Abstract
BACKGROUND
As systemic therapy has improved for locally advanced pancreatic cancer (LAPC), efforts to improve local control with optimal radiotherapy may be critical. Although conventionally fractionated radiation therapy (CFRT) has more recently shown a limited role in LAPC, stereotactic body radiation therapy (SBRT) is an emerging approach with promising results. With no studies to date comparing SBRT with CFRT for LAPC, this study used the National Cancer Data Base (NCDB) to evaluate these 2 modalities.
METHODS
With the NCDB, patients with American Joint Committee on Cancer cT2-4/N0-1/M0 adenocarcinoma of the pancreas diagnosed from 2004 to 2013 were analyzed. Radiation therapy delivered at ≤2 Gy was deemed CFRT, and radiation therapy delivered at ≥4 Gy per fraction was considered SBRT. Kaplan-Meier analysis, log-rank testing, and multivariate Cox proportional hazards regression were performed with overall survival (OS) as the primary outcome. Propensity score matching was used.
RESULTS
Among 8450 patients, 7819 (92.5%) were treated with CFRT, and 631 (7.5%) underwent SBRT. Receipt of SBRT was associated with superior OS in the multivariate analysis (hazard ratio, 0.84; 95% confidence interval, 0.75–0.93; P<.001). With propensity score matching, 988 patients in all were matched, with 494 patients in each cohort. Within the propensity-matched cohorts, the median OS (13.9 vs 11.6 months) and the 2-year OS rate (21.7% vs 16.5%) were significantly higher with SBRT versus CFRT (P=.0014).
CONCLUSIONS
In this retrospective review using a large national database, SBRT was associated with superior OS in comparison with CFRT for LAPC, and these findings remained significant in a propensity-matched analysis. Further prospective studies investigating these hypothesis-generating results are warranted.
Keywords: intensity modulated radiation therapy (IMRT), pancreatic cancer, radiation therapy (RT), stereotactic body radiation therapy (SBRT)
INTRODUCTION
Pancreatic adenocarcinoma is an aggressive malignancy: it is the fourth leading cause of cancer deaths in the United States with a 5-year overall survival (OS) rate less than 8%.1 Although complete surgical resection has historically been the only curative treatment option, only 10% to 12% of patients have potentially resectable disease, with only a portion of these patients achieving a margin-negative (R0) resection.2–4 For patients with nonmetastatic, unresectable disease, the median survival is approximately 12 to 15 months.5 The optimal treatment for these locally advanced, unresectable tumors remains unknown and represents a significant clinical challenge.
Previous landmark studies by the Gastrointestinal Tumor Study Group have established the role of multimodality therapy with radiation therapy and chemotherapy for locally advanced tumors.6,7 However, recent studies, including the LAP-07 trial, have challenged the role of radiation therapy in the treatment of these tumors because of a lack of improvement in OS.8–10 These previous radiation trials used conventionally fractionated radiation therapy (CFRT) and delivered small fractions of radiation at 1.8 to 2.0 Gy daily over 5 to 6 weeks.6,7,11
Modern systemic therapy regimens have significantly improved control of micrometastatic disease in locally advanced pancreatic cancer (LAPC).12,13 As such, strategies to optimize local control may play an increasing role in maximizing therapy. Stereotactic body radiation therapy (SBRT) uses recent advances in diagnostic imaging and radiation delivery techniques to allow the delivery of higher biological radiation doses for LAPC.14,15 SBRT offers the advantage of delivering radiation therapy over 1 to 2 weeks and thereby minimizing delays in full-dose chemotherapy delivery. SBRT also delivers smaller volumes of radiation with sharper dose gradients, and this potentially results in less irradiated normal tissue. Our institution and others have reported single-arm, prospective studies showing promising early disease-related outcomes with tolerable toxicities with SBRT.16–20
Despite these favorable findings with SBRT, there have been no studies comparing the outcomes with CFRT and SBRT for patients with locally advanced pancreatic adenocarcinoma, and SBRT has not been adopted as a standard treatment for pancreatic cancer. In this study, we used a large hospital-based registry, the National Cancer Data Base (NCDB), to investigate and compare clinical outcomes with CFRT and SBRT for patients with locally advanced, nonmetastatic pancreatic ductal adenocarcinoma. We also examined the utilization patterns of CFRT and SBRT in the United States for patients with locally advanced adenocarcinoma of the pancreas.
MATERIALS AND METHODS
Patient Cohorts
The NCDB registry for cases diagnosed between 2004 and 2012 was used to identify study subjects. The NCDB is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society and captures 70% or more of newly diagnosed malignancies in the United States annually.21,22 The database contains parameters not included in other large national registries, including detailed radiotherapy information regarding the treatment area, radiation dose (grays), and number of radiation fractions. No patient or physician identifiers are collected. Consequently, this study was granted exempt status by the Emory institutional review board.
Patients whose first and only cancer diagnosis was pancreatic adenocarcinoma (diagnosed from 2004 to 2012) and who received radiation designated for the abdomen or pancreas were analyzed. Only tumors in the pancreatic head and/or body were included; tumors in the tail of the pancreas were excluded because these are typically treated with distal pancreatectomy and may have dissimilar outcomes.23 Only patients diagnosed with histologically confirmed adenocarcinoma of the pancreas at American Joint Committee on Cancer (AJCC) clinical tumor stage 2 to 4 and nodal stage 0 to I were included. Exclusion criteria included a diagnosis of metastatic disease, receipt of radiation therapy after surgery, and brachytherapy.
Patients were grouped into the CFRT and SBRT cohorts on the basis of the prescribed dose per fraction, which was calculated as the total prescribed dose divided by the total prescribed number of fractions. Patients documented to receive radiation at a dose of ≤2 Gy per fraction were included in the CFRT cohort; patients receiving ≥4 Gy per fraction were included in the SBRT cohort. The NCDB does not specify whether the prescribed dose is to an isocenter or planning tumor volume. The fraction size of 4 Gy or more was chosen to account for possible institutional variations in SBRT practices.16–20 Patient demographics, tumor characteristics, and treatment details were also ascertained from the NCDB and included the following: age of diagnosis, sex, AJCC clinical TNM staging, tumor size (largest axial dimension), Charlson-Deyo comorbidity score, chemotherapy use, surgical procedure and margin status for patients after radical resection, and year of diagnosis. Unfortunately, the majority of the patients had missing carbohydrate antigen 19-9 values, so this laboratory value was not included in the analysis. Patients who underwent partial pancreatectomy and duodenectomy with partial gastrectomy were categorized as receiving the Whipple procedure. Those who underwent oncologic variations of the Whipple procedure, including partial pancreatectomy and duodenectomy with or without partial gastrectomy, total pancreatectomy, or total pancreatectomy and subtotal gastrectomy or duodenectomy were categorized as receiving Whipple-variant procedures.
Statistical Analyses
Descriptive statistics were compiled to summarize the patient, disease, and treatment characteristics. Associations of patient and other cancer-related variables were tested with Pearson’s chi-square test and an analysis of variance for categorical and numerical variables, respectively. OS was calculated from the date of disease diagnosis to the patient’s death or last follow-up. Univariate associations of patient or disease characteristics with OS were assessed with the log-rank test. Univariate and multivariate Cox proportional hazards regression models were fit for OS. The collinearity among the all baseline patient and disease characteristics was verified by the calculation of variance inflation factors. Variables with variance inflation factors >10 were not considered in the multivariate analysis. Statistical analyses were performed with SAS 9.4 with software macros generated at the Winship Cancer Institute’s Department of Biostatistics and Bioinformatics.24
To minimize the treatment selection bias, a propensity score matching method was used. A logistic regression model predicting CFRT treatment versus SBRT treatment was used to calculate propensity scores for covariates of interest. The covariates chosen were ones found to be significant in the multivariate analysis or ones thought to be clinically significant and included the following: patient age, AJCC clinical T and N staging, chemotherapy use, Charlson-Deyo comorbidity score, year of diagnosis, and receipt of definitive surgery. Patients from each study cohort were matched to each other at a ratio of 1:1 on the basis of the propensity score with a greedy 5-1 digit match algorithm; after matching, the covariate balance between 2 cohorts was evaluated by the standardized differences, and a value <0.1 was considered to indicate negligible imbalance.25 The effects were estimated in the matched sample with a Cox model with a robust variance estimator for OS.26
RESULTS
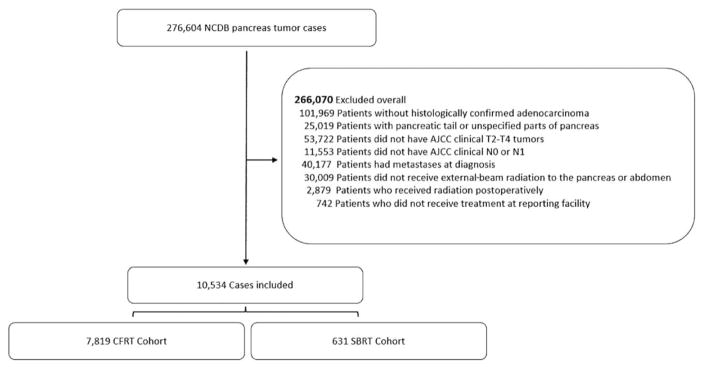
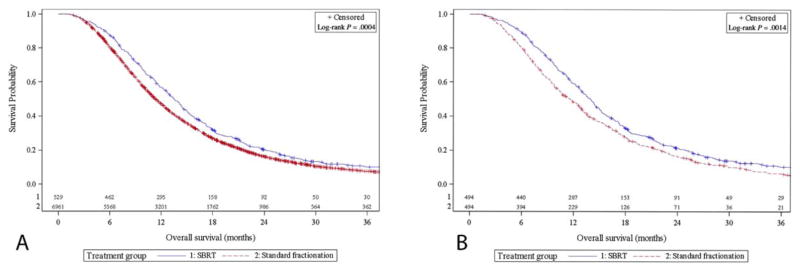
A total of 10,534 patients with pancreatic head or body ductal adenocarcinoma who met the inclusion criteria between 2004 and 2013 were identified from the NCDB. Figure 1 illustrates the patient selection and exclusion criteria. Of these patients, 7819 (92.5%) met our inclusion criteria for CFRT, and 631 (7.5%) met our criteria for SBRT. Patient and tumor characteristics by cohort are presented in Table 1. Most notably, the SBRT cohort was older (median age, 69 vs 66 y; P < .001) and had lower rates of chemotherapy utilization (87.3% vs 96.1%; P < .001). The SBRT cohort contained a lower proportion of T4 tumors (43.7% with SBRT vs 49.0% with CFRT; P = .013), although there were no significant differences in tumor size. The CFRT cohort contained a larger proportion of node-positive patients (32.3% with SBRT vs 37.0% with CFRT; P = .02) and a lower percentage of patients with a Charlson-Deyo comorbidity score of 0 (73.8% with SBRT vs 69.8% with CFRT; P = .044). The median dose per fraction and the median number of fractions for the CFRT and SBRT cohorts were 1.8 Gy and 28 fractions and 8 Gy and 5 fractions, respectively. Patients were found to undergo resection at similar rates between the 2 cohorts (10.8% with SBRT vs 9.2% with CFRT; P = .41). The rate of negative-resection margins for the subset of patients undergoing radical surgery was 92% for SBRT and 84% for CFRT (P = .062). Kaplan-Meier curves for the 2 cohorts are displayed in Figure 2A and demonstrate 2-year OS rates of 20.3% and 16.3% for the SBRT and CFRT groups, respectively (P < .001).
Figure 1.
Patient Consolidated Standards of Reporting Trials diagram. AJCC indicates American Joint Committee on Cancer; CFRT, conventionally fractionated radiation therapy; NCDB, National Cancer Data Base; SBRT, stereotactic body radiation therapy.
TABLE 1.
Patient and Clinical Characteristics and Treatment Details by Cohort Group
| Variable | SBRT (n 5631) | Standard Fractionation (n 57819) | Pa |
|---|---|---|---|
| Age at diagnosis, median, y | 69 | 66 | <.001 |
| Sex, No. (%) | |||
| Male | 311 (49.29) | 3932 (50.29) | .629 |
| Female | 320 (50.71) | 3887 (49.71) | |
| AJCC clinical T stage, No. (%) | |||
| 2 | 97 (15.37) | 1238 (15.83) | .013 |
| 3 | 258 (40.89) | 2753 (35.21) | |
| 4 | 276 (43.74) | 3828 (48.96) | |
| AJCC clinical N stage, No. (%) | |||
| 0 | 427 (67.67) | 4929 (63.04) | .02 |
| 1 | 204 (32.33) | 2890 (36.96) | |
| Tumor size | |||
| Mean, cm | 3.69 | 3.93 | .072 |
| Median, cm | 3.5 | 3.5 | |
| Unknown, No. (%) | 42 (6.67) | 875 (11.2) | |
| Charlson-Deyo score, No. (%) | |||
| 0 | 466 (73.85) | 5459 (69.82) | .044 |
| 1 | 123 (19.49) | 1867 (23.88) | |
| 2 | 42 (6.66) | 493 (6.31) | |
| Chemotherapy, No. (%) | |||
| No | 75 (12.71) | 297 (3.87) | <.001 |
| Yes | 515 (87.29) | 7381 (96.13) | |
| Surgical procedure, No. (%) | .41 | ||
| No surgery | 563 (89.22) | 7098 (90.78) | |
| Whipple | 59 (9.35) | 635 (8.12) | |
| Whipple variant | 9 (1.43) | 86 (1.1) | |
| Surgical margins, No. (%) | |||
| Negative | 60 (0.92) | 566 (0.84) | .062 |
| Positive | 5 (0.08) | 105 (0.16) | |
| Year of diagnosis, No. (%) | |||
| 2004–2008 | 205 (32.49) | 4295 (54.93) | <.001 |
| 2009–2013 | 426 (67.51) | 3524 (45.07) | |
| Fraction size received, Gy | |||
| Mean | 10.7 | 1.8 | <.001 |
| Median | 8.0 | 1.8 | |
| 10th, 90th percentiles | 5.0, 20.0 | 1.8, 1.9 | |
| No. of fractions | |||
| Mean | 5.2 | 26.9 | <.001 |
| Median | 5.0 | 28.0 | |
| 10th, 90th percentiles | 2, 5 | 21, 31 | |
Abbreviations: AJCC, American Joint Committee on Cancer; SBRT, stereotactic body radiation therapy.
Bolded P values are significant.
Unknown values for each variable omitted, if applicable.
Figure 2.
Kaplan-Meier curves demonstrating overall survival for (A) unmatched cohorts and (B) propensity-matched cohorts. SBRT indicates stereotactic body radiation therapy.
In the multivariate analysis (Table 2), treatment with SBRT was associated with significantly improved OS with a hazard ratio of 0.84 (95% confidence interval, 0.75–0.93; P < .001). In addition, as expected, the patient age at diagnosis, Charlson-Deyo comorbidity index, AJCC clinical N stage, chemotherapy use, year of diagnosis, and receipt of surgical resection all demonstrated a significant impact on OS (P < .001). The AJCC clinical T stage and the tumor size were not significant predictors of survival in the multivariate analysis.
TABLE 2.
Multivariate Subgroup Analysis of Overall Survival for the SBRT and CFRT Cohorts
| Variable | Hazard Ratio (95% CI) | Pa |
|---|---|---|
| Treatment group | ||
| Standard fractionation | — | — |
| SBRT | 0.84 (0.75–0.93) | <.001 |
| Age at diagnosis (median) | 1.01 (1.01–1.01) | <.001 |
| AJCC clinical T stage | ||
| 2 | — | — |
| 3 | 0.98 (0.90–1.07) | .701 |
| 4 | 0.92 (0.85–1.01) | .068 |
| Tumor size (cm) | 1.01 (1.00–1.01) | .268 |
| AJCC clinical N stage | ||
| 0 | — | — |
| 1 | 1.13 (1.07–1.19) | <.001 |
| Surgical procedure | ||
| No surgery | — | — |
| Whipple | 0.32 (0.29–0.36) | <.001 |
| Whipple variant | 0.30 (0.22–0.41) | <.001 |
| Charlson-Deyo score | ||
| 0 | 0.88 (0.78–0.98) | .017 |
| 1 | 0.99 (0.88–1.11) | .835 |
| 2 | — | — |
| Chemotherapy use | ||
| No | — | — |
| Yes | 0.66 (0.58–0.76) | <.001 |
| Year of diagnosis | ||
| 2004–2008 | 1.23 (1.16–1.31) | <.001 |
| 2009–2013 | — | — |
Abbreviations: AJCC, American Joint Committee on Cancer; CFRT, conventionally fractionated radiation therapy; CI, confidence interval; SBRT, stereotactic body radiation therapy.
Bolded P values are significant.
Propensity-Matched Outcomes
With matched propensity score analysis, a total of 988 patients were analyzed, with 494 patients in each cohort. Table 3 displays the propensity-matched groups for the following variables: patient age, Charlson-Deyo comorbidity score, AJCC clinical T and N staging, tumor size, chemotherapy use, year of diagnosis, and receipt of surgery. The median follow-up time was 26 months. After propensity matching, SBRT usage continued to be associated with significantly improved OS with a median survival of 13.9 months versus 11.6 months (P < .001). Kaplan-Meier curves for the propensity-matched groups are displayed in Figure 2B, and they demonstrate a significantly better OS curve for the SBRT cohort (P = .0014) with 2-year OS rates of 21.7% and 16.5% for the SBRT and CFRT groups, respectively (P = .001).
TABLE 3.
Patient and Disease Characteristics and Treatment Details for Propensity-Matched Cohort Groups
| Variable | SBRT (n = 5494) | Standard Fractionation (n = 5494) | P |
|---|---|---|---|
| Age at diagnosis, median, y | 67.91 | 68.95 | .148 |
| AJCC clinical T stage, No. (%) | |||
| 2 | 72 (14.57) | 87 (17.61) | .335 |
| 3 | 197 (39.88) | 200 (40.49) | |
| 4 | 225 (45.55) | 207 (41.9) | |
| Tumor size, median, cm | 3.67 | 3.78 | .267 |
| AJCC clinical N stage, No. (%) | |||
| 0 | 334 (67.61) | 340 (68.83) | .682 |
| 1 | 160 (32.39) | 154 (31.17) | |
| Chemotherapy, No. (%) | |||
| Yes | 429 (86.84) | 437 (88.46) | .439 |
| No | 65 (13.16) | 57 (11.54) | |
| Surgical procedure, No. (%) | |||
| No surgery | 445 (90.08) | 448 (90.69) | .81 |
| Whipple | 43 (8.7) | 42 (8.5) | |
| Whipple variant | 6 (1.21) | 4 (0.81) | |
| Charlson-Deyo score, No. (%) | |||
| 0 | 366 (74.09) | 352 (71.26) | .575 |
| 1 | 96 (19.43) | 104 (21.05) | |
| 2 | 32 (6.48) | 38 (7.69) | |
| Year of diagnosis, No. (%) | |||
| 2004–2008 | 192 (38.87) | 183 (37.04) | .555 |
| 2009–2013 | 302 (61.13) | 311 (62.96) | |
Abbreviations: AJCC, American Joint Committee on Cancer; SBRT, stereotactic body radiation therapy.
Subset Analysis
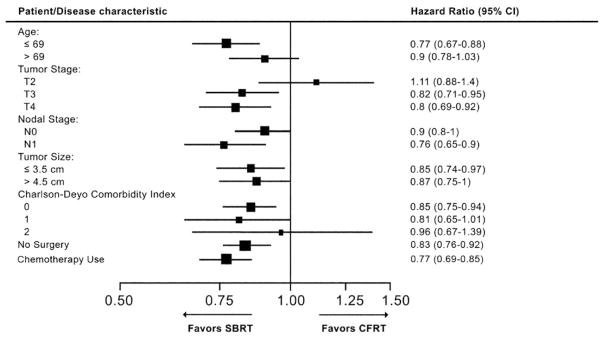
Figure 3 demonstrates multivariate subgroup analyses of the effects of patient demographics, disease characteristics, and treatment details on OS for CFRT and SBRT. Within the subset of patients who received chemotherapy, the receipt of SBRT continued to be significantly associated with improved survival (hazard ratio, 0.77; 95% confidence interval, 0.69–0.85). Similarly, for patients who did not undergo surgical resection, SBRT use was also significantly associated with better OS (hazard ratio, 0.83; 95% confidence interval, 0.76–0.92). In addition, the following subgroups all appeared to significantly benefit from SBRT versus CFRT: age ≤69 years, T3/T4 tumors, N1 disease, tumor size ≤3.5 cm, and Charlson-Deyo comorbidity index of 0. There were no subgroups of patients who appeared to significantly benefit from CFRT versus SBRT.
Figure 3.
Multivariate subgroup analyses of the effects of patient demographics, disease characteristics, and treatment details on overall survival with CFRT versus SBRT. CI indicates confidence interval; CFRT, conventionally fractionated radiation therapy; SBRT, stereotactic body radiation therapy.
DISCUSSION
The objective of this study was to compare survival outcomes for patients with locally advanced, nonmetastatic ductal adenocarcinoma of the pancreas who were treated with either SBRT or CFRT. Our data demonstrate a significant OS benefit for patients who received SBRT rather than CFRT, and these findings remained significant in a propensity-matched analysis controlled for age, comorbidities, receipt of chemotherapy, tumor stage, tumor size, and year of treatment. The increased survival remained for the subsets of patients who received chemotherapy as well as those who never underwent radical resection. We believe that the survival benefit associated with SBRT treatment cannot likely be explained by confounding factors alone because of our rigorous propensity matching as well as our strict inclusion criteria. The median OS of 13.9 months for the propensity-matched SBRT cohort is consistent with recent institutional reports of outcomes with SBRT for locally advanced disease (range, 11.8–20 months).16–19 Our study also validates patient and disease factors known to affect prognoses, such as patient age, comorbid status, nodal positivity, chemotherapy, and surgery.27
Prolonged survival after SBRT in our study may be explained by multiple factors. The median SBRT dose in this study was 40 Gy in 5 fractions, whereas the dose was 45 Gy in 28 fractions for CFRT. On the basis of the linear-quadratic model, under the assumption of an α/β value of 10 Gy for pancreatic adenocarcinoma, the median biologically effective dose (BED) was 74.6 Gy for SBRT and 59.5 Gy for CFRT. Indeed, the techniques used for CFRT deliver a homogeneous radiation dose to the pancreas as well as adjacent organs; the prescribed total dose is often limited by normal-tissue tolerance. SBRT, on the other hand, can deliver much higher doses to the target while minimizing normal-tissue exposure by using sharp dose gradients. Higher radiation doses have been associated with superior local control in pancreatic cancer.28 Moreover, previously reported local control rates for SBRT compare favorably with the local control rates of 68% to 75% reported for CFRT.7,17,18,29,30 Although we hypothesize that improved local control with SBRT affected OS, local control data cannot be ascertained from the NCDB. However, the higher rates of R0 resection after SBRT in this series support this hypothesis.
In the recently reported LAP-07 trial, CFRT was associated with improved local control but had no impact on OS. However, in this study, induction and adjuvant gemcitabine alone was used. Recent landmark trials have shown that FOLFIRINOX (5-FU, irinotecan, oxaliplatin) and gemcitabine plus nab-paclitaxel are superior to gemcitabine alone.12,13 As more patients (particularly younger patients with a good performance status) are treated with these modern regimens, local control will likely play a greater role in shaping ultimate disease outcomes in LAPC.
Initial experiences with SBRT for pancreatic cancer showed concerning rates of high-grade toxicity. Using a single fraction of 25 Gy (BED10, 87.5 Gy), Stanford investigators demonstrated promising local control (84% at 1 year) but with a grade 2 or higher toxicity rate of 25% and median survival of 6.4 months for patients with locally advanced disease.14 Maturation of the SBRT technique has led many, including our institution, to target smaller volumes and use lower doses per fraction. In our experience, when 45 Gy was delivered in 3 fractions, no grade 3 or higher toxicities were observed.20 Similarly, Herman et al31 recently reported a multi-institutional experience with gemcitabine before and after SBRT (33 Gy in 5 fractions) with acute and late grade 2 or higher gastrointestinal toxicity rates of merely 2% and 11%, respectively. Although toxicity data are unavailable in the NCDB, the 5% survival advantage of SBRT at 2 years is encouraging in that high-grade toxicity does not nullify the survival benefit of radiation dose intensification.
An additional benefit of SBRT over CFRT is the shorter treatment duration. CFRT is typically given over 5 to 6 weeks, and this leaves significant chances for interruptions and delays in radiation, chemotherapy, or both if they are given concurrently. In contrast, SBRT can typically be safely delivered in 1 to 2 weeks, and this is more convenient for patients, particularly those who live at a distance. Gurka et al32 reported SBRT to be associated with fewer interruptions in chemotherapy in comparison with CFRT. Consequently, the incorporation of SBRT into modular treatment platforms for LAPC may afford multidisciplinary care teams greater ability to deliver personalized combinations of systemic and local therapy.
This study has limitations inherent in the structure of the NCDB. A critical limitation of this analysis is that we could not control for the specific type of chemotherapy (type of agent or agents and number of cycles) in propensity matching because this information is not captured by the NCDB. This limitation presents a potential confounder in that SBRT patients tended to be treated more recently and potentially received more effective systemic therapy. We attempted to address this confounder by controlling for the year of treatment, which can be viewed as a surrogate for the dominant type of chemotherapy regimen administered. Even after the year of treatment was included in the propensity-matched analysis, SBRT treatment remained superior with respect to OS. In addition, data regarding local control and the cause of death are not captured by the NCDB, and this presents a further limitation.
Finally, this study also used a large fraction size as a surrogate to define SBRT under the assumption that practitioners also used other critical components of therapy while delivering SBRT, including organ motion management, advanced imaging, radiation delivery techniques, and patient immobilization. The NCDB does not clearly specify whether the prescribed dose was to a 3-dimensional volume or isocenter. We have provided the dose and fractionation of the SBRT cohort to serve as an internal validation of our selection parameters, and we do not encourage practitioners to use this study as a guide to ideal SBRT dosing. The decision to make 4 Gy per fraction or higher as the cutoff for SBRT was made to include institutional variations, although it could be argued that these fractionations may have been used with palliative intent. However, our data demonstrate that regardless of the intent of the radiation oncologist, patients who received the higher dose per fraction had better survival than patients who received standard fractionation.
Despite these limitations, this study encapsulates the majority of patients treated in the United States in the past decade and is the best available resource outside multicenter, randomized trials. To our knowledge, this study is the only report of a direct comparison of outcomes between SBRT and CFRT in a large patient population. Although SBRT demonstrated better survival, this treatment, along with all currently available treatments for LAPC, still has significant limitations. Currently, it is still clear that there does not exist any treatment that can be substituted for surgical resection, and better results are clearly needed in this patient population. Despite these challenges, we believe that the survival advantage seen with SBRT treatment versus CFRT provides the basis for the consideration of future randomized trials in this setting.
Acknowledgments
FUNDING SUPPORT
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Footnotes
An abstract of this study has been accepted for presentation at the 2017 Gastrointestinal Cancers Symposium; January 19–21, 2017; San Francisco, CA.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Jim Zhong: Planning and performance of the study, reporting, overall guarantor, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Kirtesh Patel: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Jeffrey Switchenko: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Richard J. Cassidy: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. William A. Hall: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Theresa Gillespie: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Pretesh R. Patel: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. David Kooby: Reporting, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript. Jerome Landry: Planning and performance of the study, reporting, overall guarantor, contributions to the manuscript, reading of the manuscript, and agreement with the contents of the manuscript.
References
- 1.Surveillance, Epidemiology, and End Results Program. [Accessed March 16, 2017];Cancer stat facts: pancreas cancer. http://seer.cancer.gov/statfacts/html/pancreas.html.
- 2.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: an appraisal of data from the National Cancer Data Base. Ann Surg Oncol. 2013;20:3634–3642. doi: 10.1245/s10434-013-3047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG., III Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. 2016 Jul 15; doi: 10.1097/SLA.0000000000001924. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 6.Gastrointestinal Tumor Study Group. Comparative therapeutic trial of radiation with or without chemotherapy in pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 1979;5:1643–1647. doi: 10.1016/0360-3016(79)90789-2. [DOI] [PubMed] [Google Scholar]
- 7.Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst. 1988;80:751–755. [PubMed] [Google Scholar]
- 8.Hammel P, Huguet F, van Laethem JL, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 9.Katz MH, Landry J, Kindler HL. Current controversies in the stage-specific multidisciplinary management of pancreatic cancer. Am Soc Clin Oncol Educ Book. 2014:e157–e164. doi: 10.14694/EdBook_AM.2014.34.e157. [DOI] [PubMed] [Google Scholar]
- 10.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 11.Rich T, Harris J, Abrams R, et al. Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12. Am J Clin Oncol. 2004;27:51–56. doi: 10.1097/01.coc.0000046300.88847.bf. [DOI] [PubMed] [Google Scholar]
- 12.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 13.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 15.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Mahadevan A, Jain S, Goldstein M, et al. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 17.Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2011;81:181–188. doi: 10.1016/j.ijrobp.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 18.Goyal K, Einstein D, Ibarra RA, et al. Stereotactic body radiation therapy for nonresectable tumors of the pancreas. J Surg Res. 2012;174:319–325. doi: 10.1016/j.jss.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moningi S, Dholakia AS, Raman SP, et al. The role of stereotactic body radiation therapy for pancreatic cancer: a single-institution experience. Ann Surg Oncol. 2015;22:2352–2358. doi: 10.1245/s10434-014-4274-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaib WL, Hawk N, Cassidy RJ, et al. A phase 1 study of stereotactic body radiation therapy dose escalation for borderline resectable pancreatic cancer after modified FOLFIRINOX ( NCT01446458) Int J Radiat Oncol Biol Phys. 2016;96:296–303. doi: 10.1016/j.ijrobp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Menck HR, Cunningham MP, Jessup JM, et al. The growth and maturation of the National Cancer Data Base. Cancer. 1997;80:2296–2304. doi: 10.1002/(sici)1097-0142(19971215)80:12<2296::aid-cncr11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 22.Partridge EE. The National Cancer Data Base: ten years of growth and commitment. CA Cancer J Clin. 1998;48:131–133. doi: 10.3322/canjclin.48.3.131. [DOI] [PubMed] [Google Scholar]
- 23.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 24.Nickleach D, Liu Y, Shrewsberry A, Ogan K, Kim S, Wang Z. SAS® macros to conduct common biostatistical analyses and generate reports. http://analytics.ncsu.edu/sesug/2013/PO-05.pdf.
- 25.Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- 26.Lin DYWL. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 27.Compton CC, Mulvihill SJ. Prognostic factors in pancreatic carcinoma. Surg Oncol Clin N Am. 1997;6:533–554. [PubMed] [Google Scholar]
- 28.Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for unresectable pancreatic cancer: a multi-institutional retrospective analysis of 144 patients. Int J Radiat Oncol Biol Phys. 2011;80:111–118. doi: 10.1016/j.ijrobp.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 29.Loehrer PJ, Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–4112. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads +5-fluorouracil), and high dose radiation +5-fluorouracil: the Gastrointestinal Tumor Study Group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–1137. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurka MK, Kim C, He AR, et al. Stereotactic body radiation therapy (SBRT) combined with chemotherapy for unresected pancreatic adenocarcinoma. Am J Clin Oncol. doi: 10.1097/COC.0000000000000118. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]