Abstract
Methamphetamine (METH) is a potent and highly addictive central nervous system (CNS) stimulant. Additionally, METH adversely impacts immunological responses, which might contribute to the higher rate and more rapid progression of certain infections in drug abusers. However no studies have shown the impact of METH on inflammation within specific organs, cellular participation and cytokine production. Using a murine model of METH administration, we demonstrated that METH modifies, with variable degrees, leukocyte recruitment and alters cellular mediators in the lungs, liver, spleen and kidneys of mice. Our findings demonstrate the pleotropic effects of METH on the immune response within diverse tissues. These alterations have profound implications on tissue homeostasis and the capacity of the host to respond to diverse insults, including invading pathogens.
Keywords: cytokines, macrophages, methamphetamine, neutrophils, organs, T cells
INTRODUCTION
Methamphetamine (METH) is an extremely addictive central nervous system (CNS) stimulant. METH abuse is a significant public health problem for many communities throughout much of the United States. It is estimated that >12.3 million Americans older than 12 years of age have used METH at least once (Gonzales, Mooney et al. 2010). METH is self-administered intravenously, by nasal inhalation, anally, and orally, in doses of 250–500 mg by occasional users to as much as 1 g by chronic abusers (Talloczy, Martinez et al. 2008). Users tend to take METH in binges, and, as the drug have a half-life of 11.4–12 h (Cho, Melega et al. 2001; Harris, Boxenbaum et al. 2003), this can lead to extremely high levels. Published studies modeling binge patterns of use in individuals show that after the fourth administration of 260 mg during a single day produces blood levels of 2.5 mg/L, and can reach 3 mg/L on the second day of such a binge (Melega, Cho et al. 2007). Thus, binge doses in the range of 260–1000 mg produce 2.5–12 mg/L blood METH levels. The estimates appear consistent with blood levels detected after fatalities (Wilson, Kalasinsky et al. 1996; Logan, Fligner et al. 1998; Karch and Stephens 2000), reaching as high as 12.5 mg/L in an individual who expired ~16 hours after METH ingestion.
Although there is substantial evidence of the effects of METH on CNS function, the effects of METH on host responses have not been extensively described. Limited studies about the effects of METH on immune function have revealed that its abuse has profound implications in host immunity. Acute and chronic rodent models of METH administration have shown a reduction in thymic and splenic cellularity via apoptosis (Freire-Garabal, Balboa et al. 1991; Iwasa, Maeno et al. 1996). Furthermore, METH is an immunosuppressive agent as it alkalizes normally acidic organelles within immune cells, inhibits antigen presentation and impairs phagocytosis (Talloczy, Martinez et al. 2008). We have also found that the microbicidal capacity of macrophages is significantly decreased after METH exposures (Talloczy, Martinez et al. 2008; Martinez, Mihu et al. 2009). Similarly, METH exposure results in mitochondrial oxidative damage and caused dysfunction of primary human T cells (Potula, Hawkins et al. 2010). Moreover, we have shown that METH negatively alters antibody and cytokine production (Martinez, Mihu et al. 2009). Together, these data indicate that METH causes immune dysfunction in mature mammals. Therefore, we hypothesized that METH alters immune cells recruitment and cytokine production, and that these effects can be tested in a murine model of chronic METH abuse. Our results demonstrate that METH modifies leukocyte infiltration and alters the production of immune response mediators in vivo.
MATERIALS AND METHODS
Methamphetamine administration
High-dose METH users initially take small amounts of the drug intermittently before progressively increasing the dose (Simon, Richardson et al. 2002). To simulate this pattern, increasing doses (2.5, 5, and 10 mg/kg/day on weeks 1, 2, and 3, respectively) of METH (Sigma, St. Louis, MO) were intraperitoneally (i.p.) administered daily to female C57BL/6 mice (age, 6–8 weeks; NCI) over 21 days. Phosphate-buffered saline (PBS)-treated animals were used as controls. All animal studies were conducted according to the experimental practices and standards approved by the Animal Welfare and Research Ethics Committee at the Albert Einstein College of Medicine.
Flow-Cytometric Analysis (FACS)
For FACS staining, primary cells were isolated from excised tissues (lung and spleen) from five mice treated with METH or PBS as described above; the cells were washed and then stained with fluorescence-labeled antibodies. Anti-Ly-6G-APC (neutrophils), anti-F4/80-Cy7 (macrophages), and anti-CD3-FITC (T cells) were purchased from Becton Dickinson (BD) Biosciences (San Diego, CA). Samples were processed on a FACSCalibur flow cytometer (BD) and were analyzed using the Cell Quest Pro software (BD).
Histological examinations
After METH administration, each mouse was euthanized and vascularly perfused with 4% paraformaldehyde (PFA) and 10% sucrose solutions in PBS (pH 7.4). Then, organs (lung, spleen, liver, and kidney) were excised and fixed in 4% PFA for 24 h. Tissues were processed, embedded in paraffin, and 4 μm vertical sections were fixed to glass slides. Hemotoxylin and Eosin (H&E), myeloperoxidase (MPO), F4/80, and CD3 staining were performed for tissue morphology, neutrophils, macrophages, and T cells, respectively. Microscopic examinations of tissues were performed by light microscopy with an Axiovert 40 CFL inverted microscope (Carl Zeiss, Germany) and photographed with an AxioCam MrC digital camera using the Zen 2011 digital imaging software (Carl Zeiss). Staining intensity indicates brown color distribution in 10X images. For cellular quantifications, leukocytes were counted and averaged per five 40X-magnification fields.
Cytokine determinations
Organs (lung, spleen, liver, and kidney) from mice were excised and homogenized in PBS with protease inhibitors (Complete Mini; Roche, Ridgefield, CT, USA). Cell debris was removed from homogenates by centrifugation at 6,000 g for 10 minutes. Samples were stored at −80°C until tested. Supernatants were tested for IFN-γ, TNF-α, IL-1β, IL-4, IL-6, IL-10, and IL-12p70r by ELISA according to the manufacturer’s protocol (BD and e-Biosciences, San Diego, CA). The limits of detection were 7.8 pg/mL for IL-4, 15.6 pg/mL for TNFα, IL-1β, and IL-6, 30 pg/mL for IL-10, 31.3 pg/mL for IFNγ, and 62.5 pg/mL for 12p70r.
Statistical Analysis
FACS, cellular counts, immunohistochemistry staining intensities, and cytokine data were subjected to statistical analysis using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). P values were calculated by Student’s t test analysis. P values lower than 0.05 were considered significant.
RESULTS
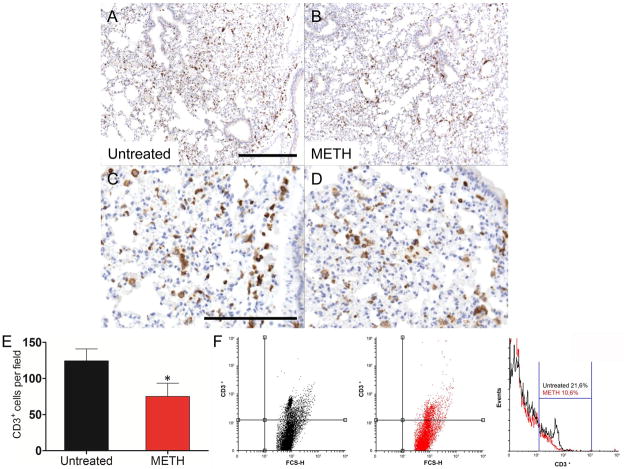
We investigated the effect of METH on leukocyte recruitment and cytokine production in the lungs. Immunohistochemistry (IHC) and FACS showed no differences in the intensity and distribution of neutrophils (Ly-6G+) and macrophages (F4/80+) between METH-treated and control animals. Conversely, the lungs of METH-treated mice displayed significantly fewer T (CD3+) cells (Fig. 1B and D) than untreated tissues (Fig. 1A and C), which may indicate reduced circulating CD3+ cells. Cell counts showed that METH-treated mice (75.2 ± 8.2) had significant reduction in CD3+ cells than controls (124.6 ± 7.3; P<0.01) (Fig. 1E). FACS analysis confirmed a significant decrease of CD3+ cells in the lungs of METH-treated mice (10.6% vs. control: 21.6%) (Fig. 1F). Lungs of METH-treated mice showed significantly higher levels of IL-6 and IL-10 and lower levels of IFN-γ when compared to untreated mice (Table 1).
Figure 1.
Analyses of the lungs of untreated or methamphetamine (METH)-injected C57BL/6 mice. Representative CD3 (T cells) stained sections of untreated (A, C) and METH-treated (B, D) lungs are shown (scale bar, 20 μm; 10X magnification for A and B, and 40X magnification for C and D). Cell counts of CD3+ cells (E) were quantified by light microscopy using a 40X objective (n=5). Solid bars denote the means and error bars denote standard deviations. P values were calculated by Student’s t test (P<0.05). * indicates decreased cellular recruitment when compared to untreated group. The expression levels of the CD3+ (F) were analyzed by flow-cytometric analysis (FACS) and a representative graph is shown. Primary cells were isolated from lungs of mice (n=5) exposed to PBS or METH (2.5, 5, and 10 mg/Kg) for 21 days. The experiments were performed twice with similar results obtained.
TABLE 1.
Cytokines levels (pg/mL) in organs of untreated and METH-treated C57BL/6 mice.
| Lung | Spleen | Liver | Kidney | |||||
|---|---|---|---|---|---|---|---|---|
| PBS | METH | PBS | METH | PBS | METH | PBS | METH | |
| IFN-γ | 2351.4 ± 5.26† | 1894.3 ± 4.32 | 31.42 ± 2.39 | 442.43 ± 2.08* | 2650.3 ± 6.65 | 3332.9 ± 6.55* | 2251.42 ± 2.37 | 2728.6 ± 3.61* |
| TNF-α | 5412.6 ± 4.36 | 5238.3 ± 2.35 | 252.51 ± 5.08 | 630.85 ± 3.02* | 9592.8 ± 5.86 | 17700.2 ± 4.73* | 8726.04 ± 3.99† | 4454.37 ± 8.59 |
| IL-1β | 889.2 ± 2.35 | 819.2 ± 1.01 | 115.2 ± 3.11 | 179.9 ± 1.53 | 1340.2 ± 5.7 | 1779.2 ± 9.2* | 877.2 ± 6.22 | 1797.9 ± 7.44* |
| IL-4 | 74.20 ± 2.31 | 79.07 ± 3.65 | 9.41 ± 4.93 | 33.05 ± 2.98 | 133.83 ± 6.74 | 250.90 ± 2.54* | 137.85 ± 7.27 | 245.95 ± 2.87* |
| IL-6 | 292.4± 1.51 | 565.4 ± 5.3* | 161.3 ± 1.41 | 299.5 ± 3.6* | 478 ± 1.35 | 760.2 ± 6.26* | 323.6 ± 1.07 | 827.6 ± 3.47* |
| IL-10 | 723.5 ± 2.87 | 864.33 ± 4.36* | 173.69 ± 5.70 | 214.83 ± 5.92 | 1183.2 ± 2.49 | 1742.3 ± 5.17* | 776.17 ± 5.39 | 1730.3 ± 5.78* |
| IL-12 | 42.19 ± 3.14 | 43.19 ± 1.36 | 73.50 ± 3.65 | 55.62 ± 6.47* | 145.50 ± 3.25 | 323.75 ± 8.21* | 145.63 ± 9.02 | 288.06 ± 4.49* |
indicates increased and decreased cytokine production, respectively, when compared to untreated group.
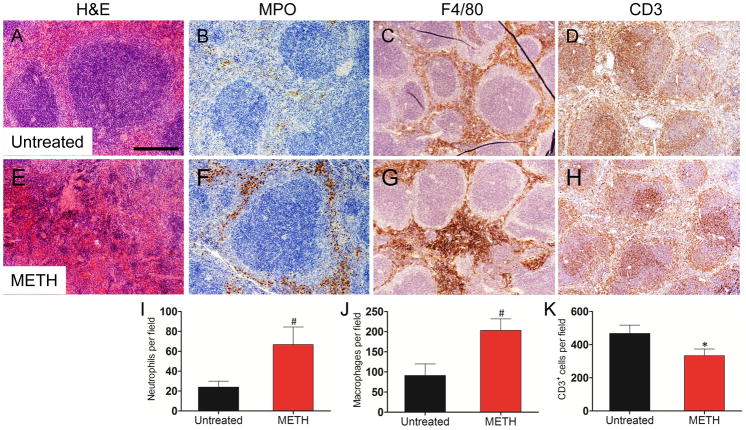
IHC analyses revealed that the spleen had the most notable inflammatory differences between untreated and METH-treated mice. H&E staining revealed severe necrosis of the spleen after 21 days of METH administration when compared to the control animals (Fig. 2A and E). METH-treated mice had increased MPO staining intensity (Fig. 2B and F). The distribution and intensity of F4/80 staining were more widely spread and of greater intensity in the red-pulp of the METH-treated mice (Fig. 2C and G). CD3 staining in the spleens of METH-treated mice was slightly reduced relative to controls (Fig. 2D and H). The distribution of neutrophils, macrophages, and T cells observed by IHC was confirmed with the cell count results. METH-treated mice displayed greater numbers of Ly-6G+ (66.8 ± 7.9) (Fig. 2I) and F4/80+ (203.4 ± 12.7) (Fig. 2J) cells than those of controls (Ly-6G+, 24 ± 2.7, P<0.001; F4/80+, 91.4 ± 12.8, P<0.001). However, CD3+ cells (Fig. 2K) were significantly reduced in METH-treated animals (333.8 ± 17.9 vs. control: 468.4 ± 22.3, P<0.001).
Figure 2.
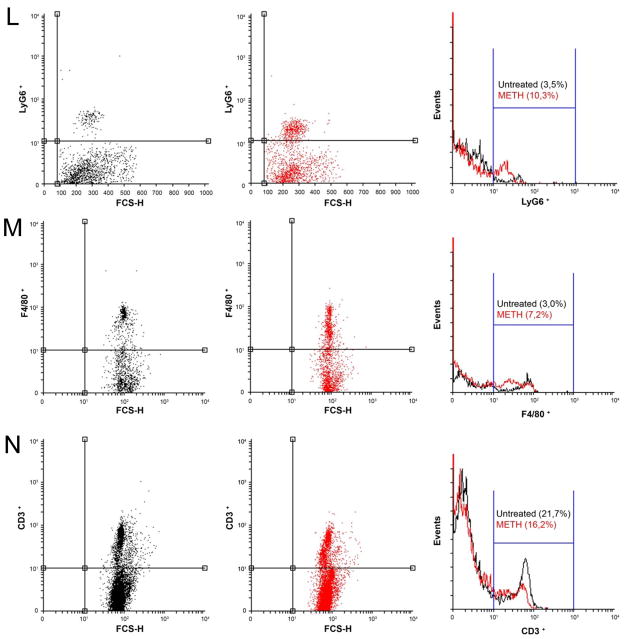
Analyses of spleens of untreated or METH-injected C57BL/6 mice. Representative H&E (morphology), MPO (neutrophils), F4/80 (macrophages), and CD3 (T cells) stained sections of untreated (A, B, C, D) and METH-treated (E, F, G, H) spleens are shown (scale bar, 20 μm; 10X magnification). Cell counts of Ly-6G+ (I), F4/80+ (J), and CD3+ (K) cells were quantified by light microscopy using a 40X objective (n=5). Solid bars denote the means and error bars denote standard deviations. P values were calculated by Student’s t test (P<0.05). # and * indicates increased and decreased cellular recruitment, respectively, when compared to untreated group. The expression levels of the Ly-6G+ (L), F4/80+ (M), and CD3+ (N) were analyzed by FACS and a representative graph for each marker is shown. Primary cells were isolated from spleens of animals (n=5) exposed to PBS or METH (2.5, 5, and 10 mg/Kg) for 21 days. The experiments were performed twice with similar results obtained.
Consistent with the IHC findings, FACS analyses revealed the presence of Ly-6G+ (10.3% vs. control: 3.5%) (Fig. 2L) and F4/80+ (7.2% vs. control: 3%) (Fig. 2M) cells were significantly increased after METH administration whereas CD3+ cells (Fig. 2N) were significantly reduced in METH-treated animals (16.2% vs. control: 21.7%). Furthermore, splenic tissue of mice treated with METH contained significantly higher quantities of TNF-α, IFN-γ, IL-6, and IL-12 than that of controls (Table 1).
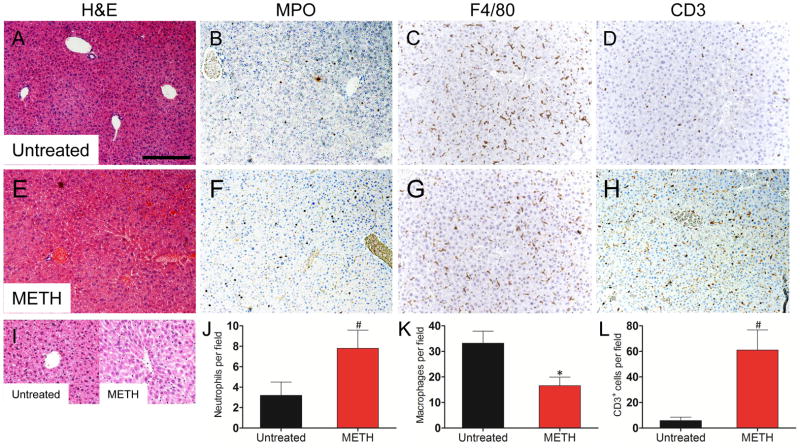
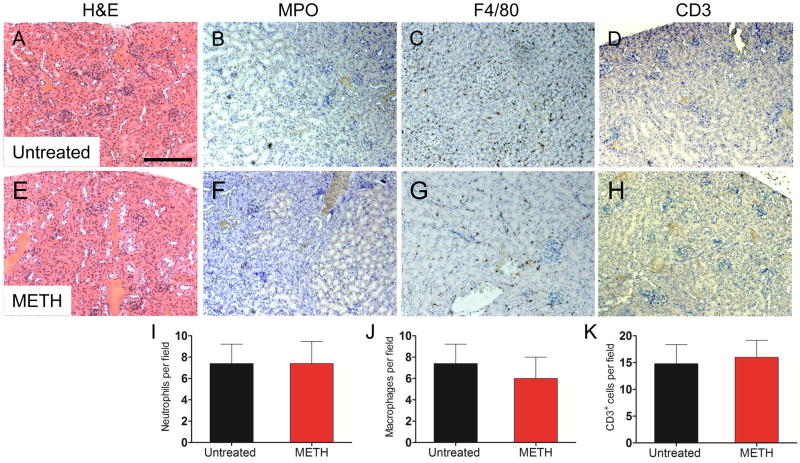
METH-treated animals had significant liver abnormalities. H&E staining showed hepatocellular atrophy in the METH-treated (Fig. 3E and I) mice (Fig. 3A and I). IHC revealed an increase in MPO staining in the livers of METH-treated animals (Fig. 3B) relative to controls (Fig. 3F). F4/80 staining was significantly reduced in the METH-treated mice (Fig. 3C and G). CD3 staining was increased in the METH-treated mice (Fig. 3D and H). To confirm the IHC qualitative findings, cell count analyses showed that neutrophils (7.8 ± 0.8 vs. control: 3.2 ± 0.6, P<0.001) (Fig. 3J) and T cells (61 ± 7.1 vs. control: 5.8 ± 1.2, P<0.001) (Fig. 3L) cells were significantly increased in the murine livers after METH administration. F4/80+ were significantly reduced in METH-treated animals (16.6 ± 1.5 vs. control: 33.2 ± 2.1, P<0.001) (Fig. 3K). Additionally, livers of METH-treated mice produced significant increased amounts of IFN-γ, TNF-α, IL-1β, IL-4, IL-6, IL-10 and IL-12 compared to controls (Table 1).
Figure 3.
Analyses of the livers of untreated or METH-injected C57BL/6 mice. Representative H&E (morphology), MPO (neutrophils), F4/80 (macrophages), and CD3 (T cells) stained sections of untreated (A, B, C, D, I) and METH-treated (E, F, G, H, I) livers are shown (scale bar, 20 μm, 10X magnification, and 40X magnification for I). Cell counts of Ly-6G+ (J), F4/80+ (K), and CD3+ (L) cells were quantified by light microscopy using a 40X objective (n=5). # and * indicates increased and decreased cellular recruitment, respectively, when compared to untreated group. Solid bars denote the means and error bars denote standard deviations. P values were calculated by Student’s t test (P<0.05). The experiments were performed twice with similar results obtained.
Although METH can cause acute kidney failure by constriction of the blood vessels that nourish the kidney, IHC and cell counts of kidneys excised from untreated and METH-treated animals showed no differences in morphology (Fig. 4A and E) and leukocyte infiltration (Fig. 4B, F, and I for neutrophils; Fig. 4C, G, and J for macrophages; Fig. 4D, H, and K for T cells). Nevertheless, METH-treated mice exhibited significantly high levels of IFN-γ, IL-1β, IL-4, IL-6, IL-10 and IL-12 and reduced levels of TNF-α when compared to controls (Table 1).
Figure 4.
Analyses of the kidneys of untreated or METH-injected C57BL/6 mice. Representative H&E (morphology), MPO (neutrophils), F4/80 (macrophages), and CD3 (T cells) stained sections of untreated (A, B, C, D) and METH-treated (E, F, G, H) kidneys are shown (scale bar, 20 μm, 10X magnification). Cell counts of Ly-6G+ (I), F4/80+ (J), and CD3+ (K) cells were quantified by light microscopy using a 40X objective (n=5). Solid bars denote the means and error bars denote standard deviations. P values were calculated by Student’s t test (P<0.05). The experiments were performed twice with similar results obtained.
DISCUSSION
METH is widely distributed throughout the body (Volkow, Fowler et al. 2010). In this study, we assess the effects of METH on leukocyte proliferation and cytokine production in the lungs, spleen, liver, and kidneys of animals treated with escalating doses of the drug over a 21 day period to mimic chronic abuse. The accumulation of METH in these organs is likely to contribute to the medical complications associated with its abuse including immunosuppressive effects that may contribute to an increased risk of infection (Talloczy, Martinez et al. 2008; Martinez, Mihu et al. 2009).
The lungs have the highest METH uptake during consumption but the drug is cleared faster than the other organs tested (Volkow, Fowler et al. 2010). METH is associated with elevated free radical formation and significant lung injury (Wells, Buford et al. 2008). However, METH administration did not alter the numbers of neutrophils or macrophages in lung tissues. This result indicates that in absence of infection, neutrophils and macrophages are undistinguishable present in the respiratory tissue which may reflect a combination of sporadic presence of the drug and the responsive migration of these cells in this tissue. Additionally, we have previously shown that METH alters innate immune cell effector functions such as phagocytosis and antigen processing. Perhaps, high accumulation of METH in the lungs may also contribute by rendering pulmonary tissue more vulnerable to infections due to these defects in macrophages and neutrophils. A recent report has revealed a greater risk for tuberculosis (TB) among METH users than non-users (Pevzner, Robison et al. 2010). Similarly a study of HIV-infected patients in Thailand reported that 40% of those also infected with TB had a history of METH use (Mankatittham, Likanonsakul et al. 2009). In mice, METH reduces T cell infiltrates in the lungs, which are important components of adaptive immunity. METH inhibits T cell proliferation diminishing the capacity of these cells to modulate a protective immune response against respiratory pathogens (Martinez, Han et al. 2009). Similarly, we have found elevated levels of early response IL-6 and anti-inflammatory IL-10 in homogenates of METH-treated mice (Table 1), which might explain the development of a non-protective Th2 response against respiratory bacterial and fungal pathogens, even when Th1 cytokines are present.
The spleen had the most notable differences in inflammatory responses between untreated and METH-treated mice. The presence of significantly elevated number of macrophages and neutrophils in the spleen may be associated with elevated free radical formation (Wells, Buford et al. 2008) that can promote the significant necrosis we identified. Additionally, increased quantities of pro-inflammatory cytokines, IFN-γ, TNF-α, IL-6, and IL-12, correlate with a sustained cellular inflammatory response and tissue injury. Similar to previous studies, METH reduced the distribution of splenic T lymphocyte and produces immunosuppression (Saito, Terada et al. 2008; Martinez, Mihu et al. 2009), which could also contribute to the higher rate of infections in METH users. In this regard, METH induces cell death of the splenic lymphocytes via apoptosis (Iwasa, Maeno et al. 1996).
Liver damage is common in METH users, often leading to hepatitis and cirrhosis (Verachai, Phutiprawan et al. 2002). Liver disease is especially severe in individuals with hepatitis C (HCV) infection. The accumulation of METH in the liver may be responsible for the hepatocellular atrophy we observed in METH-treated animals. One of the mechanisms by which METH can cause exacerbation of HCV infection is, in part, linked to a reduced number of macrophages and a mixed Th1-Th2 phenotype immune response, which is what we observed in the METH-treated animals in this study. Thus, METH might play a role in facilitating HCV replication in human hepatocytes (Ye, Peng et al. 2008). Our findings provide fundamental insights into how METH may play an important role in HCV infection-related morbidity and mortality.
In summary this study reports that METH may negatively modify the immune response, which is likely to increase susceptibility of users and contribute serious medical conditions, including acquisition of infectious diseases that affect these drug users. Insights could lead to new prophylactic or treatment strategies to manage impaired immunity and microbial diseases in METH users.
Acknowledgments
A.J.G. is supported by Fundação de Apoio a Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Pesquisa e Desenvolvimento (CNPq). J.D.N. is supported in part by an Irma T. Hirschl/Monique Weill-Caulier Trust Research award. L. R. M. is supported by the NIH-NIAID 5K22A1087817-02 and LIU-Post Faculty Research awards.
Footnotes
AUTHORSHIP
All authors contributed to the design of the experiments, analysis of the data, and writing of the manuscript. H.P. and J.A.G. performed the histopathology and cytokine analysis. A.J.G. performed the FACS.
CONFLICT OF INTEREST
The authors state no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cho AK, Melega WP, et al. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39(2):161–166. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Freire-Garabal M, Balboa JL, et al. Effects of amphetamine on T-cell immune response in mice. Life Sci. 1991;49(16):PL107–112. doi: 10.1016/0024-3205(91)90570-2. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Mooney L, et al. The methamphetamine problem in the United States. Annu Rev Public Health. 2010;31:385–398. doi: 10.1146/annurev.publhealth.012809.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DS, Boxenbaum H, et al. The bioavailability of intranasal and smoked methamphetamine. Clin Pharmacol Ther. 2003;74(5):475–486. doi: 10.1016/j.clpt.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Iwasa M, Maeno Y, et al. Induction of apoptotic cell death in rat thymus and spleen after a bolus injection of methamphetamine. Int J Legal Med. 1996;109(1):23–28. doi: 10.1007/BF01369597. [DOI] [PubMed] [Google Scholar]
- Karch SB, Stephens BG. Toxicology and pathology of deaths related to methadone: retrospective review. West J Med. 2000;172(1):11–14. doi: 10.1136/ewjm.172.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BK, Fligner CL, et al. Cause and manner of death in fatalities involving methamphetamine. J Forensic Sci. 1998;43(1):28–34. [PubMed] [Google Scholar]
- Mankatittham W, Likanonsakul S, et al. Characteristics of HIV-infected tuberculosis patients in Thailand. The Southeast Asian journal of tropical medicine and public health. 2009;40(1):93–103. [PubMed] [Google Scholar]
- Martinez LR, Han G, et al. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129(10):2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Mihu MR, et al. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200(1):131–141. doi: 10.1086/599328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melega WP, Cho AK, et al. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61(4):216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- Pevzner ES, Robison S, et al. Tuberculosis transmission and use of methamphetamines in Snohomish County, WA, 1991–2006. American journal of public health. 2010;100(12):2481–2486. doi: 10.2105/AJPH.2009.162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Hawkins BJ, et al. Methamphetamine causes mitrochondrial oxidative damage in human T lymphocytes leading to functional impairment. J Immunol. 2010;185(5):2867–2876. doi: 10.4049/jimmunol.0903691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Terada M, et al. Effects of single or repeated administrations of methamphetamine on immune response in mice. Experimental animals/Japanese Association for Laboratory Animal Science. 2008;57(1):35–43. doi: 10.1538/expanim.57.35. [DOI] [PubMed] [Google Scholar]
- Simon SL, Richardson K, et al. A comparison of patterns of methamphetamine and cocaine use. J Addict Dis. 2002;21(1):35–44. doi: 10.1300/j069v21n01_04. [DOI] [PubMed] [Google Scholar]
- Talloczy Z, Martinez J, et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4(2):e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verachai V, Phutiprawan T, et al. Prevalence and genotypes of hepatitis C virus infection among drug addicts and blood donors in Thailand. The Southeast Asian journal of tropical medicine and public health. 2002;33(4):849–851. [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, et al. Distribution and pharmacokinetics of methamphetamine in the human body: clinical implications. PloS one. 2010;5(12):e15269. doi: 10.1371/journal.pone.0015269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells SM, Buford MC, et al. Acute inhalation exposure to vaporized methamphetamine causes lung injury in mice. Inhalation toxicology. 2008;20(9):829–838. doi: 10.1080/08958370801895121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Ye L, Peng JS, et al. Methamphetamine enhances Hepatitis C virus replication in human hepatocytes. J Viral Hepat. 2008;15(4):261–270. doi: 10.1111/j.1365-2893.2007.00940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]