Abstract
Objective
This retrospective cohort study utilized 3 imaging modalities to analyze quantitatively reticular pseudodrusen (RPD) area changes in eyes that progressed from early to late age-related macular degeneration (AMD).
Methods
Subjects with AMD, unilateral choroidal neovascularization (CNV), and early AMD with RPD in the fellow eye (the study eye) were included. The study eyes underwent indocyanine green angiography (ICGA), near infrared reflectance (NIR-R), and short-wavelength autofluorescence (AF) imaging of the macula at baseline and at follow-up. Study eyes were analyzed for RPD and for the development of late AMD—CNV and/or geographic atrophy (GA). RPD area was measured at baseline and at follow-up as a percentage of the 30-degree field.
Results
During the study period (mean follow-up time 23.5 ± 5.0 months), 12/31 study eyes developed CNV and 4/31 developed GA. In the eyes that developed CNV, there was a statistically significant decrease in mean RPD area over the follow-up period as seen on AF (P<0.01) and NIR-R (P=0.01), and the decrease in mean RPD area approached statistical significance on ICGA (P=0.08).
Conclusion
Using 3 en face imaging techniques, we demonstrate that RPD undergo dynamic spatiotemporal changes in eyes that progress from early AMD to CNV, namely, a decrease in the area of lesions detected.
Keywords: reticular pseudodrusen, choroidal neovascularization, geographic atrophy, age-related macular degeneration
Introduction
Age-related macular degeneration (AMD) is the leading cause of legal blindness in developed countries and the third leading cause worldwide [1]. Traditionally, AMD has been defined on color fundus photography as either early, intermediate, or late. The late stage of the disease is divided into 2 subtypes: non-exudative (“dry”) AMD and exudative (“wet”) AMD. Non-exudative late AMD, also known as geographic atrophy (GA), refers to degeneration of the retinal pigment epithelium (RPE), which is responsible for supporting the photoreceptor cells; its degeneration ultimately leads to photoreceptor cell death. On the other hand, exudative AMD, also known as choroidal neovascularization (CNV), is the growth of abnormal blood vessels either under the RPE, breaking through the RPE, or within the retina. These abnormal blood vessels leak, creating collections of subretinal fluid, exudations, and disciform scars [2].
Recently, the importance of reticular pseudodrusen (RPD) in AMD has received increased attention. RPD were first described in 1990 by Mimoun et al. as a yellowish reticular pattern ranging from 125 to 250 μm in width on color fundus photography [3] and were first demonstrated on histopathologic study in 1995 by Arnold et al [4]. The etiology of RPD is still unclear but is possibly vascular [5]. RPD have been distinctly visualized using indocyanine green angiography (ICGA), short-wavelength autofluorescence (AF), near infrared reflectance (NIR-R), and spectral domain optical coherence tomography (SD-OCT) imaging. On AF and NIR-R, RPD appear as small, ill-defined hypoautofluorescent/hyporeflectant lesions surrounded by areas of increased AF/NIR-R signal, with sensitivity approaching 86% and 95%, respectively [6,7]. Similarly, ICGA shows RPD as hypofluorescent dots in the mid to late phase of the angiogram, with sensitivity ranging between 73% and 100% [7,8]. SD-OCT reveals that RPD localize above the RPE; therefore, they are commonly referred to as “subretinal drusenoid deposits” (SDD) [9,10]. Studies have correlated SDD in SD-OCT imaging with RPD's reticular pattern found in well-circumscribed groups in en face imaging [9,11]. Our research group has called RPD, in association with probable choroidal alterations, “reticular macular disease,” a phenotype of AMD [8]. More recently, adaptive optics scanning laser ophthalmoscopy (AOSLO) has allowed for visualization of stage 3 RPD lesions as reflective material, surrounded by a hyporeflective annular zone, and has provided greater evidence to localize RPD material in the subretinal space [12].
RPD are associated with advanced AMD. Cohen et al. showed that 24% of patients with newly diagnosed CNV had RPD on color fundus photographs [13]. In another study by Schmitz-Valckenberg et al., 62% of patients with GA had reticular lesions on scanning laser ophthalmoscopy imaging [14]. Finally, Smith et al. noted that 74% of patients with RPD had advanced AMD, with CNV being the predominant subtype [8]. To understand the role that RPD play in AMD progression, it is necessary to understand the evolution of these lesions over time. Smith et al., in 2009, and Sarks et al., in 2011, described the typical superior arcade localization of RPD and also noted fading of RPD with the onset of CNV [15,8]. Recent work has suggested that RPD may be even more common than previously reported, with one study finding 79% of patients with intermediate AMD having presence of RPD by multimodal imaging [16]. In the present study, we assessed the change in RPD area in eyes that progressed to late AMD compared to those that did not progress. This data will potentially provide us with a better understanding of the natural history of RPD and its relation to late AMD.
Material and Methods
This retrospective cohort study was conducted collaboratively by the Department of Ophthalmology at New York University (NYU) School of Medicine in the United States and the Association for Innovation and Biomedical Research on Light and Image (AIBILI) in Coimbra, Portugal. This study received approval from the Institutional Review Board of AIBILI and was conducted in accordance with the tenets of the Declaration of Helsinki. We retrospectively analyzed data from a prospective observational study, originally designed to study early predictive signs of CNV [17]. Informed consent was obtained from all study participants. Data was shared in a de-identified fashion with investigators at NYU.
Subjects
Patients were recruited at AIBILI and followed over a 32-month period. Demographic information was collected, and all patients underwent comprehensive ophthalmologic examinations by a retinal specialist, as well as retinal imaging, at the initial and final visits. Subjects older than 50 years of age with AMD, unilateral CNV, and early AMD with RPD in the fellow eye (the study eye) were included. Exclusion criteria were media opacity resulting in poor image quality, photographic artifacts, or history, in the study eye, of macular thermal laser photocoagulation, retinal vascular occlusion, retinal detachment, vitreoretinal or glaucoma surgery, macular hole, central serous chorioretinopathy, or high myopia (spherical equivalent greater than -6 diopters).
Diagnosis of CNV was based on clinical examination and confirmed by the presence of characteristic components on imaging, such as intraretinal fluid, subretinal fluid, serous pigment epithelial detachment, and hemorrhage. Diagnosis of GA was based on clinical examination and imaging findings of typical lesions >500 μm in diameter within 2 disc diameters of the fovea. The study eyes were divided into 3 groups: (1) those that progressed to CNV; (2) those that progressed to GA; and (3) those that did not progress to late AMD.
Imaging Modalities
The study eye was analyzed for the development of CNV and GA, and for the presence and extent of RPD at baseline and at follow-up, using ICGA, AF, and NIR-R imaging. All images were acquired using a confocal scanning laser ophthalmoscope (Heidelberg Retina Angiograph 2, Heidelberg Engineering, Heidelberg, Germany) in a 30-degree field of view at a resolution of 1536 pixels2. RPD were defined as hypofluorescent dots seen in the mid to late phase of the angiogram on ICGA; hyporeflectant lesions in well-defined reticular patterns against a mildly hyperreflectant background on NIR-R; and hypoautofluorescent lesions against a background of elevated fluorescence on AF. The extent of RPD was drawn independently on each modality by 2 graders (CN and CS) using ImageJ software (Version 1.4, National Institutes of Health, Bethesda, MD, USA) and calculated as the RPD area divided by the total fundus area (in pixels2). If the 2 graders obtained an area measurement differing by >15%, arbitration through open adjudication was performed. If agreement was still not achieved, resolution was achieved by a third, expert grader (RTS). The average of the 2 observer measurements was used for statistical analysis. Images were excluded if assessment was not possible due to poor quality.
Statistical Analysis
Statistical analysis was performed with SPSS software (Version 22, IBM Corp., Armonk, NY, USA). Baseline characteristics were compared using analysis of variance (ANOVA) or Fisher's exact test. Due to non-normal distribution of data, we chose to use Wilcoxon signed-rank test for non-parametric paired samples to compare RPD area between the initial and final visit. For all tests, a p-value of less than 0.05 was considered statistically significant.
Results
Demographics
A total of 31 subjects (31 study eyes) were included in this study. The mean follow-up period was 23.5 ± 5.0 months (range: 12-32 months). In total, 16/31 study eyes (52%) did not progress to late AMD, while 12/31 (39%) progressed to CNV, and 4/31 (13%) progressed to GA (one study eye developed both CNV and GA). Demographic characteristics (age and gender) were similar in all groups (Table 1).
Table 1. Baseline demographic characteristics of patients.
| Progressed to CNV (n=12)† | Progressed to GA (n=4)† | Did Not Progress to Late AMD (n=16) | P-Value* | |
|---|---|---|---|---|
| Age (Years) | 78.8 ± 7.8 | 76.8 ± 2.5 | 77.9 ± 3.7 | 0.809 |
| Follow-Up (Months) | 22.7 ± 7.8 | 25.3 ± 3.2 | 23.7 ± 1.6 | 0.665 |
| Follow-up Range (Months) | 12-32 | 23-30 | 23-25 | |
| Sex | 0.424 | |||
| Male | 5 (41.7%) | 1 (25%) | 10 (62.5%) | |
| Female | 7 (58.3%) | 3 (75%) | 6 (37.5%) |
One study eye developed both CNV and GA.
ANOVA or Fisher's exact test was used for all p-values.
RPD Area
We compared the initial and final mean RPD area within each group (Table 2). Among eyes that progressed to CNV during the study period, the mean RPD area significantly decreased on AF (P<0.01) and NIR-R (P=0.01), and the decrease approached significance on ICGA (P=0.08). Of note, the number of gradable images was 8 ICGA, 9 AF, and 8 NIR-R.
Table 2. Change in RPD area of study eye over study period.
| N# | Mean Area (%)†at Start (range) | Mean Area (%)†at End (range) | P-Value* | |
|---|---|---|---|---|
| Progressed to CNV | ||||
| ICGA | 8 | 25.36 (3.96-44.56) | 4.64 (0-26.74) | 0.08 |
| AF | 9 | 15.38 (2.89-36.54) | 0.96 (0-7.09) | <0.01 |
| NIR-R | 8 | 18.04 (3.35-47.55) | 1.74 (0-13.95) | 0.01 |
| Progressed to GA | ||||
| ICGA | 3 | 32.98 (6.47-56.83) | 23.35 (5.29-53.86) | 0.75 |
| AF | 4 | 24.09 (2.67-41.92) | 13.30 (0-39.68) | 0.38 |
| NIR-R | 0 | ‡ | ||
| Did Not Progress to Late AMD | ||||
| ICGA | 15 | 26.17 (5.36-54.79) | 22.18 (2.84-67.33) | 0.15 |
| AF | 11 | 16.47 (1.96-25.63) | 12.08 (2.61-28.51) | 0.01 |
| NIR-R | 9 | 15.14 (3.15-43.43) | 16.52 (4.25-55.20) | 0.43 |
Only gradable images were used for this analysis.
Mean area is the percentage of total fundus area in which RPD are present.
Wilcoxon signed-rank test for non-parametric paired samples was used for all p-values.
Sample size was insufficient for statistical test.
Among eyes that progressed to GA, no significant differences in mean area were found; however, the sample size was the smallest of all outcome groups, limiting analysis (number of gradable images: 3 ICGA, 4 AF, 0 NIR-R).
Among eyes that did not progress to late AMD, the mean RPD area did not change on ICGA or NIR-R but decreased significantly on AF (P=0.01). The number of gradable images was: 15 ICGA, 11 AF, and 9 NIR-R. In analyzing each eye that did not progress, either regression or expansion of RPD area could be seen.
For example, by ICGA, 6 eyes had increased RPD area, while 9 eyes decreased in RPD area. Changes were generally minimal, with 7 eyes undergoing ≤5% change in RPD area.
Discussion
The present study analyzed evolution of RPD in fellow eyes of patients with AMD and unilateral CNV over an average period of 2 years, utilizing a multimodal imaging approach. Numerous studies have shown an association between RPD and late AMD [8,13,18], highlighting the clinical relevance of these lesions. Sarks et al. qualitatively described the evolution of RPD over time in eyes that progressed to CNV [15], and here we build on that study with a quantitative assessment of RPD evolution in eyes that progressed to late AMD. By utilizing 3 en face imaging techniques commonly used in clinical practice, our study provides greater understanding of the dynamic nature of RPD. During the study period, 39% of eyes progressed to CNV, 13% progressed to GA, and 52% did not progress to late AMD (one eye progressed to both CNV and GA). These percentages are similar to recently published work by Finger et al., a study that also analyzed eyes with CNV in the fellow eye [19]. We found a significant decrease, on AF and NIR-R, in RPD area in eyes that developed CNV, with near significance on ICGA (Figure 1). Among eyes that did not progress to late AMD (Figure 2), only on AF was the change in RPD area significant (mean decrease of 4.4%, compared to the larger 14.4% decrease on progression to CNV). There was no significant change in RPD area among eyes that developed GA, though small sample size limited this analysis (Figure 3).
Fig. 1.
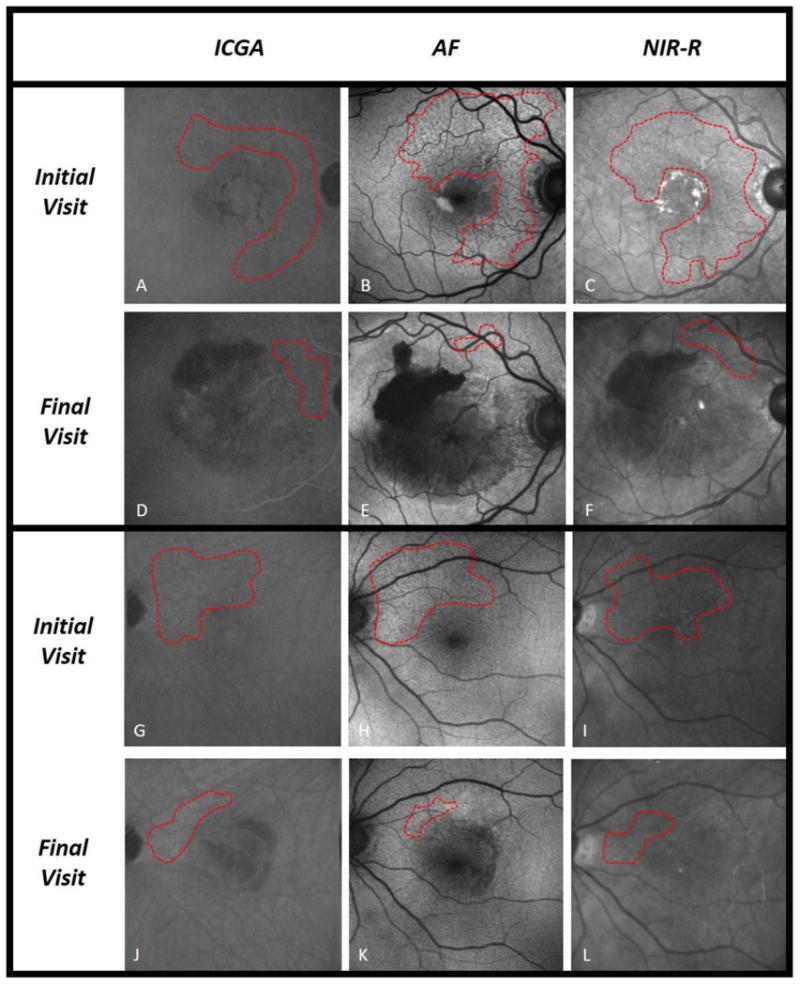
Reticular pseudodrusen (RPD) area measurements in 2 eyes with new-onset CNV. Fundus images by indocyanine green angiography (ICGA), short-wavelength autofluorescence (AF), and near infrared reflectance (NIR-R), with RPD area delineated (outlined in red) on initial and final visit. Two eyes with progression to CNV are included: Subject 1 in panels a-f, and Subject 2 in panels g-l. CNV is shown as an area of hemorrhage centered on the fovea and covering the macula. Both eyes demonstrate considerable shrinking of RPD area near the superior arcade, statistically significant by AF and NIR-R, and approaching significance by ICGA.
Fig. 2.
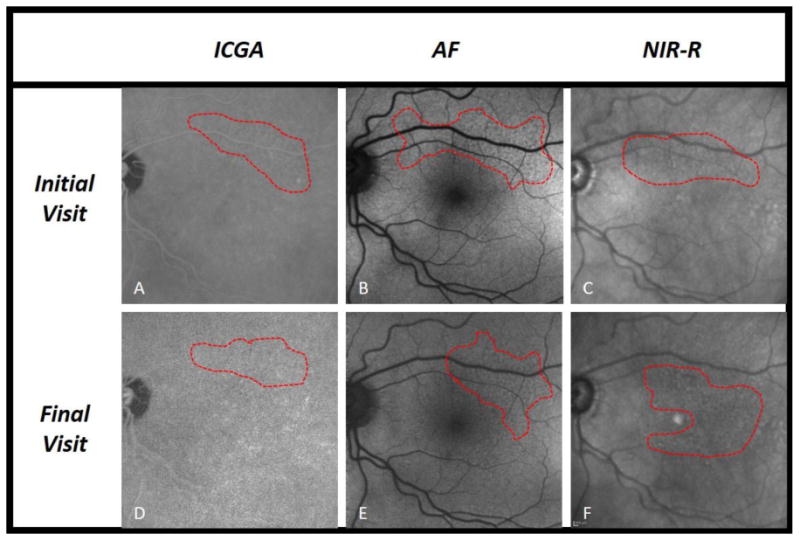
Reticular pseudodrusen (RPD) area measurements in an eye that did not progress to late AMD. Fundus images by indocyanine green angiography (ICGA), short-wavelength autofluorescence (AF), and near infrared reflectance (NIR-R), with RPD area delineated (outlined in red) on initial and final visit. One eye that did not progress to late AMD is demonstrated in panels a-f. In this eye, RPD area remained largely unchanged from initial to final visit.
Fig. 3.
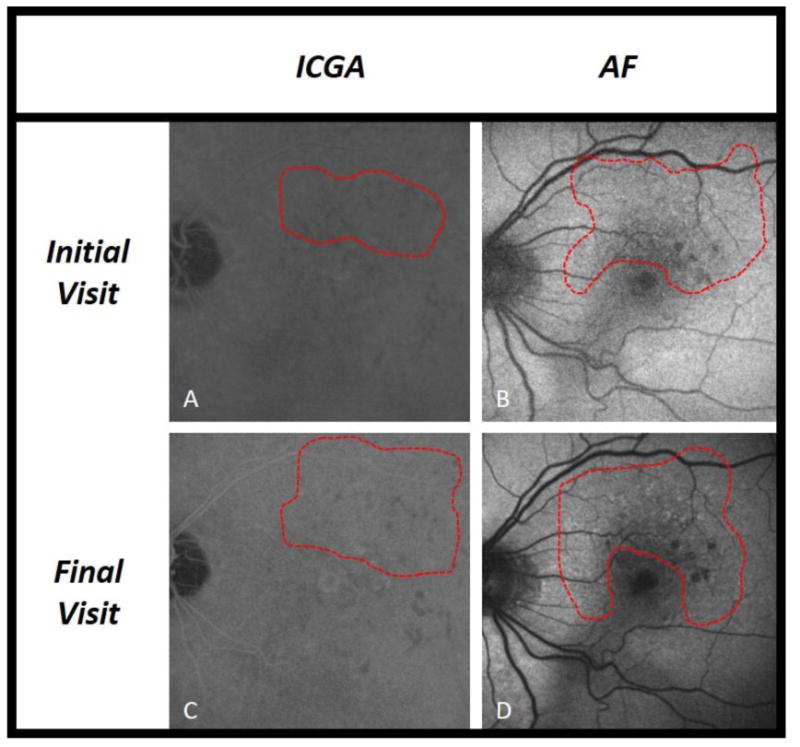
Reticular pseudodrusen (RPD) area measurements in an eye with new onset GA. Fundus images by indocyanine green angiography (ICGA) and short-wavelength autofluorescence (AF), with RPD area delineated (outlined in red) on initial and final visit. One eye that progressed to GA is demonstrated in panels a-d. In this eye, RPD area remained largely unchanged from initial to final visit.
This study adds quantification to a growing literature showing that RPD are dynamic structures, undergoing spatiotemporal changes that include a tendency to fade on imaging upon development of CNV [8,13,18,20]. The mechanism may be different than the disappearance of soft drusen in areas involved by the exudative process. In the latter case, CNV-related exudative changes, such as serous retinal detachments or subretinal hemorrhages, may directly wash out drusen material, or simply make visualization difficult. In the case of RPD, however, the lesions also fade in areas not involved by the exudative process, which requires an alternative explanation. RPD are proposed to progress through stages involving accumulation, reabsorption, and finally migration [20,21]. Thus, one explanation of our results is that RPD evolve and migrate more specifically with CNV development. What remains unclear is the mechanism by which CNV formation would correlate with the undetectability or disappearance of RPD. Perhaps the formation of CNV, which marks the disruption of the blood retinal barrier, introduces factors that stimulate their reabsorption. Conversely, perhaps RPD reabsorption and migration are related to an increase in ischemia with upregulation of vascular endothelial growth factor. Whatever the mechanism, it may be operative mostly in the macula: Sarks et al. found that RPD faded around CNV but remained visible outside the macula and nasal to the optic disc [15]. Furthermore, the mechanism appears to be specific to the development of CNV as opposed to GA. Although the number of eyes that developed GA herein was small, larger studies of the natural history of GA have shown that, while GA tends to progress in areas involved by RPD, the remaining areas of RPD are unaffected [14,22,23].
The spatiotemporal history of RPD is clearly complex; we have shown similar complexity in the remodeling of soft drusen [24]. Of note, Sarks et al. describe RPD spread, over a period of several years, generally starting near the superior temporal vessels and extending by continuity upward and even out to the mid-periphery [15], while Spaide et al. found overall expansion of RPD area but regression of RPD area within the superior macula in 9/21 eyes (43%) over a 2.9-year follow-up period [25], More work needs to be done regarding the natural course of RPD.
This study had several limitations. First, the lack of SD-OCT imaging, with its high sensitivity for detecting RPD/SDD [5,7], limited our ability to grade RPD. Second, the small number of subjects limited some analyses, especially regarding analysis of patients who progressed to GA. Lastly, analysis of an intermediate time-point was not possible with our data set, but we hope to investigate changes of RPD area at shorter follow-up intervals in future work. On the other hand, a major strength of this study was its use of 3 commonly employed en face imaging techniques, providing greater clinical applicability. In addition, the longitudinal nature of this study expands our understanding of RPD evolution.
In conclusion, our results, by quantifying changes in RPD area over time, add to the growing literature demonstrating spatiotemporal evolution of RPD lesions. Given the role of RPD as an independent risk factor for progression to late AMD [8,13,15,21], these results may have important clinical implications for the diagnosis and management of patients with this disease.
Acknowledgments
The authors wish to thank Jennifer Dalberth for her help in editing the manuscript. This work was supported by an individual investigator research award from the Foundation Fighting Blindness (RTS), National Institutes of Health/National Eye Institute grant R01 EY015520 (RTS), and unrestricted funds from Research to Prevent Blindness (RTS). The funding organizations had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit for publication.
1. Funding: This work was supported by an individual investigator research award from the Foundation Fighting Blindness (RTS), National Institutes of Health/National Eye Institute grant R01 EY015520 (RTS), and unrestricted funds from Research to Prevent Blindness (RTS). The funding organizations had no role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit for publication.
Footnotes
Compliance with Ethical Standards: Disclosures: The authors have no disclosures to report.
This article does not contain any studies with animals performed by any of the authors.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
References
- 1.Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, Mariotti SP. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. doi:/S0042-96862004001100009. [PMC free article] [PubMed] [Google Scholar]
- 2.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 3.Mimoun G, Soubrane G, Coscas G. [Macular drusen] J Fr Ophtalmol. 1990;13(10):511–530. [PubMed] [Google Scholar]
- 4.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995;15(3):183–191. [PubMed] [Google Scholar]
- 5.Saade C, Smith RT. Reticular macular lesions: a review of the phenotypic hallmarks and their clinical significance. Clin Experiment Ophthalmol. 2014;42(9):865–874. doi: 10.1111/ceo.12353. [DOI] [PubMed] [Google Scholar]
- 6.Sohrab MA, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011;52(8):5743–5748. doi: 10.1167/iovs.10-6942. [DOI] [PubMed] [Google Scholar]
- 7.Ueda-Arakawa N, Ooto S, Tsujikawa A, Yamashiro K, Oishi A, Yoshimura N. Sensitivity and specificity of detecting reticular pseudodrusen in multimodal imaging in Japanese patients. Retina. 2013;33(3):490–497. doi: 10.1097/IAE.0b013e318276e0ae. [DOI] [PubMed] [Google Scholar]
- 8.Smith RT, Sohrab MA, Busuioc M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148(5):733–743. doi: 10.1016/j.ajo.2009.06.028. e732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303–312. doi: 10.1016/j.ophtha.2009.07.014. e301. [DOI] [PubMed] [Google Scholar]
- 10.Spaide RF. Colocalization of pseudodrusen and subretinal drusenoid deposits using high-density en face spectral domain optical coherence tomography. Retina. 2014;34(12):2336–2345. doi: 10.1097/IAE.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 11.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Wang X, Rivero EB, Clark ME, Witherspoon CD, Spaide RF, Girkin CA, Owsley C, Curcio CA. Photoreceptor perturbation around subretinal drusenoid deposits as revealed by adaptive optics scanning laser ophthalmoscopy. Am J Ophthalmol. 2014;158(3):584–596. doi: 10.1016/j.ajo.2014.05.038. e581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91(3):354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmitz-Valckenberg S, Alten F, Steinberg JS, Jaffe GJ, Fleckenstein M, Mukesh BN, Hohman TC, Holz FG, Geographic Atrophy Progression Study G Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(9):5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 15.Sarks J, Arnold J, Ho IV, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95(7):979–985. doi: 10.1136/bjo.2010.194977. [DOI] [PubMed] [Google Scholar]
- 16.Zarubina AV, Neely DC, Clark ME, Huisingh CE, Samuels BC, Zhang Y, McGwin G, Jr, Owsley C, Curcio CA. Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging. Ophthalmology. 2016;123(5):1090–1100. doi: 10.1016/j.ophtha.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cachulo L, Silva R, Fonseca P, Pires I, Carvajal-Gonzalez S, Bernardes R, Cunha-Vaz JG. Early markers of choroidal neovascularization in the fellow eye of patients with unilateral exudative age-related macular degeneration. Ophthalmologica. 2011;225(3):144–149. doi: 10.1159/000321064. [DOI] [PubMed] [Google Scholar]
- 18.Pumariega NM, Smith RT, Sohrab MA, Letien V, Souied EH. A prospective study of reticular macular disease. Ophthalmology. 2011;118(8):1619–1625. doi: 10.1016/j.ophtha.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finger RP, Wu Z, Luu CD, Kearney F, Ayton LN, Lucci LM, Hubbard WC, Hageman JL, Hageman GS, Guymer RH. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology. 2014;121(6):1252–1256. doi: 10.1016/j.ophtha.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Querques G, Canoui-Poitrine F, Coscas F, Massamba N, Querques L, Mimoun G, Bandello F, Souied EH. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(3):1264–1270. doi: 10.1167/iovs.11-9063. [DOI] [PubMed] [Google Scholar]
- 21.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117(9):1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 22.Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Jr, Freund KB, Yannuzzi LA, Smith RT. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(12):7362–7369. doi: 10.1167/iovs.12-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L, Blonska AM, Pumariega NM, Bearelly S, Sohrab MA, Hageman GS, Smith RT. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850–1862. doi: 10.1097/IAE.0b013e31828991b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RT, Sohrab MA, Pumariega N, Chen Y, Chen J, Lee N, Laine A. Dynamic soft drusen remodelling in age-related macular degeneration. Br J Ophthalmol. 2010;94(12):1618–1623. doi: 10.1136/bjo.2009.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33(9):1800–1808. doi: 10.1097/IAE.0b013e31829c3765. [DOI] [PubMed] [Google Scholar]


