Abstract
Interval timing is crucial for decision-making and motor control and is impaired in many neuropsychiatric disorders, including schizophrenia – a neurodevelopmental disorder with a strong genetic component. Several gene mutations, polymorphisms or rare copy number variants have been associated with schizophrenia. L1 cell adhesion molecules (L1CAMs) are involved in neurodevelopmental processes, and in synaptic function and plasticity in the adult brain. Mice deficient in the Close Homolog to L1 (CHL1) adhesion molecule show alterations of hippocampal and thalamo-cortical neuroanatomy as well as deficits in sensorimotor gating and exploratory behavior. We analyzed interval timing and attentional control of temporal and spatial information in male CHL1 deficient (KO) mice and wild type (WT) controls. In a 20-s peak-interval timing procedure (standard and reversed), KO mice showed a maintained leftward shift of the response function relative to WT, indicative of a deficit in memory encoding/decoding. In trials with 2, 5, or 10-s gaps, KO mice shifted their peak times less than WT controls at longer gap durations, suggesting a decreased (attentional) effect of interruptions. In the spatial-temporal task, KO mice made more working and reference memory errors than controls, suggestive of impaired use of spatial and/or temporal information. When the duration spent on the central platform of the maze was manipulated, WT mice showed fewer spatial errors at the trained duration than at shorter or longer durations, indicative of discrimination based upon spatial-temporal integration. In contrast, performance was similar at all tested durations in KO mice, indicative of control by spatial cues, but not by temporal cues. These results suggest that CHL1 KO mice selectively attend to the more relevant cues of the task, and fail to integrate more complex spatial-temporal information, possibly as a result of reduced memory capacity related to hippocampal impairment, and altered temporal-integration mechanisms possibly due to thalamo-cortical anomalies.
Keywords: cell adhesion molecule, close homolog to L1, CHL1, interval timing, schizophrenia, spatial navigation
Introduction
Interval timing, or timing in the seconds-to-minutes range, is an essential process for rate estimation, planning and decision-making (Buhusi & Meck, 2005; Gallistel, 1990), as well as for animal foraging (Bateson, Healy, & Hurly, 2003; Bateson & Kacelnik, 1998). Deficits in interval timing have been reported in many human disorders, particularly in those associated with alterations in the dopaminergic system (Allman & Meck, 2012; Coull, Cheng, & Meck, 2011; Ward, Kellendonk, Kandel, & Balsam, 2012), such as Parkinson’s Disease (Malapani, Deweer, & Gibbon, 2002; Malapani et al., 1998), Huntington’s Disease (Paulsen et al., 2004), and Attention Deficit Hyperactivity Disorder (Barkley, Murphy, & Bush, 2001; Radonovich & Mostofsky, 2004). Importantly, timing impairments are also reported in schizophrenia (Braus, 2002; Green & Nuechterlein, 1999; Kimura, 2003; McDowell, Clementz, & Wixted, 1996; Volz et al., 2001). Schizophrenic patients and individuals at risk for schizophrenia are impaired at discriminating time intervals (Papageorgiou et al., 2013), at using predictive timing (Turgeon, Giersch, Delevoye-Turrell, & Wing, 2012), and show features suggestive of a faster internal clock (Carroll, O’Donnell, Shekhar, & Hetrick, 2009; Penney, Meck, Roberts, Gibbon, & Erlenmeyer-Kimling, 2005; Ward et al., 2012).
Most interval timing studies in laboratory animals use the peak-interval procedure (Catania, 1970). Changes in memory capacity and attention gating can be tested in the peak-interval (PI) procedure by inserting unexpected, brief breaks or gaps in the signal (Church, 1978; Gibbon, Church, & Meck, 1984; Roberts & Church, 1978). Observed changes in the distribution of responses in trials with gaps following behavioral (Buhusi & Meck, 2000, 2006a, 2006b) and neurobiological (Buhusi, Lamoureux, & Meck, 2008; Buhusi & Meck, 2002; Meck, 1988; Meck, Church, & Olton, 1984) manipulations are used to address the mechanisms involved in memory for timed events. For example, when rats time a visual signal in a (standard) PI procedure, the introduction of a (dark) gap prompts rats to delay their response function by an amount approximately equal to the duration of the gap, which is taken to suggest that rodents retain in working memory the pre-gap interval and resume timing after the gap where they left off before the gap, using a stop mode. In contrast, when rats time the absence of a visual signal in a so-called reversed PI procedure (Buhusi & Meck, 2000), the introduction of a reversed, illuminated gap prompts rats to delay their response function after the gap for a duration that is approximately the sum of the gap and pregap intervals. This has been taken to suggest that they restart the entire timing process after the gap, using a reset mode (reviewed in Buhusi et al., 2008; Buhusi & Meck, 2000, 2009). Such a reset mode was observed after lesions of the hippocampal system in the standard PI procedure (Eichenbaum, 2013; Meck et al., 1984; Olton, Meck, & Church, 1987; Yin & Troger, 2011), suggesting that the hippocampus is needed to retain the pregap interval in working memory.
While most interval timing studies have used rats as subjects, less is known about interval timing in the mouse, or in genetically engineered mouse models of disease (Abner, Edwards, Douglas, & Brunner, 2001; Balci et al., 2010; Balci et al., 2008; Carvalho, Silva, & Balleine, 2001; Drew et al., 2007; Meck et al., 2012). These studies were carried in various mouse background strains, raising the question of the role of the genetic background, given that differences in performance have been reported in different strains in many behavioral paradigms: Morris water maze (Crawley, 2007), fear conditioning (Smith, Gallagher, & Stanton, 2007), radial arm maze (Crawley, 2007), and social behavior (Crawley et al., 2007). Therefore, it is challenging to dissociate the deficits due to the particular gene modification from those related to the background used. Importantly, our group recently demonstrated that the C57BL/6 mice -- the strain most used for behavioral studies -- shows accurate and scalar timing in the PI procedure (Buhusi et al., 2009), and can be further used for investigations of biological mechanisms of timing.
The current studies investigate interval timing and spatial-temporal integration in a mouse model of neurodevelopmental deficits relevant to schizophrenia. Genetic case-control association studies in Japanese and Chinese populations (Sakurai, Migita, Toru, & Arinami, 2002) and analysis of rare gene copy number variants (Tam et al., 2010) have identified CHL1 as a gene associated with schizophrenia. Indeed, cell adhesion molecules of the immunoglobulin superfamily, including the close homolog to L1 (CHL1), have multiple functions in the formation of normal neuronal connections during development and in synaptic function and plasticity in the adult, processes which are thought to be disrupted in intellectual disabilities and schizophrenia. Mice deficient in the CHL1 adhesion molecule show alterations of hippocampal and thalamocortical circuitry and function, as well as behavioral anomalies such as altered exploratory behavior in novel environments (Montag-Sallaz, Baarke, & Montag, 2003; Montag-Sallaz, Schachner, & Montag, 2002) and sensorimotor gating (prepulse inhibition) (Irintchev, Koch, Needham, Maness, & Schachner, 2004; Morellini, Lepsveridze, Kahler, Dityatev, & Schachner, 2007) as also reported in schizophrenia patients (Delerue & Boucart, 2012a, 2012b; Hammer et al., 2013; Ross et al., 2013; Velasques et al., 2011; Wang et al., 2013).
Given that timing is disrupted in schizophrenic patients and individuals at risk for schizophrenia (Braus, 2002; Green & Nuechterlein, 1999; Kimura, 2003; McDowell et al., 1996; Penney et al., 2005; Volz et al., 2001), we hypothesized that timing is also disrupted in CHL1 deficient mice. Moreover, because lesions of the hippocampus impair the stop/reset of timing (Meck et al., 1984), we also hypothesized that disruptions of the stop/reset mechanism will be found in CHL1 deficient mice. Finally, given that schizophrenia is characterized by deficits in spatial-temporal integration (Herzog & Brand, 2009; Velasques et al., 2011), we hypothesized that CHL1 KO mice would also be impaired in integrating spatial and temporal information.
The current study examined the ability of CHL1-deficient mice to learn a time interval, as well as their memory capacity and attention to time, by incorporating gaps into the standard and reversed PI procedure in a manner similar to (Buhusi & Meck, 2000). We have also evaluated their capacity to integrate spatial and temporal information using a modified radial maze procedure. To our knowledge this is the first study to examine the effect of gaps on interval timing and spatial-temporal integration, in mice. Our results provide evidence of deficits in interval timing and temporal integration in CHL1-deficient mice.
Materials and methods
Timing task
Subjects
The subjects were 6 month-old male CHL1 deficient mice (KO, n=8) and wild-type controls (WT, n=12) (Montag-Sallaz et al., 2002) from a CHL1 colony maintained in a C57BL/6 background for at least 10 generations. The genotype was confirmed by PCR genotyping from tail biopsy samples. The mice were housed in groups of three or four in a temperature-controlled room under a 12-hr light– dark cycle. The mice were tested during the light period of the cycle. Water was given ad libitum in the home cages. The mice were maintained at 85% of their ad libitum weight by restricting their access to food (Rodent Diet 5001, PMI Nutrition International, Brentwood, MO). All manipulations were performed in compliance with ethical standards for the treatment of animals.
Apparatus
The apparatus consisted in 10 standard mouse operant chambers (Med Associates, St. Albans VT) equipped with a house light (to-be-timed stimulus), two levers (only the left lever was used), and a standard mouse 20-mg pellet feeder.
Standard and reversed peak-interval (PI) procedures
The mice were trained in the standard and reversed peak-interval (PI) procedures with gaps as described in Buhusi and Meck (2000). Briefly, mice received 12 fixed-interval 20s (FI) sessions, during which they were trained to time the presence of the house light, and their first lever-press 20s after the onset of the house light was reinforced by one 20-mg dustless precision pellet (BioServ, Frenchtown, NJ). Afterwards, mice received 12 peak-interval (PI) sessions during which FI trials were randomly mixed with PI trials which had the to-be-timed signal presented for 3–4 times the duration of the criterion, and no reinforcement was provided. Afterwards, mice were tested in 3 gap sessions, which included FI, PI, and gap trials; gap trials were similar to PI trials, except that 10s after the onset of the to-be-timed signal, the house light was turned off for either 2s, 5s, or 10s (standard gap), and then turned back on for the remainder of the trial. After being tested, mice were given a two week break when food was provided ad libitum and no training was done, and then re-trained in the reversed PI procedure with gaps, in which the to-be-timed signal was the absence of the house light, and the gaps were signaled by the presence of the house light (reversed gap). All temporal parameters were identical in the standard and reversed PI procedures with gaps. After being tested in 3 reversed gap sessions, mice were given another two week break with food provided ad libitum and then re-trained in the spatial-temporal task (see below).
Data analysis
Data from the 3 standard and 3 reversed gap sessions were analyzed as described in Buhusi and Meck (2000), except that the window of analysis was three times the criterion (60s). Briefly, the average response functions were fitted by a Gaussian curve to estimate the peak time. A shift in peak time between gap trials and PI trials was computed by subtracting the estimated peak time in PI trials and the gap duration from the estimated peak time in gap trials. The estimated peak time in PI trials and the estimated shift time between gap trials and PI trials were submitted to repeated measures ANOVAs with genotype (WT, KO) as the between-group variable and condition (Standard, Reversed) and gap length (2s, 5s, 10s) as the repeated measure (Buhusi and Meck, 2000). All statistical analyses were conducted with an alpha-level of 0.05.
Spatial-temporal task
Subjects
The subjects were the same mice used in the timing task, minus one WT mouse which did not complete the spatial-temporal task study: CHL1 deficient mice (KO, n=8) and wild-type controls (WT, n=11). The mice were housed as described for the timing study. They were maintained at 85% of their ad libitum weight by restricting their access to food (Rodent Diet 5001, PMI Nutrition International, Brentwood, MO).
Apparatus
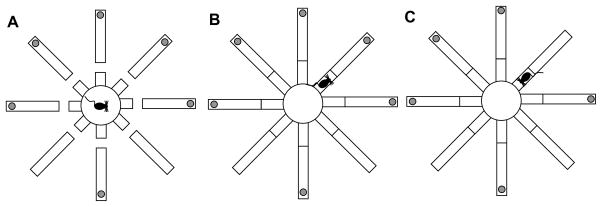
The apparatus was an elevated eight-arm radial maze, similar to that described in (Roullet & Lassalle, 1995). The center platform is separated from the arms by “bridges” (Fig. 1A). When raised, the bridges allow the mice to explore the arms, collect the food (if available) (Fig. 1B) and return to the center platform (Fig. 1C) (Roullet & Lassalle, 1995). The maze was shown to prevent mice from using response strategies or taking advantage of intra-maze olfactory trails or other proximal cues (Mohler, 2002; Roullet & Lassalle, 1995): In our setting, we used the maze to explore integration of spatial and temporal cues, by varying the confinment duration as shown below.
Figure 1.
Spatial-temporal task. (A) The mouse is confined to the center platform for a specified duration, with bridges lowered. (B) Bridges are raised to allow the mouse to explore one arm, and possibly collect food. (C) The mouse returns to the center platform, the bridges are lowered, and the mouse is confined again to the center platform. The sequence is repeated until all reinforcement is collected.
Pre-training
Mice were accustomed to the maze (with raised bridges) in groups of six, for three 30-min daily sessions, during which food was replenished on a continuous basis at the end of all eight arms.
Spatial-temporal training procedure
Six of the eight arms were baited with one piece of Fruity Pebbles (Post Holdings, Inc., Battle Creek, MI); six baiting patterns were used, counter-balanced across the subjects; the pattern was maintained for each subject throughout training and testing. Each mouse was placed on the center platform, with the bridges lowered (Fig. 1A). After being confined to the center-platform for a 10-s criterion interval (Fig. 1A), the bridges were raised, and the mouse was allowed to explore one of the arms (Fig. 1B), consume the bait (if available), and eventually return to the center-platform (Fig. 1C), where it was confined for another 10-s criterion interval before the next choice (Fig. 1A). The procedure was repeated until all six baited arms were visited. Between subjects, the maze was cleaned to eliminate olfactory trails. This training procedure was repeated for 30 daily sessions.
Testing procedure
Afterwards, mice underwent six test sessions similar to training, except that the confinement interval was manipulated as being either shorter than (3s), identical to (10s) or longer (30s) than the criterion interval for the entire testing session. The six daily sessions were pseudo-randomly intermixed, two test sessions for each confinement interval.
Rotation test
To evaluate whether the mice used extra-maze (spatial) cues or intra-maze cues (e.g., odor trails) 2 sessions of a rotation test were administered as follows: Mice were allowed to make three correct choices, as described above. Afterwards, mice were removed from the center platform and placed in their home cage for 30s, during which time the maze was rotated. After the rotation, the mice were placed on the center platform, and allowed to complete the maze as usual. Three rotation patterns were used: no rotation (accommodation), one arm rotation clockwise, and one arm rotation counterclockwise. The no-rotation pattern was used first during the rotation session to allow mice to accommodate to the procedure; afterwards, the two other patterns were used, in a counterbalanced order between subjects.
Data analysis
The choices, number of choices, the latency to make six choices, the number of working-memory errors (re-entrances in previously visited arms, WM) and reference-memory errors (entrances in arms that were never baited, RM) were recorded for each subject. The total number of choices, the number of WM and the number of RM errors were averaged over 3-sessions blocks and submitted to repeated measures ANOVAs with genotype as the between-subject variable, and block of sessions as the repeated measure. The numbers of WM and RM errors from the test sessions were averaged over the two test sessions with each confinement interval and submitted to repeated measures ANOVAs with genotype as the between-subject variable and confinement interval (3s, 10s, 30s) as the repeated measure. Under the hypothesis that mice learn to make fewer errors resulting in a faster time to completion of the session, errors (both WM and RM) were computed based on a strategy using external cues and on a strategy using internal cues. Our assumption was that the strategy used by each mouse was the one that resulted in the fewest errors. The number of choices, and the number of WM and RM errors were averaged over the 2 sessions of rotation test and submitted to repeated measures ANOVAs with genotype as the between-subject variable and cues (extra-maze, intra-maze) as the repeated measure All statistical analyses were conducted with an alpha-level of 0.05.
Results
CHL1 deficient mice are sensitive to temporal information
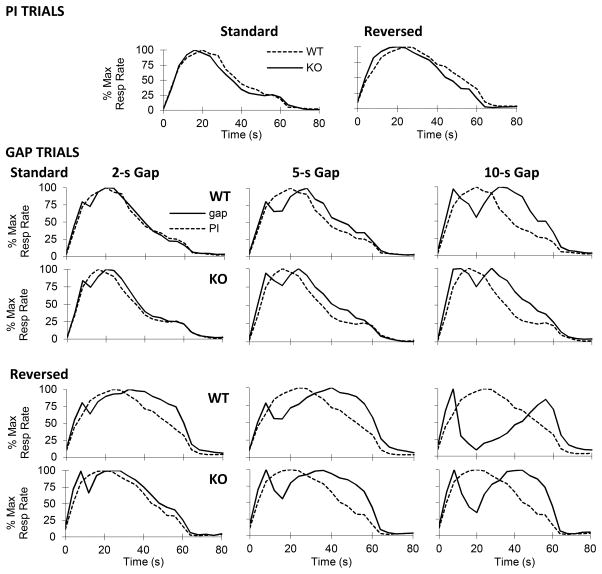
As shown in the upper panel of Fig. 2, CHL1 deficient mice and WT controls showed temporal control both when the to-be-timed signal was the presence (Standard) and in the absence of a visual signal (Reversed). Moreover, under both conditions, the timing functions peak around the 20-s criterion for both the CHL1 deficient mice and WT controls, indicating that mice acquired the timing task.
Figure 2.
Normalized mean response rate functions in CHL1 deficient mice (KO) and wild-type controls (WT) in the peak-interval procedure with gaps, when timing the presence (Standard condition) or absence (Reversed condition) of a visual cue. First row: PI trials. Lower 4 rows: Gap trials, with 2-s gaps (left), 5-s gaps (center) and 10-s gaps (right) in the Standard (upper 2 rows) and Reversed condition (bottom 2 rows).
CHL1 deficient mice show altered encoding of durations
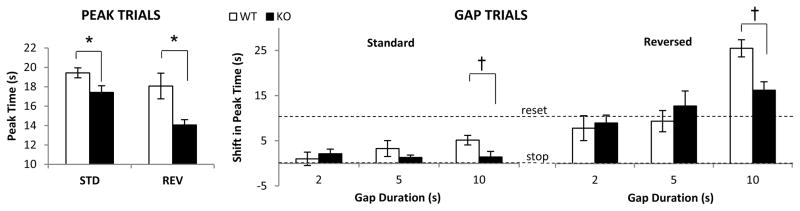
As can be seen in the upper panel of Fig. 2, the timing functions of CHL1 deficient mice peaked to the left of the timing functions of the WT mice. Analyses shown in the left panel of Fig. 3 confirmed that under both the Standard and Reversed conditions, the timing functions of WT mice peaked at the criterion duration, 20s (Standard: t(11)=1.11, p>0.29, Reversed: t(11)=1.46, p>0.17. In contrast, the estimated peak time in the timing functions of CHL1 deficient mice was reliably shorter than the estimated peak time in WT controls (Standard: F(1,18)=5.79, p<0.05, Reversed: F(1,18)=5.61, p<0.05). The observation of maintained leftward shifts in the 20-s PI functions may reflect alterations in encoding / decoding temporal information (Balci, Meck, Moore, & Brunner, 2009; Buhusi & Oprisan, 2013; Meck, 1996, 2001; Oprisan & Buhusi, 2011).
Figure 3.
Altered timing in CHL1 deficient mice. Left panel: Mean (±SEM) estimated peak time in PI trials; CHL1 deficient mice (KO) show earlier peaks than wild-type controls (WT) in both the standard and reversed PI procedure. Right panel: Mean (±SEM) estimated shift in peak time in gap trials; mice stop timing during dark gaps (standard) and reset timing after illuminated gaps (reversed). CHL1 deficient mice shift less than WT controls at longer gap durations. * = p<0.05. † = p<0.01.
Differential effect of gaps in CHL1 deficient mice relative to WT controls
The right panel of Fig. 3 shows the estimated shift in peak time in gap trials relative to PI trials in CHL1 deficient mice and WT controls. A null shift indicates that mice retained the pre-gap duration in working memory, and resumed timing after the gap (stop). A 10-s shift indicates that mice restarted timing all over after the gap (reset). Similar to results from rats reported previously (Buhusi and Meck, 2000), mice shifted less during a dark gap (Standard condition) relative to an illuminated gap (Reversed condition) (F(1,18)=50.88, p<0.01). However, while WT controls modulated their response according to gap duration (F(1,18)=2,36=9.92, p<0.01), CHL1 deficient mice were less able to modulate their response after a gap. For example, in the Standard condition, both CHL1 deficient mice and WT controls stopped timing for 2-s gap and 5-s gap (ts < 2.05, ps>0.08). In contrast, at the 10-s gap WT controls reliably resumed timing after the gap (t(11)=4.85, p<0.01), while CHL1 deficient mice continued to stop (t(7)=1.17, p>0.28). Similarly, in the Reversed condition, both CHL1 deficient mice and WT controls reset timing for 2-s gap and 5-s gap (ts < 0.99, ps>0.34). In contrast, at the 10-s gap WT controls resumed timing after the gap significantly more than CHL1 deficient mice (F(1,18)=11.09, p<0.01). Interestingly, for 10-s reversed gap, both CHL1 deficient mice and WT controls delayed more than reset (ts>3.03, p>0.05): CHL1 deficient mice shifted 15.34s, while WT controls shifted 24.12s. In summary, WT controls modulated their response to the gap (stopping at short gap durations, and resetting at longer gap durations) while CHL1 deficient mice were less able to modulate their responses after gap retention intervals.
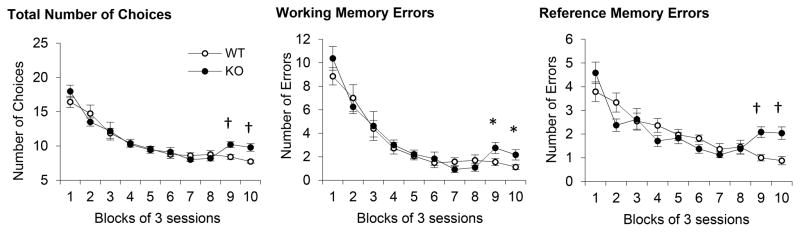
Deficits in acquiring the spatial-temporal task
As shown in Fig. 4, both CHL1 deficient mice and WT controls reliably decreased their number of choices (F(9,153)=40.07, p<0.01), working-memory (WM) errors (F(9,153)=42.08, p<0.01) and reference-memory (RM) errors (F(9,153)=21.39, p<0.01) during training. However, differences in number of choices, WM errors and RM errors between genotypes became reliable in the last two 3-session blocks of training (Fs(1,17)>4.94, ps<0.05), indicating that unlike CHL1 deficient mice, WT controls continued to improve their performance, possibly by integrating temporal information (criterion confinement interval, 10s) into the spatial task.
Figure 4.
Mean (±SEM) number of choices (left), working memory errors (center) and reference memory errors (right) during learning the spatial-temporal task (8-arm radial arm maze with confinement interval). CHL1 deficient mice and WT controls acquire the task similarly, except for the last 2 3-session blocks, when CHL1 deficient mice are reliably impaired. * = p<0.05. † = p<0.01.
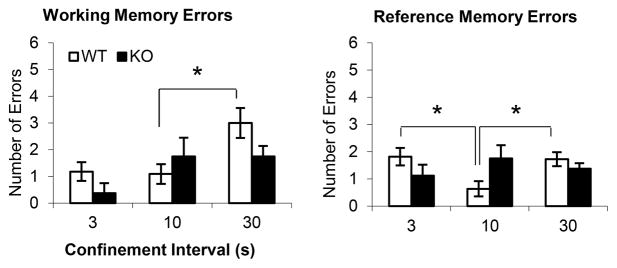
CHL1 deficient mice failed to integrate spatial and temporal information
Fig. 5 shows the results from test sessions where the confinement interval was manipulated to be either shorter (3s), longer (30s), or identical to the training interval (10s). WT controls showed more WM errors when the confinement interval was increased to 30s (F(1,17)=6.04, p<0.05); they also showed more RM errors at both shorter (F(1,17)=7.79, p<0.05) and longer (F(1,17)=6.89, p<0.05) confinement intervals than at the training interval, suggesting that they integrated the temporal (confinement duration) and spatial information. In contrast, CHL1 deficient mice failed to show any differences in WM and RM errors when the confinement duration was either shorter or longer than the training duration (Fs(1,17)<2.89, p>0.11), suggesting that they failed to integrate the temporal and spatial information. This failure to integrate information may explain their deficits in acquiring the spatial-temporal task (Fig. 4): Presumably, throughout training mice minimized the errors based on spatial information, and only in the last two blocks of 3 sessions did they optimize the task by integrating temporal information with spatial information. While WT controls continued to improve during the last two blocks by integrating the temporal information with the spatial information, CHL1 deficient mice failed to integrate temporal information and made more errors because they failed to learn to wait during the confinement interval before choosing the next arm.
Figure 5.
Mean (± SEM) working memory errors (left) and reference memory errors (right) in the spatial-temporal task (8-arm radial arm maze with confinement interval) when manipulating the confinement interval. Variations in confinement interval have effects in wild-type control mice (WT), but not in CHL1 deficient mice (KO). * = p<0.05.
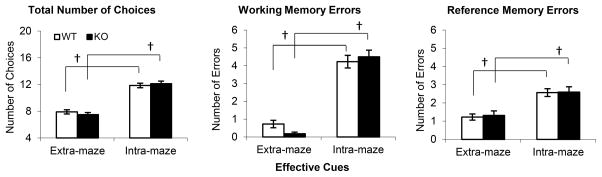
Both CHL1 deficient mice and WT control use extra-maze (spatial) cues rather than intra-maze cues
To evaluate whether mice used extra-maze (spatial) cues rather than intra-maze cues (e.g., odor trails or small variations in the maze arms) to solve the task, we performed a rotation test, in which extra-maze and intra-maze cues were put in conflict. The errors made during the rotation test were computed under two scenarios: relative to extra-maze and intra-maze cues. Because during training mice have learned to minimize errors, the scenario that yielded the fewest errors was thought to indicate which cues have mice used to solve the test. Fig. 6 shows the results of the rotation test, computed under the two scenarios. As seen in Fig. 6, all mice made fewer choices to find all food location, and fewer WM and RM errors when choices/errors were computed relative to extra-maze cues (Fs(1,17)>23.90, ps<0.01), suggesting that all mice effectively used the extra-maze cues to solve the task.
Figure 6.
Mean (± SEM) number of choices (left), working memory errors (center) and reference memory errors (right) in a rotation test, where extra-maze (spatial) cues where put in conflict with intra-maze cues (e.g., odor trials). Both CHL1 deficient mice (KO) and wild-type controls (WT) made fewer choices and fewer errors relative to extra-maze rather than intra-maze cues.
Discussion
We investigated temporal processing, and spatial-temporal integration in mice deficient in CHL1 (Montag-Sallaz et al., 2002), a gene associated with schizophrenia (Chen et al., 2005; Sakurai et al., 2002; Tam et al., 2010). Because timing is disrupted in schizophrenic patients and individuals at risk for schizophrenia (Braus, 2002; Green & Nuechterlein, 1999; Kimura, 2003; McDowell et al., 1996; Penney et al., 2005; Volz et al., 2001), we hypothesized that timing would be also disrupted in CHL1 deficient mice. Moreover, because CHL1 mice show alterations in hippocampal circuitry (Montag-Sallaz et al., 2003; Montag-Sallaz et al., 2002), and given that lesions of the hippocampus impair the stop/reset mechanisms of timing (Meck et al., 1984), we also hypothesized that disruptions of the stop/reset mechanism would be also found in CHL1 deficient mice. Finally, because schizophrenia is characterized by deficits in spatial-temporal integration (Herzog & Brand, 2009; Velasques et al., 2011), we also hypothesized that CHL1 KO mice would also be impaired in integrating spatial and temporal information.
All these predictions were confirmed experimentally. Although CHL1 deficient mice acquired the timing task, their timing functions were shifted leftward relative to WT controls (Fig. 2), suggesting an alterations in their encoding / decoding of temporal information. When their timing was interrupted by either standard (dark) or reversed (illuminated) gaps, CHL1 deficient mice failed to modulate their start/stop mechanism according to the duration of the gap. In contrast, WT controls tended to stop for shorter gaps and reset for longer gaps (Fig. 2). Moreover, although CHL1 deficient mice acquired the spatial-temporal task (Fig. 4), they showed reliably more choices, working-memory (WM) errors and reference-memory (RM) errors in the last training sessions (Fig. 4), possibly due to failure to integrate spatial and temporal information. For example, during early training mice might have acquired mainly spatial information. However, in the last two blocks of training sessions, errors could not decrease further unless mice incorporated temporal information as well. Based on results from the timing experiment, it is very likely that at this stage, WT mice acquired a veridical criterion time, and continued to improve, while KO mice incorporated a distorted temporal criterion, or failed to integrate the temporal information whatsoever, such that their errors increased in these sessions. As a result of that, KO mice might have been unable to integrate temporal information, or might have given up using temporal information because the use of the (distorted) temporal information failed to be useful in reducing errors. In support of this interpretation, while changes in the temporal parameters of the task determined an increase in the WM and RM errors in WT controls, they had no effect on CHL1 deficient mice (Fig. 5), suggesting the latter do not use temporal information in the task. These results cannot be explained by a general failure to attend to the task, or by a difference in what spatial features of the task were used, because a rotation test affected both genotypes equally, suggesting all mice effectively used extra-maze spatial information to solve the task. In summary, CHL1 deficient mice showed impairments in their internal clock, in their stop/reset mechanism, and in integrating temporal information with other (spatial) features of the task at hand.
An explanation of the increase in WM and RM errors in WT mice when the confinment interval was manipulated, is that, during the confinement period, the mice mapped the past experiences or intentional spatial sequence action to a timeline (spatial-temporal integration). In other words, the mice started timing immediately upon being placed in the central confinement of the maze, or immediately upon the raising of the bridges, and that these cues were used to plan out the spatial action sequence based on past experiences. When the duration of the confinement period was altered, the spatial-temporal mapping was distorted, hence impairing the performance in the WT mice. WT mice made more RM errors both when the confinement interval was increased and decreased, but made WM errors only when the confinement was increased, and not when it was decreased. Therefore, the confinement interval manipulation seemed to have had a larger effect on RM than WM, suggesting that the task involved encoding (integrating) temporal and spatial information in one unique engram. In contrast, due to interval timing deficits (as revealed in the timing task), the spatial-temporal mapping may have been distorted in CHL1 deficient mice in the first place. For example, during training of the spatial-temporal task, in the last blocks of training, the (reliable) spatial cues were in fact put in conflict with the (distorted) temporal cues, such that the performance of the CHL1 deficient mice worsened. As a result, CHL1 KO mice might have used only the most reliable cues (spatial), and discarded the temporal cues, as revealed by the confinement interval manipulation. The failure to encode and/or use both (integrate) temporal and spatial information in CHL1 mice, is in accord with the deficits in encoding temporal information in the timing task, and may reflect a more general memory deficit in CHL1 mice, possibly related to neuroanatomical defects of the hippocampus (Montag-Sallaz et al., 2003; Montag-Sallaz et al., 2002).
The results obtained with CHL1 deficient mice bear a striking resemblance with those reported for rats that were made deficient in the nutrient choline prenatally (Buhusi et al., 2008). Both prenatal choline deficient rats and CHL1 deficient mice show less sensitivity to contextual manipulations: they did not reset in the standard condition, they did not over-reset in the reversed condition, and were not affected by changes in the temporal parameters of the task, thus demonstrating limited attentional control over the starting and resetting of an internal clock (Buhusi et al., 2008; Buhusi & Meck, 2000). Finally, both prenatal choline-deficient rats and CHL1-deficient mice shifted their timing functions less than control animals when their timing was disrupted by gaps (Buhusi et al., 2008).
In both choline-deficient rats and CHL1-deficient mice, these similarities may be explained by deficits in hippocampal function. CHL1 deficient mice show alterations of hippocampal circuit organization (Montag-Sallaz et al., 2003; Montag-Sallaz et al., 2002) and function (Leshchyns’ka et al., 2006; Nikonenko et al., 2006). On the other hand, changes in hippocampal cholinergic input following prenatal choline manipulations are accompanied by modifications in acetylcholine turnover and choline transporter expression in the septum and hippocampus (Cermak et al., 1999), modulation of hippocampal neurogenesis, (Glenn et al., 2007; Sandstrom & Williams, 2001; Wong-Goodrich et al., 2011), MAPK and CREB activation (Mellott, Williams, Meck, & Blusztajn, 2004), changes in dendritic fields and spine density in CA1 and dentate gyrus (DG) regions of the hippocampus (Meck, Williams, Cermak, & Blusztajn, 2007), as well as modification of the long-term potentiation (LTP) in the hippocampus (reviewed in Buhusi et al., 2008). Interestingly, perinatal choline supplementation was shown to be involved in timely development of cerebral inhibition, a pathophysiological brain deficit related to poor sensory gating and attention, with relevance to subsequent schizophrenia risk (Ross et al., 2013). Taken together with early reports that hippocampal function is required for the normal functioning of the stop/reset mechanism of the internal clock (Meck et al., 1984), and for the feedback control of timing (Meck, 1988), these data suggest that the hippocampus is a critical component of the internal clock mechanism, and that alterations in hippocampal function have wide-spread effects on timing.
A description of the neurobiological mechanisms involved in interval timing is currently provided by the Striatal Beat-Frequency (SBF) model, which ascribes a role for detecting event durations to medium spiny neurons within the dorsal striatum (Buhusi & Cordes, 2011; Buhusi & Meck, 2005; Matell & Meck, 2004; Oprisan & Buhusi, 2011). These striatal neurons have a set of functional properties that place them in an ideal position to detect behaviorally relevant patterns of afferent cortical input and reflect alteration in clock speed (reviewed by Coull et al., 2011; Matell & Meck, 2004). Briefly, the SBF model posits that medium spiny neurons in the dorsal striatum become entrained to fire in response to oscillating, coincident cortical inputs that become active at previously trained event durations. This timing model is particularly useful insofar as the striatal neurons modeled using the SBF framework behave as they do when assessed using multiunit electrical recordings during interval-timing procedures (Matell, Meck, & Nicolelis, 2003). However, the exact nature of the horizontal shifts in timing functions as a result of attentional and/or clock speed manipulations, prenatal choline availability, or CHL1 deficiency is as yet unresolved within the context of the SBF model. One proposal is that tonic dopamine levels within the striatum modulate the oscillatory frequency within the cortex through cortico-striato-thalamo-cortical feedback mechanisms (Buhusi & Cordes, 2011; Buhusi & Meck, 2009; Matell & Meck, 2004; Oprisan & Buhusi, 2011). This hypothesis is supported by known disruptions in thalamo-cortical projections both in CHL1 mice (Montag-Sallaz et al., 2003; Montag-Sallaz et al., 2002) and schizophrenia patients (Welsh, Chen, & Taylor, 2010; Woodward, Karbasforoushan, & Heckers, 2012). The observation that timing and attention to time are sensitive to CHL1 deficiency further supports the view that differential activation of cortical, hippocampal, and striatal systems is critical for contextual processing and the control of an internal clock.
Acknowledgments
This work was made possible by grant AG038767 from the National Institutes of Health to MB, by grants MH065561 and MH075357 from the National Institutes of Health to CVB, and by a NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation to CVB. We would like to thank Drs. Melitta Schachner and Patricia Maness for kindly providing a pair of CHL1 deficient mice.
References
- Abner RT, Edwards T, Douglas A, Brunner D. Pharmacology of temporal cognition in two mouse strains. International Journal of Comparative Psychology. 2001;14:189–210. [Google Scholar]
- Allman MJ, Meck WH. Pathophysiological distortions in time perception and timed performance. Brain. 2012;135(Pt 3):656–677. doi: 10.1093/brain/awr210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balci F, Ludvig EA, Abner R, Zhuang X, Poon P, Brunner D. Motivational effects on interval timing in dopamine transporter (DAT) knockdown mice. Brain Res. 2010;1325:89–99. doi: 10.1016/j.brainres.2010.02.034. [DOI] [PubMed] [Google Scholar]
- Balci F, Meck WH, Moore H, Brunner D. Timing deficits in aging and neuropathology. In: Bizon JL, Woods A, editors. Animal models of human cognitive aging. Totowa, NJ: Humana Press; 2009. pp. 161–201. [Google Scholar]
- Balci F, Papachristos EB, Gallistel CR, Brunner D, Gibson J, Shumyatsky GP. Interval timing in genetically modified mice: a simple paradigm. Genes Brain Behav. 2008;7(3):373–384. doi: 10.1111/j.1601-183X.2007.00348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Bush T. Time perception and reproduction in young adults with attention deficit hyperactivity disorder. Neuropsychology. 2001;15(3):351–360. doi: 10.1037//0894-4105.15.3.351. [DOI] [PubMed] [Google Scholar]
- Bateson M, Healy SD, Hurly TA. Context-dependent foraging decisions in rufous hummingbirds. Proc Biol Sci. 2003;270(1521):1271–1276. doi: 10.1098/rspb.2003.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson M, Kacelnik A. Risk-sensitive foraging: decision making in variable environments. In: Dukas R, editor. Cognitive ecology: The evolutionary ecology of information processing and decision making. Chicago: Chicago University Press; 1998. [Google Scholar]
- Braus DF. Temporal perception and organisation, neuronal synchronisation and schizophrenia. Fortschr Neurol Psychiatr. 2002;70(11):591–600. doi: 10.1055/s-2002-35172. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Aziz D, Winslow D, Carter RE, Swearingen JE, Buhusi MC. Interval timing accuracy and scalar timing in C57BL/6 mice. Behav Neurosci. 2009;123(5):1102–1113. doi: 10.1037/a0017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Cordes S. Time and number: the privileged status of small values in the brain. Front Integr Neurosci. 2011;5:67. doi: 10.3389/fnint.2011.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Lamoureux JA, Meck WH. Prenatal choline supplementation increases sensitivity to contextual processing of temporal information. Brain Res. 2008;1237:204–213. doi: 10.1016/j.brainres.2008.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Timing for the Absence of a Stimulus: The Gap Paradigm Reversed. Journal of Experimental Psychology: Animal Behavior Processes. 2000;26(3):305–322. doi: 10.1037//0097-7403.26.3.305. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Differential effects of methamphetamine and haloperidol on the control of an internal clock. Behavioral Neuroscience. 2002;116(2):291–297. doi: 10.1037//0735-7044.116.2.291. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nature Reviews Neuroscience. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Interval Timing With Gaps and Distracters: Evaluation of the Ambiguity, Switch, and Time-Sharing Hypotheses. Journal of Experimental Psychology: Animal Behavior Processes. 2006a;32(3):329–338. doi: 10.1037/0097-7403.32.3.329. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Time sharing in rats: A peak-interval procedure with gaps and distracters. Behavioural Processes. 2006b;71:107–115. doi: 10.1016/j.beproc.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. Relative time sharing: new findings and an extension of the resource allocation model of temporal processing. Philos Trans R Soc Lond B Biol Sci. 2009;364(1525):1875–1885. doi: 10.1098/rstb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi CV, Oprisan SA. Time-scale invariance as an emergent property in a perceptron with realistic, noisy neurons. Behavioural Processes. 2013 doi: 10.1016/j.beproc.2013.02.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009;71(3):345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho OM, Silva AJ, Balleine BW. Evidence of selective learning deficits on tests of pavlovian and instrumental conditioning in α-CaMKII(T286A) mutant mice. International Journal of Comparative Psychology. 2001;14(3–4):161–174. [Google Scholar]
- Catania AC. Reinforcement schedules and psychophysical judgements: a study of some temporal properties of behavior. In: Schoenfeld WN, editor. The Theory of Reinforcement Schedules. New York: Appleton-Centruy-Crofts; 1970. pp. 1–42. [Google Scholar]
- Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald CM, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. [Research Support, U.S. Gov’t, P.H.S.] Dev Neurosci. 1999;21(2):94–104. doi: 10.1159/000017371. 17371. [DOI] [PubMed] [Google Scholar]
- Chen QY, Chen Q, Feng GY, Lindpaintner K, Chen Y, Sun X, … He L. Case-control association study of the close homologue of L1 (CHL1) gene and schizophrenia in the Chinese population. Schizophr Res. 2005;73(2–3):269–274. doi: 10.1016/j.schres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Church RM. The internal clock. In: Hulse SH, Fowler H, Honig WK, editors. Cognitive processes in animal behavior. Hillsdale, NJ: Erlbaum; 1978. pp. 277–310. [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36(1):3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s wrong with my mouse? Behavioral phenotyping of transgenic and knockout mice. 2. Hoboken, NJ: 2007. [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, … Young WS. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41(3):145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Delerue C, Boucart M. The relationship between visual object exploration and action processing in schizophrenia. [Research Support, Non-U.S. Gov’t] Cogn Neuropsychiatry. 2012a;17(4):334–350. doi: 10.1080/13546805.2011.646886. [DOI] [PubMed] [Google Scholar]
- Delerue C, Boucart M. Visual exploration and action processing in schizophrenia. Cogn Neuropsychiatry. 2012b doi: 10.1080/13546805.2012.683245. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, … Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27(29):7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. Memory on time. Trends Cogn Sci. 2013;17(2):81–88. doi: 10.1016/j.tics.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallistel CR. The organization of behavior. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gibbon J, Church RM, Meck WH. Scalar timing in memory. Annals of the New York Academy of Sciences. 1984;423:52–77. doi: 10.1111/j.1749-6632.1984.tb23417.x. [DOI] [PubMed] [Google Scholar]
- Glenn MJ, Gibson EM, Kirby ED, Mellott TJ, Blusztajn JK, Williams CL. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. [Research Support, N.I.H., Extramural] Eur J Neurosci. 2007;25(8):2473–2482. doi: 10.1111/j.1460-9568.2007.05505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: timing is of the essence. Arch Gen Psychiatry. 1999;56(11):1007–1008. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Skimminge A, Aggernaes B, Ebdrup BH, Glenthoj B, Baare W. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. [Research Support, Non-U.S. Gov’t] J Psychiatry Neurosci. 2013;38(1):34–42. doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog MH, Brand A. Pitting temporal against spatial integration in schizophrenic patients. Psychiatry Res. 2009;168(1):1–10. doi: 10.1016/j.psychres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029(1):131–134. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Kimura B. Disturbance of timing and selfhood in schizophrenia. Seishin Shinkeigaku Zasshi. 2003;105(6):729–732. [PubMed] [Google Scholar]
- Leshchyns’ka I, Sytnyk V, Richter M, Andreyeva A, Puchkov D, Schachner M. The adhesion molecule CHL1 regulates uncoating of clathrin-coated synaptic vesicles. Neuron. 2006;52(6):1011–1025. doi: 10.1016/j.neuron.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Malapani C, Deweer B, Gibbon J. Separating storage from retrieval dysfunction of temporal memory in Parkinson’s disease. Journal of Cognitive Neuroscience. 2002;14(2):311–322. doi: 10.1162/089892902317236920. [DOI] [PubMed] [Google Scholar]
- Malapani C, Rakitin B, Levy R, Meck WH, Deweer B, Dubois B, Gibbon J. Coupled temporal memories in Parkinson’s disease: a dopamine-related dysfunction. Journal of Cognitive Neuroscience. 1998;10(3):316–331. doi: 10.1162/089892998562762. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH. Cortico-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cognitive Brain Research. 2004;21(2):139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Matell MS, Meck WH, Nicolelis MA. Interval timing and the encoding of signal duration by ensembles of cortical and striatal neurons. Behavioral Neuroscience. 2003;117:760–773. doi: 10.1037/0735-7044.117.4.760. [DOI] [PubMed] [Google Scholar]
- McDowell JE, Clementz BA, Wixted JT. Timing and amplitude of saccades during predictive saccadic tracking in schizophrenia. Psychophysiology. 1996;33(1):93–101. doi: 10.1111/j.1469-8986.1996.tb02112.x. [DOI] [PubMed] [Google Scholar]
- Meck WH. Hippocampal function is required for feedback control of an internal clock’s criterion. Behavioral Neuroscience. 1988;1102:54–60. doi: 10.1037//0735-7044.102.1.54. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cognitive Brain Research. 1996;3(3–4):227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH. Interval timing and genomics: What makes mutant mice tick? International Journal of Comparative Psychology. 2001;14:211–231. [Google Scholar]
- Meck WH, Cheng RK, MacDonald CJ, Gainetdinov RR, Caron MG, Cevik MO. Gene-dose dependent effects of methamphetamine on interval timing in dopamine-transporter knockout mice. [Research Support, Non-U.S. Gov’t] Neuropharmacology. 2012;62(3):1221–1229. doi: 10.1016/j.neuropharm.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behavioral Neuroscience. 1984;98(1):3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- Meck WH, Williams CL, Cermak JM, Blusztajn JK. Developmental periods of choline sensitivity provide an ontogenetic mechanism for regulating memory capacity and age-related dementia. Front Integr Neurosci. 2007;1:7. doi: 10.3389/neuro.07.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellott TJ, Williams CL, Meck WH, Blusztajn JK. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004;18(3):545–547. doi: 10.1096/fj.03-0877fje. [DOI] [PubMed] [Google Scholar]
- Mohler EG. PhD. Duke University; Durham, NC: 2002. Nutritional and genetic influences on behavioral phenotype: Prenatal choline supplementation and apolipoprotein E deficiency. (Doctoral dissertation) Retrieved from Dissertations and Theses database ProQuest, UMI No. 3066568). database. [Google Scholar]
- Montag-Sallaz M, Baarke A, Montag D. Aberrant neuronal connectivity in CHL1-deficient mice is associated with altered information processing-related immediate early gene expression. J Neurobiol. 2003;57(1):67–80. doi: 10.1002/neu.10254. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Mol Cell Biol. 2002;22(22):7967–7981. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morellini F, Lepsveridze E, Kahler B, Dityatev A, Schachner M. Reduced reactivity to novelty, impaired social behavior, and enhanced basal synaptic excitatory activity in perforant path projections to the dentate gyrus in young adult mice deficient in the neural cell adhesion molecule CHL1. Mol Cell Neurosci. 2007;34(2):121–136. doi: 10.1016/j.mcn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Nikonenko AG, Sun M, Lepsveridze E, Apostolova I, Petrova I, Irintchev A, … Schachner M. Enhanced perisomatic inhibition and impaired long-term potentiation in the CA1 region of juvenile CHL1-deficient mice. Eur J Neurosci. 2006;23(7):1839–1852. doi: 10.1111/j.1460-9568.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- Olton DS, Meck WH, Church RM. Separation of hippocampal and amygdaloid involvement in temporal memory dysfunctions. Brain Research. 1987;404:180–188. doi: 10.1016/0006-8993(87)91369-2. [DOI] [PubMed] [Google Scholar]
- Oprisan SA, Buhusi CV. Modeling pharmacological clock and memory patterns of interval timing in a striatal beat-frequency model with realistic, noisy neurons. Front Integr Neurosci. 2011;5:52. doi: 10.3389/fnint.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papageorgiou C, Karanasiou IS, Kapsali F, Stachtea X, Kyprianou M, Tsianaka EI, … Papadimitriou GN. Temporal processing dysfunction in schizophrenia as measured by time interval discrimination and tempo reproduction tasks. Prog Neuropsychopharmacol Biol Psychiatry. 2013;40:173–179. doi: 10.1016/j.pnpbp.2012.07.017. [DOI] [PubMed] [Google Scholar]
- Paulsen JS, Zimbelman JL, Hinton SC, Langbehn DR, Leveroni CL, Benjamin ML, … Rao SM. fMRI Biomarker of Early Neuronal Dysfunction in Presymptomatic Huntington’s Disease. American Journal of Neuroradiology. 2004;25(10):1715–1721. [PMC free article] [PubMed] [Google Scholar]
- Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58(1):109–118. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Radonovich KJ, Mostofsky SH. Duration judgments in children with ADHD suggest deficient utilization of temporal information rather than general impairment in timing. Child Neuropsychol. 2004;10(3):162–172. doi: 10.1080/09297040409609807. [DOI] [PubMed] [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:318–337. [Google Scholar]
- Ross RG, Hunter SK, McCarthy L, Beuler J, Hutchison AK, Wagner BD, … Freedman R. Perinatal Choline Effects on Neonatal Pathophysiology Related to Later Schizophrenia Risk. Am J Psychiatry. 2013 doi: 10.1176/appi.ajp.2012.12070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Lassalle JM. Radial maze learning using exclusively distant visual cues reveals learners and nonlearners among inbred mouse strains. Physiol Behav. 1995;58(6):1189–1195. doi: 10.1016/0031-9384(95)02066-7. [DOI] [PubMed] [Google Scholar]
- Sakurai K, Migita O, Toru M, Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Mol Psychiatry. 2002;7(4):412–415. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behav Neurosci. 2001;115(2):384–393. [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14(9):597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam GW, van de Lagemaat LN, Redon R, Strathdee KE, Croning MD, Malloy MP, … Grant SG. Confirmed rare copy number variants implicate novel genes in schizophrenia. Biochem Soc Trans. 2010;38(2):445–451. doi: 10.1042/BST0380445. [DOI] [PubMed] [Google Scholar]
- Turgeon M, Giersch A, Delevoye-Turrell Y, Wing AM. Impaired predictive timing with spared time interval production in individual with schizophrenia. Psychiatry Res. 2012;197(1–2):13–18. doi: 10.1016/j.psychres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Velasques B, Machado S, Paes F, Cunha M, Sanfim A, Budde H, … Ribeiro P. Sensorimotor integration and psychopathology: motor control abnormalities related to psychiatric disorders. World J Biol Psychiatry. 2011;12(8):560–573. doi: 10.3109/15622975.2010.551405. [DOI] [PubMed] [Google Scholar]
- Volz HP, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12(2):313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Wang ZR, Tan YL, Yang FD, Zhang WF, Zou YZ, Tan SP, … Zhou DF. Impaired prepulse inhibition of acoustic startle in Chinese patients with first-episode, medication-naive schizophrenia. Chin Med J (Engl) 2013;126(3):526–531. [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2012;62(3):1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36(4):713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Goodrich SJ, Glenn MJ, Mellott TJ, Liu YB, Blusztajn JK, Williams CL. Water maze experience and prenatal choline supplementation differentially promote long-term hippocampal recovery from seizures in adulthood. Hippocampus. 2011;21(6):584–608. doi: 10.1002/hipo.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Troger AB. Exploring the 4th dimension: hippocampus, time, and memory revisited. Front Integr Neurosci. 2011;5:36. doi: 10.3389/fnint.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]