Abstract
Up to 5% of the US population has suffered anaphylaxis. Fatal outcome is rare, such that even for people with known venom or food allergy, fatal anaphylaxis constitutes less than 1% of total mortality risk. The incidence of fatal anaphylaxis has not increased in line with hospital admissions for anaphylaxis. Fatal drug anaphylaxis may be increasing, but rates of fatal anaphylaxis to venom and food are stable. Risk factors for fatal anaphylaxis vary according to cause. For fatal drug anaphylaxis, previous cardiovascular morbidity and older age are risk factors, with beta-lactam antibiotics, general anesthetic agents, and radiocontrast injections the commonest triggers. Fatal food anaphylaxis most commonly occurs during the second and third decades. Delayed epinephrine administration is a risk factor; common triggers are nuts, seafood, and in children, milk. For fatal venom anaphylaxis, risk factors include middle age, male sex, white race, cardiovascular disease, and possibly mastocytosis; insect triggers vary by region. Upright posture is a feature of fatal anaphylaxis to both food and venom. The rarity of fatal anaphylaxis and the significant quality of life impact of allergic conditions suggest that quality of life impairment should be a key consideration when making treatment decisions in patients at risk for anaphylaxis.
Key words: Anaphylaxis, Mortality, Insect sting, Food allergy, Drug allergy
Abbreviation used: ICD, International Classification of Diseases
Information for Category 1 CME Credit.
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the following instructions.
Method of Physician Participation in Learning Process: The core material for these activities can be read in this issue of the Journal or online at the JACI: In Practice Web site: www.jaci-inpractice.org/. The accompanying tests may only be submitted online at www.jaci-inpractice.org/. Fax or other copies will not be accepted.
Date of Original Release: September 1, 2017. Credit may be obtained for these courses until August 31, 2018.
Copyright Statement: Copyright © 2017-2019. All rights reserved.
Overall Purpose/Goal: To provide excellent reviews on key aspects of allergic disease to those who research, treat, or manage allergic disease.
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates this journal-based CME activity for 1.00 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Paul J. Turner, MD, PhD, Elina Jerschow, MD, Thisanayagam Umasunthar, MD, Robert Lin, MD, Dianne E. Campbell, MD, PhD, and Robert J. Boyle, MB, ChB, PhD (authors); Scott H. Sicherer, MD (editor)
Learning objectives:
-
1.
To communicate risk estimates for fatal or near-fatal anaphylaxis to patients and caregivers.
-
2.
To evaluate a patient's risk for fatal or near-fatal anaphylaxis.
-
3.
To discuss the uncertainties in understanding fatal anaphylaxis.
Recognition of Commercial Support: This CME has not received external commercial support.
Disclosure of Relevant Financial Relationships with Commercial Interests: P. J. Turner has received research support from the Medical Research Council, NIHR/Imperial BRC, and EU FP7 Programme; and has received consultancy fees from UK Food Standards Agency. T. Umasunthar has received research support from Lincoln Medical. D. E. Campbell is employed by NSW Health; has received research support from the National Health and Medical Research Council, Australian Food Allergy Foundation, and the Allergy and Immunology Foundation of Australasia; and has received travel support from DBV. R. J. Boyle has received consultancy fees from Oval Technoloties and ALK Abello; and has provided expert testimony for Squitieri and Fearon. The rest of the authors declare that they have no relevant conflicts of interest. S. H. Sicherer disclosed no relevant financial relationships.
Between 1.6% and 5.1% of US citizens are estimated to have experienced anaphylaxis,1 a systemic hypersensitivity reaction that can be rapidly fatal. An estimated, 1% of hospitalizations and 0.1% of emergency department attendances for anaphylaxis have a fatal outcome.2 Groups at risk of anaphylaxis include those with IgE-mediated food allergy (approximately 5% to 8% of US children and 2% to 3% of adults) and those with IgE-mediated drug or insect venom allergy.3, 4 For these at-risk groups, the unpredictable possibility of fatal anaphylaxis can lead to significant anxiety and restriction of daily activities. The aim of this review is to provide clinicians with information that can be used to identify and counsel those individuals at risk of fatal anaphylaxis. We review the incidence and time trends of fatal anaphylaxis due to the 3 main causes (drugs, food, and insect venom) from recent studies and summarize risk factors for fatal anaphylaxis associated with these triggers.
Fatal Drug Anaphylaxis
Epidemiology
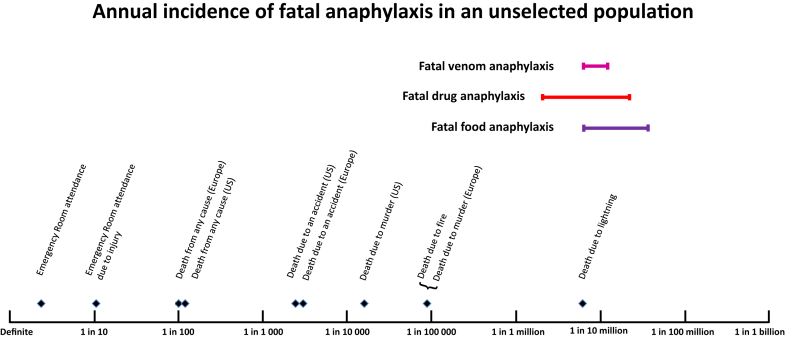
Drugs are the most common reported cause of fatal anaphylaxis in several countries, including Australia, New Zealand, United Kingdom, Brazil, and United States.5, 6, 7, 8, 9, 10 Recent epidemiological data are summarized in Table I. Rates of fatal drug-induced anaphylaxis estimated from national death certification data,15 or defined anaphylaxis registries,14, 16, 17 show inconsistent evidence of increasing incidence, in contrast to other causes of fatal anaphylaxis. In the United States, using International Classification of Diseases-10 (ICD-10) categorization, the estimated fatal drug anaphylaxis rate increased significantly from 0.27 per million population in 1999-2001 to 0.51 per million population in 2008-2010.6 The year 1999 was the first year when ICD-10 codes were used to record deaths in the US National Mortality database, raising the possibility of a code shift underlying the reporting increase.6 A significant increase was also noted in an Australian ICD-10-based report, between 1997 and 2005,7 and an overall rate of increase of 5.6% per year over the period 1997-2013.18 In contrast, an increase has not been reported in the United Kingdom, according to data from a national fatal anaphylaxis registry.14 Problems with current ICD-10-based anaphylaxis mortality coding have been recently detailed.15 Fig 1 shows the range of estimates for fatal drug anaphylaxis incidence. The risk of fatal drug anaphylaxis is seen to be low compared with other population mortality risks.
Table I.
Population-based data for rate of fatal anaphylaxis triggered by drugs
| Region | Data Source | Time period | Total deaths | Rate of fatal drug anaphylaxis (per million/year) | Age | Gender predominance | Leading causal drugs | Risk factors identified | Authors |
|---|---|---|---|---|---|---|---|---|---|
| Australia | Australian Bureau of Statistics and National Coroners Information System | 1997-2013 | 147 cases in total 84 (57%) triggered by drugs ICD code T88.6 |
1997: 0.05 2013: 0.13 |
Median 66 (IQR 52-73; range 26-94) | Male > female | Antibiotics 43% General anesthetic 35% Radiocontrast 18% |
Age Cardiovascular disease 71% Known penicillin allergy 11% (33% of beta-lactam fatalities) |
Mullins et al 201611 |
| Canada (Ontario) | Ontario Coroner's database | 1986-2011 | 92 total 16 (17%) drugs Coroner reports searched; ICD codes not used |
0.1 | Mean 65 (range 39-86) | 38% male | Antibiotics 44% Radiocontrast 25% |
Age Known allergy to the drug in 1 of 5 cases with data available (20%) |
Xu et al 201412 |
| France | French National Pharmacovigilance Database∗ | 2000-2011 | 84 (0.04% of total anaphylaxis cases) Pharmacovigilance Database | Not calculated | Mean age 59 | Male > female | Not stated | Male gender Hypertension and cardiovascular comorbidities Obesity Beta-blocker use |
Reitter et al 201413 |
| United Kingdom | National fatal anaphylaxis registry | 1992-2012 | 479 total 263 drugs (55% of total) ICD code T88.6 |
1992: 0.24 2012: 0.24 |
Median 58 (range 56-61) | Not stated | Not stated | Older age | Turner et al 201514 |
| United States | National Center for Health Statistics MCDD | 1999-2010 | 2458 total 1446 (59% of total) ICD codes T78.2 or T88.6 |
1999: 0.27 2010: 0.51 |
Median 60 (IQR 47-73) | None | Antibiotics (mostly beta-lactams) Contrast agents Antineoplastic drugs |
African American ethnicity Older age |
Jerschow et al 20146 |
ICD, International Classification of Diseases; IQR, interquartile range; MCDD, National Center for Health Statistics' Multiple Cause of Death Data.
Reported data were only on neuromuscular blocking agents.
Figure 1.
Estimated rates of fatal drug, food, and venom anaphylaxis compared with other risks for the general population. Reference risks are for the US population, unless otherwise stated. Bars represent the range of estimates from recent population-based studies of fatal anaphylaxis.
Not all drug anaphylaxis studies report fatalities, and it is unclear whether the drugs causing nonfatal anaphylaxis are the same as those causing fatal or near-fatal anaphylaxis. Hospitalization could be viewed as a marker of anaphylaxis severity, but fatal drug anaphylaxis events may occur because of in-patient use of medication rather than as a consequence of drug-induced anaphylaxis in the community; and even for hospitalized anaphylaxis, fatalities are uncommon.14, 19, 20 In Australia, the ratio of deaths to hospitalizations relating to non-food anaphylaxis was 11:1000.7 The precise proportion of drug anaphylaxis that results in a fatal outcome is not known. In Denmark, the 30-day mortality of patients admitted for anaphylactic shock (any cause) was less than 1%, and vasopressor use or mechanical ventilation was reported in less than 3% of admissions.20 The latter interventions can be considered as evidence for severe anaphylaxis21 (and possibly “near-fatal” anaphylaxis, but there is no consensus over these definitions).
The causative agent in drug-induced anaphylaxis may differ by country and method of data collection. Antibiotics (predominantly penicillins and cephalosporins)6, 22, 23, 24 are often the most common drugs associated with fatal drug anaphylaxis, although in the United Kingdom general anesthetics are the most common identified group, of which neuromuscular blocking agents are the leading trigger.10, 14 Radiocontrast agents are the leading cause in at least one hospital-based study in South Korea, and also feature prominently in recent studies from Australia and Canada.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 Similarly, a recent US report found that radiocontrast agents caused more fatal drug anaphylaxis than penicillin and cephalosporins combined.6 This suggested that radiocontrast administration may carry a relatively high “per injection” fatality risk compared with these frequently used antibiotics.36 Although nonsteroidal anti-inflammatory drugs are frequently associated with anaphylaxis, they do not appear to be a common trigger of fatal anaphylaxis.18
Risk factors
Older age has been consistently associated with higher fatal drug anaphylaxis rates.6, 7, 14 In the United Kingdom, the mean age for fatal drug anaphylaxis was 58 years,14 and in Australia, most drug anaphylactic fatalities occurred between 55 and 85 years.7 This may be related to increased prevalence of drug allergy due to increased drug exposure, and increased cardiovascular vulnerability, in older age groups. No consistent gender predilection has been noted in studies on drug-associated anaphylaxis; however in the United States, a significant association with African American ethnicity has been noted.6 The role of comorbidities as a purported risk for fatal drug anaphylaxis has not been supported in many studies, and such morbidities are of course common in older people. However, one recent study reported 71% of fatal drug anaphylaxis occurred in people with known cardiovascular disease, and 39% in those with known asthma or emphysema.11 In a French study of neuromuscular blocker-associated severe anaphylaxis (N = 1247), male gender, hypertension, cardiovascular disease, obesity, and beta-blocker use were all associated with fatal outcome, and respiratory disorders were not associated with fatal outcome.13 Neuromuscular blocking agent anaphylaxis may be promoted by cross-sensitization induced by the use of a cough medicine, pholcodine.37, 38, 39, 40, 41 This hypothesis is supported by the reduction of general anesthetic anaphylaxis in Norway after pholcodine was removed from the market.39 Although antihypertensive drugs are considered risk factors for severe anaphylaxis,42, 43, 44 this class of drugs was not prominent among confirmed fatal drug anaphylaxis cases.6, 11, 14
A small but significant number of fatalities occur due to a drug administration error, that is, the patient was already known to be allergic to the relevant drug, or a closely related drug.45 This was most clearly reported in Australia, where 9 of 27 cases of fatal penicillin or cephalosporin anaphylaxis were known to be penicillin-allergic.11
Practical implications of fatal drug anaphylaxis data
-
•
Drug-induced anaphylaxis is the most common cause of fatal anaphylaxis in most regions where data are available, but is rare relative to nonanaphylactic causes of mortality.
-
•
The incidence of fatal drug anaphylaxis may be increasing, in contrast to other causes of fatal anaphylaxis.
-
•
People older than 50 years with pre-existing cardiovascular morbidity appear to be at highest risk for fatal drug anaphylaxis, and drug administration errors account for a significant proportion of cases.
-
•
Beta-lactam antibiotics, muscle relaxants given at general anesthesia, and injected radiocontrast medium are the commonest reported triggers of fatal drug anaphylaxis.
Fatal Food Anaphylaxis
Epidemiology
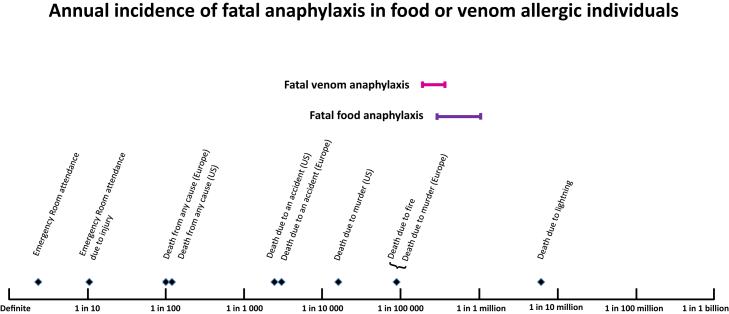
Despite consistent reports of increased incidence in nonfatal food anaphylaxis over recent decades, a parallel increase in fatalities has not, in general, been reported,2, 6, 11, 12, 14 with the exception of one recent Australian study.18 Recent epidemiological data are summarized in Table II. There are unexplained regional variations, with United Kingdom and Australia reporting almost double the rate of fatal, food-related anaphylaxis to that in the United States. Overall, although food-related anaphylaxis is relatively common, fatalities remain rare with a reported range of approximately 0.03 to 0.3 deaths per million person years in the general population (Fig 1). Case fatality rate is up to 1%, for medically coded food anaphylaxis, but varies significantly according to the definition of anaphylaxis used.46, 47 The estimated incidence of fatal food anaphylaxis for an individual with food allergy (Fig 2) is low and adds little to overall mortality risk.46 This low level of risk may nevertheless be important for individuals with food allergy and their carers.49
Table II.
Population-based data for rate of fatal anaphylaxis triggered by food
| Region | Data Source | Time period | Total deaths | Rate of fatal food anaphylaxis (per million/year) | Age | Gender predominance | Leading causal foods | Risk factors identified | Authors |
|---|---|---|---|---|---|---|---|---|---|
| Australia | Australian Bureau of Statistics and National Coronial Information System (NCIS) | 1997-2013 | 324 (119 with known cause) 23 (19%) food ICD codes 995.6, T78 |
1997: 0 2014: 0.09 |
Median 28 (range 4-66) | No | Seafood 50% Nuts 32% |
Known food allergy 91% Asthma 68% Alcohol or recreational drugs 27% Upright posture 68% Delayed use of epinephrine |
Mullins et al 201611 |
| Canada (Ontario) | Ontario Coroner's database | 1986-2011 | 92 total 40 (43%) food Coroner reports searched; ICD codes not used |
1986: 0.32 2011: 0.08 |
Mean 32 (range 9-78) | No | Peanut | Delayed use of epinephrine Known allergy to the culprit food in all 34 cases where this information was available (100%) |
Xu et al 201412 |
| United Kingdom | National fatal anaphylaxis registry | 1992-2008 | 479 total 124 (26%) food ICD codes T78, and registry |
1992: 0.10 2012: 0.12 |
Mean 25 Median 20 (range 4-85) |
Male (under 15 y) Female (over 15 y) |
Peanut or Tree nut 73% |
Known food allergy 69% Asthma 78% Change in posture |
Turner et al 201514 |
| United States | 3 national databases (NIS, NEDS, MCDD) | 1999-2009 | 2229 total approximately 122 (5%) food ICD codes 995.6, T78 |
1999: 0.03 2009: 0.04 |
Not stated | Not stated | Not stated | Not stated | Ma et al 20142 |
| United States | National Center for Health Statistics MCDD | 1999-2010 | 2458 total 164 (7%) food ICD codes T78 |
0.05 | Median 40 (IQR 20-60) | Male > female | Not stated | African American ethnicity | Jerschow et al 20146 |
ICD, International Classification of Diseases; IQR, interquartile range; MCDD, National Center for Health Statistics' Multiple Cause of Death Data; NCIS, Australian National Coronial Information System; NEDS, Nationwide Emergency Department Sample, from the Healthcare Cost and Utilization Project; NIS, Nationwide Inpatient Sample, from the Healthcare Cost and Utilization Project.
Figure 2.
Estimated rates of fatal food and venom anaphylaxis for people with known food allergy or insect venom allergy. Reference risks are for the US population, unless otherwise stated. Data shown for individuals with food allergy are the 95% confidence interval of fatal food anaphylaxis risk, derived from the systematic review of Umasunthar et al.46 Data shown for individuals with insect venom allergy were calculated using the range of estimates from recent population-based studies of fatal venom anaphylaxis, and an estimated 3% population prevalence of insect venom allergy.48
Regional variations are also seen in the precise triggers responsible: peanut and tree nuts are the most common reported triggers in most series; however, recent data suggest that seafood is a more common cause in Australia.11 In children, cow's milk is one of the most common causes in the United Kingdom, perhaps due to its ubiquitous role in the diet.14 Despite egg being the most common food allergy in young children in Australia,50 United Kingdom,51 and possibly the United States, it is under-represented in documented anaphylaxis fatalities.
Risk factors—food
Several risk factors or “coassociations” reported in fatal food anaphylaxis series are specific to food-triggered cases (Table I, Table II, Table III). Risk factors are often identified by individual case reviews, but variable recording of mortality data, and the absence of suitable controls limits the ability to reliably distinguish associations from risk factors, and thus stratify food-allergic individuals according to risk. Although infants and young children have the highest reported rates of food-related anaphylaxis and subsequent hospitalization, fatal food anaphylaxis in this age group is very rare indeed.2, 6, 11, 14 Overall, there appears to be an age-related predisposition to fatal outcomes in the second and third decade in some but not all studies, which is currently unexplained, and is specific to fatal food anaphylaxis. Most fatal food anaphylaxis occurs in people with known food allergy, but in many cases prior reactions were not severe.14 This may partly be because initial reactions usually occur during the first decade, when reaction severity appears to be lower than in the second and third decades. The delayed use of epinephrine, identified as a significant feature in several reports of fatal food anaphylaxis,11, 12, 52, 53, 54 is perhaps the risk factor most amenable to modification. This has, in part, driven the widespread provision of epinephrine autoinjectors for the management of anaphylaxis, although controversy exists as to their use in less severe, nonanaphylactic allergic reactions.55 Although epinephrine is an essential treatment modality in anaphylaxis, there is no formal controlled trial evidence that epinephrine or epinephrine autoinjectors effectively prevent fatal outcome.56 Fatal reactions occur despite timely epinephrine administration,16 which may relate to the need for more intensive administration in severe reactions beyond that which can be administered by autoinjector devices.57, 58
Table III.
Population-based data for rate of fatal anaphylaxis triggered by insect venom
| Region | Data Source | Time period | Total deaths | Rate of fatal venom anaphylaxis (per million/year) | Age | Gender predominance | Leading causal insects | Risk factors identified | Authors |
|---|---|---|---|---|---|---|---|---|---|
| Australia | Australian Bureau of Statistics and National Coronial Information System (NCIS) | 1997-2013 | 324 (119 with known cause) 41 (13%) insect X23, X25 |
0.09 | Median 50 (range 19-79) | 90% male | Honeybee 73% Ants 9% Ticks 9% Wasp 6% |
Age Male sex Cardiovascular disease 45% Upright posture 30% Known venom allergy 48% Squeezing tick bites associated with death in all tick cases |
Mullins et al 201611 |
| Canada (Ontario) | Ontario Coroner's database | 1986-2011 | 92 total 30 (33%) insect Coroner reports searched; ICD codes not used. |
0.1 | Mean 54 (range 25 to 77) | 80% male | Not stated | Age Male sex Known venom allergy in 11 of 21 (52%) cases where this information was available |
Xu et al 201412 |
| United Kingdom | National fatal anaphylaxis registry | 1992-2008 | 479 total 92 (19%) insect X23 |
0.09 | Mean 59 (95% CI 56-63) | Not stated | Not stated | Not stated | Turner et al 201514 |
| United States | 3 national databases (NIS, NEDS, MCDD) | 1999-2009 | 2229 total 295 (13%) insect X23 |
0.09 | Not stated | Not stated | Not stated | Not stated | Ma et al 20142 |
| United States | National Center for Health Statistics MCDD | 1999-2010 | 2458 total 374 (15%) insect X23, X25, T63.4 |
0.17 in Southern states 0.11 to 0.13 in other areas |
Median 52 y | 80% male 88% white |
Not stated | Age White race Male sex |
Jerschow et al 20146 |
ICD, International Classification of Diseases; MCDD, National Center for Health Statistics' Multiple Cause of Death Data; NCIS, Australian National Coronial Information System; NEDS, Nationwide Emergency Department Sample, from the Healthcare Cost and Utilization Project; NIS, Nationwide Inpatient Sample, from the Healthcare Cost and Utilization Project.
Asthma is a well-documented feature in fatal food anaphylaxis series, affecting approximately 70% to 75% of fatalities in recent UK and Australian series.11, 14 Most fatal food anaphylaxis is associated with severe respiratory symptoms, with cardiovascular compromise thought to be secondary to respiratory failure.18 For example, acute dyspnea was noted in 64% of cases in one study.11 It therefore seems sensible to optimize asthma management in individuals at risk of food anaphylaxis. However, asthma is common in food-allergic individuals, and there are no good data to differentiate risk on the basis of asthma control. Indeed, in the UK registry, there is little evidence for an association with poor asthma control or worsening asthma symptoms leading up to the fatal event.
The presence of ethanol or recreational drugs and upright posture (eg, during assessment or while in transit to a health care facility) have been reported as potential risk factors in Australia, and the latter in the United Kingdom.11, 59 Both are biologically plausible: ethanol or recreational drugs may, through disinhibition, increase the likelihood of accidental allergen exposure, mask the early warning signs of anaphylaxis, or suppress physiological responses to hypotension.60 Ethanol may also increase absorption of food allergens through increased intestinal permeability, a mechanism that may also be relevant to the effects of exercise. Upright posture has been associated with both fatal food and fatal venom anaphylaxis, suggesting significant cardiovascular compromise in both cases.
Other proposed risk factors, although lacking consistent evidence, are race (increased risk in African Americans, and UK-resident South Asians),6, 14 allergy to multiple foods,61, 62 exercise, and intercurrent illness.60 Low serum platelet-activating factor acetyl hydrolase activity was associated with fatal outcome in acute samples analyzed in one study of peanut allergy.63 However, this finding has not be replicated elsewhere, and may reflect increased levels of platelet-activating factor release during severe reactions.
Practical implications of fatal food anaphylaxis data
-
•
Fatal food anaphylaxis is rare, such that it adds little to overall mortality risk, even in young people known to have food allergy.
-
•
Reliable identification of patients at increased risk of fatal food anaphylaxis is not currently possible, but patients with isolated egg allergy or no asthma appear to be at lowest risk, and risk is highest in the second and third decades of life.
-
•
Features of food anaphylaxis and its management associated with fatal outcome are upright posture and delayed use of epinephrine.
-
•
Given the rarity of fatal food anaphylaxis, our inability to reliably stratify risk, and the limited evidence that specific interventions reduce fatality risk—quality of life considerations should play a key role in driving treatment decisions for people with food allergy.
Fatal Venom Anaphylaxis
Epidemiology
In common with food- and drug-induced anaphylaxis, hospital admission rates for venom anaphylaxis have increased over the last decade in most regions where data are available. There was an overall 12% increase per annum in the United Kingdom between 1998 and 2012,14 and data from Rochester, Minnesota, showed a significant 59% increase in emergency department visits for venom anaphylaxis between 2005 and 2014.64 Overall insect stings account for 10% to 20% of anaphylaxis in these and other studies,35, 65 but up to 50% in a European registry of severe anaphylaxis.17 Population rates of fatal insect venom anaphylaxis in recent studies from 4 geographic regions6, 11, 12, 14 are summarized in Table III. A consistent finding is that fatal insect venom anaphylaxis occurs at a rate of approximately 0.1 cases per million population in Australia, Canada (Ontario), United Kingdom, and United States. Some geographic variation was noted in the United States, with a higher rate in Southern states,6 something that was not reported for fatal food or drug anaphylaxis. Another consistent finding across these studies is the absence of a significant change in the rate of fatal venom anaphylaxis over time, despite the increases in emergency department attendance and hospitalizations noted above. This is consistent with an earlier report of stable fatal venom anaphylaxis rates from the 1960s to the 1980s in the United States.66 The estimated incidence of fatal venom anaphylaxis for an individual with venom allergy (Fig 2) is low and adds little to overall mortality risk. However, this risk may be higher for specific groups, as discussed below. Mullins et al11 reported honeybee to be the dominant cause of fatal insect venom anaphylaxis, although this may relate to the relatively high prevalence of allergy to this insect in Australia. Wasps were the commonest cause of fatal venom anaphylaxis in the United Kingdom,10 and in a large European registry of nonfatal anaphylaxis.17
Risk factors—venom
Key risk factors from recent studies of fatal venom anaphylaxis, including a total of 535 cases, are summarized in Table III. Consistent findings are that fatal insect venom allergy is a disease of adult males, with 80% to 90% of cases occurring in men, at an average age of 50 to 60 years. Two studies reported that only half of cases occur in people known to have had a prior systemic allergic reaction to the same insect. This may limit the impact that venom immunotherapy can have on fatal venom anaphylaxis rates. White race appears to be a risk factor for fatal venom anaphylaxis in the United States. In Australia, upright posture and pre-existing cardiovascular disease were cited as common features. For anaphylaxis triggered by tick bites, as opposed to insect venom, squeezing ticks for removal was a common feature. This has led to Australian recommendations to freeze ticks with an ether-containing spray, rather than remove them by squeezing. Cardiovascular disease is thought to be an important risk factor for fatal venom anaphylaxis, and consistent with this postural changes have been reported in fatal cases.14, 67
Other cofactors such as exercise, alcohol, nonsteroidal anti-inflammatory drugs, acute infections, stress, and perimenstrual status are thought to increase the risk for anaphylaxis and severe anaphylaxis in general. Little direct evidence for these risk factors is available in relation to insect venom anaphylaxis, and alcohol use was not found to be a risk factor in one study.14 Mastocytosis is associated with nonfatal insect venom anaphylaxis,68 and has been associated with a specific clinical presentation of hypotensive anaphylaxis in the absence of skin symptoms.69 Although there is particular interest in the relationship between systemic mastocytosis and reaction severity, systemic mastocytosis has only been specifically identified as a risk factor for fatal venom anaphylaxis in case reports.70 Low platelet-activating factor acetyl hydrolase has been associated with increased risk,71 but these data require further validation.
A health economic analysis undertaken for the UK National Institute for Health and Care Excellence found that a highly effective treatment for venom allergy, subcutaneous venom immunotherapy, was only cost effective if quality of life improvement occurred, or in specific high-risk groups with frequent stings and frequent reactions, such as beekeepers.72, 73, 74 This was due to the rarity of costly outcomes such as death or disability in patients with known venom allergy. Disability is not widely reported as an outcome of venom anaphylaxis, but anecdotal evidence suggests that persistent vegetative state after hypoxic encephalopathy in near-fatal venom anaphylaxis is a significant risk, and this may impact on health economic analyses. The UK health economic analysis does suggest that quality of life impact is an important factor to consider when making treatment decisions with venom allergic patients.75
Practical implications of fatal venom anaphylaxis data
-
•
The risk of fatal venom anaphylaxis for venom allergic individuals is low, approximately 3 to 6 cases per million person years.
-
•
Risk factors for fatal venom anaphylaxis are middle age, male sex, white race, pre-existing cardiovascular disease, and possibly specific immunological disorders such as mastocytosis.
-
•
Fatal venom anaphylaxis is associated with upright posture, and fatal tick bite anaphylaxis is associated with squeezing ticks for removal.
-
•
These risk factors should be considered, together with quality of life impairment, when making treatment decisions in venom allergic patients.
Conclusions
We have summarized key clinical indicators of increased risk for fatal anaphylaxis and highlighted information that might be used for stratifying risk and making treatment decisions in at-risk patients. Published reports of fatal anaphylaxis have generally been obtained from national registers of death certificate data, and these data are subject to underreporting, miscoding, and significant discrepancies in coronial notification and subsequent investigation of suspected fatal anaphylaxis. In some regions, death from food or insect anaphylaxis may be coded as due to “natural causes.” In most published datasets, a significant proportion of fatal anaphylaxis cases are classified as “unspecified cause.” Improved diagnostic codes for anaphylaxis, and the maintenance of fatal anaphylaxis registries, are important to ensure data quality. With these caveats in mind, the available data do suggest that fatal anaphylaxis is a very rare event, and although fatal drug anaphylaxis may be increasing, data do not consistently support a change in incidence of fatal food or venom anaphylaxis in recent years. This may in part be due to improved delivery of emergency medical care and increased availability of epinephrine autoinjectors limiting any potential increase in anaphylaxis fatalities.14 Risk factors for fatal anaphylaxis are mainly cause-specific, although increased age and cardiovascular comorbidity are common risk factors for fatal venom and drug anaphylaxis, and upright posture during anaphylaxis is a feature of fatal venom and food reactions. Further work should focus on improving our ability to identify those at risk and prevent fatal anaphylaxis, amongst populations with known allergy to drugs, food, and venom.
Footnotes
PJT is in receipt of a Clinician Scientist award funded by the UK Medical Research Council (reference MR/K010468/1). Both PJT and RJB are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) based at Imperial College Healthcare NHS Trust and Imperial College London. The views expressed are those of the author(s) and not necessarily those of the NHS, NIHR, or the Department of Health.
Conflicts of interest: P. J. Turner has received research support from the Medical Research Council, NIHR/Imperial BRC, and EU FP7 Programme; and has received consultancy fees from UK Food Standards Agency. T. Umasunthar has received research support from Lincoln Medical. D. E. Campbell is employed by NSW Health; has received research support from the National Health and Medical Research Council, Australian Food Allergy Foundation, and the Allergy and Immunology Foundation of Australasia; and has received travel support from DBV. R. J. Boyle has received consultancy fees from Oval Technoloties and ALK Abello; and has provided expert testimony for Squitieri and Fearon. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Wood R.A., Camargo C.A., Jr., Lieberman P., Sampson H.A., Schwartz L.B., Zitt M. Anaphylaxis in America: the prevalence and characteristics of anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:461–467. doi: 10.1016/j.jaci.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 2.Ma L., Danoff T.M., Borish L. Case fatality and population mortality associated with anaphylaxis in the United States. J Allergy Clin Immunol. 2014;133:1075–1083. doi: 10.1016/j.jaci.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rona R.J., Keil T., Summers C., Gislason D., Zuidmeer L., Sodergren E. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120:638–646. doi: 10.1016/j.jaci.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Branum A.M., Lukacs S.L. Food allergy among children in the United States. Pediatrics. 2009;124:1549–1555. doi: 10.1542/peds.2009-1210. [DOI] [PubMed] [Google Scholar]
- 5.Tanno L.K., Ganem F., Demoly P., Toscano C.M., Bierrenbach A.L. Undernotification of anaphylaxis deaths in Brazil due to difficult coding under the ICD-10. Allergy. 2012;67:783–789. doi: 10.1111/j.1398-9995.2012.02829.x. [DOI] [PubMed] [Google Scholar]
- 6.Jerschow E., Lin R.Y., Scaperotti M.M., McGinn A.P. Fatal anaphylaxis in the United States, 1999-2010: temporal patterns and demographic associations. J Allergy Clin Immunol. 2014;134:1318–1328.e1317. doi: 10.1016/j.jaci.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liew W.K., Williamson E., Tang M.L. Anaphylaxis fatalities and admissions in Australia. J Allergy Clin Immunol. 2009;123:434–442. doi: 10.1016/j.jaci.2008.10.049. [DOI] [PubMed] [Google Scholar]
- 8.Low I., Stables S. Anaphylactic deaths in Auckland, New Zealand: a review of coronial autopsies from 1985 to 2005. Pathology. 2006;38:328–332. doi: 10.1080/00313020600820831. [DOI] [PubMed] [Google Scholar]
- 9.Poulos L.M., Waters A.M., Correll P.K., Loblay R.H., Marks G.B. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993-1994 to 2004-2005. J Allergy Clin Immunol. 2007;120:878–884. doi: 10.1016/j.jaci.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Pumphrey R.S. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy. 2000;30:1144–1150. doi: 10.1046/j.1365-2222.2000.00864.x. [DOI] [PubMed] [Google Scholar]
- 11.Mullins R.J., Wainstein B.K., Barnes E.H., Liew W.K., Campbell D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin Exp Allergy. 2016;46:1099–1110. doi: 10.1111/cea.12748. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y.S., Kastner M., Harada L., Xu A., Salter J., Waserman S. Anaphylaxis-related deaths in Ontario: a retrospective review of cases from 1986 to 2011. Allergy Asthma Clin Immunol. 2014;10:38. doi: 10.1186/1710-1492-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reitter M., Petitpain N., Latarche C., Cottin J., Massy N., Demoly P. Fatal anaphylaxis with neuromuscular blocking agents: a risk factor and management analysis. Allergy. 2014;69:954–959. doi: 10.1111/all.12426. [DOI] [PubMed] [Google Scholar]
- 14.Turner P.J., Gowland M.H., Sharma V., Ierodiakonou D., Harper N., Garcez T. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992-2012. J Allergy Clin Immunol. 2015;135:956–963. doi: 10.1016/j.jaci.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanno L.K., Simons F.E.R., Annesi-Maesano I., Calderon M.A., Aymé S., Demoly P. Fatal anaphylaxis registries data support changes in the WHO anaphylaxis mortality coding rules. Orphanet J Rare Dis. 2017;12:8. doi: 10.1186/s13023-016-0554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grabenhenrich L.B., Dölle S., Moneret-Vautrin A., Köhli A., Lange L., Spindler T. Anaphylaxis in children and adolescents: the European Anaphylaxis Registry. J Allergy Clin Immunol. 2016;137:1128–1137.e1. doi: 10.1016/j.jaci.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Worm M., Moneret-Vautrin A., Scherer K., Lang R., Fernandez-Rivas M., Cardona V. First European data from the network of severe allergic reactions (NORA) Allergy. 2014;69:1397–1404. doi: 10.1111/all.12475. [DOI] [PubMed] [Google Scholar]
- 18.Turner P.J., Campbell D.E. Epidemiology of severe anaphylaxis: can we use population-based data to understand anaphylaxis? Curr Opin Allergy Clin Immunol. 2016;16:441–450. doi: 10.1097/ACI.0000000000000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin R.Y., Anderson A.S., Shah S.N., Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990-2006. Ann Allergy Asthma Immunol. 2008;101:387–393. doi: 10.1016/S1081-1206(10)60315-8. [DOI] [PubMed] [Google Scholar]
- 20.Jeppesen A.N., Christiansen C.F., Froslev T., Sorensen H.T. Hospitalization rates and prognosis of patients with anaphylactic shock in Denmark from 1995 through 2012. J Allergy Clin Immunol. 2016;137:1143–1147. doi: 10.1016/j.jaci.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Ring J., Messmer K. Incidence and severity of anaphylactoid reactions to colloid volume substitutes. Lancet. 1977;1:466–469. doi: 10.1016/s0140-6736(77)91953-5. [DOI] [PubMed] [Google Scholar]
- 22.Li Z.D., Liu W.G., Zhao Z.Q., Shen Y.W., Chen Y.J. Analysis of 59 anaphylactic death cases. Fa Yi Xue Za Zhi. 2015;31:206–210. [PubMed] [Google Scholar]
- 23.Shen Y., Li L., Grant J., Rubio A., Zhao Z., Zhang X. Anaphylactic deaths in Maryland (United States) and Shanghai (China): a review of forensic autopsy cases from 2004 to 2006. Forensic Sci Int. 2009;186:1–5. doi: 10.1016/j.forsciint.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Yilmaz R., Yuksekbas O., Erkol Z., Bulut E.R., Arslan M.N. Postmortem findings after anaphylactic reactions to drugs in Turkey. Am J Forensic Med Pathol. 2009;30:346–349. doi: 10.1097/PAF.0b013e3181c0e7bb. [DOI] [PubMed] [Google Scholar]
- 25.Gurrieri C., Weingarten T.N., Martin D.P., Babovic N., Narr B.J., Sprung J. Allergic reactions during anesthesia at a large United States referral center. Anesth Analg. 2011;113:1202–1212. doi: 10.1213/ANE.0b013e31822d45ac. [DOI] [PubMed] [Google Scholar]
- 26.Hitti E.A., Zaitoun F., Harmouche E., Saliba M., Mufarrij A. Acute allergic reactions in the emergency department: characteristics and management practices. Eur J Emerg Med. 2015;22:253–259. doi: 10.1097/MEJ.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 27.Jares E.J., Baena-Cagnani C.E., Sánchez-Borges M., Ensina L.F., Arias-Cruz A., Gómez M. Drug-induced anaphylaxis in Latin American Countries. J Allergy Clin Immunol Pract. 2015;3:780–788. doi: 10.1016/j.jaip.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlen J.L., Jr., Camargo C.A., Jr., Balekian D.S., Blumenthal K.G., Guyer A., Morris T. Antibiotics are the most commonly identified cause of perioperative hypersensitivity reactions. J Allergy Clin Immunol Pract. 2016;4:697–704. doi: 10.1016/j.jaip.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renaudin J.M., Beaudouin E., Ponvert C., Demoly P., Moneret-Vautrin D.A. Severe drug-induced anaphylaxis: analysis of 333 cases recorded by the Allergy Vigilance Network from 2002 to 2010. Allergy. 2013;68:929–937. doi: 10.1111/all.12168. [DOI] [PubMed] [Google Scholar]
- 30.Saff R.R., Camargo C.A., Jr., Clark S., Rudders S.A., Long A.A., Banerji A. Utility of ICD-9-CM codes for identification of allergic drug reactions. J Allergy Clin Immunol Pract. 2016;4:114–119.e111. doi: 10.1016/j.jaip.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Smit D.V., Cameron P.A., Rainer T.H. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–388. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 32.Tang R., Xu H.-Y., Cao J., Chen S., Sun J.-L., Hu H. Clinical characteristics of inpatients with anaphylaxis in China. Biomed Res Int. 2015;2015:429534. doi: 10.1155/2015/429534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Worm M., Eckermann O., Dölle S., Aberer W., Beyer K., Hawranek T. Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014;111:367–375. doi: 10.3238/arztebl.2014.0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang M.S., Lee S.H., Kim T.W., Kwon J.W., Lee S.M., Kim S.H. Epidemiologic and clinical features of anaphylaxis in Korea. Ann Allergy Asthma Immunol. 2008;100:31–36. doi: 10.1016/S1081-1206(10)60401-2. [DOI] [PubMed] [Google Scholar]
- 35.Ye Y.M., Kim M.K., Kang H.R., Kim T.B., Sohn S.W., Koh Y.I. Predictors of the severity and serious outcomes of anaphylaxis in Korean adults: a multicenter retrospective case study. Ann Allergy Asthma Immunol Res. 2015;7:22–29. doi: 10.4168/aair.2015.7.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheinfeld M.H., Sprayregen S., Jerschow E., Dym R.J. Contrast is the new penicillin, and possibly worse. J Am Coll Radiol. 2015;12:942–943. doi: 10.1016/j.jacr.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 37.Florvaag E., Johansson S.G. The pholcodine story. Immunol Allergy Clin North Am. 2009;29:419–427. doi: 10.1016/j.iac.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Florvaag E., Johansson S.G. Pholcodine in cough medicines and IgE-sensitization in the EU: an urgent task. Allergy. 2012;67:581–582. doi: 10.1111/j.1398-9995.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- 39.Florvaag E., Johansson S.G., Irgens A., de Pater G.H. IgE-sensitization to the cough suppressant pholcodine and the effects of its withdrawal from the Norwegian market. Allergy. 2011;66:955–960. doi: 10.1111/j.1398-9995.2010.02518.x. [DOI] [PubMed] [Google Scholar]
- 40.Johansson S.G., Florvaag E., Oman H., Poulsen L.K., Mertes P.M., Harper N.J. National pholcodine consumption and prevalence of IgE-sensitization: a multicentre study. Allergy. 2010;65:498–502. doi: 10.1111/j.1398-9995.2009.02193.x. [DOI] [PubMed] [Google Scholar]
- 41.Johansson S.G., Oman H., Nopp A., Florvaag E. Pholcodine caused anaphylaxis in Sweden 30 years ago. Allergy. 2009;64:820–821. doi: 10.1111/j.1398-9995.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- 42.Greenberger P.A. Fatal and near-fatal anaphylaxis: factors that can worsen or contribute to fatal outcomes. Immunol Allergy Clin North Am. 2015;35:375–386. doi: 10.1016/j.iac.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Lee S., Hess E.P., Nestler D.M., Bellamkonda Athmaram V.R., Bellolio M.F., Decker W.W. Antihypertensive medication use is associated with increased organ system involvement and hospitalization in emergency department patients with anaphylaxis. J Allergy Clin Immunol. 2013;131:1103–1108. doi: 10.1016/j.jaci.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Nassiri M., Babina M., Dolle S., Edenharter G., Rueff F., Worm M. Ramipril and metoprolol intake aggravate human and murine anaphylaxis: evidence for direct mast cell priming. J Allergy Clin Immunol. 2015;135:491–499. doi: 10.1016/j.jaci.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Corre K.A., Spielberg T.E. Adverse drug reaction processing in the United States and its dependence on physician reporting: zomepirac (Zomax) as a case in point. Ann Emerg Med. 1988;17:145–149. doi: 10.1016/s0196-0644(88)80300-7. [DOI] [PubMed] [Google Scholar]
- 46.Umasunthar T., Leonardi-Bee J., Hodes M., Turner P.J., Gore C., Habibi P. Incidence of fatal food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2013;43:1333–1341. doi: 10.1111/cea.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umasunthar T., Leonardi-Bee J., Turner P.J., Hodes M., Gore C., Warner J.O. Incidence of food anaphylaxis in people with food allergy: a systematic review and meta-analysis. Clin Exp Allergy. 2015;45:1621–1636. doi: 10.1111/cea.12477. [DOI] [PubMed] [Google Scholar]
- 48.Golden D.B. Anaphylaxis to insect stings. Immunol Allergy Clin North Am. 2015;35:287–302. doi: 10.1016/j.iac.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 49.Hu W., Grbich C., Kemp A. When doctors disagree: a qualitative study of doctors' and parents' views on the risks of childhood food allergy. Health Expect. 2008;11:208–219. doi: 10.1111/j.1369-7625.2008.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Osborne N.J., Koplin J.J., Martin P.E., Gurrin L.C., Lowe A.J., Matheson M.C. Prevalence of challenge-proven IgE-mediated food allergy using population-based sampling and predetermined challenge criteria in infants. J Allergy Clin Immunol. 2011;127:668–676.e1-2. doi: 10.1016/j.jaci.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 51.Perkin M.R., Logan K., Tseng A., Raji B., Ayis S., Peacock J. Randomized trial of introduction of allergenic foods in breast-fed infants. N Engl J Med. 2016;374:1733–1743. doi: 10.1056/NEJMoa1514210. [DOI] [PubMed] [Google Scholar]
- 52.Sampson H.A., Mendelson L., Rosen J.P. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327:380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 53.Bock S.A., Muñoz-Furlong A., Sampson H.A. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 54.Pumphrey R.S., Gowland M.H. Further fatal allergic reactions to food in the United Kingdom, 1999-2006. J Allergy Clin Immunol. 2007;119:1018–1019. doi: 10.1016/j.jaci.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Turner P.J., DunnGalvin A., Hourihane J.O. The emperor has no symptoms: the risks of a blanket approach to using epinephrine autoinjectors for all allergic reactions. J Allergy Clin Immunol Pract. 2016;4:1143–1146. doi: 10.1016/j.jaip.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sheikh A., Shehata Y.A., Brown S.G., Simons F.E. Adrenaline for the treatment of anaphylaxis: cochrane systematic review. Allergy. 2009;64:204–212. doi: 10.1111/j.1398-9995.2008.01926.x. [DOI] [PubMed] [Google Scholar]
- 57.Brown S.G. Anaphylaxis: clinical concepts and research priorities. Emerg Med Australas. 2006;18:155–169. doi: 10.1111/j.1742-6723.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 58.Smith P.L., Kagey-Sobotka A., Bleecker E.R., Traystman R., Kaplan A.P., Gralnick H. Physiologic manifestations of human anaphylaxis. J Clin Invest. 1980;66:1072–1080. doi: 10.1172/JCI109936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pumphrey R., Sturm G. Risk factors for fatal anaphylaxis. In: Moneret-Vautrin D.A., editor. Advances in Anaphylaxis Management. Future Medicine; London: 2014. pp. 32–48. [Google Scholar]
- 60.Turner P.J., Baumert J.L., Beyer K., Boyle R.J., Chan C.H., Clark A.T. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy. 2016;71:1241–1255. doi: 10.1111/all.12924. [DOI] [PubMed] [Google Scholar]
- 61.Mehr S., Turner P.J., Joshi P., Wong M., Campbell D.E. Safety and clinical predictors of reacting to extensively heated cow's milk challenge in cow's milk-allergic children. Ann Allergy Asthma Immunol. 2014;113:425–429. doi: 10.1016/j.anai.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 62.Turner P.J., Mehr S., Joshi P., Tan J., Wong M., Kakakios A. Safety of food challenges to extensively heated egg in egg-allergic children: a prospective cohort study. Pediatric Allergy Immunol. 2013;24:450–455. doi: 10.1111/pai.12093. [DOI] [PubMed] [Google Scholar]
- 63.Vadas P., Gold M., Perelman B., Liss G.M., Lack G., Blyth T. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 64.Motosue M.S., Bellolio M.F., Van Houten H.K., Shah N.D., Campbell R.L. Increasing emergency department visits for anaphylaxis, 2005-2014. J Allergy Clin Immunol Pract. 2017;5:171–175.e3. doi: 10.1016/j.jaip.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Manuyakorn W., Benjaponpitak S., Kamchaisatian W., Vilaiyuk S., Sasisakulporn C., Jotikasthira W. Pediatric anaphylaxis: triggers, clinical features, and treatment in a tertiary-care hospital. Asian Pac J Allergy Immunol. 2015;33:281–288. doi: 10.12932/AP0610.33.4.2015. [DOI] [PubMed] [Google Scholar]
- 66.Graft D.F. Insect sting allergy. Med Clin North Am. 2006;90:211–232. doi: 10.1016/j.mcna.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 67.Lieberman P., Simons F.E. Anaphylaxis and cardiovascular disease: therapeutic dilemmas. Clin Exp Allergy. 2015;45:1288–1295. doi: 10.1111/cea.12520. [DOI] [PubMed] [Google Scholar]
- 68.Alvarez-Twose I., Zanotti R., Gonzalez-de-Olano D., Bonadonna P., Vega A., Matito A. Nonaggressive systemic mastocytosis (SM) without skin lesions associated with insect-induced anaphylaxis shows unique features versus other indolent SM. J Allergy Clin Immunol. 2014;133:520–528. doi: 10.1016/j.jaci.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 69.Zanotti R., Lombardo C., Passalacqua G., Caimmi C., Bonifacio M., De Matteis D. Clonal mast cell disorders in patients with severe Hymenoptera venom allergy and normal serum tryptase levels. J Allergy Clin Immunol. 2015;136:135–139. doi: 10.1016/j.jaci.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 70.Vos B.J.P.R., van Anrooij B., van Doormaal J.J., Dubois A.E.J., Oude-Elberink J.N.G. Fatal anaphylaxis to yellow jacket stings in mastocytosis: options for identification and treatment of at risk patients. J Allergy Clin Immunol Pract. 2017;5:1264–1271. doi: 10.1016/j.jaip.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 71.Pravettoni V., Piantanida M., Primavesi L., Forti S., Pastorello E.A. Basal platelet-activating factor acetylhydrolase: prognostic marker of severe Hymenoptera venom anaphylaxis. J Allergy Clin Immunol. 2014;133:1218–1220. doi: 10.1016/j.jaci.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 72.Boyle R.J., Dickson R., Hockenhull J., Cherry M.G., Elremeli M. Immunotherapy for hymenoptera venom allergy: too expensive for European health care? Allergy. 2013;68:1341–1342. doi: 10.1111/all.12210. [DOI] [PubMed] [Google Scholar]
- 73.Boyle R.J., Elremeli M., Hockenhull J., Cherry M.G., Bulsara M.K., Daniels M. Venom immunotherapy for preventing allergic reactions to insect stings. Cochrane Database Syst Rev. 2012;10:CD008838. doi: 10.1002/14651858.CD008838.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hockenhull J., Elremeli M., Cherry M.G., Mahon J., Lai M., Darroch J. A systematic review of the clinical effectiveness and cost-effectiveness of Pharmalgen for the treatment of bee and wasp venom allergy. Health Technol Assess. 2012;16 doi: 10.3310/hta16120. III-IV,1-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oude Elberink J.N., De Monchy J.G., Van Der Heide S., Guyatt G.H., Dubois A.E. Venom immunotherapy improves health-related quality of life in patients allergic to yellow jacket venom. J Allergy Clin Immunol. 2002;110:174–182. doi: 10.1067/mai.2002.125827. [DOI] [PubMed] [Google Scholar]