Abstract
Introduction
Peripheral nerve injuries (PNI) are among the leading causes of physical disability in the United States. The majority of injuries occur in the upper extremities, and functional recovery is often limited. Robust animal models are critical first steps for developing effective therapies to restore function after PNI.
Methods
We developed an automated behavioral assay that provides quantitative measurements of volitional forelimb strength in rats. Multiple forelimb PNI models involving the median and ulnar nerves were used to assess forelimb function for up to 13 weeks postinjury.
Results
Despite multiple weeks of task-oriented training following injury, rats exhibit significant reductions in multiple quantitative parameters of forelimb function, including maximal pull force and speed of force generation.
Conclusions
This study demonstrates that the isometric pull task is an effective method of evaluating forelimb function following PNI and may aid in development of therapeutic interventions to restore function.
Keywords: behavior, forelimb, nerve transection, peripheral nerve injury, rat, volitional strength
Peripheral nerve injuries (PNI) are a major cause of physical disability in the United States. An estimated 20 million Americans are affected by these injuries.1–5 PNI most commonly occur in the upper extremities, resulting in impairment of sensory and motor function of the arm and hand.6,7 Disruption of nerve integrity often leads to severely diminished muscle strength, limb coordination, tactile discrimination, and skilled hand use.8–10 Despite advances in repair techniques and rehabilitative strategies, fewer than 50% of patients regain satisfactory function of the affected limb.8,11 Experimental techniques to augment recovery hold promise, but must be rigorously examined and developed in preclinical models.12–18
Despite the prevalence of nerve injuries in the upper extremities and consequently arm and hand dysfunction in patients, the preponderance of preclinical rodent PNI studies focus on the sciatic nerve of the hind limb. Recent studies demonstrate that forelimb PNI results in significant impairment on standard behavioral measures in rats, including pellet retrieval, grip strength, and horizontal ladder.12,19–23 These behavioral assays have provided valuable insight into forelimb function and recovery after PNI, but additional assays are needed to model other aspects of forelimb dysfunction. Weakness is a significant contributor to disability after PNI,6,24,25 and volitional strength in the forearm is critical to performing many activities of daily living. To date, no preclinical methods quantitatively measure volitional forelimb strength. In conjunction with standard measures of forelimb function, assessment of volitional strength would provide greater insight into function after PNI. Here, we evaluate the isometric pull task, an automated, quantitative measure of volitional forelimb strength, in multiple PNI forelimb models.
MATERIALS AND METHODS
Subjects
Twenty-one adult female Sprague-Dawley rats were studied, each weighing approximately 250 g when they entered the study. All rats were maintained above 85% of their ideal body weight for age. The rats were housed in a 12:12 reversed light cycle environment, and behavioral training was performed during the dark cycle to increase daytime activity levels. All handling, housing, surgical procedures, and behavioral training were approved by the University of Texas at Dallas Institutionl Animal Care and Use Committee.
Behavioral Apparatus and Software
The behavioral apparatus and software were used as previously described.26 The behavioral chamber consists of a clear acrylic cage (30 cm × 13 cm × 25 cm) with a 1-cm-wide slot on the right edge of the front wall (MotoTrak Base Cage, Vulintus, Inc., Dallas, Texas). The slot restricts use to the right forelimb while allowing full range of movement during interaction with the device (Fig. 1A). An aluminum pull handle was centered in the slot at a height of 6 cm from the cage floor. The pull handle was mounted on a metal slide which allowed the device to be placed at various fixed distances relative to the inside wall of the cage (Pull Behavior Module, Vulintus, Inc., Dallas, Texas). A force transducer measured the force applied to the pull handle with a resolution of 0.1 grams. Custom MATLAB software was used to control the task. A microcontroller sampled the force transducer at a frequency of 100 HZ, and the signal was passed to the computer for data display, control of behavioral sessions, and data storage for analysis.
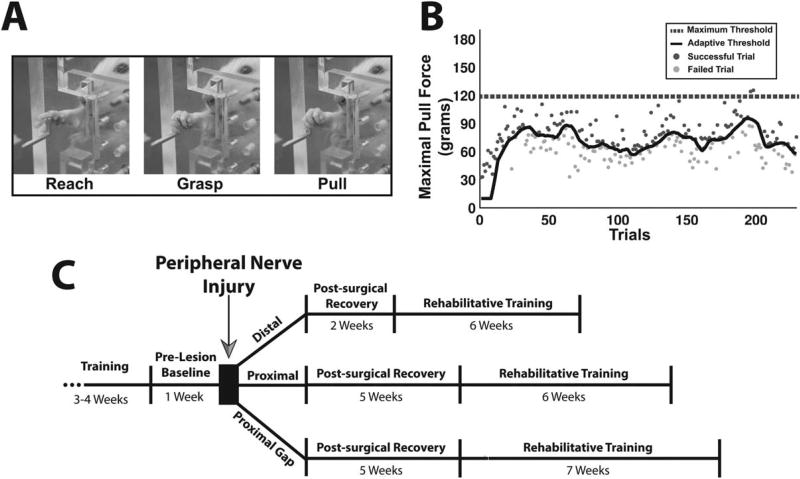
FIGURE 1.
Isometric pull task and experimental design. (A) An animal performing the isometric pull task by firmly grasping and pulling on the handle. (B) Maximal pull force of individual trials taken from a behavioral session. Each point represents the maximal pull force of a single trial, with dark gray points indicating successful trials and light gray ones indicating unsuccessful trials. The black line indicates the adaptive threshold, and the dashed black line indicates the maximum threshold (120 grams). (C) Timeline of experiment.
Behavioral Training
Continuous force transducer data were collected and stored on a trial-by-trial basis for each animal. Trial initiation occurred when the animal generated 10 grams of force on the pull handle. Animals were required to exceed the predetermined force threshold within 2 s of trial initiation to receive a reward pellet and record a successful trial. If the pull force did not exceed the threshold within 2 s, the trial was recorded as a failure, and no reward pellet was given. Each trial was followed by a 2-s timeout, during which a trial could not be initiated. All activity 1 s before and 4 s following trial initiation was recorded for analysis. Reward pellets (45 mg dustless chocolate precision pellet, BioServ, Frenchtown, New Jersey) were delivered from pellet dispensers (Pellet Dispenser, Vulintus, Inc., Dallas, Texas) upon successful completion of a trial.
Animals underwent two 30-min behavioral training sessions daily, 5 days per week, with at least a 2-h interval between training periods. During the initial phases of training, the pull handle was placed 0.5 inches inside the cage wall, and the reward threshold was set to 10 grams. An experimenter encouraged animal interaction with the handle using ground pellet dust. When the animal began to interact with the handle independently, the handle was retracted outside the cage in 0.25-inch increments to a final location of 0.75 inches outside relative to the inner cage wall. After that, behavioral testing continued using an adaptive thresholding program.
The adaptive threshold algorithm used the median of the peak pull force of the immediately preceding 10 trials to calculate the current trial threshold, with programmable minimum and maximum adaptive threshold bounds. Using this algorithm, the threshold was progressively scaled throughout a behavioral session based on performance (Fig. 1B). All animals in this study trained with a reward threshold minimum of 10 grams and a maximum of 120 grams (i.e., the success threshold for any trial was never <10 grams or >120 grams). Success rate in this study was defined as the percentage of trials greater than the maximum threshold. Training continued until animals achieved a ≥85% success rate averaged across 10 consecutive training sessions. Data from the 10 sessions were used for the “Pre” time point in all analyses. At this point, animals underwent the appropriate PNI procedure.
No behavioral testing was conducted during the postsurgical recovery phase. Animals that received a distal injury were given 2 weeks of recovery, and animals receiving proximal or gap injuries were given 5 weeks of recovery during which they remained in their home cage and did not perform the task. The recovery times for each injury were determined by performing weekly postoperative assessments on the isometric pull task until animals were able to independently perform the task. Following this recovery period, behavioral testing continued twice daily for 6 weeks for animals that received distal and proximal injuries and 7 weeks for animals that received gap injuries (Fig. 1C). Analyses were split in to 1-week epochs, with each weekly time point consisting of 10 consecutive sessions (2 sessions per day for 5 weekdays).
Peripheral Nerve Lesions
All injuries were performed on the median and/or ulnar nerves, as appropriate, of the trained (right) forelimb. Three distinct injuries were performed in different cohorts of subjects. The distal injury (N = 6) involved complete transection of the median nerve distal to the elbow. A small incision on the forelimb 2 cm distal to the elbow was made, and the surrounding muscles were carefully retracted to expose the muscular branch of the median nerve. The nerve was completely transected with micro-scissors and repaired with 2 epineural stitches with 9-0 suture (Micro AROSuture, Argosurgical Instruments, Newport Beach, California) to join the nerve stumps. The proximal injury (N = 6) involved transection of both the median and ulnar nerves proximal to the elbow. A small incision was made 1 cm proximal to the elbow, and the pectoralis muscle was carefully retracted to expose the median and ulnar nerves. Both the median and ulnar nerves were fully transected. End-to-end repair of the median and ulnar nerves was immediately performed with 2 epineural stitches for both the median and ulnar nerves. The proximal gap injury (N = 9) was performed in the same location as the proximal injury. Both the median and ulnar nerves were fully transected 1 cm proximal to the elbow. Immediately following transection, the proximal and distal stumps of each nerve were sutured 1 mm from the ends of a 7-mm saline filled polyurethane tube (Micro-Renathane 0.095-inch inside diameter 0.066-inch outside diameter, Braintree Scientific, Inc., Braintree, Massachusetts), resulting in a 5-mm gap between nerve stumps.
Following each procedure, skin incisions were sutured and treated with antibiotic ointment. All animals were given Baytril (7.5 mg/kg) immediately following surgery and sustained release buprenorphine (1.2 mg/kg) for 6 days following injury. All animals were placed in Elizabethan collars for 5 days following injury to prevent chewing on the denervated limb. No animals in this study displayed autophagia.
Statistics
All data are shown as mean ± SEM. All comparisons were planned in the experimental design a priori, and significant differences were determined using 1-way analyses of variance (ANOVAs), 1-way repeated measures ANOVAs, and 2-tailed t-tests, where appropriate. Effect sizes were determined using Cohen’s d. Alpha level was set to 0.05 for single comparisons. A Bonferroni-corrected alpha for multiple comparisons of 0.0083 was used for the distal and proximal injury groups, and an alpha of 0.0071 for the gap injury group
RESULTS
Isometric Pull Task Acquisition
Animals became highly proficient at the isometric pull task, reaching a ≥85% success rate averaged across 10 consecutive sessions within 16 ± 2 days. During the final 10 sessions of preinjury training, animals demonstrated an average maximal pull force of 166 ± 3.4 grams and average success rate of 91% ± 0.6% across all groups.
Performance after Distal Median Nerve Transection and End-to-End Repair
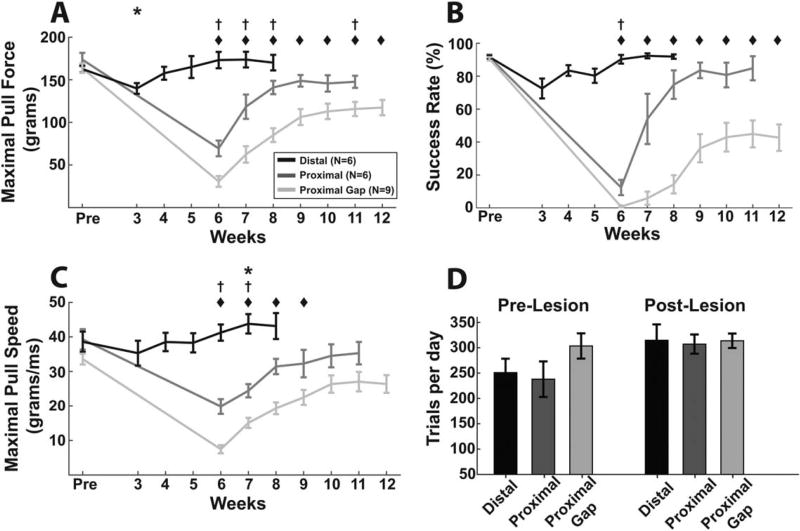
Median nerve transection and end-to-end repair 2 cm distal to the elbow transiently worsened performance on the isometric pull task. A repeated measures 1-way ANOVA showed a significant effect of distal lesion on maximal pull force [Fig. 2A; F(7,56) = 10.28; P = 3.46 × 10−6] and success rate [Fig. 2B; F(6,30) = 7.24; P = 7.59 × 10−5]. Post hoc comparisons indicated that maximal pull force was significantly decreased compared with prelesion on Week 3, but was not significantly reduced in the subsequent weeks (Fig. 2A; Pre vs. Weeks 3–8; paired t-test; P < 0.0083 for Week 3). Success rate (Fig. 2B) followed a similar trend, but post hoc comparisons failed to reach significance during any week. Speed of force generation (Fig. 2C) was not significantly decreased after distal lesion. Together, these findings indicate that distal median nerve transection with end-to-end repair resulted in a transient reduction in volitional forelimb strength.
FIGURE 2.
Multiple PNI models resulted in varying degrees of transient and chronic impairments in metrics of forelimb function. Maximal pull force (A), success rate (B), and maximal pull speed (C) each show varying degrees of impairment dependent on lesion. (D) Animals performed several hundred trials per day across groups, with no reduction in trials performed following lesion. All plots show group averages, and error bars indicate ± SEM. *P < 0.0083 for the distal group, †P < 0.0083 for the proximal group, ◆P < 0.0071 for the proximal gap group for each time point compared with prelesion levels in panels A–C.
Performance after Proximal Median and Ulnar Nerve Transection and End-to-End Repair
This injury model resulted in substantially reduced forelimb function over the course of several weeks. A repeated measures 1-way ANOVA revealed a significant effect of lesion on maximal pull force [Fig. 2A; F(6,30) = 41.47; P < 3.29 × 10−13] and success rate [Fig. 2B; F(6,30) = 23.99; P < 3.39 × 10−10]. Post hoc comparisons demonstrated that maximal pull force was significantly reduced on most weeks postlesion despite extensive task-oriented training (Fig. 2A; Pre vs. Weeks 6–11; paired t-test; P < 0.0083 for Weeks 6–8 and 11), suggesting a chronic impairment of volitional strength. Success rate demonstrated a significant reduction compared with prelesion at Week 6 (Fig. 2B; Pre vs. Weeks 6–11; paired t-test; P < 0.0083 for Week 6).
Speed of force generation was significantly reduced by a proximal lesion [Fig. 2C; repeated measures 1-way ANOVA; F(6,30) = 11.65; P = 1.02 × 10−6], consistent with weakness associated with forelimb impairment.27 Post hoc comparisons revealed a significant reduction compared with prelesion during Weeks 6 and 7 (Fig. 2C; Pre vs. Weeks 6–11; paired t-test; P < 0.0083 for Weeks 6 and 7). The effect size (Cohen’s d) of maximal force between Pre and the final week of testing (Week 11) was 1.42, and apparent asymptotic recovery was observed during the final 4 weeks of training [Fig. 2A: Repeated measures 1-way ANOVA; Maximal Pull Force, Weeks 8–11; F(3,15) = 1.1867; P = 0.35]. These results demonstrate that median and ulnar nerve transection proximal to the elbow with end-to-end repair impairs multiple parameters of volitional forelimb function.
Performance after Proximal Median and Ulnar Nerve Transection and Tubular Repair with a 5-mm Gap
Volitional forelimb function was markedly diminished following gap injury. A repeated measures 1-way ANOVA of maximal pull force revealed a significant effect of lesion [Fig. 2A; F(7,56) = 49.65; P < 9.76 × 10−22]. Post hoc comparisons indicated that maximal pull force was significantly reduced at every week postinjury (Fig. 2A; Pre vs. Weeks 6–12; paired t-test; all P < 0.007), suggesting a chronic impairment of volitional strength. Success rate was also significantly impaired by the gap injury [Fig. 2B; Repeated measures 1-way ANOVA; F(7,56) = 33.42; P < 8.73 × 10−18]. Post hoc comparisons on success rate indicated significant reductions at all postlesion time points compared with prelesion (Fig. 2B; Pre vs. Weeks 6–12; paired t-test; all P < 0.007). Speed of force generation was significantly reduced during Weeks 6–9 [Fig. 2C; F(7,56) = 35.25; P < 2.68 × 10−18; post hoc paired t-tests, Pre vs. Weeks 6–9; P < 0.007]. The effect size (Cohen’s d) of maximal force between Pre and Week 12 was 2.13. Similarly to the proximal injury, asymptotic performance was observed across the final 4 weeks of rehabilitation [Fig. 2A: repeated measures 1-way ANOVA; Maximal Pull Force, Weeks 9–12; F(3,24) = 1.71; P = 0.19]. Together, these results indicate that the gap injury results in a marked, chronic impairment of volitional forelimb strength.
Performance in the 3 injury groups was compared to determine if the isometric pull task could discriminate performance deficits between injury models. A 1-way ANOVA on maximal pull force during the first week of rehabilitative training revealed a significant effect of lesion [1-way ANOVA; maximal pull force: Distal Week 3, Proximal Week 6, Proximal Gap Week 6; F(2,18) = 58.69, P=1.29 × 10−8]. Post hoc comparisons revealed that each injury group was significantly different from the others (Unpaired t-test; Distal vs. Proximal, P = 8.66 × 10−5; Distal vs. Proximal Gap, P = 2.75 × 10−8; Proximal vs. Proximal Gap, P = 0.0032). Similarly, a 1-way ANOVA revealed a significant effect of lesion on maximal pull force during the final week of rehabilitative training [1-way ANOVA; Maximal Pull Force: Distal Week 8, Proximal Week 11, Proximal Gap Week 12; F(2,18) = 9.44; P = 0.0016]. Post hoc comparisons revealed significant effects between the Distal and Proximal Gap injury group, and Proximal and Proximal Gap injury groups (Unpaired t-test; Distal vs. Proximal, P=0.08; Distal vs. Proximal Gap; P = 0.0017; Proximal vs. Proximal Gap; P = 0.03). These results indicate that the isometric pull task can detect graded deficits in performance across PNI models of differing severity.
Number of Isometric Pull Task Trials Performed during Study
We investigated whether lesion severity affected ability to perform trials. Trial counts of the 3 injury groups were not significantly different [Fig. 2D; 2-way ANOVA; Group × Time, F(2,18) = 1.11; P = 0.35] despite the substantial difference in forelimb performance, indicating the nerve injuries did not impair the animal’s capability to perform trials. These results suggest that differences in the number of trials performed following lesions cannot account for the differences in functional impairment across lesion models.
DISCUSSION
While previous studies in rodents have provided thorough characterization of motor performance following forelimb peripheral nerve injuries,19–22,28–31 additional assays are needed to thoroughly model the complex functional consequences of these injuries. The isometric pull task provides an additional efficient method to evaluate volitional forelimb strength in rats and yields a large quantitative dataset. Unlike grip strength assessment, the isometric pull task provides automated measures of forelimb strength without experimenter intervention during testing. A previous study demonstrated that the isometric pull task correlates well with standard forelimb assessments including pellet reaching and pasta matrix tasks, but yields a larger effect size and decreases experimenter oversight.32 This study suggests that the isometric pull task is sensitive to multiple models of PNI and could be combined with additional motor and sensory assays to gain a more comprehensive understanding of forelimb dysfunction following injury.
Three distinct nerve injuries were performed to examine the behavioral effects on the isometric pull task. The distal median nerve injury resulted in transient forelimb grasping dysfunction. This injury was performed distal to the median nerve bifurcation, targeting the muscular branch of the median nerve. The muscular branch supplies partial innervation to the digit flexors of the forelimb (flexor digitorum sublimis, flexor digitorum profundus, and palmaris longus) and was fully transected. The spared branch, the volar interosseous, supplies innervation to the pronator quadratus and the remaining heads of the flexor digitorum profundus. Additionally, multiple intrinsic muscles of the forepaw supplied by the muscular branch were also damaged.33 The model produced minor impairments in maximal pull force, and all measured parameters returned to prelesion levels within 4 weeks of the injury. This model may prove useful in short-term studies due to the transient deficit.
The proximal injury produced a more severe functional impairment compared with the distal injury. Proximal peripheral nerve injuries require reinnervation over greater distances and consequently increased time, resulting in less successful reinnervation of target end-organs. Axonal misdirection is also increased following proximal injuries, leading to impaired functional recovery.34 Furthermore, the ulnar nerve was included in this model, because previous studies have shown combined ulnar and median injuries reduce performance on the staircase and grip strength tasks compared with median only injuries.12,20,21 The proximal injuries were performed 1cm above the elbow, resulting in complete denervation of all extrinsic and intrinsic digit flexors.33 Previous studies have characterized reinnervation patterns following end-to-end repair of the median and ulnar nerves.12,20,21,29,35,36 Using the isometric pull task, animals with proximal median and ulnar nerve injuries exhibit significant impairments in maximal force, success rate, and pull speed during postinjury training. These results suggest that this injury model may be used for long-term studies.
Similar to the proximal injury, the gap injury resulted in complete denervation of digit flexors of the forelimb. Saline filled tubes are often used as controls to other complex interventions to promote growth37; therefore, saline-filled polyurethane tubes were used to investigate loss of function and recovery under control conditions. Additionally, nerve reinnervation is known to occur following short saline-filled tubular gaps.16,38,39 Following gap injury, all measured parameters reflecting volitional forelimb function were significantly reduced for the entirety of postinjury training. This injury model produces a substantial chronic behavioral impairment and provides a framework to assess therapeutic interventions.
The 3 distinct nerve injuries described above resulted in a wide range of behavioral impairments. Due to the variability in forelimb function postlesion, the adaptive thresholding program allowed for simple, flexible software-controlled postinjury training. All animals in this study were tested on the same training stage with adaptively scaled 10–120 gram lower and upper bounds, encompassing a wide dynamic range of forces. This range allowed for a variety of lesion severities to be accurately measured, reducing experimenter involvement with training progression and thus limiting experimenter bias. No animals in this study were excluded due to variability of postinjury impairment (i.e., impairment that was too severe to be measured, or too mild and resulted in no task deficit). Additionally, the large number of trials performed per day ensures that performance data can be reliably assessed before and after lesioning.
The isometric pull task allows efficient, quantitative analysis of forelimb function, providing a rich dataset of continuous high-resolution force measurements. Along with recent reports in stroke, traumatic brain injury, and spinal cord injury, this study demonstrates the flexibility and sensitivity of the isometric pull task to assay forelimb function after neurological injury.26,40,41 The isometric pull task may also be useful in investigating additional forelimb PNI models, including nerve crush and brachial plexus injury. This study demonstrates that the isometric pull task may provide a framework for investigators to study peripheral nerve injuries and aid in development of therapeutic strategies to restore function.
Acknowledgments
Funding: This work was sponsored in part by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) ElectRx program under the auspices of Dr. Doug Weber through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 (R.L.R., M.P.K., and S.A.H.) and by NIH NINDS R01 NS094384-01 (S.A.H.) and R01 NS085167-01 (R.L.R. and M.P.K.)
The authors thank Andrew Sloan for engineering assistance, Patrick Ganzer for his valuable input on the project, and Nicole Robertson, Katherine Adcock, and Justin James for assistance with surgical procedures and postoperative care. We also thank Rachel Choi, Tania Kader, Sania Khan, Jennifer Putman, Preston D’Souza, Sanketh Kichena, Sina Kashef, Ai Hua, Roshan Manuel, Ami Shah, Sana Arni, Hansaim Jeong, Shreya Permanaki, Helena Zhang, Rachel Liao, Roja Todigala, and Tim Park for their assistance in behavioral testing.
Abbreviations
- ANOVA
analysis of variance
- PNI
peripheral nerve injury
Footnotes
Conflicts of Interest: R.L.R. own shares in Vulintus, Inc., which is developing products based on this research. Vulintus, Inc., did not have any role in data collection, analysis, or the decision to publish. The remaining authors have no conflicts of interest.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45:116–122. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 2.Liu W, Ren Y, Bossert A, Wang X, Dayawansa S, Tong J, et al. Allotransplanted neurons used to repair peripheral nerve injury do not elicit overt immunogenicity. PLoS One. 2012;7:e31675. doi: 10.1371/journal.pone.0031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selecki BR, Ring IT, Simpson DA, Vanderfield GK, Sewell MF. Trauma to the central and peripheral nervous systems. Part II. A statistical profile of surgical treatment New South Wales 1977. Aust N Z J Surg. 1982;52:111–116. doi: 10.1111/j.1445-2197.1982.tb06081.x. [DOI] [PubMed] [Google Scholar]
- 4.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:1–13. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor CA, Braza D, Rice JB, Dillingham T. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87:381–385. doi: 10.1097/PHM.0b013e31815e6370. [DOI] [PubMed] [Google Scholar]
- 6.Kouyoumdjian JA. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34:785–788. doi: 10.1002/mus.20624. [DOI] [PubMed] [Google Scholar]
- 7.McAllister RM, Gilbert SE, Calder JS, Smith PJ. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J Hand Surg Br. 1996;21:4–13. doi: 10.1016/s0266-7681(96)80004-0. [DOI] [PubMed] [Google Scholar]
- 8.Jaquet JB, Luijsterburg AJ, Kalmijn S, Kuypers PD, Hofman A, Hovius SE. Median, ulnar, and combined median-ulnar nerve injuries: functional outcome and return to productivity. J Trauma. 2001;51:687–692. doi: 10.1097/00005373-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Duff SV. Impact of peripheral nerve injury on sensorimotor control. J Hand Ther. 2005;18:277–291. doi: 10.1197/j.jht.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Brandsma JW, Schreuders TA. Sensible manual muscle strength testing to evaluate and monitor strength of the intrinsic muscles of the hand: a commentary. J Hand Ther. 2001;14:273–278. doi: 10.1016/s0894-1130(01)80005-3. [DOI] [PubMed] [Google Scholar]
- 11.Ruijs ACJ, Jaquet J-B, Kalmijn S, Giele H, Hovius SER. Median and ulnar nerve injuries: a meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast Reconstr Surg. 2005;116:484–494. doi: 10.1097/01.prs.0000172896.86594.07. [DOI] [PubMed] [Google Scholar]
- 12.Galtrey CM, Asher RA, Nothias F, Fawcett JW. Promoting plasticity in the spinal cord with chondroitinase improves functional recovery after peripheral nerve repair. Brain. 2007;130(Pt 4):926–939. doi: 10.1093/brain/awl372. [DOI] [PubMed] [Google Scholar]
- 13.Ghizoni MF, Bertelli JA, Grala CG, da Silva RM. The anabolic steroid nandrolone enhances motor and sensory functional recovery in rat median nerve repair with long interpositional nerve grafts. Neurorehabil Neural Repair. 2013;27:269–276. doi: 10.1177/1545968312465190. [DOI] [PubMed] [Google Scholar]
- 14.Arbat-Plana A, Torres-Espín A, Navarro X, Udina E. Activity dependent therapies modulate the spinal changes that motoneurons suffer after a peripheral nerve injury. Exp Neurol. 2015;263:293–305. doi: 10.1016/j.expneurol.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Ma CHE, Omura T, Cobos EJ, Latrémolière A, Ghasemlou N, Brenner GJ, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemp SWP, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229:460–470. doi: 10.1016/j.expneurol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Udina E, Puigdemasa A, Navarro X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve. 2011;43:500–509. doi: 10.1002/mus.21912. [DOI] [PubMed] [Google Scholar]
- 18.English AW, Wilhelm JC, Sabatier MJ. Enhancing recovery from peripheral nerve injury using treadmill training. Ann Anat. 2011;193:354–361. doi: 10.1016/j.aanat.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papalia I, Tos P, Stagno d’Alcontres F, Battiston B, Geuna S. On the use of the grasping test in the rat median nerve model: a re-appraisal of its efficacy for quantitative assessment of motor function recovery. J Neurosci Methods. 2003;127:43–47. doi: 10.1016/s0165-0270(03)00098-0. [DOI] [PubMed] [Google Scholar]
- 20.Galtrey CM, Fawcett JW. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J Peripher Nerv Syst. 2007;12:11–27. doi: 10.1111/j.1529-8027.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Sorenson EJ, Spinner RJ, Windebank AJ. Electrophysiologic findings and grip strength after nerve injuries in the rat forelimb. Muscle Nerve. 2008;38:1254–1265. doi: 10.1002/mus.20971. [DOI] [PubMed] [Google Scholar]
- 22.Ronchi G, Nicolino S, Raimondo S, Tos P, Battiston B, Papalia I, et al. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J Neurosci Methods. 2009;179:51–57. doi: 10.1016/j.jneumeth.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Pagnussat AS, Michaelsen SM, Achaval M, Ilha J, Hermel EES, Back FP, et al. Effect of skilled and unskilled training on nerve regeneration and functional recovery. Braz J Med Biol Res. 2012;45:753–762. doi: 10.1590/S0100-879X2012007500084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- 25.Schreuders TAR, Roebroeck ME, Jaquet J-B, Hovius SER, Stam HJ. Measuring the strength of the intrinsic muscles of the hand in patients with ulnar and median nerve injuries: reliability of the Rotterdam Intrinsic Hand Myometer (RIHM) J Hand Surg Am. 2004;29:318–324. doi: 10.1016/j.jhsa.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Hays SA, Khodaparast N, Sloan AM, Hulsey DR, Pantoja M, Ruiz AD, et al. The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J Neurosci Methods. 2013;212:329–337. doi: 10.1016/j.jneumeth.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Canning CG, Ada L, O’Dwyer N. Slowness to develop force contributes to weakness after stroke. Arch Phys Med Rehabil. 1999;80:66–70. doi: 10.1016/s0003-9993(99)90309-x. [DOI] [PubMed] [Google Scholar]
- 28.Bertelli JA, Mira JC. The grasping test: a simple behavioral method for objective quantitative assessment of peripheral nerve regeneration in the rat. J Neurosci Methods. 1995;58:151–155. doi: 10.1016/0165-0270(94)00169-h. [DOI] [PubMed] [Google Scholar]
- 29.Papalia I, Tos P, Scevola A, Raimondo S, Geuna S. The ulnar test: a method for the quantitative functional assessment of posttraumatic ulnar nerve recovery in the rat. J Neurosci Methods. 2006;154:198–203. doi: 10.1016/j.jneumeth.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Speck AE, Ilha J, do Espírito Santo CC, Aguiar AS, Dos Santos ARS, Swarowsky A. The IBB forelimb scale as a tool to assess functional recovery after peripheral nerve injury in mice. J Neurosci Methods. 2014;226:66–72. doi: 10.1016/j.jneumeth.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Spinner RJ, Sorenson EJ, Windebank AJ. Measurement of forelimb function by digital video motion analysis in rat nerve transection models. J Peripher Nerv Syst. 2008;13:92–102. doi: 10.1111/j.1529-8027.2008.00162.x. [DOI] [PubMed] [Google Scholar]
- 32.Sloan AM, Fink MK, Rodriguez AJ, Lovitz AM, Khodaparast N, Rennaker RL, et al. A within-animal comparison of skilled forelimb assessments in rats. PLoS One. 2015;10:e0141254. doi: 10.1371/journal.pone.0141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene EC. Anatomy of the rat. Philadelphia: American Philosophical Society; 1935. [Google Scholar]
- 34.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Bontioti E, Kanje M, Lundborg G, Dahlin LB. End-to-side nerve repair in the upper extremity of rat. J Peripher Nerv Syst. 2005;10:58–68. doi: 10.1111/j.1085-9489.2005.10109.x. [DOI] [PubMed] [Google Scholar]
- 36.Sinis N, Guntinas-Lichius O, Irintchev A, Skouras E, Kuerten S, Pavlov SP, et al. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Exp Brain Res. 2008;185:469–483. doi: 10.1007/s00221-007-1174-y. [DOI] [PubMed] [Google Scholar]
- 37.Deumens R, Bozkurt A, Meek MF, Marcus MAE, Joosten EAJ, Weis J, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92:245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Bodine-Fowler SC, Meyer RS, Moskovitz A, Abrams R, Botte MJ. Inaccurate projection of rat soleus motoneurons: a comparison of nerve repair techniques. Muscle Nerve. 1997;20:29–37. doi: 10.1002/(sici)1097-4598(199701)20:1<29::aid-mus4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Boyd JG, Gordon T. Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol. 2003;183:610–619. doi: 10.1016/s0014-4886(03)00183-3. [DOI] [PubMed] [Google Scholar]
- 40.Pruitt D, Hays S, Schmid A, Choua C, Kim L, Trieu J, et al. Controlled-cortical impact reduces volitional forelimb strength in rats. Brain Res. 2014;1582:91–98. doi: 10.1016/j.brainres.2014.07.039. [DOI] [PubMed] [Google Scholar]
- 41.Ganzer PD, Meyers EC, Sloan AM, Maliakkal R, Ruiz A, Kilgard MP, et al. Awake behaving electrophysiological correlates of forelimb hyperreflexia, weakness and disrupted muscular synchronization following cervical spinal cord injury in the rat. Behav Brain Res. 2016;307:100–111. doi: 10.1016/j.bbr.2016.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]