking KB220 variants. KB220 variants alone for rapid detoxification the average number of days of compliance calculated on 1000 patients is 37 days (T. J. Chen et al., 2004).
Statistical analysis of the survey results demonstrated that stress reduction lead to improved sleep, enhanced energy, and improved focus and performance, reduced appetite, loss of unwanted weight, decreased body inches, and enhanced well-being (Blum et al., 2006).
Results after 1 year include building up to relapse scores and ability to refrain from drug-seeking behavior both significantly improved. The dropout rate for alcohol users 7% and psychostimulant users 73% (T. J. Chen, Blum, Waite, et al., 2007).
Emotional and behavioral recovery scores significantly improved after administration of oral and intravenous KB220 variants. Mean reductions for craving, depression, anxiety, anger, fatigue, lack of energy and crisis were all significantly greater than 50% (p<0.001) (Blum et al., 2007).
In carriers of the DRD2 A2 genotype, weight loss and other changes in body composition were significant (T. J. Chen, Blum, Kaats, et al., 2007).
Significant results were observed for weight loss, sugar craving reduction, appetite suppression, snack reduction, reduction of late night eating, increased energy, etc (Blum, Chen, Chen, Rhoades, Prihoda, Downs, Waite, et al., 2008).
In the 41-day period, we found a trend in weight loss whereby 71.4% of subjects lost weight (Blum, Chen, Chen, Rhoades, Prihoda, Downs, Bagchi, et al., 2008).
Patients receiving KB220 variants had a significantly reduced stress response as measured by SCL, compared to patients receiving placebo (Blum et al., 2009).
After one year, the 58-year-old patient's BMT decreased from 32 to 25.4kg/m2 representing a 6.9kg/m2 reduction. His body fat composition decreased from 36.91% to 17.8% as measured by the Hologic DEXA scanner. (Braverman, Braverman, Arcuri, … Blum, 2010).
We report that the qEEGs of an alcoholic and a heroin abuser with existing abnormalities during protracted abstinence are significantly normalized by the administration of 1 intravenous dose of KB220 variants (Miller, Bowirrat, Manka, … Blum, 2010).
Oral KB220 variants showed an increase of Alpha activity and an increase low Beta activity similar to 10–20 sessions with Neurofeedback (Blum, Stice, et al., 2011).
The results showed that the insomnia and withdrawal scores were significantly improved over time in participants in the intervention group as compared with those in the control group. (D. Chen, Liu, He, Wang, & Wang, 2012).
A two-year follow-up in a subset of 23 patients showed: 21(91%) were sober at 6 months with 19(82%) having no relapse; 19 (82% were sober at one year with 18 (78%) having no relapse; 21(91%) were sober at two-years post-treatment with 16(70%) having no relapse. (Miller et al., 2012).
After over four decades of development, KB220 variants can provide clinical benefits for impairments due to either genetics or environmental influences on the Brain Reward Cascade will result in a reduced amount of dopamine release in the brain reward site (Blum et al., 2012).
We presented a case study of a 35-year-old female with a history of chronic pain from reflex sympathetic dystrophy and fibromyalgia and observed that she was opioid free with KB220 variants maintenance at 432 days post Suboxone® withdrawal (Blum et al., 2013).
We present two cases of dramatic alleviation of terrifying lucid dreams in patients with PTSD revealing changes in the frequency, intensity and nature of these dreams after KB220 variants were added to the first patient's regimen (McLaughlin et al., 2015).
In eight clinical cases, the administration of KB220 variants was associated with the elimination of unpleasant and/or terrifying, lucid dreams in 87.5% of the cases presented, whereas one very heavy cocaine abuser showed a minimal response (McLaughlin et al., 2015).
KB220 variants induced an increase in BOLD activation in caudate-accumbens-dopaminergic pathways compared to placebo following 1-hour acute administration in abstinent heroin addicts. Increased functional connectivity was observed in a putative network (Blum, Liu, et al., 2015).
We found following seeding of the dorsal hippocampus, enhanced connectivity volume across several regions of Interest (ROI), with the exception of the pre- frontal cortex (McLaughlin et al., 2016).
The resultant z-scores, averaged across Eyes Closed, Eyes Open and Working Memory conditions, increased for each frequency band, in the anterior, dorsal and posterior cingulate regions, as well as the right dorsolateral prefrontal cortex during Working Memory, with KB220 variants. These scores are consistent with other human and animal neuroimaging studies that demonstrated increased connectivity volumes in reward circuitry and may offer a new approach to ADHD treatment (Steinberg, Blum, McLaughlin, … Badgaiyan, 2016).
Prior to 2010, no real evidence existed to explain these clinical benefits, except to suggest that by mirroring the BRC, the glutaminergic-dopaminergic complex balances the reward circuitry of the brain, leading to a regulated neuronal release of dopamine at the VTA.
In summary, through the use of neuroimaging tools, KB220z acts on neurobiological mechanistic targets to restore “Dopamine Homeostasis” and is best described as “Glutaminergic-Dopaminergic Optimization Complex” [GDOC]. This is based on important, revealing studies that have unraveled the long-standing mystery of Neuroadaptogen Amino-Acid Therapy (NAT).
Neuroimaging Studies
Braverman and Blum (1996) systematically revealed that among the substance and non-substance use disorders as well as among the psychiatrically ill (PI), the psychiatrically ill Substance Dependence (PI/SUD) groups had considerably more and diverse brain mapping irregularities that were seen comparative to an evaluated control population. Furthermore, with regard to EEG spectral analysis, ANOVA was statistically significant at a P<0.038, and a weighted linear trend of augmented irregular total spectral analysis (P<0.0113), was found in the substance use group compared to controls. In fact, among the psychiatrically ill (PI) and psychiatrically ill with co-morbid Substance Use Disorder (PI/SUD) groups, considerably more total evoked potential (EP) brain trap irregularities were witnessed relative to a characterized standard population (P<0.0023) with growing irregularities as caused by substance use disorder measured by a weighted linear trend (P<0.0022). In order to establish the location of the EPS irregularities, the authors evaluated these abnormalities by location. In this regard, they found all temporal and frontal lobe abnormalities among the PI and PI/SD groups to be considerably greater relative to an evaluated standard population. Importantly, the PI/SD group had significantly more abnormalities compared to the PI group. These results underscore the exacerbation of drug-related abnormalities of the reward circuitry and therefore, argue that treatment must address such abnormalities especially in recovery.
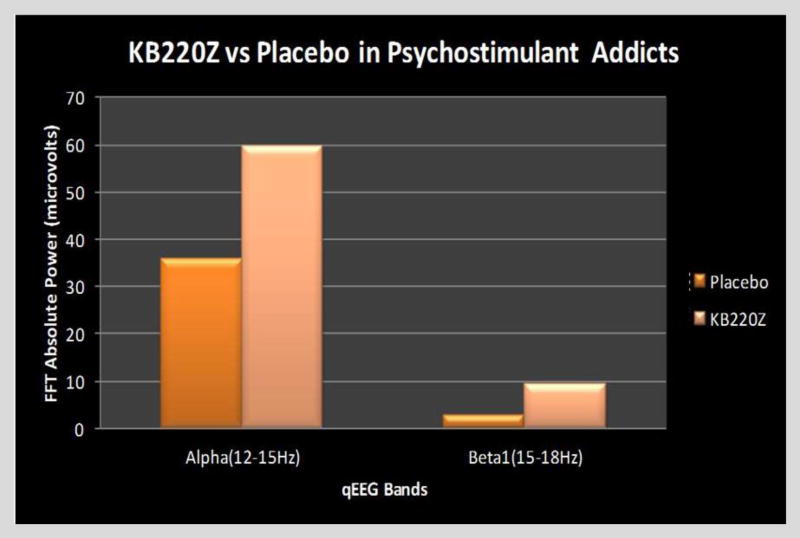
Our first glimpse of such SUD electrophysiological abnormalities came from our qEEG studies in 2010 (Blum et al., 2010) involving a cohort of abstinent psychostimulant addicts. In this study, we found that one hour following a dose of KB220z, there was actually reduced widespread theta activity, especially in the PFC (pre-frontal cortex) as well as in the cingulate gyrus (a region involved in executive function and decision making), as well as increased alpha bands as well as and low beta bands which induced a calming effect, comparable to that which requires between 10–20 neurofeedback sessions. These results were also supported by similar findings using qEEG in both alcoholics and heroin dependent patients (Miller et al., 2010) (Figure 2).
Figure 2.
Illustrates the effect of KB220Z taken one-hour prior to qEEG in ten psycho-stimulant abstinent abusers compared to the placebo group in a cross-over study. The FFT Absolute Power shown in microvolts, revealed that compared to placebo, the KB220z complex increased alpha (12–15 Hz) as well as low beta at 15–18 Hz [Blum et al., 2012 – with permission].
It is quite notable that we recently published a paper with our Chinese associates on the effects of KB220z on abstinent Chinese heroin addicts in a randomized placebo-controlled crossover study. The robust findings, using fMRI, revealed a widespread distribution of dopamine BOLD activity in the brain. Simply, it has been found that KB220z has been shown to gently activate (light up) dopamine across the reward circuitry of the brain in abstinent heroin addicts, including regulating brain abnormalities in the cingulate gyrus (the decision-making part of the brain responsible for relapse or drug reinstatement) in psychostimulant and alcohol abstinent addicts (Blum et al., 2015).
The reason why we could state that BOLD activation is indeed linked to a dopaminergic effect has been underscored by the work of others (Tye et al., 2013; Ferenczi & Deisseroth, 2016). To understand the importance of these findings, a group from Seattle spearheaded by Willuhn et al. (2014) reported that another drug of abuse, namely, cocaine, as well as non-substance related addictive behaviors increased, suggesting that dopaminergic function is reduced (meaning Hypodopaminergic). Long-term cocaine exposure has been linked to reductions in D2/D3 receptors as well as poorer activation to drug cues in occipital cortex and cerebellum, in a recent PET study by Tomasi et al. (2015). Therefore, treatment strategies like pro-dopamine regulation therapy that could preserve dopamine activity at a normal level and serve as a method for relapse prevention, involving psychoactive drug and behavioral addictions. With this aim in mind, the authors assessed the outcome of KB220Z (Blum et al., 2015) on reward circuitry in 10 heroin addicts who had undergone prolonged abstinence (median = 16.9 months). In a KB220Z randomized placebo-controlled cross-over study, five participants finished a triple-blinded experiment, in which the participant, the individual overseeing the treatment, and the individual assessing the reaction to treatment were blinded to the treatment that any specific participant was receiving. In preliminary reports, KB220Z stimulated a rise in BOLD activation involving the caudate-accumbens-dopaminergic pathways relative to placebo, following a 1-hour acute administration. Additionally, KB220Z also decreased resting-state activity in the putamen of abstinent heroin addicts. In the second stage of this pilot study, in all 10 abstinent heroin-dependent participants, the authors detected three brain areas of interest that were considerably activated compared to the q-EEG resting state by KB220Z when compared to the placebo (p < 0.05). Improved functional connectivity was also seen in a putative neuronal network involving the dorsal anterior cingulate, medial frontal gyrus, nucleus accumbens, posterior cingulate, occipital cortical regions, and cerebellum.
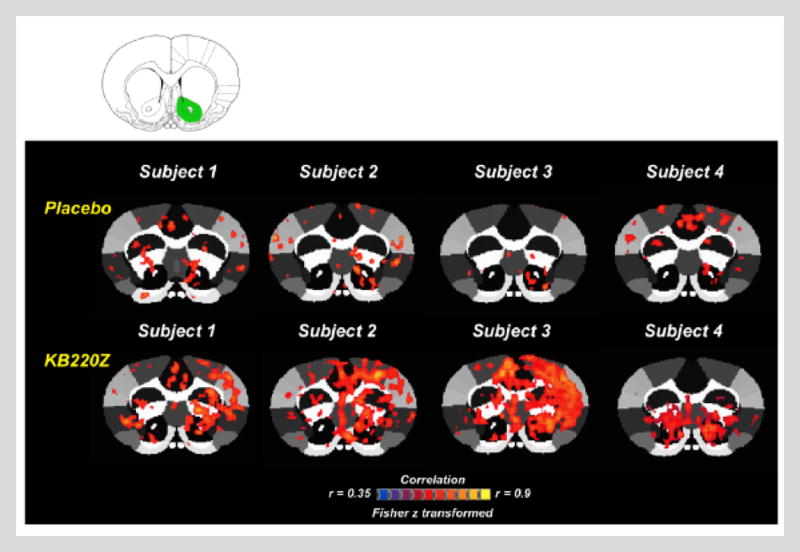
These findings provide important evidence that KB220Z activates important dopamine pathways in the reward circuit of the brain and seems to cause a balancing of glutaminergic-dopaminergic systems, which can be seen in Figure 3. These findings have led our laboratory to repeat fMRI studies on naïve rodents at the University of Florida. In essence, in unpublished research, we found that not only does KB220Z induce an increase of resting state functional connectivity across important reward brain regions similar to what we have seen in our human studies, but we also observed an increase in connectivity volume. Figure 4 represents activation of the NAc by KB220Z compared to placebo in naïve rats in a crossover study. The increase in connectivity volume suggests that 5–15 minutes after rodents receive a KB220Z dose, an unexplained increase of neuronal firing (more neurons are recruited) in selected parts of the brain involved in memory, recall, executive function, decision-making, craving, and even somatosensory processing (an area Volkow from NIDA (W. Chen, Liu, Volkow, Pan, & Du, 2015) considers important to drug seeking) occurs. The latter effect may suggest an important brain mechanism called “neuroplasticity.” While the authors have, to date, published two papers showing that KB220z eliminates life-long, frequently terrifying lucid dreams, our most recent findings show a prolonged effect of KB220Z in the elimination of these nightmares, and potential conversion to happy lucid dreams up until 12 months. These clinical findings suggest the possibility of KB220Z-induced for neuroplasticity (McLaughlin, Blum, Oscar-Berman, Febo, Agan, et al., 2015; McLaughlin, Blum, Oscar-Berman, Febo, Demetrovics, et al., 2015).
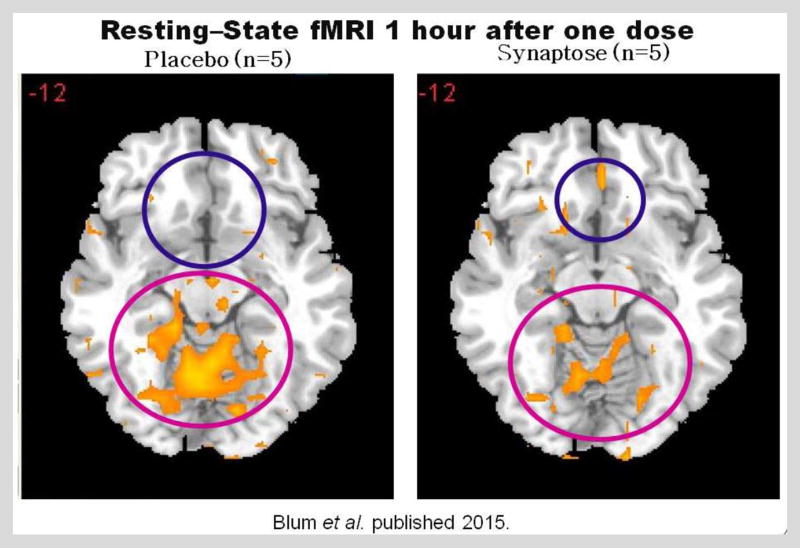
Figure 3.
Represents a fMRI cross-over study in five abstinent heroin addicts receiving either placebo or KB220z (Synaptose) one–hour prior to testing. What is noteworthy is that following the KB220Z, there was BOLD activation in the NAc and an attenuation of high BOLD activation in the putamen. This illustration suggests a balancing of dopamine function in the brain at the reward site [Blum et al. 2012 – with permission].
Figure 4. Representative cross-correlation maps show 4 subjects: placebo compared to KB220Z treated rats.
Maps correspond to resting state connectivity for the left nucleus accumbens (highlighted in green in the atlas map above the figure). Note the distributed, but significant connectivity between various brain regions and the nucleus accumbens in the placebo subjects. KB220Z improved connectivity, especially between left-right accumbens, dorsal striatum, and limbic cortical areas, including the anterior cingulate, prelimbic, and infralimbic regions. Correlation maps for characteristic participants presented at a threshold between 0.35≤ r ≥0.9 (Fisher’s z transformed). With Permission [Febo & Blum, 2016].
In addition, the newest version of KB220z, a liquid nano, was evaluated in an ADHD patient. To reiterate, Attention Deficit-Hyperactivity Disorder (ADHD) frequently continues into adulthood (Biederman, 1998). Recent neuroimaging studies found lowered baseline dopamine tone in the brains of affected individuals (Badgaiyan et al., 2015). Both genders, with or without comorbidities in ADHD, are at risk for Substance Use Disorder (SUD) and children treated with psychostimulants are not protected from long-term SUD (Biederman et al., 2007). In an observational case study using a non-addictive glutaminergic-dopaminergic optimization complex KB200Z using low-resolution electromagnetic tomography (LORETA), the authors evaluated the effects of KB220Z in a 72-year-old male with ADHD, at baseline and one hour following administration of the nutraceutical. The results indicated that z-scores, averaged across Eyes Closed, Eyes Open, and Working Memory conditions, increased for each frequency band in the anterior, dorsal, and posterior cingulate regions, as well as right dorsolateral prefrontal cortex during Working Memory with KB220Z. These scores are consistent with other human and animal neuroimaging studies that demonstrated increased connectivity volumes in reward circuitry and may offer a new approach to ADHD treatment.
Summary
In the United States, 8–10% of individuals ages ≥12 years (approximately 20–22 million persons nationwide) are addicted to alcohol or other drugs of abuse. The abuse of tobacco, alcohol, and illicit drugs in the United States causes greater than $700 billion per year in costs associated with crime, lost work throughput, and health care. With almost one trillion dollars in annual productivity costs as well as thousands of individuals dying every day in America (25,000 deaths in 2015), it behooves us to understand that all addictive behaviors result from a real brain disorder (Rowe et al., 2015).
Most recently, Volkow from NIDA, Koob from NIAAA, and others, have provided clear evidence linking addiction to neurobiology (Volkow & Koob, 2015). These authorities have adequately assessed evidence about the desensitization of reward circuits, which reduces the capability to feel pleasure and the subsequent drive to chase addictio-alleviating undertakings. These authors assessed the rising power of habituated reactions and stress reactivity, which increases cravings for alcohol and other drugs as well as inducing adverse emotions when these cravings are not satiated. These same authors also noted that the atrophy of brain areas involved in executive actions such as decision-making, inhibitory control, and self-regulation, which eventually led to relapse. The take home message, based on Volkow, Koob, and McLellan’s (2016) review, suggests the need to balance the variability of brain region activity seen before and after substance addiction in an attempt to help regulate the “normalization” of brain function. This approach re-enforces the need for a better understanding of high genetic risk in addiction as well as the need to modulate these susceptibilities even at birth in individuals suffering from either DNA gene polymorphisms or chromatin epigenetic alterations (environmental), which are passed from one generation to another. See our model below (Figure 5).
Figure 5.
The figure portrays “Dopamine homeostasis.” It involves balancing the brain to reduce a hypodopaminergic trait/state by targeting both genetic polymorphisms and epigenetics (methylation). [With permission Blum & Gold, 2016]
The steep increase in prescription opioids in the United Sates, and across the world has led to a significantly paralleled increase in opioid and heroin misuse and fatal overdoses. Unfortunately, there has also been a dramatic increase in the number of infants born with neonatal abstinence syndrome (NAS). Moreover, here at home, where approximately 14–22% of pregnant women receive these opioids legally, the rise in NAS may be the direct result of prescription opioids.
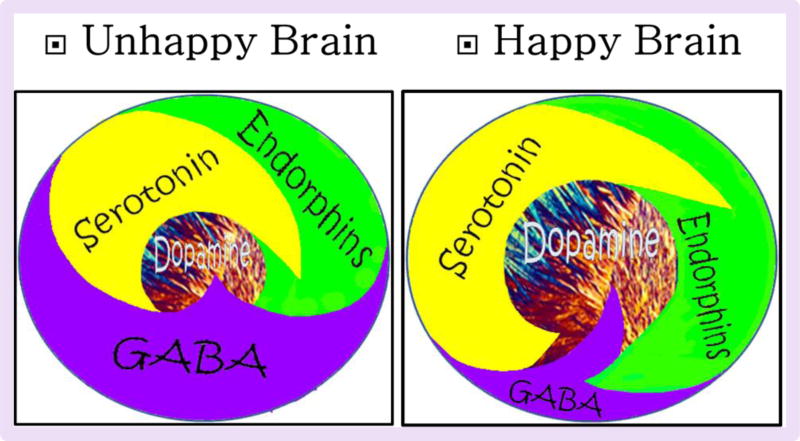
We encourage the scientific community to, as suggested in the DSM-5, treat acute opiate/opioid abstinence in the short-term by focusing on withdrawal symptoms. Additionally, the model we are proposing concentrates at the same time on treating the etiology of RDS: the long-term “hypo-dopaminergic” trait/state as demonstrated by the reduced phasic dopamine tone. Through required additional research, we hope to find new ways to optimize of the glutaminergic/dopaminergic systems and thereby induce “dopamine homeostasis” in spite of either a “hypo” or “hyper” trait/state. Our goal is to convert the unhappy brain to a happy one as a result of more in-depth research (see Figure 6).
Figure 6.
Schematic of Brain Reward Cascade: Normal and Abnormal Representation [With permission Blum et al. 2014].
Our final hope is the attainment of one important goal for treatment of all RDS behaviors, which is to develop a standard of care plan termed, Reward Deficiency Solution System (RDSS). This solution will include the use of Genetic Addiction Risk Score (GARS); Comprehensive Analysis of Reported Drugs (CARD); and a Glutaminergic-Dopaminergic Optimization Complex (KB220Z) based on both pre-clinical studies in animals and important human clinical trials mentioned above.
Acknowledgments
Funding
This work was supported by the National Institutes of Health grants 1R01NS073884 and 1R21MH073624, awarded to Dr. Rajendra D. Badgaiyan. Dr. Marcelo Febo is the recipient of R01DA019946 and R21DA038009.
Footnotes
Declaration of interest
Dr. Kenneth Blum owns stock in LaVita RDS and is owner of Synaptamine, which holds patents on KB220Z. Drs. Marcelo Febo and Rajendra D. Badgaiyan are on the Scientific Advisory Board of LaVita RDS.
There are no other conflicts to reports.
References
- Badgaiyan RD, Sinha S, Blum K. Do We Really Need to Continue Pharmacotherapy for Opioid Use Disorder (OUD) Indefinitely? J Reward Defic Syndr. 2015;1:16–19. [Google Scholar]
- Badgaiyan RD, Sinha S, Sajjad M, Wack DS. Attenuated Tonic and Enhanced Phasic Release of Dopamine in Attention Deficit Hyperactivity Disorder. PLoS One. 2015;10:e0137326. doi: 10.1371/journal.pone.0137326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biederman J. Attention-deficit/hyperactivity disorder: a life-span perspective. J Clin Psychiatry. 1998;59(Suppl 7):4–16. [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Spencer T, Wilens TE, Macpherson HA, Faraone SV. Stimulant therapy and risk for subsequent substance use disorders in male adults with ADHD: a naturalistic controlled 10-year follow-up study. Am J Psychiatry. 2008;165:597–603. doi: 10.1176/appi.ajp.2007.07091486. [DOI] [PubMed] [Google Scholar]
- Blum K, Allison D, Trachtenberg MC, Williams RW, et al. Reduction of both drug hunger and withdrawal against advice rate of cocaine abusers in a 30 day inpatient treatment program by the neuronutrient Tropamine. Current Therapeutic Research. 1988;43:1204–1214. [Google Scholar]
- Blum K, Briggs AH, Elston SF, DeLallo L, Sheridan PJ, Sar M. Reduced leucine-enkephalin--like immunoreactive substance in hamster basal ganglia after long-term ethanol exposure. Science. 1982;216:1425–1427. doi: 10.1126/science.7089531. [DOI] [PubMed] [Google Scholar]
- Blum K, Briggs AH, Trachtenberg MC, Delallo L, Wallace JE. Enkephalinase inhibition: regulation of ethanol intake in genetically predisposed mice. Alcohol. 1987;4:449–456. doi: 10.1016/0741-8329(87)90084-x. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen AL, Chen TH, Bowirrat A, Waite RL, Kerner M, Blum SH, Downs BW, Savarimuthu S, Rhoades P, Reinking J, Braverman ER, Braverman D, DiNubile N, Oscar-Berman M. Putative targeting of dopamine D2 receptor function in Reward deficiency Syndrome (RDS) by Synaptamine Complex Variant (KB220): clinical trial showing anti-anxiety effects. Gene Therapy Molecular Biology. 2009;13:214–230. [Google Scholar]
- Blum K, Chen AL, Chen TJ, Rhoades P, Prihoda TJ, Downs BW, Waite RL, Williams L, Braverman ER, Braverman D, Arcuri V, Kerner M, Blum SH, Palomo T. LG839: anti-obesity effects and polymorphic gene correlates of reward deficiency syndrome. Advances in Therapy. 2008;25:894–913. doi: 10.1007/s12325-008-0093-z. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Bailey J, Bowirrat A, Femino J, Chen AL, Oscar-Berman M. Can the chronic administration of the combination of buprenorphine and naloxone block dopaminergic activity causing anti-reward and relapse potential? Mol Neurobiol. 2011;44:250–268. doi: 10.1007/s12035-011-8206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Chen TJH, Chen ALC, Rhoades P, Prihoda TJ, Downs BW, Bagchi D, Bagchi M, Blum SH, Williams L, Braverman ER, Kerner M, Waite RL, Quirk B, White L, Reinking J. Dopamine D2 Receptor Taq A1 allele predicts treatment compliance of LG839 in a subset analysis of pilot study in the Netherlands. Gene Therapy Molecular Biology. 2008;12:129–140. [Google Scholar]
- Blum K, Chen TJH, Downs BW, Meshkin B, Blum SH, Martinez-Pons M, Mengucci JF, Waite RL, Arcuri V, Varshofsiky M, Braverman ER. Synaptamine (SG8839), TM An Amino-Acid Enkephalinase Inhibition Nutraceutical Improves Recovery of Alcoholics, A Subtype of Reward Deficiency Syndrome (RDS) Trends in Applied Sciences Research. 2007;2:132–138. [Google Scholar]
- Blum K, Chen TJ, Meshkin B, Downs BW, Gordon CA, Blum S, Mengucci JF, Braverman ER, Arcuri V, Varshavskiy M, Deutsch R, Martinez-Pons M. Reward deficiency syndrome in obesity: a preliminary cross-sectional trial with a Genotrim variant. Adv Ther. 2006;23:1040–1051. doi: 10.1007/BF02850224. [DOI] [PubMed] [Google Scholar]
- Blum K, Chen TJ, Morse S, Giordano J, Chen AL, Thompson J, Braverman ER. Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: part 2. Postgrad Med. 2010;122:214–226. doi: 10.3810/pgm.2010.11.2237. [DOI] [PubMed] [Google Scholar]
- Blum K, Cull JG, Chen TJH, Swan SG, Holder JM, Wood R, Braverman ER, Bucci LR, Trachenberg MG. Clinical evidence for effectiveness of Phencal™ in maintaining weight loss in an open-label, controlled, 2-year study Blum K, Cull JG, Chen TJH, et al. Clinical evidence for effectiveness of Phencal™ in maintaining weight loss in an open-label, controlled, 2-year study. Current Therapeutic Research. 1997;55:745–763. [Google Scholar]
- Blum K, Elston SF, DeLallo L, Briggs AH, Wallace JE. Ethanol acceptance as a function of genotype amounts of brain [Met]enkephalin. Proc Natl Acad Sci U S A. 1983;80:6510–6512. doi: 10.1073/pnas.80.21.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Febo M, Fahlke C, Archer T, Berggren U, Demetrovics Z, Badgaiyan RD. Hypothesizing Balancing Endorphinergic and Glutaminergic Systems to Treat and Prevent Relapse to Reward Deficiency Behaviors: Coupling D-Phenylalanine and N-Acetyl-L-Cysteine (NAC) as a Novel Therapeutic Modality. Clin Med Rev Case Rep. 2015;2 doi: 10.23937/2378-3656/1410076. pii: 076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Femino J, Teitelbaum S, Giordano J, Oscar-Berman M, Gold M. Springer Briefs in Neuroscience. New York: Springer; 2014. Molecular Neurobiology of Addiction Recovery: The 12 Steps Program and Fellowship. [Google Scholar]
- Blum K, Liu Y, Wang W, Wang Y, Zhang Y, Oscar-Berman M, Gold MS. rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts. Postgrad Med. 2015;127:232–241. doi: 10.1080/00325481.2015.994879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Oscar-Berman M, Femino J, Waite RL, Benya L, Giordano J, Borsten J, Downs WB, Braverman ER, Loehmann R, Dushaj K, Han D, Simpatico T, Hauser M, Barh D, McLaughlin T. Withdrawal from Buprenorphine/Naloxone and Maintenance with a Natural Dopaminergic Agonist: A Cautionary Note. Journal of Addiction Research and Therapy. 2013:4. doi: 10.4172/2155-6105.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Oscar-Berman M, Jacobs W, McLaughlin T, Gold MS. Buprenorphine Response as a Function of Neurogenetic Polymorphic Antecedents: Can Dopamine Genes Affect Clinical Outcomes in Reward Deficiency Syndrome (RDS)? J Addict Res Ther. 2014;5:pii: 1000185. doi: 10.4172/2155-6105.1000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Oscar-Berman M, Stuller E, Miller D, Giordano J, Morse S, Simpatico T. Neurogenetics and Nutrigenomics of Neuro-Nutrient Therapy for Reward Deficiency Syndrome (RDS): Clinical Ramifications as a Function of Molecular Neurobiological Mechanisms. J Addict Res Ther. 2012;3:139. doi: 10.4172/2155-6105.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum K, Stice E, Liu Y, Giordano J, Morse S, Downs B, Waite RL, Madigan MA, Braverman ER, Kerner M, Oscar-Berman M, Miller D, Stokes S, Gant C, Thompson T, Allen C, Smolen A, Bowirrat A, Gold MS. “Dopamine Resistance” in brain reward circuitry as a function of DRD2 gene receptor polymorphisms in RDS: Synaptamine complex variant (KB220) induced “Dopamine Sensitivity” and enhancement of happiness; Paper presented at the XIX World Congress of Psychiatric Genetics; Washington D.C. 2011. Sep, [Google Scholar]
- Blum K, Thanos PK, Badgaiyan RD, Febo M, Oscar-Berman M, Fratantonio J, Gold MS. Neurogenetics and gene therapy for reward deficiency syndrome: are we going to the Promised Land? Expert Opin Biol Ther. 2015;15:973–85. doi: 10.1517/14712598.2015.1045871. [DOI] [PubMed] [Google Scholar]
- Blum K, Trachtenberg MC, Cook DW. Neuronutrient effects on weight loss in carbohydrate bingers: an open clinical trial. Curr Ther Res. 1990;48:217–233. [Google Scholar]
- Blum K, Trachtenberg MC, Elliott CE, Dingler ML, Sexton RL, Samuels AI, Cataldie L. Enkephalinase inhibition and precursor amino acid loading improves inpatient treatment of alcohol and polydrug abusers: double-blind placebo-controlled study of the nutritional adjunct SAAVE. Alcohol. 1988;5:481–493. doi: 10.1016/0741-8329(88)90087-0. [DOI] [PubMed] [Google Scholar]
- Blum K, Trachtenberg MC, Ramsay JC. Improvement of inpatient treatment of the alcoholic as a function of neurotransmitter restoration: a pilot study. Int J Addict. 1988;23:991–998. doi: 10.3109/10826088809058853. [DOI] [PubMed] [Google Scholar]
- Braverman ER, Blum K. Substance use disorder exacerbates brain electrophysiological abnormalities in a psychiatrically-ill population. Clin Electroencephalogr. 1996;27:5–27. doi: 10.1177/1550059496027s0402. [DOI] [PubMed] [Google Scholar]
- Braverman ER, Braverman D, Acrui V, Kerner M, Downs BW, Blum K. Sustainable Weight Loss and Muscle Gain Utilizing the Rainbow Diet™: Targeting Noradrenergic and dopaminergic Mechanistic Sites, Hormonal Deficiency Repletion Therapy and Exercise: A case report. The American Journal of Bariatric Medicine. 2010;25:18–28. [Google Scholar]
- Brown RJ, Blum K, Trachtenberg MC. Neurodynamics of relapse prevention: a neuronutrient approach to outpatient DUI offenders. Psychoactive Drugs. 1990;22:173–187. doi: 10.1080/02791072.1990.10472542. [DOI] [PubMed] [Google Scholar]
- Caruso V, Bahari H, Morris MJ. The beneficial effects of early short-term exercise in the offspring of obese mothers are accompanied by alterations in the hypothalamic gene expression of appetite regulators and FTO (fat mass and obesity associated) gene. J Neuroendocrinol. 2013;25:742–752. doi: 10.1111/jne.12053. [DOI] [PubMed] [Google Scholar]
- Chen D, Liu Y, He W, Wang H, Wang Z. Neurotransmitter-precursor-supplement intervention for detoxified heroin addicts. Journal of Huazhong University Science and Technology [Medical Sciences] 2012;32:422–427. doi: 10.1007/s11596-012-0073-z. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Chen AL, Bowirrat A, Downs WB, Madigan MA, Braverman ER. Neurogenetics and clinical evidence for the putative activation of the brain reward circuitry by a neuroadaptagen: proposing an addiction candidate gene panel map. J Psychoactive Drugs. 2011;43:108–127. doi: 10.1080/02791072.2011.587393. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Payte JT, Schoolfield J, Hopper D, Stanford M, Braverman ER. Narcotic antagonists in drug dependence: pilot study showing enhancement of compliance with SYN-10, amino-acid precursors and enkephalinase inhibition therapy. Medical Hypotheses. 2004;63:538–548. doi: 10.1016/j.mehy.2004.02.051. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Blum K, Waite RL, Meshkin B, Schoolfield J, Downs BW, Braverman EE, Arcuri V, Varshavskiy M, Blum SH, Mengucci J, Reuben C, Palomo T. Gene\Narcotic Attenuation Program attenuates substance use disorder, a clinical subtype of reward deficiency syndrome. Advances in Therapy. 2007;24:402–414. doi: 10.1007/BF02849910. [DOI] [PubMed] [Google Scholar]
- Chen TJH, Blum K, Kaats G, Braverman ER, Eisenberg A, Sherman M, Davis K, Comings DE, Wood R, Pullin D, Arcuri V, Varshavski M, Mengucci JF, Blum SH, Downs BW, Meshkin B, Waite RL, Williams L, Schoolfield J, Prihoda TJ, White L. Chromium Picolinate (Crp) A putative Anti-Obesity Nutrient Induces Changes In Body Composition As Function Of The Taq1 Dopamine D2 Receptor Gene. Gene Ther Molbiol. 2007;11:161–170. [Google Scholar]
- Chen W, Liu P, Volkow ND, Pan Y, Du C. Cocaine attenuates blood flow but not neuronal responses to stimulation while preserving neurovascular coupling for resting brain activity. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cold JA. NeuRecover-SATM in the Treatment of Cocaine Withdrawal and Craving: A Pilot Study. Clinical Drug Investigation. 1996;12:1–7. [Google Scholar]
- Dasgupta N, Creppage K, Austin A, Ringwalt C, Sanford C, Proescholdbell SK. Observed transition from opioid analgesic deaths toward heroin. Drug Alcohol Depend. 2014;145:238–241. doi: 10.1016/j.drugalcdep.2014.10.005. [DOI] [PubMed] [Google Scholar]
- DeFrance JF, Hymel C, Trachtenberg MC, Ginsberg LD, Schweitzer FC, Estes S, Chen TJ, Braverman ER, Cull JG, Blum K. Enhancement of attention processing by Kantroll in healthy humans: a pilot study. Clinical Electroencephalography. 1997;28:68–75. doi: 10.1177/155005949702800204. [DOI] [PubMed] [Google Scholar]
- Demetrovics Z, Farkas J, Csorba J, Németh A, Mervó B, Szemelyácz J, Rácz J. Early experience with Suboxone maintenance therapy in Hungary. Neuropsychopharmacol Hung. 2009;11:249–257. [PubMed] [Google Scholar]
- Elman I, Borsook D. Common Brain Mechanisms of Chronic Pain and Addiction. Neuron. 2016;89:11–36. doi: 10.1016/j.neuron.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Ferenczi E, Deisseroth K. Illuminating next-generation brain therapies. Nat Neurosci. 2016 doi: 10.1038/nn.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Badgaiyan RD, Blum K. A Shared Molecular and Genetic Basis for Food and Drug Addiction: Overcoming Hypodopaminergic Trait/State by Incorporating Dopamine Agonistic Therapy in Psychiatry. Psychiatr Clin North Am. 2015;38:419–462. doi: 10.1016/j.psc.2015.05.011. [DOI] [PubMed] [Google Scholar]
- Gorelick DA, Gardner EL, Xi ZX. Agents in development for the management of cocaine abuse. Drugs. 2004;64:1547–1573. doi: 10.2165/00003495-200464140-00004. [DOI] [PubMed] [Google Scholar]
- Häkkinen M, Heikman P, Ojanperä I. Parenteral buprenorphine-naloxone abuse is a major cause of fatal buprenorphine-related poisoning. Forensic Sci Int. 2013;232:11–15. doi: 10.1016/j.forsciint.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Hill E, Han D, Dumouchel P, Dehak N, Quatieri T, Moehs C, Blum K. Long term Suboxone™ emotional reactivity as measured by automatic detection in speech. PLoS One. 2013;8:e69043. doi: 10.1371/journal.pone.0069043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillemacher T, Frieling H, Buchholz V, Hussein R, Bleich S, Meyer C, Rumpf HJ. Alterations in DNA-methylation of the dopamine-receptor 2 gene are associated with abstinence and health care utilization in individuals with a lifetime history of pathologic gambling. Prog Neuropsychopharmacol Biol Psychiatry. 2015;63:30–34. doi: 10.1016/j.pnpbp.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Hirth N, Meinhardt MW, Noori HR, Salgado H, Torres-Ramirez O, Uhrig S, Hansson AC. Convergent evidence from alcohol-dependent humans and rats for a hyperdopaminergic state in protracted abstinence. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1506012113. pii: 201506012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanate D, Folk D, Cirone S, Gordon J, Kirlew M, Veale T, Kelly L. Community-wide measures of wellness in a remote First Nations community experiencing opioid dependence: evaluating outpatient buprenorphine-naloxone substitution therapy in the context of a First Nations healing program. Can Fam Physician. 2015;61:160–165. [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Charney DS, Price LH, Morgan CH, Kleber HD. Opioid antagonist challenges in buprenorphine maintained patients. Drug Alcohol Depend. 1990;25:73–78. doi: 10.1016/0376-8716(90)90144-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Blum K, Oscar-Berman M, Febo M, Agan G, Fratantonio JL, Gold MS. Putative dopamine agonist (KB220Z) attenuates lucid nightmares in PTSD patients: role of enhanced brain reward functional connectivity and homeostasis redeeming joy. J Behav Addict. 2015;4:106–115. doi: 10.1556/2006.4.2015.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Blum K, Oscar-Berman M, Febo M, Demetrovics Z, Agan G, Gold MS. Using the Neuroadaptagen KB200z™ to Ameliorate Terrifying, Lucid Nightmares in RDS Patients: the Role of Enhanced, Brain-Reward, Functional Connectivity and Dopaminergic Homeostasis. J Reward Defic Syndr. 2015;1:24–35. doi: 10.17756/jrds.2015-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Febo M, Badgaiyan R, Barh D, Dushaj K, Braverman ER, Li M, Madigan MA, Blum K. KB220Z™ a Pro-Dopamine Regulator Associated with the Protracted, Alleviation of Terrifying Lucid Dreams. Can We Infer Neuroplasticity-induced Changes in the Reward Circuit? Journal of Reward Deficiency Syndrome & Addiction Science. 2016;2:3–13. doi: 10.17756/jrdsas.2016-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DK, Bowirrat A, Manka M, Miller M, Stokes S, Manka D, Blum K. Acute intravenous synaptamine complex variant KB220™ "normalizes" neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports. Postgrad Med. 2010;122:188–213. doi: 10.3810/pgm.2010.11.2236. [DOI] [PubMed] [Google Scholar]
- Miller M, Chen AL, Stokes SD, Silverman S, Bowirrat A, Manka M, Manka D, Miller DK, Perrine K, Chen TJ, Bailey JA, Downs WB, Waite RL, Madigan MA, Braverman ER, Damle U, Kerner M, Giordano J, Morse S, Oscar-Berman M, Barh D, Blum K. Early Intervention of Intravenous KB220IV- Neuroadaptagen Amino-Acid Therapy (NAAT)™ Improves Behavioral Outcomes in a Residential Addiction Treatment Program: A Pilot Study. Journal of Psychoactive Drugs. 2012;44:398–409. doi: 10.1080/02791072.2012.737727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SG, Willet J, Monico LB, James A, Rudes DS, Viglioni J, Friedmann PD. Community correctional agents' views of medication-assisted treatment: Examining their influence on treatment referrals and community supervision practices. Subst Abus. 2016;37:127–133. doi: 10.1080/08897077.2015.1129389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, Montaner JS. A call for evidence-based medical treatment of opioid dependence in the United States and Canada. Health Aff (Millwood) 2013;32:1462–1469. doi: 10.1377/hlthaff.2012.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J. Amino-acid precursor and enkephalinase inhibition therapy: evidence for effectiveness in treatment of “Reward Deficiency Syndrome (RDS) with particular emphasis on eating disorders. Mol Psychiatry. 2001;6:S1–8. [Google Scholar]
- Rowe C, Santos GM, Behar E, Coffin PO. Correlates of overdose risk perception among illicit opioid users. Drug Alcohol Depend. 2015 doi: 10.1016/j.drugalcdep.2015.12.018. pii: S0376-8716(15)01833-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg B, Blum K, McLaughlin T, Lubar J, Febo M, Braverman E, Badgaiyan R. Low-Resolution Electromagnetic Tomography (LORETA) of changed Brain Function Provoked by Pro-Dopamine Regulator (KB220z) in one Adult ADHD case. Open Journal of Clinical & Medical Case Reports. 2016:2. [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Wang GJ, Wang R, Caparelli EC, Logan J, Volkow ND. Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors. Hum Brain Mapp. 2015;36:120–136. doi: 10.1002/hbm.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier BB, Steimer T, Millet P, Moulin-Sallanon M, Vallet P, Ibañez V, Ginovart N. Innately low D2 receptor availability is associated with high novelty-seeking and enhanced behavioural sensitization to amphetamine. Int J Neuropsychopharmacol. 2013;16:1819–1834. doi: 10.1017/S1461145713000205. [DOI] [PubMed] [Google Scholar]
- Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob G. Brain disease model of addiction: why is it so controversial? Lancet Psychiatry. 2015;2:677–9. doi: 10.1016/S2215-0366(15)00236-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat Neurosci. 2014;17:704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Kabbaj M. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci. 2015;35:8948–8958. doi: 10.1523/JNEUROSCI.5227-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]