Abstract
Background
Over 40% of renal cell carcinoma (RCC) cases in the US are attributed to excessive body weight. Moreover, obesity may also be linked to RCC prognosis. However, the molecular mechanism underlying these associations are unclear. In the present study, we evaluated the role of promoter methylation in obesity-related genes in RCC tumorigenesis and recurrence.
Methods
Paired tumors (TU) and normal adjacent (N-Adj) tissues of 240 newly diagnosed and previously untreated Caucasian RCC patients were examined. For the discovery phase, 63 RCC-pairs were analyzed. Additional 177 RCC-pairs were evaluated for validation. Pyrosequencing was used to determine CpG methylation in 20 candidate obesity-related genes. An independent TCGA dataset was also analyzed for functional validation. Association between methylation and recurrence was analyzed using multivariate Cox proportional hazards models and Kaplan-Meier survival analysis.
Results
Methylation in NPY, LEP and LEPR was significantly higher in TU compared with N-Adj tissues (p<0.0001) in both discovery and validation groups. High methylation in LEPR was associated with increased risk of recurrence (HR=3.15; 95%CI: 1.23–8.07; p=0.02). Patients with high-methylation in LEPR had shorter recurrence-free survival than low-methylation group (Log-Rank p=2.25E-03). Additionally, high LEPR methylation in TU was associated with more advanced features (p≤0.05). Consistent with our findings, lower LEPR expression in TU compared with N-Adj tissues (p=1.00E-03) was found in TCGA data.
Conclusions
Somatic alterations of promoter methylation in NPY, LEP and LEPR genes are involved in RCC-tumorigenesis. Furthermore, LEPR methylation is associated with RCC recurrence. Future research to elucidate the biology underlying this association is warranted.
Keywords: LEPR, obesity, methylation, recurrence, kidney cancer
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 2–3% of all malignancies in adults and comprises 85% of adult kidney cancer1. Despite improved diagnosis, a third of patients undergoing nephrectomy progress to metastasis or experience local recurrence and distant metastasis during follow-up2. It is important to be able to predict RCC recurrence early and intervene accordingly3, 4. Obesity, measured by body mass index (BMI), influences RCC development. More than 40% of RCC cases in US are indeed associated with excessive body weight5. Previous studies have revealed the association of overweight and obesity with increased RCC risk6; however, patients with higher BMI had a significantly better RCC prognosis compared with normal weight patients, a phenomenon well known as the “obesity paradox”7. Although strong associations between obesity and RCC were found, limited studies have studied the molecular mechanism linking obesity and RCC tumorigenesis5. Epigenetic changes have been suggested as a molecular mechanism mediating this interplay8–11. To date, there are well-established obesity-related genes whose expressions are regulated through epigenetic mechanisms (e.g., DNA methylation, histone modification and miRNAs). Animal models and human studies have clearly demonstrated methylation changes in promoters of various genes that are implicated in obesity (LEP, LEPR, POMC, MC4R, UBASH3A, TRIM3), appetite control and metabolism (NPY, POMC), insulin signaling (IGF-2, IRS-1) and inflammation (ADIPOQ, ATP10A, TNF). However, the role of DNA methylation in obesity-related genes in RCC prognosis has yet to be elucidated12.
Previous studies have shown that aberrant DNA methylations contribute to RCC tumorigenesis13, 14 and clinical outcomes15–18. The methylations of several genes, including DAL-1/4.1B19, COL14A120, SFRP121, GREM1, NEURL, LAD1 and NEFH22, and DAB2IP23, have been shown as independent prognostic factors for RCC. For example, van Vlodrop et al.22 recently identified four methylation markers, GREM1, NEURL, LAD1 and NEFH, that individually predicted prognosis of ccRCC patients. The four markers combined were associated with poorer survival in two independent patient series and a third series of ccRCC cases from TCGA. No study has reported DNA methylation in obesity-related genes as prognostic markers for RCC patients.
Leptin (LEP) has been suggested as a biological link between cancer and obesity. LEP exerts its action through leptin receptor (LEPR), a class I cytokine receptor; disrupted LEP/LEPR signaling has been associated with RCC invasion24–27.
In the current study, we sought to investigate the potential role of obesity-related gene methylation in RCC tumorigenesis and recurrence. The methylation of promoter-associated CpG islands of obesity-related genes was assessed by pyrosequencing in RCC tissue pairs of tumor (TU) and normal adjacent tissue (N-Adj) samples and its association with clinicopathologic characteristics and prognosis were evaluated.
MATERIAL AND METHODS
Study population and human tissue samples
This is an ongoing study that has been recruiting RCC patients from the University of Texas MD Anderson Cancer Center in Houston, Texas, since 2002. The study design was described previously28. Briefly, all recruited cases were patients with newly diagnosed (within 1 year of diagnosis), histologically confirmed, and previously untreated RCC. A total of 240 Caucasian RCC patients were included in the present study. For the discovery population, 63 tissue pairs of TU and N-Adj from the surrounding kidney were collected and for the validation population, 177 tissue pairs were included. The RCC tissues were collected during the surgery. The study was approved by the MD Anderson Institutional Review Board. All participants provided written informed consent before participating in the study.
An independent dataset for gene expression including 64 RCC tissue pairs of TU and N-Adj was downloaded from The Cancer Genome Atlas (TCGA) to provide confirmatory evidence for our methylation findings29.
Epidemiologic and clinical data collection
Epidemiological data were collected by MD Anderson interviewers in a 45-min structured in-person interview. Data including information regarding history of hypertension (yes/no), smoking status and pack-years of smoking, physical activity and usual weight, weight at age 20 and 40 years was recorded. An individual who had never smoked or had smoked <100 cigarettes in his or her lifetime was defined as a never smoker. An individual who had smoked at least 100 cigarettes in his or her lifetime but had quit at least 12 months before diagnosis was classified as a former smoker. Current smokers were those who were currently smoking or quit <12 months before diagnosis. The number of pack-years was calculated as the average number of cigarettes smoked per day divided by 20 cigarettes and then multiplied by smoking years. Body mass index (BMI; kg/m2) was calculated through self-reported usual height and weight. BMI was categorized according to the standard classifications of the World Health Organization (normal<25 kg/m2; overweight= 25–29.9 kg/m2; obese≥30 kg/m2). Participants also reported the average frequency they spent on five broad groups of physical activities in the year before the interview. A metabolic equivalent (MET) value was assigned to each activity group and categorized into low (MET<27 per week), medium (MET 27–44.9 per week) and intensive (MET≥45 per week)30.
The clinicopathologic information was abstracted from patient medical records, including pathologic stage, Fuhrman grade and histology. The pathologic stage was determined according to the 2009 American Joint Committee on Cancer TNM staging system. Tumor cell differentiation was assessed according to the Fuhrman nuclear grade and patients were group in low-grade (Fuhrman grade 1 and 2) and high-grade (Fuhrman grade 3 and 4). The tumor histological subtypes were classified according to the 2004 WHO classification. All study participants were followed on treatments and recurrence. Recurrence was defined as local or distant metastatic disease occurring after nephrectomy. The endpoint of this study was recurrence free survival (RFS), defined as the time from the date of nephrectomy to the date of recurrence or last follow-up.
DNA extraction and bisulfite pyrosequencing
Genomic DNA was extracted using a QIAamp DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. DNA concentration was assessed with an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). Bisulfite conversion treatment of genomic DNA from each DNA sample was done using the EZ DNA Methylation Kit (Zymo Research, Orange, CA), which converts unmethylated cytosines to uracil but leaves methylated cytosines unchanged. We composed a list of obesity-related genes according to literature search, online database of obesity, obesity-related pathways and at the end we have restricted the gene list to those obesity-related genes whose expression has been reported to be regulated by methylation. The methylation status of the CpG islands in the promoter regions of 20 obesity-related genes in TU and N-Adj samples was analyzed by pyrosequencing at the DNA Methylation Analysis Core, MD Anderson Cancer Center. PCR primers for the genomic area proximal to the transcription start site of the following genes: ADIPOQ, ADRB3, ATP10A, CREB3L3, CTSZ, FASN, IGF2, INS, IRS1, LEP, LEPR, MC4R, NPY, POMC, PPARG, TNF, TRIM3, UCP1, FTO and UBASH3A were designed using the PyroMark Assay Design 1.0 software, Qiagen (Hilden, Germany) (Supplementary Table S1). The pyrosequencing was performed using PSQ HS 96 system (Biotage AB, Uppsala, Sweden) following the manufacturer’s instruction. Controls for high methylation (SssI-treated DNA), low methylation (WGA- amplified DNA), partial methylation (equimolar mixture of SssI-treated and WGA-amplified DNA) and a blank control without DNA were included in each reaction. The methylation level was calculated using the Pyro-Q CpG 1.0.9v software (Biotage AB, Uppsala, Sweden). The methylation percentage of each gene was computed as the average of all the assayed CpG sites in the gene.
Statistical analysis
The χ2 test or Fisher’s exact test was applied separately to compare the distribution of selected demographic and clinical variables by recurrence status. The distribution of each categorical variable was summarized in terms of frequencies and percentages. Differences in continuous variables were evaluated using the Student’s t-test. To describe weather higher methylation is associated with higher age and BMI in normal kidney tissues, we measured standardized b-coefficients in normal kidney tissues. A positive estimate (ß-coefficient) of the correlation between the two variables reflects an increasing methylation response to the age and/or BMI factors and a negative estimate reflects a diminishing response to the factors.
Cox proportional hazard models were used to examine the association between obesity-related gene methylation and recurrence risk. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated. The multivariate regression model was adjusted by age, gender, pathologic stage, grade, smoking status, BMI, hypertension and histology. RFS curves were determined by the Kaplan-Meier analysis and compared by the Log-Rank test. We also performed independent analyses focusing on clear cell RCC (ccRCC) histology subtype only. All statistical analyses were conducted using STATA version 9.0 (Stata corporation, College Station, TX). All tests were two-sided and a P value of ≤0.05 was considered statistically significant.
To examine the gene expression, we analyzed the data from TCGA portal. We downloaded level 3 normalized mRNA-seq data of ccRCC from TCGA and after quality control by removing ineligible samples, the analytic dataset consisted of 64 ccRCC tissue pairs of TU and N-Adj samples. The normalized counts were further log2 transformed. Paired t-test was performed to compare the expression levels of selected genes between the tumor and normal samples.
RESULTS
Methylation Levels of obesity-related genes in RCC tumors and normal-adjacent tissues
The characteristics of the 63 RCC patients in the discovery phase are shown in Table 1. The mean age of the population was 60 years old, largely males, ~50% were never smokers. Most of the cases were pathologic stage I (65.1%), high-grade (63.5%), and ccRCC (76.2%) (Table 1).
Table 1.
Host Characteristics of RCC patients in the Discovery and Validation Populations
|
Discovery set (n=63)
|
Validation set (n=177)
|
|
|---|---|---|
| Variable | n(%) | n(%) |
| Age Mean (SD) | 60.2(9.9) | 59.5(11.4) |
| Pack-years Mean (SD) | 25.3(21.3) | 28.1(29.0) |
| BMI Mean (SD) | 28.9(6.6) | 31.0(6.6) |
| Gender | ||
| Male | 41(65.1) | 121(68.4) |
| Female | 22(34.9) | 56(31.6) |
| Smoking Status | ||
| Never | 28(44.4) | 91(51.4) |
| Former | 25(39.7) | 61(34.5) |
| Current | 10(15.9) | 25(14.1) |
| Pack-years | ||
| 0~30 | 22(34.9) | 48(27.1) |
| ≥30 | 12(19.0) | 35(19.8) |
| Missing | 29(46.0) | 94(53.1) |
| Hypertension | ||
| Yes | 34(54.0) | 90(50.8) |
| No | 29(46.0) | 87(49.2) |
| BMI | ||
| Normal | 21(33.3) | 30(16.9) |
| Overweight | 20(31.7) | 51(28.8) |
| Obese | 20(31.7) | 93(52.5) |
| Missing | 2(3.2) | 3(1.7) |
| Pathologic Stage | ||
| I | 41(65.1) | 105(59.3) |
| II | 6(9.5) | 11(6.2) |
| III | 16(25.4) | 61(34.5) |
| Fuhrman grade | ||
| Low | 22(34.9) | 79(44.6) |
| High | 40(63.5) | 97(54.8) |
| Missing | 1(1.6) | 1(0.6) |
| Physical Activity | ||
| Low | 33(55.0) | 60(33.9) |
| Medium | 17(28.3) | 34(19.2) |
| Intensive | 10(16.7) | 83(46.9) |
| BMI at age 20 | ||
| Normal | 46(73.0) | 82(46.3) |
| Overweight | 10(15.9) | 27(15.3) |
| Obese | 4(6.4) | 6(3.4) |
| Missing | 3(4.7) | 62(35.0) |
| BMI at age 40 | ||
| Normal | 23(36.5) | 30(16.9) |
| Overweight | 25(39.7) | 50(28.2) |
| Obese | 11(17.5) | 31(17.5) |
| Missing | 4(6.3) | 66(37.3) |
| Histology | ||
| Clear Cell | 48(76.2) | 143(80.8) |
| Other | 15(23.8) | 34(19.2) |
| Recurrence | ||
| No | 48(76.2) | 146(82.5) |
| Yes | 15(23.8) | 31(17.5) |
| Dead | ||
| No | 52(82.5) | 152(85.9) |
| Yes | 11(17.5) | 25(14.1) |
SD: standard deviation, BMI: body mass index
The mean methylation level of the promoter-associated CpG sites in the 20 measured genes in TU and N-Adj samples are shown in Table 2. For each gene, we defined the mean methylation level of all the CpG sites within the promoter region as this gene’s final DNA methylation value. We set a criteria of statistical significance (p<0.05) and a minimum Δ-mean of methylation greater than 10% between TU and N-Adj to select methylated genes for further validation. NPY, LEP and LEPR were the most significantly hypermethylated genes in TU than in N-Adj tissues. The methylation percentage of NPY, LEP and LEPR were 39.91±22.07, 34.12±14.42 and 16.66±16.32, respectively, in tumor tissues compared to 17.68±12.45, 22.62±6.12 and 5.20±4.13, respectively, in normal adjacent tissues (p<0.0001 for all three genes). We then validated these three genes in a large validation set of TU and paired N-Adj tissues. Again, we observed significantly higher methylation in NPY (40.21±22), LEP (35.21±14.20) and LEPR (14.12±11.89) in TU compared with N-Adj tissues where we observed lower methylation in NPY (14.38±6.74), LEP (22.58±4.84) and LEPR (4.63±2.74) (p<0.0001 for all three genes) (Table 2).
Table 2.
Methylation and expression levels in RCC patients in the Discovery and Validation Populations
| Discovery set (n=63) | Validation set (n=177) | Functional Validation TCGA-set (n=64) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Mean Methylation Level, % (SD) | Mean Methylation Level, % (SD) | Mean Expression Level, (SD)* | |||||||||
|
|
|||||||||||
| Marker | Normal | Tumor | p value | q value | Normal | Tumor | p value | Normal | Tumor | p value | |
| ADIPOQ | 88.20(2.13) | 87.21(9.28) | 0.41 | 0.27 | |||||||
| ADRB3 | 10.68(5.08) | 17.38(10.31) | 1.94E-05 | 4.45E-05 | |||||||
| ATP10A | 3.20(3.81) | 7.01(9.92) | 1.70E-03 | 2.17E-03 | |||||||
| CREB3L3 | 16.11(4.45) | 11.72(8.31) | 5.29E-04 | 7.58E-04 | |||||||
| CTSZ | 0.55(0.43) | 1.52(4.22) | 0.08 | 0.07 | |||||||
| NPY | 17.68(12.45) | 39.91(22.07) | 1.33E-08 | 1.52E-07 | 14.38(6.74) | 40.21(22.00) | 5.682E-35 | 0.11(1.61) | −0.32(1.17) | 0.81 | |
| FASN | 2.95(0.49) | 2.98(0.47) | 0.66 | 0.40 | |||||||
| IGF2 | 18.40(4.34) | 19.52(10.38) | 0.42 | 0.27 | |||||||
| INS | 79.72(4.31) | 71.33(11.60) | 9.45E-07 | 2.71E-06 | |||||||
| LEP | 22.62(6.12) | 34.12(14.42) | 4.11E-08 | 2.36E-07 | 22.58(4.84) | 35.21(14.20) | 1.796E-22 | 0.94(2.44) | 0.85(1.76) | 0.84 | |
| IRS1 | 10.61(5.98) | 16.36(12.44) | 1.93E-03 | 2.21E-03 | |||||||
| LEPR | 5.20(4.13) | 16.66(16.32) | 5.61E-07 | 2.14E-06 | 4.63(2.74) | 14.12(11.89) | 1.073E-20 | 9.68(0.7) | 9.23(0.87) | 1.00E-03 | |
| MC4R | 1.73(0.90) | 2.70(1.89) | 2.12E-04 | 3.47E-04 | |||||||
| POMC | 9.18(1.52) | 14.62(10.55) | 1.29E-04 | 2.46E-04 | |||||||
| PPARG | 2.15(5.25) | 1.83(2.34) | 0.41 | 0.27 | |||||||
| TNF | 39.51(8.60) | 42.48(19.34) | 0.23 | 0.18 | |||||||
| TRIM3 | 2.86(2.35) | 4.60(3.88) | 2.86E-03 | 2.98E-03 | |||||||
| UCP1 | 2.77(0.80) | 5.34(8.07) | 0.01 | 0.01 | |||||||
| FTO | 1.54(0.98) | 1.54(0.86) | 0.97 | 0.56 | |||||||
| UBASH3A | 78.99(6.26) | 75.15(8.96) | 4.05E-03 | 3.87E-03 | |||||||
The normalized counts were log2 transformed. Paired t-test was performed to compare the expression levels of selected genes between the tumor and normal samples
Additionally, we analyzed the effect of demographics and lifestyle factors such as age and BMI on NPY, LEP and LEPR methylation in normal kidney tissues. We used standardized ß-coefficients to measure the estimates of the correlation. In normal kidney, DNA methylation levels of these three genes were not significantly associated with BMI. However, there was a significant positive correlation between age and LEP methylation (Rho=0.26, p=3.58E-05) and between age and LEPR methylation (Rho=0.43, p=4.86E-12) (Supplementary Table S2).
Methylation Levels of obesity-related genes and Recurrence in RCC Patients
Discovery-Set
We evaluated the associations of the methylation of these three differentially methylated genes with recurrence risk. Among the patients, 15 (23.8%) had recurrence. The median follow up time for patients who did not recur was 75.5 months. The distribution of demographic and clinical variables for RCC patients by recurrence status is presented in Table 3.
Table 3.
Host characteristics by Recurrence Status in RCC patients in the Discovery and Validation Populations
| Discovery set (n=63) | Validation set (n=177) | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Variable | Recurrence n(%) |
No Recurrence n(%) |
p value | Recurrence n(%) |
No Recurrence n(%) |
p value |
| Age Mean (SD) | 62.27(11.06) | 59.50(9.52) | 0.35 | 63.61(7.92) | 58.62(11.83) | 0.03 |
| Pack-years Mean (SD) | 28.41(23.05) | 24.00(20.90) | 0.59 | 42.67(44.66) | 25.18(24.20) | 0.04 |
| BMI Mean (SD) | 31.47(7.91) | 28.08(6.02) | 0.08 | 28.88(4.35) | 31.46(6.95) | 0.05 |
| Gender | ||||||
| Male | 13(86.7) | 28(58.3) | 22(71.0) | 99(67.8) | ||
| Female | 2(13.3) | 20(41.7) | 0.04 | 9(29.0) | 47(32.2) | 0.73 |
| Smoking Status | ||||||
| Never | 4(26.7) | 24(50.0) | 17(54.8) | 74(50.7) | ||
| Former | 9(60.0) | 16(33.3) | 10(32.3) | 51(34.9) | ||
| Current | 2(13.3) | 8(16.7) | 0.17 | 4(12.9) | 21(14.4) | 0.91 |
| Pack-years | ||||||
| 0~30 | 5(33.3) | 17(35.4) | 7(22.6) | 41(28.1) | ||
| ≥30 | 5(33.3) | 7(14.6) | 7(22.6) | 28(19.2) | ||
| Missing | 5(33.3) | 24(50.0) | 0.25 | 17(54.8) | 77(52.7) | 0.52 |
| Hypertension | ||||||
| Yes | 10(66.7) | 24(50.0) | 23(74.2) | 67(45.9) | ||
| No | 5(33.3) | 24(50.0) | 0.26 | 8(25.8) | 79(54.1) | 4.20E-03 |
| BMI | ||||||
| Normal | 2(13.3) | 19(39.6) | 5(16.1) | 25(17.1) | ||
| Overweight | 6(40.0) | 14(29.2) | 15(48.4) | 36(24.7) | ||
| Obese | 7(46.7) | 13(27.1) | 10(32.3) | 83(56.8) | ||
| Missing | 0 | 2(4.2) | 0.13 | 1(3.2) | 2(1.4) | 0.02 |
| Pathologic Stage | ||||||
| I | 4(26.7) | 37(77.1) | 5(16.1) | 100(68.5) | ||
| II | 3(20.0) | 3(6.3) | 2(6.5) | 9(6.2) | ||
| III | 8(53.3) | 8(16.7) | 1.68E-03 | 24(77.4) | 37(25.3) | 1.16E-07 |
| Fuhrman grade | ||||||
| Low | 1(6.7) | 21(43.8) | 2(6.5) | 77(53.1) | ||
| High | 14(93.3) | 26(54.2) | 29(93.5) | 68(46.9) | ||
| Missing | 0 | 1(2.1) | 7.38E-03 | 0 | 1(0.7) | 2.14E-06 |
| Histology | ||||||
| Clear Cell | 13(86.7) | 35(72.9) | 0.28 | 24(77.4) | 119(81.5) | 0.60 |
| Other | 2(13.3) | 13(27.1) | 7(22.6) | 27(18.5) | ||
SD: standard deviation, BMI: Body mass index
We used Cox proportional hazard model-adjusted for known and suspected risk factors and confounders to elucidate the association of promoter methylation level in NPY, LEP and LEPR with recurrence risk in RCC patients. Patients were dichotomized into high and low methylation groups according to the median value for each promoter. Multivariate Cox model adjusted by age, gender, pathologic stage, grade, smoking status, BMI, hypertension and histology identified high methylation levels in LEP as predictor of recurrence in RCC patients (HR=5.14; 95% CI: 1.07–24.66; p=0.04) as well as in the ccRCC subset (HR=5.96; 95% CI: 1.02–34.76; p=0.05) (Table 4).
Table 4.
Methylation levels in Selected Genes and RCC recurrence Risk
| Discovery Set | Validation Set | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| RCC patients (n=63) | RCC patients (n=177) | |||||||
|
| ||||||||
| Marker | Recurrence n(%) |
No Recurrence n(%) |
HR*(95%CI) | p value | Recurrence n(%) |
No Recurrence n(%) |
HR*(95%CI) | p value |
| NPY | ||||||||
| Low | 7(22.58) | 24(77.42) | 1(ref) | 17(19.32) | 71(80.68) | 1(ref) | ||
| High | 8(25.00) | 24(75.00) | 0.94(0.31–2.80) | 0.91 | 14(15.73) | 75(84.27) | 1.16(0.53–2.54) | 0.71 |
| LEP | ||||||||
| Low | 2(6.25) | 30(93.75) | 1(ref) | 11(12.36) | 78(87.64) | 1(ref) | ||
| High | 13(41.94) | 18(58.06) | 5.14(1.07–24.66) | 0.04 | 20(22.73) | 68(77.27) | 1.48(0.66–3.32) | 0.34 |
| LEPR | ||||||||
| Low | 3(9.38) | 29(90.63) | 1(ref) | 8(9.09) | 80(90.91) | 1(ref) | ||
| High | 12(38.71) | 19(61.29) | 1.02(0.14–7.71) | 0.98 | 23(25.84) | 66(74.16) | 3.15(1.23–8.07) | 0.02 |
|
| ||||||||
| ccRCC patients (n=48) | ccRCC patients (n=143) | |||||||
|
| ||||||||
| NPY | ||||||||
| Low | 7(29.17) | 17(70.83) | 1(ref) | 14(19.72) | 57(80.28) | 1(ref) | ||
| High | 6(25.00) | 18(75.00) | 1.70(0.39–7.40) | 0.48 | 10(13.89) | 62(86.11) | 1.05(0.42–2.62) | 0.91 |
| LEP | ||||||||
| Low | 2(8.33) | 22(91.67) | 1(ref) | 9(12.50) | 63(87.50) | 1(ref) | ||
| High | 11(45.83) | 13(54.17) | 5.96(1.02–34.76) | 0.05 | 15(21.13) | 56(78.87) | 1.41(0.58–3.42) | 0.44 |
| LEPR | ||||||||
| Low | 2(8.33) | 22(91.67) | 1(ref) | 6(8.33) | 66(91.67) | 1(ref) | ||
| High | 11(45.83) | 13(54.17) | 1.59(0.22–11.59) | 0.65 | 18(25.35) | 53(74.65) | 6.00(1.92–18.82) | 2.00E-03 |
HR* Multivariate regression model adjusted by age, gender, stage, grade, smoking status, BMI, hypertension and histology
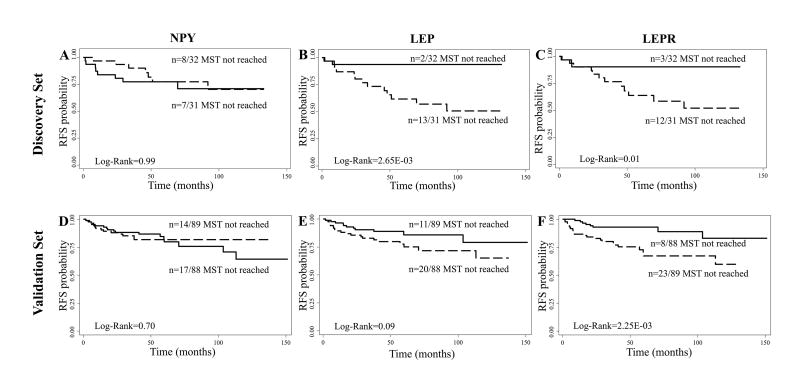
Subsequently, we evaluated the association between methylation in NPY, LEP and LEPR and RFS. Kaplan-Meier curves showed a significant association between methylation in LEP and LEPR (low vs. high methylation) and RFS (Log-Rank p=2.65E-03 and p=0.01, respectively) (Figure 1B and 1C). No significant differences were found for NPY methylation and RFS (p=0.99) (Figure 1A).
Figure 1.
Kaplan-Meier estimates of recurrence free survival (RFS) for RCC patients stratified by methylation levels (Low-solid line vs High-dashed line). RFS of RCC patients by NPY, LEP and LEPR methylation levels in (A–C) discovery set and (D–F) validation set. MST indicates median event-free survival times (in months).
Validation-Set
Then, we used an additional 177 RCC tissue pairs to validate our findings. The characteristics of RCC patients are displayed in Table 1. Among the patients, 31 (17.5%) patients had recurrence. The median follow-up time for patients who did not recur was 49.4 months. The distribution of demographic and clinical variables for RCC patients by recurrence status is presented in Table 3. Among demographic and clinicopathologic variables, higher pathologic stage (HR=7.77; 95% CI: 2.50–24.10; p=4.00E-04) and higher Fuhrman grade (HR=5.52; 95% CI: 1.20–25.28; p=0.03) were associated with increased risks of recurrence.
In the multivariate Cox proportional hazards model, patients with high methylation levels in LEPR had an increased risk of recurrence (HR=3.15; 95% CI: 1.23–8.07; p=0.02) as compared with low methylation group in RCC patients as well in the ccRCC subset (HR=6.00; 95% CI: 1.92–18.82; p=2.00E-03) (Table 4).
Kaplan-Meier analysis and Log-Rank test confirmed the prognostic significance of LEPR in this independent set. Patients with high LEPR methylation in the tumor tissue had shorter RFS than low LEPR methylation group (p=2.25E-03) (Figure 1F). The 5-year RFS rate was estimated at 67% (95% CI: 53–78) for patients with high LEPR methylation compared with 93% (95% CI: 85–97) for patients with low LEPR methylation (p=5.00E-04). There was no significant association between NPY and LEP methylation and RFS in RCC patients (Log-Rank p=0.70 and p=0.09, respectively) (Figure 1D and 1E).
External independent TCGA data set
The methylation of CpG islands in gene promoter regions has been widely studied and this epigenetic event is often linked to gene silencing and loss of tumor suppressor functions during tumorigenesis. To provide indirect but confirmatory evidence for our methylation findings, we examined the mRNA expression of NPY, LEP and LEPR in an external independent dataset consisted of 64 RCC tissue pairs of TU and N-Adj samples downloaded from the TCGA portal.
We analyzed NPY, LEP and LEPR expression in 64 RCC tissue pairs of TU and N-Adj samples from TCGA portal. We observed a significantly lower LEPR expression in TU compared with N-Adj (p=1.00E-03) (Table 2), consistent with our data of higher promoter methylation of LEPR in TU than N-Adj tissues. This result suggests that the hypermethylation of the CpG islands in the promoter region of the LEPR may be a mechanism downregulating its expression in RCC tumors.
The data found in TCGA portal regarding NPY and LEP expression did not provide confirmatory evidence for our methylation results in RCC tissue pairs, as no significant differences were found in NPY and LEP expression between TU and N-Adj tissues (p=0.81 and p=0.84, respectively) (Table 2).
Association of LEPR methylation levels and clinicopathologic characteristics in RCC patients
To determine whether LEPR promoter methylation level is associated with demographic and clinicopathologic characteristics in RCC patients, we dichotomized the patients into high and low methylation groups according to the same median cut-off point of LEPR promoter described previously and analyzed the association between LEPR methylation level and host characteristics. We found a significant correlation between high LEPR methylation and high pathologic stage (p=1.77E-04) and a borderline significant correlation between LEPR methylation and high Fuhrman grade (p=0.05) (Supplementary Table S3). These data indicate that LEPR methylation is an event present in pathogenesis of RCC and is associated with patient poor prognosis.
DISCUSSION
Our study demonstrates that methylation in NPY, LEP and LEPR promoters is involved in RCC tumorigenesis. Additionally, the comparison of methylation data between tumor and normal adjacent tissues revealed that hypermethylation in these particular obesity-related genes was specific for renal cell carcinoma tumors; besides NPY, LEP and LEPR showed to be low or unmethylated in normal tissues from the surrounding tissues. Our studies revealed aberrations in DNA methylation that clearly distinguished RCC from normal tissues. Moreover, high methylation in LEPR and the clinicopathologic data indicates that promoter hypermethylation in LEPR methylation might be a late event in kidney tumorigenesis and tumor differentiation.
Promoter hypermethylation in VHL, p16INK4a, p14ARF, APC, GSTP1, MGMT, RASSF1A, RARβ2, E-Cadherin and TIMP3 have been evaluated in kidney tumors; Dulaimi et al, demonstrated that aberrant promoter hypermethylation in tumor suppressor and cancer genes may disrupt critical pathways, and thus, play an important role in kidney tumorigenesis31. Recent high-resolution epigenomic and genomic map of RCC tumors have reported a significantly increased number of hypermethylated loci in RCC tumors compared with controls; the majority of DMRs (differentially methylated regions) in RCC were localized on enhancer regions of the kidney genome32. Although a great number of hypermethylated loci have been identified in RCC14, to date, only a few subset of CpG island methylation has been clinically characterized and the association of hypermethylation and disease free survival in RCC has been identify in a small number of genes15, 17, 19, 33, 34, but not for obesity-related genes.
To our knowledge, this is the first study in RCC using paired tumor and normal tissue to evaluate the role of obesity-related gene methylation in RCC tumorigenesis and to associate high methylation in LEPR with risk of recurrence in RCC patients.
In our investigation, a borderline significance was obtained for high methylation levels in LEPR in poorly differentiated cancers, which indicates that gene methylation of LEPR may be a late event during RCC tumorigenesis. There has been strong evidence suggesting an association between LEPR expression and tumor aggressiveness, invasion, metastasis and clinical outcome35, 36. Furthermore, a recent study reported that LEPR displayed distinct expression patterns in different histological subtypes of thyroid carcinoma and positive LEPR expression was associated with longer disease-free survival in anaplastic thyroid carcinoma patients37. In addition to this evidence and consistent with our findings, a previous study reported that down-regulation of LEPR expression increases the risk of metastasis38. Moreover, low LEPR was associated with more aggressive tumors38, 39. Biologically, methylation in the promoter-associated CpG sites of LEPR presumably down-regulates LEPR expression in RCC tumors, and this assumption is supported indirectly by the information reported in TCGA portal regarding LEPR expression in tissue pairs of RCC tumor and normal-adjacent tissues. Epigenetic regulation of LEPR expression has been previously suggested in thyroid cancer cells40. Nevertheless, direct experimental data showing promoter hypermethylation leading to reduced LEPR expression in RCC cells is warranted to provide biological insights into the role of LEPR methylation in RCC prognosis.
RCC patients with high methylation in LEPR have a higher risk of recurrence and shorter RFS time. Regarding LEPR function and biology, we speculated that LEPR produced by cancer cells is able to inhibit cell migration and exhibit anti-metastatic effect through STAT3 (Signal Transducer and Activator of Transcription 3) activation which consequently augments TIMP1 (TIMP Metallopeptidase Inhibitor 1) expression, an endogenous inhibitor of MMP2 (Matrix Metalloproteinase 2). In RCC tumors, increased MMP2/9 expression was strongly associated with clinical stage and poor prognosis41. Up-regulation of TIMP-1 has been reported to inhibit metastasis in hepatocellular carcinoma42. These data support our hypothesis that LEPR may inhibit cell migration. Further research regarding the underlying molecular mechanism of LEPR and RCC recurrence is warranted.
Even though our findings suggest an association between epigenetic alteration in obesity-related genes and RCC recurrence, the potential clinical relevance and the interaction of LEPR methylation with obesity/BMI are not fully understood due to the complexity of metabolic cancer pathways in particular leptin/leptin receptor signaling. Additionally, the relationship between obesity and RCC clinical outcome remains uncertain. Multiple studies, including a recent meta-analysis, have suggested that having a higher BMI is associated with improved outcomes in RCC7. However, a recent study did not find extreme obesity as an independent predictor of worse recurrence or survival in a multivariate analysis of surgically treated RCC patients43
Since epigenetics changes are more tissue-specific and the blood cell methylation profile may not reveal the epigenetic state of the tumor, one of the strengths of this study is the possibility of performed the methylation analysis in RCC paired tissue samples, enlightening the important role of methylation in RCC tumorigenesis and clinical outcome. Another strength of this study is that we performed the methylation analysis through a quantitative evaluation of DNA methylation such as pyrosequencing, which may be more optimal for exploring the clinical significance of a given aberrant promoter methylation because qualitative evaluation may have overvalued low-level methylation, which has less clinical significance. Another advantage was the relatively large number of samples analyzed with discovery and validation phases. The present study has also limitations and for prognostic purpose in a clinical setting, epigenetic analysis should be detectable in easily accessible samples such as peripheral blood, because of this the identification and validation of this marker has to be evaluated in other cell-based samples such as paraffin-embedded tissues or circulating cell-free DNA samples. The present study only considered limited CpG sites for each gene promoter regions, we cannot exclude the possibility that other methylation marks may exist and could exhibit significant associations with RCC tumorigenesis and clinicopathologic characteristics.
In conclusion, our findings demonstrates that methylation in NPY, LEP and LEPR promoters is involved in RCC tumorigenesis. In particular, our results suggest that novel methylation marker LEPR is an independent factor of recurrence in RCC patients. This prognostic significance may constitute a promising tool to improve individualized therapy risk stratification. Further research to elucidate the mechanisms and biology underlying the role of LEPR methylation and RCC tumorigenesis and recurrence are needed.
Supplementary Material
Précis.
In this study of 240 tumor-normal pairs from renal cell carcinoma (RCC) patients, we found methylation in obesity-related genes was involved in RCC tumorigenesis and prognosis. Specifically, high methylation in leptin receptor (LEPR) gene was associated with more advanced tumor features and correlated with short recurrence-free survival.
Acknowledgments
Financial support: This work was supported in part by the National Institutes of Health (grant R01 CA170298) and the Center for Translational and Public Health Genomics, Duncan Family Institute for Cancer Prevention, The University of Texas MD Anderson Cancer Center.
Footnotes
Conflict of interest: No potential conflicts of interest are disclosed
Author contribution:
Julia Mendoza-Pérez: conception and design, data acquisition and analysis, manuscript writing and revision; Jian Gu: manuscript writing and revision; Louis A. Herrera: manuscript revision; Nizar M. Tannir: resource provision; Shanyu Zhang: statistical analysis; Surena Matin: resource provision; Jose A. Karam: resource provision; Christopher G. Wood: resource provision; Xifeng Wu: administration and supervision; conception and design, data analysis, funding support, manuscript revision.
References
- 1.Purdue MP, Moore LE, Merino MJ, et al. An investigation of risk factors for renal cell carcinoma by histologic subtype in two case-control studies. Int J Cancer. 2013;132:2640–2647. doi: 10.1002/ijc.27934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii49–56. doi: 10.1093/annonc/mdu259. [DOI] [PubMed] [Google Scholar]
- 3.Sun M, Shariat SF, Cheng C, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol. 2011;60:644–661. doi: 10.1016/j.eururo.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 4.Eichelberg C, Junker K, Ljungberg B, Moch H. Diagnostic and prognostic molecular markers for renal cell carcinoma: a critical appraisal of the current state of research and clinical applicability. Eur Urol. 2009;55:851–863. doi: 10.1016/j.eururo.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.McGuire BB, Fitzpatrick JM. BMI and the risk of renal cell carcinoma. Curr Opin Urol. 2011;21:356–361. doi: 10.1097/MOU.0b013e32834962d5. [DOI] [PubMed] [Google Scholar]
- 6.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 7.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–634. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard L, Rabasa-Lhoret R, Faraj M, et al. Differential epigenomic and transcriptomic responses in subcutaneous adipose tissue between low and high responders to caloric restriction. Am J Clin Nutr. 2010;91:309–320. doi: 10.3945/ajcn.2009.28085. [DOI] [PubMed] [Google Scholar]
- 9.Campion J, Milagro FI, Goyenechea E, Martinez JA. TNF-alpha promoter methylation as a predictive biomarker for weight-loss response. Obesity (Silver Spring) 2009;17:1293–1297. doi: 10.1038/oby.2008.679. [DOI] [PubMed] [Google Scholar]
- 10.Cordero P, Campion J, Milagro FI, et al. Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J Physiol Biochem. 2011;67:463–470. doi: 10.1007/s13105-011-0084-4. [DOI] [PubMed] [Google Scholar]
- 11.Milagro FI, Campión J, Cordero P, et al. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25:1378–1389. doi: 10.1096/fj.10-170365. [DOI] [PubMed] [Google Scholar]
- 12.Lopomo A, Burgio E, Migliore L. Epigenetics of Obesity. Prog Mol Biol Transl Sci. 2016;140:151–84. doi: 10.1016/bs.pmbts.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Ellinger J, Holl D, Nuhn P, et al. DNA hypermethylation in papillary renal cell carcinoma. BJU Int. 2011;107:664–669. doi: 10.1111/j.1464-410X.2010.09468.x. [DOI] [PubMed] [Google Scholar]
- 14.Ricketts CJ, Hill VK, Linehan WM. Tumor-specific hypermethylation of epigenetic biomarkers, including SFRP1, predicts for poorer survival in patients from the TCGA Kidney Renal Clear Cell Carcinoma (KIRC) project. PloS One. 2014;9:e85621. doi: 10.1371/journal.pone.0085621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eggers H, Steffens S, Grosshennig A, et al. Prognostic and diagnostic relevance of hypermethylated in cancer 1 (HIC1) CpG island methylation in renal cell carcinoma. Int J Oncol. 2012;40:1650–1658. doi: 10.3892/ijo.2012.1367. [DOI] [PubMed] [Google Scholar]
- 16.Patrício P, Ramalho-Carvalho J, Costa-Pinheiro P, et al. Deregulation of PAX2 expression in renal cell tumours: mechanisms and potential use in differential diagnosis. J Cell Mol Med. 2013;17:1048–1058. doi: 10.1111/jcmm.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiao W, Wang J, Li H, et al. Fibulin-1 is down-regulated through promoter hypermethylation and suppresses renal cell carcinoma progression. J Urol. 2013;190:291–301. doi: 10.1016/j.juro.2013.01.098. [DOI] [PubMed] [Google Scholar]
- 18.He W, Li X, Xu S, et al. Aberrant methylation and loss of CADM2 tumor suppressor expression is associated with human renal cell carcinoma tumor progression. Biochem Biophys Res Commun. 2013;435:526–532. doi: 10.1016/j.bbrc.2013.04.074. [DOI] [PubMed] [Google Scholar]
- 19.Yamada D, Kikuchi S, Williams YN, et al. Promoter hypermethylation of the potential tumor suppressor DAL-1/4.1B gene in renal clear cell carcinoma. Int J Cancer. 2006;118:916–923. doi: 10.1002/ijc.21450. [DOI] [PubMed] [Google Scholar]
- 20.Morris MR, Ricketts C, Gentle D, et al. Identification of candidate tumour suppressor genes frequently methylated in renal cell carcinoma. Oncogene. 2010;29:2104–2117. doi: 10.1038/onc.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atschekzei F, Hennenlotter J, Janisch S, et al. SFRP1 CpG island methylation locus is associated with renal cell cancer susceptibility and disease recurrence. Epigenetics. 2012;7:447–457. doi: 10.4161/epi.19614. [DOI] [PubMed] [Google Scholar]
- 22.van Vlodrop IJ, Joosten SC, de Meyer T, et al. A four-gene promoter methylation marker panel consisting of GREM1, NEURL, LAD1 and NEFH predicts survival of clear cell renal cell cancer patients. Clin Cancer Res. 2016 Oct 18; doi: 10.1158/1078-0432.CCR-16-1236. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZR, Wei JH, Zhou JC, et al. Validation of DAB2IP methylation and its relative significance in predicting outcome in renal cell carcinoma. Oncotarget. 2016;7:31508–19. doi: 10.18632/oncotarget.8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horiguchi A, Sumitomo M, Asakuma J, et al. Leptin promotes invasiveness of murine renal cancer cells via extracellular signal-regulated kinases and rho dependent pathway. J Urol. 2006;176:1636–1641. doi: 10.1016/j.juro.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 25.Drabkin HA, Gemmill RM. Obesity, cholesterol, and clear-cell renal cell carcinoma (RCC) Adv Cancer Res. 2010;107:39–56. doi: 10.1016/S0065-230X(10)07002-8. [DOI] [PubMed] [Google Scholar]
- 26.Horiguchi A, Sumitomo M, Asakuma J, et al. Increased serum leptin levels and over expression of leptin receptors are associated with the invasion and progression of renal cell carcinoma. J Urol. 2006;176:1631–1635. doi: 10.1016/j.juro.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Spyridopoulos TN, Petridou ET, Dessypris N, et al. Inverse association of leptin levels with renal cell carcinoma: results from a case-control study. Hormones. 2009;8:39–46. doi: 10.14310/horm.2002.1220. [DOI] [PubMed] [Google Scholar]
- 28.Clague J, Lin J, Cassidy A, et al. Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer Epidemiol Biomarkers Prev. 2009;18:801–807. doi: 10.1158/1055-9965.EPI-08-0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Cancer Genome Atlas. TCGA portal overview. https://tcga-data.nci.nih.gov/tcga/. Accessed January, 2016.
- 30.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 31.Dulaimi E, Ibanez de Caceres I, Uzzo RG, et al. Promoter hypermethylation profile of kidney cancer. Clin Cancer Res. 2004;10:3972–3979. doi: 10.1158/1078-0432.CCR-04-0175. [DOI] [PubMed] [Google Scholar]
- 32.Hu CY, Mohtat D, Yu Y, et al. Kidney cancer is characterized by aberrant methylation of tissue specific enhancers that are prognostic for overall survival. Clin Cancer Res. 2014;20:4349–60. doi: 10.1158/1078-0432.CCR-14-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmad ST, Arjumand W, Seth A, Saini AK, Sultana S. Methylation of the APAF-1 and DAPK-1 promoter region correlates with progression of renal cell carcinoma in North Indian population. Tumour Biol. 2012;33:395–402. doi: 10.1007/s13277-011-0235-9. [DOI] [PubMed] [Google Scholar]
- 34.Peters I, Gebauer K, Dubrowinskaja N, et al. GATA5 CpG island hypermethylation is an independent predictor for poor clinical outcome in renal cell carcinoma. Oncol Rep. 2014;31:1523–1530. doi: 10.3892/or.2014.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu L, Li C, Ai H. Correlation analysis between the expressions of leptin and its receptor (ObR) and clinicopathology in endometrial cancer. Cancer Biomark. 2014;14:353–359. doi: 10.3233/CBM-140415. [DOI] [PubMed] [Google Scholar]
- 36.Milosevic VS, Vukmirovic FC, Krstic MS, Zindovic MM, Lj Stojanovic D, Jancic SA. Involvement of leptin receptors expression in proliferation and neoangiogenesis in colorectal carcinoma. J BUON. 2015;20:100–108. [PubMed] [Google Scholar]
- 37.Fan YL, Li XQ. Expression of leptin and its receptor in thyroid carcinoma: distinctive prognostic significance in different subtypes. Clin Endocrinol. 2015;83:261–267. doi: 10.1111/cen.12598. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues PR, Maia LL, Santos M, et al. Leptin receptor expression and Gln223Arg polymorphism as prognostic markers in oral and oropharyngeal cancer. Genet Mol Res. 2015;14:14979–14988. doi: 10.4238/2015.November.24.5. [DOI] [PubMed] [Google Scholar]
- 39.Menghi F, Orzan FN, Eoli M, et al. DNA microarray analysis identifies CKS2 and LEPR as potential markers of meningioma recurrence. Oncologist. 2011;16:1440–1450. doi: 10.1634/theoncologist.2010-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng SP, Liu CL, Hsu YC, Chang YC, Huang SY, Lee JJ. Regulation of leptin receptor expression in human papillary thyroid cancer cells. Biomed Pharmacother. 2012;66:469–473. doi: 10.1016/j.biopha.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 41.Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun HJ, Ringert RH. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol. 1998;160:1914–1918. [PubMed] [Google Scholar]
- 42.Wang N, Zhu M, Tsao SW, Man K, Zhang Z, Feng Y. Up-regulation of TIMP-1 by genipin inhibits MMP-2 activities and suppresses the metastatic potential of human hepatocellular carcinoma. PloS One. 2012;7:e46318. doi: 10.1371/journal.pone.0046318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blute ML, Zorn K, Grimes M, et al. Extreme Obesity Does Not Predict Poor Cancer Outcomes Following Surgery for Renal Cell Cancer. BJU Int. 2016;118:399–407. doi: 10.1111/bju.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.