Abstract
TGF-β is a multifunctional cytokine affecting many cell types and implicated in tissue remodeling processes. Due to its many functions and cell-specific effects, the consequences of TGF-β signaling are process-and stage-dependent, and it is not uncommon that TGF-β exerts distinct and sometimes opposing effects on a disease progression depending on the stage and on the pathological changes associated with the stage. The mechanisms underlying cell- and process-specific effects of TGF-β are poorly understood.
We are describing a novel pathway that mediates induction of angiogenesis in response to TGF-β1. We found that in endothelial cells (EC) TSP-4, a secreted extracellular matrix (ECM) protein is upregulated in response to TGF-β1 and mediates the effects of TGF-β1 on angiogenesis.
Upregulation of TSP-4 does not require the synthesis of new protein, is not caused by decreased secretion of TSP-4, and is mediated by activation of SMAD3. Using Thbs4−/− mice and TSP-4 shRNA, we found that TSP-4 mediated pro-angiogenic functions on cultured EC and angiogenesis in vivo in response to TGF-β1. We observed ~ 3-fold increases in tumor mass and levels of angiogenesis markers in animals injected with TGF-β1, and these effects did not occur in Thbs4−/− animals. Injections of an inhibitor of TGF-β1 signaling SB431542 also decreased the weights of tumors and cancer angiogenesis.
Our results from in vivo angiogenesis models and cultured EC document that TSP-4 mediates upregulation of angiogenesis by TGF-β1. Upregulation of pro-angiogenic TSP-4 and selective effects of TSP-4 on EC may contribute to stimulation of tumor growth by TGF-β despite the inhibition of cancer cell proliferation.
Keywords: angiogenesis, TGF-β1, thrombospondin-4
Introduction
TGF-β is a cytokine with important functions in physiological and pathological processes associated with tissue remodeling, e.g., organ fibrosis, atherosclerosis, aortic aneurysm, diabetic vascular complications, and cancer (1–8). A large body of literature suggests that TGF-β, its receptors, and mediators of its downstream signaling are attractive targets for therapeutic interventions (6, 9–13). However, TGF-β has a number of cell-type-specific and process-specific functions that are still poorly understood. The information regarding the consequences of TGF-β signaling remains incomplete and controversial. Cell-specific action of TGF-β manifests as distinct and sometimes opposite effects on disease progression, depending on the stage and location of a pathological process (6). For example, in cancer, TGF-β suppresses tumorigenesis during the early stages by inducing cancer cell apoptosis and cell cycle arrest and by supporting cell differentiation. However, at the later stages associated with increased angiogenesis, TGF-β effects can be pro-angiogenic, and TGF-β promotes tumor growth and metastasis by activation of specific pathways in vascular cell types and increasing angiogenesis. The context- and disease-stage-dependent effects of TGF-β manifest in other diseases as well, e.g., in aortic aneurysm, muscular dystrophy, and connective tissue disorders (14).
Angiogenesis is a critical modifier of every tissue remodeling process. Most tissue remodeling depends on and induces angiogenesis; the fate of remodeling is often defined by the ability to support angiogenesis. Here, we report that TGF-β1 upregulates the production of TSP-4, a pro-angiogenic extracellular matrix protein (15), in cultured endothelial cells (EC). Despite recent observations of critical roles for TSP-4 in heart, blood vessels, cancer (16–21), and the nervous system (22–26), stimuli upregulating TSP-4 are still unknown. Our results suggest that modulation of TSP-4 levels is a mechanism activated by TGF-β1 to promote angiogenesis.
TGF-β is one of the major regulators of the ECM. It induces profound changes in the composition and the amount of vascular matrix (27, 28), which causes cellular changes and, as a result, enhances angiogenesis. Angiogenesis critically depends on the composition of ECM and is regulated by ECM (29–31). TGF-β exerts its effects on ECM remodeling mainly by upregulating the expression of ECM proteins through SMAD signaling (32). SMADs are thought to act primarily at a transcriptional level, but also can regulate post-transcriptional events (33–39).
Our observations suggest that TGF-β1 promotes angiogenesis by altering the composition of the ECM in favor of pro-angiogenic cues and that upregulation of pro-angiogenic TSP-4 is EC-specific, plays a central role in acceleration of angiogenesis in response to TGF-β1, and may contribute to cell- and disease-stage-specific effects of TGF-β.
Results
TGF-β1 induces TSP-4 production in EC
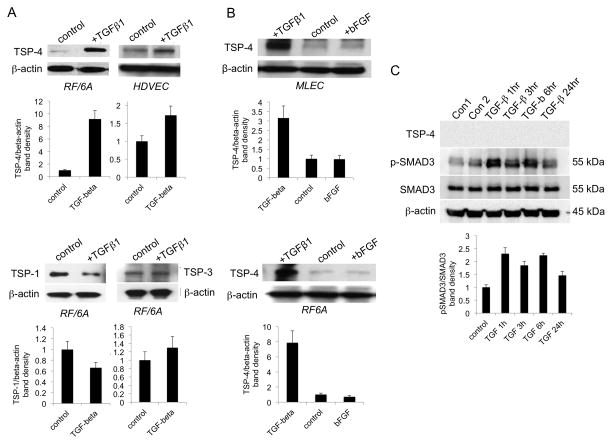
Microvascular EC (mouse lung EC, MLEC; human dermal vascular EC, HDMEC; and RF/6A, a monkey eye EC line) cells were treated with TGF-β1 (10 ng/ml) for 24 hrs. Western blotting of cell lysates revealed increases in TSP-4 levels upon TGF-β1 stimulation (Fig. 1A top, 1B). Upregulation was selective: TSP-1 levels were decreased in response to TGF-β1, and TSP-3 levels were not affected (Fig. 1A, bottom). TSP-4 upregulation was cell-specific: macrovascular arterial EC (human umbilical vein EC, HUVEC) did not respond to TGF-β1 (not shown), and there was no effect of TGF-β1 on TSP-4 production in vascular smooth muscle cells (VSMC) (Fig. 1C) although they responded to TGF-β1 stimulation by increasing phosphorylation of SMAD3. The response of microvascular EC was stimulus-specific: bFGF did not increase TSP-4 levels (Fig. 1B).
Figure 1. Treatment of microvascular EC with TGF-β1 increases TSP-4 levels.
A: RF/6A cells and HDMEC were stimulated with 10 ng/ml TGF-β1 for 24 h. TSP-4, TSP-1, TSP-3, and β-actin were visualized with corresponding antibodies in Western blotting as described in the Methods. B: MLEC and RF/6A cells were treated with 10 ng/ml TGF-β1 or bFGF; TSP-4 and β-actin were detected in Western blotting. C: Human VSMC were treated with TGF-β1 for indicated time periods; TSP-4, total SMAD3, phosphorylated SMAD3, and β-actin were detected in Western blots.
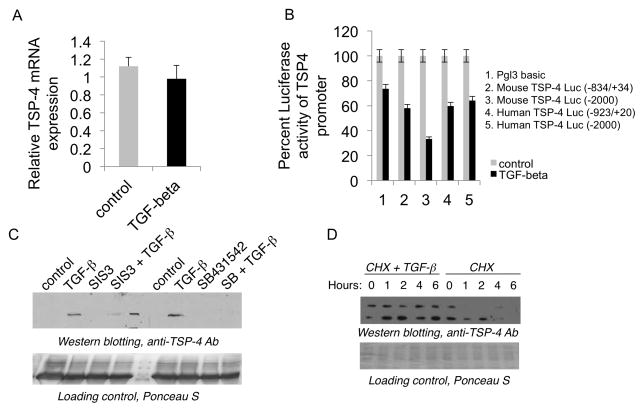
The levels of TSP-4 mRNA were not upregulated in RF/6A in response to TGF-β1 at 3 – 24 hours (Fig. 2A). TGF-β1 failed to activate mouse and human THBS4 promoter; reporter production was not increased in response to TGF-β1 in RF/6A cells transiently transfected with constructs expressing luciferase under two mouse Thbs4 and two human THBS4 promoters (Fig. 2B).
Figure 2. TGF-β1 regulates TSP-4 at the level of protein stability.
A: TSP-4 mRNA in cells stimulated with TGF-β1. RF/6A microvascular EC were stimulated with 10 ng/ml of TGF-β1 for 6 hours, and TSP-4 mRNA levels were analyzed by Quantitative RT-PCR. B: Promoter-reporter constructs were transiently transfected to RF/6A cells as described in Methods, and cells were stimulated with TGF-β1 the next day for 24 hours. Luciferase activity was measured in cell lysates. C: Cell culture supernatants (60 μl) form RF/6A stimulated with TGF-β1 were analyzed by Western blotting with anti-TSP-4 antibody. SIS3 = cells pre-treated with SIS3 for 30 min as described in Methods; SB and SB431542 = cells pre-treated with SIS3 for 30 min as described in Methods. D: RF/6A cells were pre-treated with 10 μg/ml cyclohexamide for 30 min and stimulated with TGF-β1 and analyzed by Western blotting with anti-TSP-4.
Increased levels of TSP-4 were detected in cell culture supernatants of RF/6A cells 24 hours after TGF-β1 stimulation (Fig. 2C), suggesting that decreased secretion was not causing the increased levels of TSP-4 in cell lysates. The effect of TGF-β1 on the levels of TSP-4 in supernatants was inhibited by a TGF-β1 receptor inhibitor SB-431542 and a SMAD3 inhibitor SIS3.
When we inhibited the protein synthesis in RF/6A with 10 μg/ml cyclohexamide, the levels of TSP-4 were still upregulated by TGF-β1 added to the cells 30 min after pretreatment with cyclohexamide (Fig. 2D). In cells treated with cyclohexamide alone the levels of TSP-4 rapidly declined, and the protein was undetectable by 4 hours.
TGF-β1 induces TSP-4 production via SMAD3
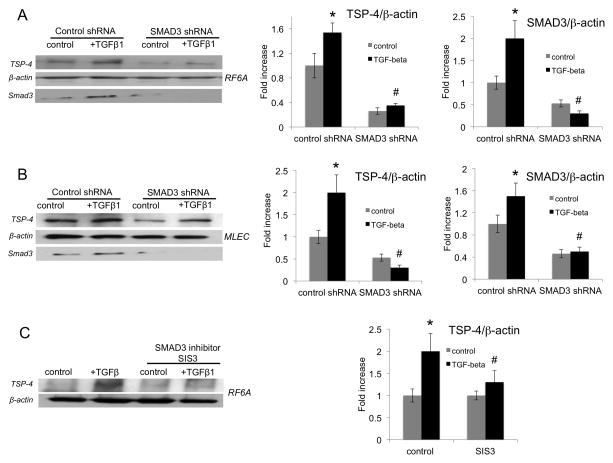
MLEC and RF/6A were infected with lentiviral particles expressing SMAD3 shRNA (25,000 IFU/ml) and stimulated with TGF-β1 for 24 hours. Levels of TSP-4 in cells expressing SMAD3 shRNA were compared to the levels of TSP-4 in cells expressing control shRNA in cell lysates using Western blotting (Fig. 3A,B). The levels of TSP-4 were decreased in both non-stimulated and TGF-β1-stimulated cells expressing SMAD3 shRNA. The knockdown of SMAD3 was confirmed in Western blotting using anti-SMAD3 antibody, and equal loading of cell lysates was verified and normalized using anti-β-actin.
Figure 3. SMAD3 mediates upregulation of TSP-4 in response to TGF-β1.
A, B: RF/6A and MLEC were transfected with lentiviral particles expressing SMAD3 shRNA (see Methods). Cells were stimulated with TGF-β1 for 24 h and lysed, and proteins were separated in SDS-PAGE followed by Western blotting. TSP-4, β-actin, and SMAD3 were detected using specific antibodies. C: RF/6A cells were pre-treated with the inhibitor of SMAD3 SIS3 as described in the Methods and stimulated with TGF-β1 and analyzed by Western blotting with anti-TSP-4.
When SMAD3-specific Stealth RNAi duplexes were transfected into RF/6A cells followed by stimulation with TGF-β1 for 24 hours, the effect of the alternative siRNA oligonucleotides was identical: SMAD3 was knocked down, and this resulted in reduced levels of TSP-4 (Suppl. Fig. 1).
In a complementary alternative approach, we inhibited SMAD3 using a specific SMAD3 inhibitor SIS3 (40). SIS3 inhibited the increase in TSP-4 levels in cell lysates in response to TGF-β1 in RF/6A (Fig. 3C). The specificity of SIS3 in endothelial cells was confirmed using antibodies for other receptor-activated SMADs, SMAD2 and SMAD1/5/9 (Suppl. Fig. 2).
TSP-4 mediates an increase in EC adhesion and migration in response to TGF-β1
We recently reported that TSP-4 upregulates adhesion, migration, and proliferation of EC (15). These responses are modulated by TGF-β1, and we sought to determine whether TSP-4 mediates these changes in cellular functions in response to TGF-β1. Accordingly, we examined the responses to TGF-β1 in cultured microvascular EC (MLEC) and macrovascular EC (mouse aortic EC, MAEC) from WT and Thbs4−/− (KO) mice (Fig. 4).
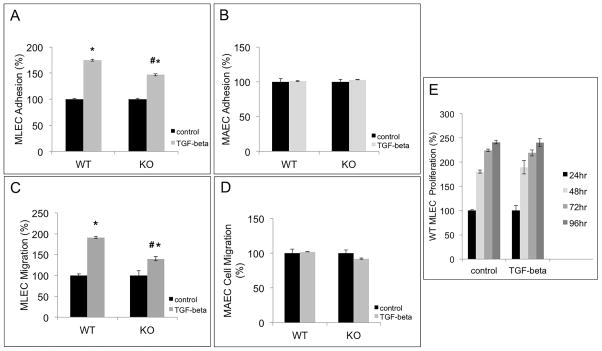
Figure 4. TSP-4 mediates increased adhesion and migration of microvascular EC in response to TGF-β1.
A: TGF-β1-stimulated MLEC from WT and Thbs4−/− (KO) mice were seeded into 24-well plates coated with fibronectin and incubated at 37 °C for 1 h. Unattached cells were removed by washing, and the remaining DNA in the wells was measured using the Cyquant reagent. Adhesion of TGF-β1-stimulated MLEC was compared to the adhesion of non-stimulated cells. *p<0.05 compared to non-stimulated cells, # p<0.05 compared to cells from WT mice; n=3. B: Experiments identical to the ones described in panel A were performed with cultured MAEC; n=3. C: Migration of TGF-β1-stimulated MLEC from WT and Thbs4−/− mice was measured in non-coated Boyden chambers with 8 μm pores and compared to migration of non-stimulated MLEC. DNA in the lower chamber was measured after 4 h at 37 °C. The values are expressed as percent of average migration of non-stimulated cells. *p < 0.05 compared to control (non-stimulated cells), # p<0.05 compared to cells from WT mice; n=3. D: Experiments identical to the ones described in A were performed in MAEC from WT and Thbs4−/− mice. E: Proliferation of TGF-β1-stimulated EC: 12-well plates were coated with fibronectin overnight at 4 °C. EC from WT mice were added and allowed to proliferate at 37 °C for 24, 48, 72, and 96 h; n=3. The amount of DNA in the wells was measured at 24, 48, 72, and 96 h after seeding the cells. The average values of fluorescence are presented as % of average values of fluorescence in control wells with non-stimulated cells.
Adhesion was measured at 1 hour after seeding the cells pre-stimulated for 24 hours with 10 ng/ml TGF-β1 on fibronectin-coated plates. Treatment with TGF-β1 significantly increased adhesion, but the response was blunted in microvascular EC from TSP-4 KO mice (Fig. 4A). MAEC did not change their adhesion in response to TGF-β1 (Fig. 4B).
Migration was assessed in Boyden chambers 4 hours after seeding TGF-β1-stimulated cells onto fibronectin-coated transwells using 20% FBS as a migratory stimulus. Migration was quantified by measuring DNA of cells on the bottom side of the filter and in lower chamber. Treatment with TGF-β1 significantly increased migration, and the response was blunted in TSP-4 KO cells (Fig. 4C). MAEC migration was not changed by TGF-β1 (Fig. 4D).
Proliferation was measured in the presence of 10 ng/ml TGF-β1 supplemented daily for up to 96 hours. There was no effect of TGF-β1 (Fig. 4E).
TSP-4 mediates angiogenesis in response to TGF-β1 in vivo in Matrigel plugs
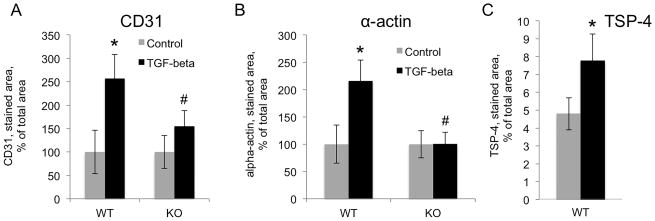
Anesthetized WT and KO mice, 20–22 weeks of age (10 per group), were injected subcutaneously with sterile liquid Matrigel (0.5ml) containing heparin (25 U/ml) and bFGF (10 ng/ml) and TGF-β1 (20 ng/ml) or with vehicle control. The plugs were removed 14 days later. Angiogenesis markers CD31 and α-actin were used to visualize and quantify the blood vessel formation and growth in the Matrigel plugs. TGF-β1 significantly increased the levels of markers in the Matrigel plugs of WT mice, but not in KO mice (Fig. 5). Immunostaining of CD31, a marker of endothelial cells, revealed 2.5-fold increase in TGF-β1-supplemented plugs in WT mice, but there was no significant change in KO mice (Fig. 5A). Staining of α-actin demonstrated significant increase in TGF-β1-supplemented plugs in WT mice (> 2-fold), but no increase in KO animals (Fig. 5B). In plugs from the WT mice, TSP-4 levels were analyzed by immunohistochemistry (Fig. 5C). TGF-β1 injections significantly increased the levels of TSP-4 protein in the plugs.
Figure 5. TSP-4 mediates increased angiogenesis in response to TGF-β1 in Matrigel plug angiogenesis model.
20 ng/ml TGF-β1 was added to Matrigel, and Matrigel was injected subcutaneously to WT and Thbs4−/− (KO) mice. A: Quantification of anti-CD31 staining (EC) in Matrigel plugs in WT and Thbs4−/− mice (n=10). B: Quantification of anti-α-actin staining (SMC) in Matrigel plugs in WT and Thbs4−/− mice (n=10). The stained area was quantified using ImagePro6.1. *p < 0.05 compared to control (non-stimulated cells), # p<0.05 compared to cells from WT mice. C: Quantification of TSP-4 (n=10), *p < 0.05.
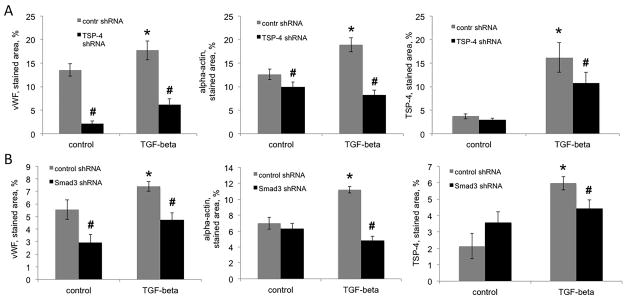
To achieve suppression of TSP-4 production by a complementary method, we used lentiviral particles expressing TSP-4 shRNA mixed into Matrigel at the concentration 25,000 IFU/ml. As the cells migrated into the Matrigel plug, they became infected with the lentivirus and expressed TSP-4 shRNA. Control shRNA-expressing lentiviral particles were used for comparison. Mice were injected with TGF-β1 (500 ng/kg) daily, starting on the day of the Matrigel implantation.
TGF-β1 injections increased the levels of markers of angiogenesis in Matrigel plugs (Fig. 6A, left and middle panel) and the levels of TSP-4 (Fig. 6A, right panel). TSP-4 shRNA significantly decreased the levels of both angiogenesis markers and the level of TSP-4 in mice injected with TGF-β1. In mice injected with PBS, the levels of vWF and of α-actin were also significantly reduced, consistent with pro-angiogenic function of TSP-4 (15).
Figure 6. Effect of TSP-4 and SMAD3 shRNA on angiogenesis in response to TGF-β1 in Matrigel plug angiogenesis model.
Matrigel supplemented with TSP-4-, SMAD3-, or control-shRNA-expressing lentiviral particles was injected subcutaneously to WT mice as described in the Methods. TGF-β1 was administered in daily IP injections. A: Quantification of anti-vWF (left panel), anti-α-actin (middle panel), and anti-TSP-4 (right panel) staining in Matrigel plugs of mice injected with Matrigel containing TSP-4 shRNA or control shRNA (n=10). B: Quantification of anti-vWF (left panel), anti-α-actin (middle panel), and anti-TSP-4 (right panel) staining in Matrigel plugs of mice injected with Matrigel containing SMAD3 shRNA or control shRNA (n=10). The stained area was quantified using ImagePro6.1. *p < 0.05 compared to control shRNA, # p<0.05 compared to control (injected with PBS) mice.
In cultured EC, we found that SMAD3 mediates upregulation of TSP-4 by TGF-β1. To demonstrate that this pathway is active in vivo, we used lentiviral particles expressing SMAD3 shRNA to knockdown SMAD3 in vascular cells in the Matrigel plug. The SMAD3 shRNA significantly decreased the levels of angiogenesis markers and the level of TSP-4 in mice injected with TGF-β1 (Fig. 6B). In mice injected with PBS, the levels of vWF, a marker of EC, was reduced while the levels of α-actin and TSP-4 were not affected.
TSP-4 mediates angiogenesis and cancer growth in response to TGF-β1
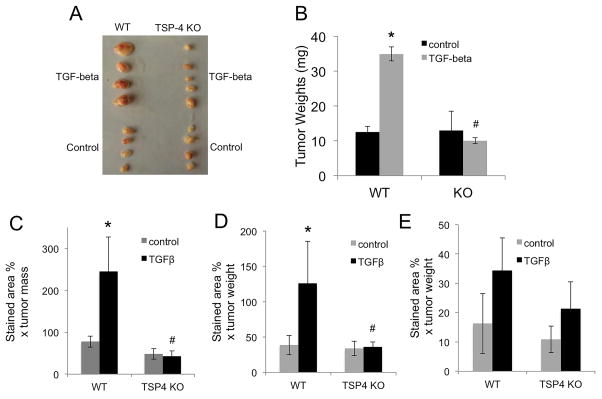
To demonstrate that TSP-4 mediates the pro-angiogenic effect of TGF-β1 in vivo in a remodeling tissue, we used cancer angiogenesis model. WT or KO mice (10 per group) were injected in the mammary fat pad with EMT6 mouse breast cancer cells. Mice received daily injections of TGF-β1 or PBS, and tumors were harvested after 14 days (Fig. 7A). Tumors were weighed (Fig. 7B), and frozen sections of tumors were stained with antibodies to CD31 (Fig. 7C), α-actin (Fig. 7D), and TSP-4 (Fig. 7E).
Figure 7. TSP-4 mediates angiogenesis in response to TGF-β1 in a cancer model.
A: Mouse breast cancer EMT6 cells were injected into mammary fat pad of WT or Thbs4−/− (KO) mice. Mice received daily IP injections of TGF-β1. A: representative tumors from one of the experiments. B: tumor weight, n=10, *p < 0.05 compared to control mice injected with PBS; # p<0.05 compared to WT mice. C: Angiogenesis marker CD31 (EC) was visualized by immunohistochemistry in frozen sections of tumors. D: α-actin (SMC) was visualized by immunohistochemistry in frozen sections of tumors from WT and Thbs4−/− mice. E: TSP-4 was visualized by immunohistochemistry in frozen sections of tumors from WT and Thbs4−/− mice. A–D: Mean stained area, % × mean tumor weight, n = 10, *p < 0.05 compared to WT, # p<0.05 compared to WT mice.
TGF-β1 had a profound effect on the size (Fig. 7A) and the weight of the tumors: tumor weight was significantly increased in mice receiving TGF-β1 injections (Fig. 7B). TSP-4 KO completely abolished the increase in tumor size in response to TGF-β1.
Increase in tumor size and weight was consistent with increase in angiogenesis markers: the levels of both CD31 and α-actin were dramatically increased by TGF-β1, but the increase was completely prevented in TSP-4 KO animals (Fig. 7C,D). In mice injected with TGF-β1, TSP-4 levels in KO mice were not statistically different from the levels in mice injected with PBS. As we previously reported, a small amount of TSP-4 is produced in vivo by cancer cells, and cancer-cell-produced TSP-4 is sufficient to support the basal angiogenesis in tumors [15]. The tumor-cell-produced TSP-4 in KO mice injected with TGF-β1 was decreased compared to WT mice (Fig. 7E), but the difference did not reach statistical significance.
Endogenous TGF-β1 regulates angiogenesis and cancer growth
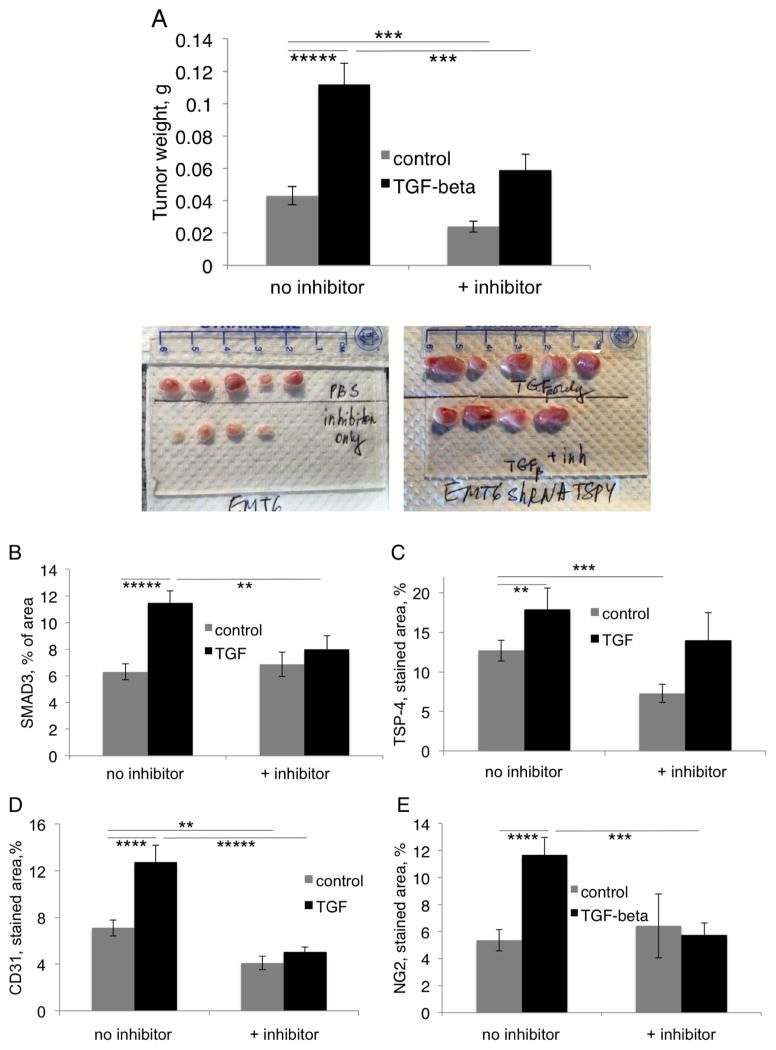
To evaluate the role of endogenous TGF-β1 in cancer growth and angiogenesis, WT mice (n = 10) were injected with SB-431542 (4.2 mg/kg weight in 0.1 ml PBS) or with PBS 30 min before the daily TGF-β1 or PBS injection (Fig. 8). The effect of the inhibitor was evaluated by assessing tumor weights (Fig. 8A) and levels of CD31 and NG2 in tumors (Fig. 8B and C). Tumor growth was inhibited by SB-431542 in the animals injected with TGF-β1 and in control animals not injected with TGF-β1, suggesting a role for endogenous TGF-β1 in stimulation of cancer growth. The inhibitor reduced CD31 levels in the animals injected with TGF-β1 and in control animals, but the levels of NG2 were reduced only in mice injected with TGF-β1 (Fig. 8C). Consistent with these observations, TSP-4 levels were downregulated by the inhibitor in animals injected with TGF-β1 and with PBS (Fig. 8E). The levels of phosphorylated SMAD3 were significantly decreased by the inhibitor in animals that received TGF-β1 injections but not in control animals (Fig. 8D).
Figure 8. Effect of inhibitor of TGF-β receptors SB-431542 on cancer growth and angiogenesis.
In the experiment similar to those described in Fig. 7, 4.2 mg/kg SB-431542 in 0.1 ml of PBS or PBS alone was injected IP daily 30 min before the injection of TGF-β1. A: EMT6 tumor weight and representative tumors, n=10, ***p<0.005, *****p<0.00005. B: Levels of CD31 in tumors, IHC, n=10. C: Levels of NG2 in tumors, IHC, n=10. D: Levels of phospho-SMAD3 in tumors, IHC, n=10. E: Levels of TSP-4 in tumors, IHC, n=10.
Discussion
Increased levels of TGF-β are associated with tissue remodeling (1–8). The role of TGF-β in remodeling is dependent on its ability to regulate the composition and deposition of the ECM. Here, we report that TGF-β1 induces upregulation of TSP-4 in EC. Increased production of TSP-4 has been reported in several pathological conditions associated with increased TGF-β levels, but this is the first report of a specific signal inducing upregulation of TSP-4. Although TSP-4 is influential in several cardiovascular pathologies and associated with critical intracellular processes (22–26), the literature is lacking in information regarding signaling mechanisms leading to its regulation.
We found that TGF-β1 induces TSP-4 in microvascular EC of different origins: in an immortalized EC line from the retina of Macaca mulatta, in human dermal microvascular EC, and in mouse lung EC. The upregulation of TSP-4 appears to be specific: two other TSPs were either downregulated (TSP-1) or unaffected (TSP-3). bFGF failed to upregulate TSP-4, and there was no increase in TSP-4 levels in macrovascular cells; arterial EC did not respond to TGF-β1 (not shown), while vascular SMC responded to TGF-β1 but failed to produce TSP-4. The specificity of the response suggests that TSP-4 upregulation may explain some of the cell-specific and stage-specific effects of TGF-β (6, 14) in pathological conditions associated with angiogenesis.
We found that upregulation of TSP-4 by TGF-β1 is mediated by SMAD3: both the specific inhibitor of SMAD3 and SMAD3 shRNA prevented it. Although SMAD proteins are usually associated with transcriptional regulation, we confirmed that upregulation of TSP-4 is post-transcriptional: levels of TSP-4 mRNA were not affected by TGF-β1 treatment of cells, and four THBS4 and Thbs4 promoter-reporter constructs were not activated by TGF-β1. We also excluded the possibility of decreased secretion: the levels of TSP-4 were increased in cell culture supernatants after the stimulation with TGF-β1. Further analysis of cells, in which the synthesis of new protein was blocked, revealed that TGF-β1 causes TSP-4 protein to increase independent of protein synthesis. This is an interesting mechanism that warrants further investigation of signaling and molecular events, especially because of a clear involvement of SMAD3. However, it is unclear at this point how the stability of TSP-4 protein is regulated: whether a protease involved in TSP-4 turnover is downregulated or TSP-4 is sequestered in response to TGF-β1.
Microvascular EC are the key cells in angiogenesis. We recently reported that TSP-4 promotes angiogenesis in remodeling tissues and regulates pro-angiogenic functions of cultured microvascular EC (15). To find out whether TSP-4 mediates the pro-angiogenic effects of TGF-β1, we examined its role in three functions of EC important in angiogenesis: adhesion, migration, and proliferation. TSP-4 mediated the increased adhesion and migration of MLEC in response to TGF-β1: both were significantly blunted in microvascular EC from TSP-4 KO mice. Aortic EC did not respond to TGF-β1.
We have reported the effect of TSP-4 on the proliferation of microvascular EC: recombinant TSP-4 accelerated proliferation of MLEC, and cells isolated from TSP-4 KO mice demonstrated significantly slower proliferation (15). To our surprise, we did not detect increased proliferation of MLEC in response to TGF-β1, suggesting that multiple counterbalancing mechanisms controlling the proliferation of microvascular EC may be activated by TGF-β1. Consistent with this explanation, both increased and the decreased EC proliferation in response to TGF-β1 have been reported (41–43).
A subcutaneous Matrigel plug provides a convenient angiogenesis model that allows for examining the behavior of vascular cells in the absence of surrounding tissues. The composition of the matrix can be readily modified to include proteins and growth factors, as well as nucleic acids and viral particles. As vascular cells migrate into the plug, the modifying agents become available to the cells and change their functions as well as the developing vasculature. We used this model to test the effect of three agents: TGF-β1, viral particles expressing TSP-4 shRNA, and viral particles expressing SMAD3 shRNA. TGF-β1 mixed into Matrigel had a significant effect on angiogenesis: the levels of both CD31 and α-actin were dramatically increased in plugs supplemented with TGF-β1. However, in the absence of TSP-4 in KO mice, there was no effect of TGF-β1 on angiogenesis in Matrigel plugs. Alternatively, we delivered TGF-β1 in daily IP injections and detected upregulation of angiogenesis markers and TSP-4 in response to TGF-β1. TSP-4 shRNA prevented angiogenesis and TSP-4 upregulation in this model.
In cultured cells, we found that upregulation of TSP-4 by TGF-β1 was mediated by SMAD3. To test the effect of SMAD3 in our in vivo angiogenesis model, we added lentiviral particles expressing SMAD3 shRNA to the matrix. SMAD3 shRNA significantly decreased upregulation of TSP-4 by TGF-β1 as well as overall angiogenesis in response to TGF-β1, confirming our in vitro observation of the importance of SMAD3 in this novel pathway.
To expand our observation into a more pathologically relevant model of angiogenesis, where growing vessels interact with other tissue cells, we examined the effect of TGF-β1 on angiogenesis in a mouse breast cancer model. In this model, as we reported, there was no difference in cancer growth or angiogenesis between WT and KO mice, unless TSP-4 in cancer cells was knocked down as previously described (15). The lack of differences in cancer growth and angiogenesis was due to the expression of TSP-4 by EMT6 cancer cells in vivo (15). TGF-β1 injections caused dramatic increase in tumor weight and size in WT mice, but not in KO mice, confirming that EC TSP-4 is a mediator of the pro-angiogenic activity of TGF-β1. The effects of TGF-β1 on angiogenesis was measured as the levels of angiogenic markers CD31 and α-actin: there was a dramatic increase in the levels of both markers in tumors grown in WT animals, but the levels of markers in the tumors from KO mice were not changed by TGF-β1 injections. Inhibition of the TGF-β receptor using SB-431542 revealed the role of endogenous TGF-β in tumor growth, regulation of TSP-4 levels, and angiogenesis: the inhibitor reduced the weight of tumors, TSP-4 and phopsho-SMAD3 levels, as well as CD31 levels in tumors. Interestingly, the levels of NG2 were not regulated by endogenous TGF-β. Therefore, the increase in NG2 levels in mice that received TGF-β1 injections appears to be secondary to and resulting from the effects on EC. We did not detect any effect of the inhibitor on the basal levels of phosphorylated SMAD3. The lack of the effect may be due to our lack of understanding of timing and kinetics of the events induced by the endogenous TGF-β. Unlike the levels of TSP-4 and angiogenesis markers, the levels of phospho-SMAD3 are rapidly changing, and our analyses represented only a snapshot of a single moment in time.
Our in vivo and in vitro results clearly identified a novel pathologically important pathway that offers explanation for distinct pathological-stage-specific effects of TGF-β on angiogenesis and cancer growth. TGF-β is known to suppress cancer cell proliferation; however, at the later stages, it promotes tumor growth in vivo (6, 14) due to the loss of response in cancer cells and increased angiogenesis. Cell-specific effects of TGF-β1 on TSP-4 production may prove to be the pathway explaining this paradox that is not limited to cancer growth, but is also observed in other remodeling tissues (14). Upregulation of TSP-4, a novel pro-angiogenic protein, appears to be cell-type-specific. Thus, only at a stage when tissue remodeling depends on angiogenesis does this pathway become influential and determine the outcome of a process. Although our results and our previous observations (15) suggest a direct effect of TSP-4 on pro-angiogenic functions of EC, it is possible that multiple complementary mechanisms activated by TGF-β regulate angiogenesis in vivo. TGF-β induces a variety of changes in tissues, including fibrosis, and these changes may lead to activation of angiogenesis.
Our results delineate a novel pathway of regulation of angiogenesis by TGF-β1. This pathway starts with TGF-β1 signaling in microvascular EC, is mediated by SMAD3, and results in upregulation of TSP-4, a protein that affects the functions of microvascular EC and promotes angiogenesis.
Materials and Methods
Animals
Mice were of the C57BL/6 background. Both genders were used (there were no gender-specific differences). Thbs4−/− mice were described previously (23, 24, 44). Control wild type (WT) mice were from the same mouse colony as Thbs4−/− mice. For experiments exclusively using WT mice, WT C57BL/6 mice were purchased from the Jackson Laboratories. Animals were housed in the AAALAC approved animal facilities of the Cleveland Clinic. Animal procedures were approved by the IACUC and were in agreement with the NIH Guide for Animal Use. Ketamine (80mg/kg)/Xylazine (5mg/kg) was used for anesthesia to immobilize the mice for subcutaneous injections.
Isolation of EC and cell culture
Mouse Lung Endothelial Cells (MLEC) were isolated from WT and Thbs4−/− mice and cultured in DMEM/F-12 (Sigma)/20% fetal bovine serum (FBS; HiClone)/80 mg/L heparin (Sigma)/40 mg/L endothelial cell growth supplement (ECGS) purified from bovine brain tissue (45). RF/6A cells, Macaca Mulatta retinal microvascular EC, were purchased from ATCC and cultured in DMEM/F-12/10% FBS. Mouse breast cancer EMT6 cells (ATCC) were grown in DMEM/F12/10% FBS and harvested using trypsin on the day of injection. Human dermal microvascular EC were from ATCC, human vascular smooth muscle cells from Cambrex and grown in DMEM/F12/10% FBS. Human umbilical vein EC, a kind gift from Dr. DiCorleto (Cleveland Clinic), were grown in HUVEC medium supplemented with 20% FBS.
RNA isolation and Real-Time Quantitative RT-PCR
RNA was isolated using Trizol reagent (Invitrogen). cDNA was synthesized using iScript cDNA synthesis kit (Bio-Rad). Expression of TSP-4 mRNA was quantified using specific TSP-4 primers from TaqMan (Applied Biosystems) and TaqMan Universal PCR Master Mix. β-actin was chosen for normalization by the 2−ΔΔCt method. PCR was done in triplicate with a fluorescence-based, real-time detection method (ABI PRISM 7900 Sequence Detection System; Applied Biosystems).
Preparation of cell lysates and Western blotting
Confluent EC were treated with DMSO or human TGF-β1 (R&D, 10ng/ml) for 24 hours. Cells were washed twice with ice-cold PBS and lysed using RIPA Biffer (Thermo Scientific, 25mM Tris•HCl pH 7.6, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS).
30 μg of protein was separated using 8% SDS-PAGE and transferred to PVDF membrane (Bio-Rad). Membranes were incubated with the anti-TSP-4 (R&D); anti-TSP-1 (Thermo Fisher); anti-phospho-SMAD3, anti-phospho-SMAD2, anti-phospho-SMAD1/5/9, and anti-SMAD1 (Cell Signaling); anti-β-actin (Sigma) primary antibodies followed by horseradish peroxidase–linked IgG; and visualized by chemiluminescence using Super Signal West Pico Chemiluminescence substrate (Thermo Fisher).
Cells were pre-treated with 2.5 μM SIS3 (Calbiochem), 10 μM SB431542 (Cayman Chemical) and cyclohexamide (Sigma-Aldrich, 10 μg/ml) for 30 min before TGF-β1 stimulation.
Transient transfection of RF/6A cells with plasmids expressing promoter-reporter constructs
Confluent cells in 24-well plates were transiently transfected with 500 ng of either human or mouse TSP-4 promoter–luciferase reporter construct plasmids: pThbs4 −834/+34, pThbs4 −2000/+34, pTHBS4 −923/+20, and pTHBS4 −2000/+20. Fragments of the mouse and human promoters were amplified from the genomic DNA by PCR and cloned into pGL3 Basic vector. 24 h after transfection, cells were treated with DMSO or TGF-β1 for 2, 4, 6, 8, 12 and 24 h, and reporter assays were performed using the Dual-Luciferase Reporter Assay System (Promega).
In vitro migration assays
MLEC were treated with 10 ng/ml TGF-β1 for 24 h, trypsinized, and 0.1 × 106 EC were resuspended in the serum-free DMEM and transferred into the trans-well chambers (Corning). 20% FBS was used as a chemo-attractant in the bottom chambers. The cells were incubated at 37 °C for 4 hours, the medium was aspirated, and attached cells were removed from the surface of the upper chamber using Q-tips. The plates were frozen at −80 °C for 3 hours, and DNA of remaining cells was quantified using CyQuant reagent (Invitrogen).
In vitro adhesion assays
Adhesion assays were performed as previously described (46, 47). EC were treated with 10 ng/ml TGF-β1 for 24 hours, trypsinized, and 5 × 103 cells were added for 1 h at 37 °C to wells of 24-well plates (Corning) pre-coated with fibronectin (Sigma Aldrich). Plates were washed with PBS and stored at −80 °C for 3 hours. CyQuant reagent was used to quantify cell DNA in wells.
In vitro proliferation assays
Proliferation assays were performed in 12-well cell culture plates (Costar, St. Louis, Missouri) coated with fibronectin for 1 hour at 37 °C in DMEM/5% serum. Cells (5 × 103) were plated per well, treated with 10 ng/ml TGF-β1, and left for 24, 48, 72, and 96 h. The wells were washed with PBS once and stored at −80 °C for 3 hours. DNA was quantified using CyQuant reagent.
Matrigel plug angiogenesis model
Twenty- to twenty-two-week-old mice were anesthetized by IP injection of a Ketamine (80mg/kg)/Xylazine (5mg/kg) mixture, and the neck area was shaved and swabbed with 70% ethanol. Mice were injected subcutaneously in the upper dorsal side with 750 μL of Matrigel Matrix Basement Membrane (BD Biosciences) supplemented with 20 ng/ml basic FGF (bFGF). In Matrigel plug angiogenesis assays in WT and KO mice, TGF-β1 was added to the Matrigel (20 ng/ml). In experiments testing the effects of shRNA, mice were injected daily with 500 ng/kg of human TGF-β1 (R&D Systems) or saline (control group).
In the experiments testing the effect of TSP-4 and SMAD3 shRNA on Matrigel plug angiogenesis, 25,000 IFU/ml of lentiviral particles expressing shRNA (Santa Cruz) was added to the Matrigel. Lentiviral particles expressing shRNA without a target (Santa Cruz) were used as control. Two SMAD3-specific Stealth RNAi duplexes and Stealth RNAi Negative control duplex (Invitrogen, Thermo Fisher) were transfected into the cells using Lipofectamine (Invitrogen, Thermo Fisher) according to the manufacturer’s directions.
Plugs were removed after 14 days and frozen in OCT in liquid nitrogen. 10 μm sections were stained using antibodies against CD31, α-actin, NG2, vWF, and TSP-4 as described (48).
Cancer angiogenesis model
Fifteen- to sixteen-week-old mice were anesthetized by IP injection of Ketamine (80mg/kg)/Xylazine (5mg/kg) mixture. Mice were injected in the mammary fat pad with 1.5 × 106 EMT6 cells (ATCC) in 100 μl of saline. TGF-β1 (500ng/kg) injections were given IP daily for 14 days. Injections of SB-431542 (4.2 mg/kg weight in 0.1 ml PBS) were given daily 30 min before the injection of TGF-β1. Tumors were frozen in OCT in liquid nitrogen. The characterization of angiogenesis in tumor sections was performed as described for Matrigel plugs.
Immunohistochemistry, imaging, and quantification of angiogenesis markers
Sections of Matrigel plugs and tumors were stained primary anti-vWF antibody (Accurate Chem), rat anti-mouse CD31 antibody (BD Biosciences), rabbit polyclonal anti-α-actin (Abcam) antibody, goat anti-TSP-4 (R&D Systems), or anti-NG2 antibody (Millipore) using Vecta Stain ABC Kit (23, 44, 48, 49). Visualization after staining with the antibodies was performed using a high-resolution slide scanner (Leica SCN400FL, Leica microsystems, GmbH, Wetzlar, Germany) at 20X magnification. High-resolution images of the whole section were generated and quantified to determine the percentage of the stained area using ImagePro 6.1 software. The person performing quantification was blinded to the assignment of animals between groups.
Statistical analysis
Group size was calculated based on the previous data obtained in angiogenesis models (15).
Analyses of the data were performed using Sigma Plot Software: Student’s t-test and one-way ANOVA were used to determine the significance of parametric data, and Wilcoxon rank-sum test was used for nonparametric data. The significance level was set at p<0.05. The data are presented as mean ± SEM, number of biological repeats are listed in each legend.
Supplementary Material
Acknowledgments
This work was supported by NIH R01HL117216 (O.S-A. and E.P.) and NIH CA177771 (O.S-A.). Isolation of HUVECs was supported by UL1TR000439.
Source of support: This work was supported by NIH R01HL117216 (O.S-A. and E.P.) and NIH CA177771 (O.S-A.). HUVECs were provided by a grant awarded to Clinical and Translational Science Collaborative of Cleveland, a grant from the National Center for Advancing Translational Sciences (UL1TR000439) component of the National Institutes of Health, and National Institutes of Health Roadmap for Medical Research.
Abbreviations
- bFGF
basic fibroblasts growth factor
- DMSO
dimethyl sulfoxide
- ECM
extracellular matrix
- HDMEC
human dermal microvascular endothelial cells
- HUVEC
human umbilical vein endothelial cells
- IP
intra-peritoneal
- KO
knock-out
- MAEC
mouse aortic endothelial cells
- MLEC
mouse lung endothelial cells
- OCT
OCT embedding cryoembedding Matrix
- shRNA
small hairpin RNA
- VSMC
vascular smooth muscle cells
- vWF
von Willebrand factor
- WT
wild type
Footnotes
Conflict of interests: none
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- 1.Hubmacher D, Apte SS. The biology of the extracellular matrix: novel insights. Curr Opin Rheumatol. 2013;25(1):65–70. doi: 10.1097/BOR.0b013e32835b137b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samarakoon R, Overstreet JM, Higgins PJ. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal. 2013;25(1):264–8. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan HY, Chung AC. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32(3):236–43. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez IE, Eickelberg O. The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9(3):111–6. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 5.Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdiscip Rev Dev Biol. 2013;2(1):47–63. doi: 10.1002/wdev.86. [DOI] [PubMed] [Google Scholar]
- 6.Katz LH, Li Y, Chen JS, Munoz NM, Majumdar A, Chen J, et al. Targeting TGF-beta signaling in cancer. Expert Opin Ther Targets. 2013;17(7):743–60. doi: 10.1517/14728222.2013.782287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toma I, McCaffrey TA. Transforming growth factor-beta and atherosclerosis: interwoven atherogenic and atheroprotective aspects. Cell Tissue Res. 2012;347(1):155–75. doi: 10.1007/s00441-011-1189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SN, Burch ML, Tannock LR, Evanko S, Osman N, Little PJ. Transforming growth factor-beta regulation of proteoglycan synthesis in vascular smooth muscle: contribution to lipid binding and accelerated atherosclerosis in diabetes. J Diabetes. 2010;2(4):233–42. doi: 10.1111/j.1753-0407.2010.00089.x. [DOI] [PubMed] [Google Scholar]
- 9.Prendes MA, Harris A, Wirostko BM, Gerber AL, Siesky B. The role of transforming growth factor beta in glaucoma and the therapeutic implications. Br J Ophthalmol. 2013;97(6):680–6. doi: 10.1136/bjophthalmol-2011-301132. [DOI] [PubMed] [Google Scholar]
- 10.Joseph JV, Balasubramaniyan V, Walenkamp A, Kruyt FA. TGF-beta as a therapeutic target in high grade gliomas - promises and challenges. Biochem Pharmacol. 2013;85(4):478–85. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Yanagita M. Inhibitors/antagonists of TGF-beta system in kidney fibrosis. Nephrol Dial Transplant. 2012;27(10):3686–91. doi: 10.1093/ndt/gfs381. [DOI] [PubMed] [Google Scholar]
- 12.Perrot CY, Javelaud D, Mauviel A. Overlapping activities of TGF-beta and Hedgehog signaling in cancer: therapeutic targets for cancer treatment. Pharmacol Ther. 2013;137(2):183–99. doi: 10.1016/j.pharmthera.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Araujo-Jorge TC, Waghabi MC, Bailly S, Feige JJ. The TGF-beta pathway as an emerging target for Chagas disease therapy. Clin Pharmacol Ther. 2012;92(5):613–21. doi: 10.1038/clpt.2012.102. [DOI] [PubMed] [Google Scholar]
- 14.Dietz HC. TGF-beta in the pathogenesis and prevention of disease: a matter of aneurysmic proportions. J Clin Invest. 2010;120(2):403–7. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muppala S, Frolova E, Xiao R, Krukovets I, Yoon S, Hoppe G, et al. Proangiogenic Properties of Thrombospondin-4. Arterioscler Thromb Vasc Biol. 2015;35(9):1975–86. doi: 10.1161/ATVBAHA.115.305912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17(7):1850–7. doi: 10.1158/1078-0432.CCR-10-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Errico M, de Rinaldis E, Blasi MF, Viti V, Falchetti M, Calcagnile A, et al. Genome-wide expression profile of sporadic gastric cancers with microsatellite instability. Eur J Cancer. 2009;45(3):461–9. doi: 10.1016/j.ejca.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, et al. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1(2):203–9. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- 19.Ma XJ, Wang Z, Ryan PD, Isakoff SJ, Barmettler A, Fuller A, et al. A two-gene expression ratio predicts clinical outcome in breast cancer patients treated with tamoxifen. Cancer Cell. 2004;5(6):607–16. doi: 10.1016/j.ccr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu X, Wang ZC, Iglehart JD, Zhang X, Richardson AL. Predicting features of breast cancer with gene expression patterns. Breast Cancer Res Treat. 2008;108(2):191–201. doi: 10.1007/s10549-007-9596-6. [DOI] [PubMed] [Google Scholar]
- 22.Congote LF, Difalco MR, Gibbs BF. The C-terminal peptide of thrombospondin-4 stimulates erythroid cell proliferation. Biochem Biophys Res Commun. 2004;324(2):673–8. doi: 10.1016/j.bbrc.2004.09.107. [DOI] [PubMed] [Google Scholar]
- 23.Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, et al. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ Res. 2010;107(11):1313–25. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mustonen E, Ruskoaho H, Rysa J. Thrombospondin-4, tumour necrosis factor-like weak inducer of apoptosis (TWEAK) and its receptor Fn14: novel extracellular matrix modulating factors in cardiac remodelling. Ann Med. 2012;44(8):793–804. doi: 10.3109/07853890.2011.614635. [DOI] [PubMed] [Google Scholar]
- 25.Lynch JM, Maillet M, Vanhoutte D, Schloemer A, Sargent MA, Blair NS, et al. A thrombospondin-dependent pathway for a protective ER stress response. Cell. 2012;149(6):1257–68. doi: 10.1016/j.cell.2012.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cingolani OH, Kirk JA, Seo K, Koitabashi N, Lee DI, Ramirez-Correa G, et al. Thrombospondin-4 is required for stretch-mediated contractility augmentation in cardiac muscle. Circ Res. 2011;109(12):1410–4. doi: 10.1161/CIRCRESAHA.111.256743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loeys BL, Mortier G, Dietz HC. Bone lessons from Marfan syndrome and related disorders: fibrillin, TGF-B and BMP at the balance of too long and too short. Pediatr Endocrinol Rev. 2013;10(Suppl 2):417–23. [PubMed] [Google Scholar]
- 28.Yokoyama H, Deckert T. Central role of TGF-beta in the pathogenesis of diabetic nephropathy and macrovascular complications: a hypothesis. Diabet Med. 1996;13(4):313–20. doi: 10.1002/(SICI)1096-9136(199604)13:4<313::AID-DIA56>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3(8):a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eming SA, Hubbell JA. Extracellular matrix in angiogenesis: dynamic structures with translational potential. Exp Dermatol. 2011;20(7):605–13. doi: 10.1111/j.1600-0625.2011.01309.x. [DOI] [PubMed] [Google Scholar]
- 31.Kostourou V, Papalazarou V. Non-collagenous ECM proteins in blood vessel morphogenesis and cancer. Biochim Biophys Acta. 2014;1840(8):2403–13. doi: 10.1016/j.bbagen.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Verrecchia F, Mauviel A. Transforming growth factor-beta signaling through the Smad pathway: role in extracellular matrix gene expression and regulation. J Invest Dermatol. 2002;118(2):211–5. doi: 10.1046/j.1523-1747.2002.01641.x. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Zhou F, Fang Z, Wang L, Li Z, Sun L, et al. Post-transcriptional and post-translational regulation of PTEN by transforming growth factor-beta1. J Cell Biochem. 2009;106(6):1102–12. doi: 10.1002/jcb.22100. [DOI] [PubMed] [Google Scholar]
- 34.Hoover LL, Kubalak SW. Holding their own: the noncanonical roles of Smad proteins. Sci Signal. 2008;1(46):pe48. doi: 10.1126/scisignal.146pe48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia R, Nistal JF, Merino D, Price NL, Fernandez-Hernando C, Beaumont J, et al. p-SMAD2/3 and DICER promote pre-miR-21 processing during pressure overload-associated myocardial remodeling. Biochim Biophys Acta. 2015;1852(7):1520–30. doi: 10.1016/j.bbadis.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 36.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chou YT, Yang YC. Post-transcriptional control of Cited2 by transforming growth factor beta. Regulation via Smads and Cited2 coding region. J Biol Chem. 2006;281(27):18451–62. doi: 10.1074/jbc.M601720200. [DOI] [PubMed] [Google Scholar]
- 38.Blanco FF, Sanduja S, Deane NG, Blackshear PJ, Dixon DA. Transforming growth factor beta regulates P-body formation through induction of the mRNA decay factor tristetraprolin. Mol Cell Biol. 2014;34(2):180–95. doi: 10.1128/MCB.01020-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blahna MT, Hata A. Smad-mediated regulation of microRNA biosynthesis. FEBS Lett. 2012;586(14):1906–12. doi: 10.1016/j.febslet.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinnin M, Ihn H, Tamaki K. Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression. Mol Pharmacol. 2006;69(2):597–607. doi: 10.1124/mol.105.017483. [DOI] [PubMed] [Google Scholar]
- 41.Krishnan S, Szabo E, Burghardt I, Frei K, Tabatabai G, Weller M. Modulation of cerebral endothelial cell function by TGF-beta in glioblastoma: VEGF-dependent angiogenesis versus endothelial mesenchymal transition. Oncotarget. 2015;6(26):22480–95. doi: 10.18632/oncotarget.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nature biotechnology. 2010;28(2):161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petroll WM, Jester JV, Bean JJ, Cavanagh HD. Myofibroblast transformation of cat corneal endothelium by transforming growth factor-beta1, -beta2, and -beta3. Invest Ophthalmol Vis Sci. 1998;39(11):2018–32. [PubMed] [Google Scholar]
- 44.Frolova EG, Sopko N, Blech L, Popovic ZB, Li J, Vasanji A, et al. Thrombospondin-4 regulates fibrosis and remodeling of the myocardium in response to pressure overload. FASEB J. 2012;26(6):2363–73. doi: 10.1096/fj.11-190728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahabeleshwar GH, Somanath PR, Byzova TV. Methods for isolation of endothelial and smooth muscle cells and in vitro proliferation assays. Methods Mol Med. 2006;129:197–208. doi: 10.1385/1-59745-213-0:197. [DOI] [PubMed] [Google Scholar]
- 46.Soloviev DA, Pluskota E, Plow EF. Cell adhesion and migration assays. Methods Mol Med. 2006;129:267–78. doi: 10.1385/1-59745-213-0:267. [DOI] [PubMed] [Google Scholar]
- 47.Stenina OI, Desai SY, Krukovets I, Kight K, Janigro D, Topol EJ, et al. Thrombospondin-4 and its variants: expression and differential effects on endothelial cells. Circulation. 2003;108(12):1514–9. doi: 10.1161/01.CIR.0000089085.76320.4E. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya S, Sul K, Krukovets I, Nestor C, Li J, Adognravi OS. Novel tissue-specific mechanism of regulation of angiogenesis and cancer growth in response to hyperglycemia. J Am Heart Assoc. 2012;1(6):e005967. doi: 10.1161/JAHA.112.005967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharyya S, Marinic TE, Krukovets I, Hoppe G, Stenina OI. Cell type-specific post-transcriptional regulation of production of the potent antiangiogenic and proatherogenic protein thrombospondin-1 by high glucose. J Biol Chem. 2008;283(9):5699–707. doi: 10.1074/jbc.M706435200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.