Abstract
Objectives
The therapeutic efficacy of cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with peritoneal carcinomatosis (PC) from colorectal cancer (CRC) is still under debate. This meta-analysis and systematic review of published literature on this comprehensive strategy aims to evaluate its efficacy on CRC patients with PC.
Methods
A systemic review with meta-analysis of published literatures on treatment of CRS plus HIPEC for patients with PC from CRC was performed. In addition, a summary of study results of published literatures concerning CRS plus HIPEC treating patients with PC from CRC was also conducted.
Results
A total of 76 studies were selected, including 1 randomized controlled trial, 14 non-randomized controlled studies, and 61 non-controlled studies. The pooled hazard ratios (HRs) for overall survival (OS) in the 15 researches for meta-analysis was 2.67 (95% CI, 2.21-3.23, I2= 0%, P < 0.00001), and no significant evidence of publication bias was found. The difference of chemotherapy regimens of HIPEC was not associated with OS and DFS (disease-free survival) after CRS and HIPEC, with no significant difference of heterogeneity (P = 0.27, I2 = 24.1%). In both groups of mitomycin C based HIPEC group and oxaliplatin group, patients received HIPEC had significant better survival (P < 0.00001). The mean mortality and morbidity for HIPEC program were 2.8% and 33.0%, respectively.
Conclusions
This meta-analysis revealed that comprehensive therapeutic strategy of CRS plus HIPEC could bring survival benefit for selected patients with PC from CRC with acceptable safety.
Keywords: colorectal cancer, peritoneal carcinomatosis, cytoreductive surgery, hyperthermic intraperitoneal chemotherapy, meta-analysis
INTRODUCTION
Peritoneal carcinomatosis (PC), as a lethal regional progression for patients with colorectal cancer (CRC), has long been considered as a terminal condition with few effective treatments. In the past, the median overall survival (OS) of PC from colorectal cancer is 4 to 7 months after palliative surgery or 5-FU-based systemic chemotherapy with best supportive care [1-3]. Current systemic chemotherapy focusing on new chemotherapeutic agents such as oxaliplatin and irinotecan, along with anti-angiogenesis molecular targeting agents cetuximab and bevacizumab [4-7], could extend the median OS up to about 12 months [5]. However, long-term survival is still hard to be achieved by systemic chemotherapy alone.
Researches on treatment of CRC PC did not reveal promising progress until the development of a comprehensive treatment strategy including cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) and perioperative chemotherapy.[8-15] This new comprehensive treatment improves the median OS of selected patients with CRC PC up to 21-63 months, and 5-year survival rate up to approximately 40% [16-28], or even 58% according to the American Society of Peritoneal Surface Malignancies (ASPSM) multi-institution study [29]. It has been widely recognized in North America, Europe, Australia, and Japan [14, 24, 26, 30-32]. In the 9th International Congress on Peritoneal Surface Malignancies in Amsterdam in 2014, peritoneal surface oncology group international (PSOGI) reached a consensus that CRS+HIPEC should be considered as the standard therapy for the selected patients with mild-to-moderate CRC PC [33].
Nevertheless, therapeutic efficacy of this comprehensive treatment strategy for CRC PC patient remains controversial due to insufficient convincing evidence. Therefore, we conducted this meta-analysis of published clinical studies to verify the efficacy of this strategy against CRC PC.
RESULTS
Basic characteristics of all data
Results of literature search
Literature search identified 326 researches, 76 of which met the inclusion criteria, including 1 randomized controlled trail (RCT) (87 patients) [12], 14 non-randomized controlled studies (3,092 patients) [13-15, 26, 28, 29, 34-40, 99], and 61 non-controlled studies (6,857 patients) [16, 19-21, 41-92, 100-104]. The other 250 studies were excluded for miscellaneous reasons, and the flowchart of search strategy is showed in Figure 1. We conducted a meta-analysis on the 15 controlled studies (3,179 patients) and a summary of 76 HIPEC-related studies (10,036 patients).
Figure 1. Study flowchart of systematic reviews and meta-analyses.
Study characteristics
The characteristics of 15 controlled studies [8-15, 26, 28, 29, 34-40, 99] were shown in Table 1-5, and all 76 selected studies [12-16, 19-21, 26, 28, 29, 34-92, 99-104] were summarized in Table 6-10. All these studies were published between 1993 and 2016 as full texts, performed in 19 countries and regions (Table 11-19). Fifty-eight studies were single-center studies [12, 16, 19, 21, 35, 36, 38-43, 45, 43-53, 55-57, 60-63, 66-71, 74-83, 86-92, 99, 100, 102-104], and the other 18 were multicenter studies (participating institutions from 2 to 28) [13-15, 28, 29, 34, 37, 44, 46, 54, 58, 59, 64, 65, 72, 73, 84, 85, 101]. In these multicenter studies, 6 studies were performed by over 10 participating institutions included studies conducted by Glehen et al (n = 28, a central database) [13], Glehen et al (n = 25, a central database) [54], Elias et al (n = 25, a central database) [14], Esquivel et al (n = 21, The American Society of Peritoneal Surface Malignancies (ASPSM)) [29], and Prada-Villaverde et al (n = 15) [72]. A total of 63 articles were retrospective studies, in which 11 articles were included in this meta-analysis [13-16, 19-21, 28, 29, 34, 37-40, 43-48, 50-52, 54-57, 59-66, 68-72, 74-88, 91, 99-104]. Thirteen articles were prospective studies, in which 4 were included in this meta-analysis [12, 26, 35, 36, 42, 49, 53, 58, 67, 73, 89, 90, 92]. According to the North-England evidence-based guidelines [34, 35], there was one evidence level Ib in this meta-analysis [12], the rest cohort studies or “outcome” researches were evidence level II.[13-15, 26, 28, 29, 34-40, 99]
Table 1. Major Characteristics of Fifteen Controlled Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) versus Surgery alone with Systemic Chemotherapy (SC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
| Author/ Year/ Country | Participating Institutions |
Study Period | Design | Level of Evidence | Number of CRC PC | Treatment strategy | |
|---|---|---|---|---|---|---|---|
| HIPEC group | Control group | ||||||
| Chua TC/ 2009/ Australia [34] |
2 | 1997-2008 | retrospective | IIb | 15 (15/33) | CRS+HIPEC 7 pts; HIPEC: MMC (10-20 mg/m2) for 90 min at 42°C using the closed abdomen technique. No EPIC. SC: FOLFOX and Bevacizumab |
SC 8 pts SC: FOLFOX and Bevacizumab No HIPEC No EPIC |
| Chua TC/ 2011/ Australia [15] |
3 | 1988-2009 | retrospective | IIa | 294 (294/294) | CRS+HIPEC+SC 110 pts HIPEC: MMC (10-20 mg/m2) for 90 min at 42°C using the Coliseum technique. No EPIC SC: 5-FU + LV; 5-FU + LV or CBP with L-OHP or CPT-11; or Regimen 2 + BEV, C225, or PAN |
Surgery and/or SC 184 pts SC: 5-FU + LV; 5-FU + LV or CBP with L-OHP or CPT-11; or Regimen 2 + BEV, C225, or PAN No EPIC No HIPEC |
| Chua TC/ 2013/ Australia [26] |
1 | 1996-2011 | prospective | IIa | 75 (75/98) | CRS+HIPEC with/without EPIC 75pts HIPEC: MMC (10–12.5 mg/m2) or L-OHP (460 mg/m2) for 90 min at 42°C using the closed abdomen technique; Before starting HIEPC, oxaliplatin, 5-FU (400 mg/m2) and LV (20 mg/m2) by intravenous perfusion. EPIC: 5-FU (650–800 mg/m2/d) on Day 1-5 after surgery SC (not reported) |
EPIC alone 23 pts EPIC: 5-FU (650–800 mg/m2/d) on Day 1-5 after surgery SC (not reported) No HIPEC |
Note: CRC: colorectal cancer; PC: peritoneal carcinomatosis; Pts: patients; MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; Cap: capecitabine; C225: cetuximab; CPT: camptothecin; BEV: bevacizumab; DXL: docetaxel; CBP: carboplatin; PAN: panitumumab;
Table 5. Major Characteristics of Fifteen Controlled Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) versus Surgery alone with Systemic Chemotherapy (SC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
| Author/ Year/ Country | Participating Institutions |
Study Period | Design | Level of Evidence | Number of CRC PC | Treatment strategy | |
|---|---|---|---|---|---|---|---|
| HIPEC group | Control group | ||||||
| Huang CQ/ 2014/ China [39] | 1 | 2004-2013 | retrospective | IIa | 62 (62/62) | CRS+HIPEC+SC with/without PIC 33 pts HIPEC: MMC (30 mg) + DDP (120 mg) for 90 min at 43.0±0.5°C using the Coliseum technique EPIC: DXL (75 mg/m2, on day 1, every 3 weeks) and CBP (at Calvert formula: area under the curve, AUC 5; on day 1, every 3 weeks) SC: FOLFOX or FOLFIRI |
CRS+ SC with/without PIC 29 pts SC: FOLFOX or FOLFIRI EPIC: DXL (75 mg/m2, on day 1, every 3 weeks) and CBP (at Calvert formula: AUC 5; on day 1, every 3 weeks) No HIPEC |
| Passot G/ 2014/ France [40] | 1 | 2005-2012 | retrospective | IIa | 82 (82/115) | Neoadjuvant SC+CRS+HIPEC 82 pts Neoadjuvant SC: 1. FOLFIRI with/without BEV or C225; 2. FOLFOX with/without BEV or C225; 3. Others regimens. HIPEC: L-OHP (360 mg/m2) for 30 min using the closed abdomen technique, not reported the perfusion temperature. No EPIC No SC |
Neoadjuvant SC + Surgery + SC 33 pts Neoadjuvant SC: 1. FOLFIRI with/without BEV or C225; 2. FOLFOX with/without BEV or C225; 3. Others regimens. No EPIC SC (uncertainty) |
| Verwaal VJ/ 2003 /Netherlands [12] | 1 | 1998-2001 | prospective | Ib | 87 (87/105) | CRS+HIPEC with/without SC 54 pts HIPEC: MMC (17.5 mg/m2) for 90 min between 42-44°C using the Coliseum technique No EPIC SC: 1. 5-FU (400 mg/m2) + LV (80 mg/m2); 2. FU + CPT-11 (350 mg/m2) |
Surgery and/or SC 51 pts SC: 1. 5-FU (400 mg/m2) + LV (80 mg/m2); 2. FU + CPT-11 (350 mg/m2) No EPIC No HIPEC |
Note: CRC: colorectal cancer; PC: peritoneal carcinomatosis; Pts: patients; MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; Cap: capecitabine; C225: cetuximab; CPT: camptothecin; BEV: bevacizumab; DXL: docetaxel; CBP: carboplatin; PAN: panitumumab;
Table 6. Major Characteristics of Sixty-one Single Arm Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
| Author/ Years/ Country | Participating Institutions |
Study Period | Design | Level of evidence | Number of CRC PC | HIPEC |
|---|---|---|---|---|---|---|
| Alzahrani/ 2015/ Australia [41] | 1 | 1996-2014 | retrospective | III | 205 (205/675) | Before HIPEC, 5-FU (400 mg/m2) were delivered by systemic i.v., L-OHP (350 mg/m2) for 30 min at 43°C using coliseum technique. |
| Beaujard/ 2000/ France [42] | 1 | 1991-1997 | prospective | IIb | 27 (27/86) | MMC (10 mg/L) for 90 min at inflow temperature 46-49 °C using the closed abdomen technique. |
| Bijelic/ 2008/ Australia [43] | 1 | 1981-2004 | retrospective | III | 70 (70/472) | MMC (10 mg/m2 for females and 12.5 mg/m2 for males) for 90 min at about 42 °C using the coliseum technique. |
| Braam/ 2014/ Australia [44] | 2 | 2005-2013 | retrospective | III | 132 (132/132) | MMC (17.5 mg/m2 an additional 8.8 mg/m2 at an interval of 30 and 60 min) for 90 min at 42 °C using the coliseum technique. |
| Cao/ 2009/ Australia [45] | 1 | 1995-2008` | retrospective | III | 52 (52/467) | MMC (10-12.5 mg/m2) for 90 min at 42 °C using coliseum technique. |
| Cavaliere/ 2006/ Italy [46] | 6 | 1996-2005 | retrospective | III | 120 (120/120) | MMC (3.3 mg/m2/L) + DDP (25 mg/m2/L) for 60-90 min at 41.5-43 °C using the coliseum or closed abdomen technique. After intravenous administration of 5-FU (400 mg/m2) and LV (20 mg/m2), L-OHP (460 mg/m2) for 30 min at 43 °C using the coliseum or closed abdomen technique. |
| Ceelen/ 2014/ Belgium [47] | 1 | 2002-2012 | retrospective | III | 152 (152/166) | Before HIPEC, LV (20 mg/m2) and 5-FU (400 mg/m2) were delivered by systemic i.v. L-OHP (460 mg/m2) or MMC (35 mg/m2) for 30-90 min at 41 °C using coliseum technique. |
| Desantis/ 2014/ France [48] | 1 | 1999-2011 | retrospective | III | 74 (74/356) | MMC (10 mg/m2 for females and 12.5 mg/m2 for males) for 90 min at 43°C using coliseum or closed abdomen technique. |
| Elias/ 2004/ France [49] | 1 | 1998-2001 | prospective | IIb | 24 (24/24) | One hour before HIPEC, LV (20 mg/m2) and 5-FU (400 mg/m2) were delivered by systemic i.v. HIPEC: L-OHP (460 mg/m2) for 30 min at 43 °C using the coliseum technique. |
| Elias/ 2014/ France [50] | 1 | 1995-2010 | retrospective | III | 119 (119/443) | MMC (5, 8, or 10 mg/L) for 1 h between 41 °C and 44 °C using the coliseum technique. MMC (20 mg/m2) + DDP (200 mg/m2) for 1 h between 41 °C and 44 °C using the coliseum technique. L-OHP 460 mg/m2 over 30 min at 43°C using the coliseum technique. MMC (12.9+/-3.8 mg/m2) for 90 min between 41 °C and 42 °C using closed abdomen technique. |
Note: MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; NR: not reported
Table 10. Major Characteristics of Sixty-one Single Arm Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
| Author/ Years/ Country | Participating Institutions |
Study Period | Design | Level of evidence | Number of CRC PC | HIPEC |
|---|---|---|---|---|---|---|
| Tabrizian/ 2014/ America [78] | 1 | 2007-2012 | retrospective | III | 51 (51/170) | MMC (total dose 40 mg) for 90 min at 41-43 °C using closed abdomen technique. |
| Teo/ 2013/ Singapore [79] |
1 | 2001-2012 | retrospective | III | 28 (28/100) | MMC for 60 min at 42 °C using closed abdomen technique. |
| Teo/ 2014/ Singapore [80] |
1 | 2001-2012 | retrospective | III | 35 (35/35) | MMC for 60 min at 42 °C using closed abdomen technique. |
| Ung/ 2013/ Australia [81] |
1 | 2000-2012 | retrospective | III | 125 (125/211) | MMC (12.5 mg/m2) for 90 min at 42 °C using coliseum technique. |
| Vaira/ 2010/ Italy [82] | 1 | 2002-2008 | retrospective | III | 40 (40/72) | MMC (16 mg/m2) + DDP (100 mg/m2) for 60 min at 41.5 °C using semi-closed abdomen technique. Before HIPEC, LV (20 mg/m2) and 5-FU (400 mg/m2) were delivered by systemic i.v. L-OHP (460 mg/m2) for 30 min at 42 °C using semi-closed abdomen technique. |
| van Leeuwen / 2008/ Sweden [83] | 1 | 2003-2006 | retrospective | III | 38 (38/103) | Before HIPEC, LV (30 mg/m2) and 5-FU (500 mg/m2) were delivered by systemic i.v. HIPEC: L-OHP (460 mg/m2) for 30 min at 42-44 °C using the coliseum technique. |
| van Oudheusden/ 2014/ Netherlands [84] | 2 | 2005-2013 | retrospective | III | 113 (113/149) | MMC (35 mg/m2) for 90 min at 41-42 °C using coliseum technique. |
| van Oudheusden / 2015/ Netherlands [85] | 2 | 2005-2013 | retrospective | III | 252 (252/351) | MMC (35 mg/m2) for 90 min at 41.1 °C using open-coliseum technique. |
| Varban/ 2009/ America [86] |
1 | 1991-2007 | retrospective | III | 128 (128/142) | MMC (total dose 30 mg) for 60 or 90 min at 42.5 °C using closed abdomen technique. MMC (total dose 40 mg) for 120 min at 42.5 °C using closed abdomen technique. |
| Verwaal/ 2005/ Netherlands [19] | 1 | 1995-2003 | retrospective | III | 117 (117/117) | MMC (35 mg/m2) for 90 min at 40-41 °C using coliseum technique. |
| Votanopoulos/ 2013/ America [87] | 1 | 1993-2011 | retrospective | III | 217 (217/217) | MMC for 90-120 min at 40.5-43 °C using closed abdomen technique. |
| Winer/ 2014/ America [88] |
1 | 2001-2011 | retrospective | III | 30 (30/67) | MMC (total dose 40 mg) for 100 min at 42 °C using closed abdomen technique. |
| Witkamp/ 2001/ Netherlands [89] | 1 | 1995-1997 | prospective | IIb | 29 (29/29) | MMC (15-40 mg/m2 initially; 35 mg/m2 majority) for 90 min at 40-41 °C using closed abdomen technique. |
| Yan/ 2006/ Australia [90] |
1 | 1997-2006 | prospective | IIb | 30 (30/30) | MMC (10-12.5 mg/m2) for 90 min at 42 °C using coliseum technique. |
| Yan/ 2008/ Australia [91] |
1 | 1997-2007 | retrospective | III | 50 (50/50) | MMC (10-12.5 mg/m2) for 90 min at 42 °C using coliseum technique. |
| Zanon/ 2006/ Italy [92] | 1 | 1998-2004 | prospective | III | 25 (25/25) | MMC (15 mg/m2) for 60 min at 42 °C using closed abdomen technique. |
Note: MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; NR: not reported
Table 11. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| USA, 17 | Wake Forest University of Baptist Medical Center [13, 20, 68, 76, 86, 87] | ||||||||
| Subtotal/Median/Range | >709 | C | 30 mg | 40.75 (38.5-43) | 90 (60-90) | ||||
| University of Pittsburgh Medical Center (University of Pittsburgh) [37, 52, 56, 88] | |||||||||
| Subtotal/Median/Range | 190 | C | 40 mg | 42 (40-42) | 100 (90-100) | ||||
| Washington Hospital Center [13, 43, 55] | |||||||||
| Subtotal/Median/Range | >81 | C | 10 or 12.5 mg/m2 | 42 (40-43) | 90 (30-90) | ||||
| Cancer Treatment Centers of America [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Loma Linda University Medical Center [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Medical College of Wisconsin [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Mercy Medical Center [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Moores Cancer Center, University of California [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Rutgers University [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| St Agnes Hospital [15, 34] | |||||||||
| Subtotal/Median/Range | >30 | O/C | 10-20 mg/m2 | 42 | 90 | ||||
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 19. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| Serbia, 1 | Institute for Oncology and Radiology of Serbia [70] | ||||||||
| Subtotal | 61 | C | 410 mg/m2 | 41 | 30-60 | ||||
| Singapore, 1 | National Cancer Centre Singapore [79, 81] | ||||||||
| Subtotal | 63 | C | Y | 42 | 60 | ||||
| Sweden, 1 | Akademiska Sjukhuset, Uppsala University Hospital [83] | ||||||||
| Subtotal | 38 | O | 460 mg/m2 | 42-44 | 30 | ||||
| Total | 73 | ≈6,500 | O (n = 63) C (n = 51) |
n = 64 30-50 mg/m2 10-12.5 mg/m2 35 mg/m2 10-20 mg/m2 |
n = 24 30-50 mg/m2 + 50-100 mg/m2 |
n = 43 460 mg/m2 360-460 mg/m2 |
MMC+CPT-11, 5-FU L-OHP + CPT-11 MMC + CPT-11 |
41.5 (40-43) /43 (40-43) | 90 (60-90) / 60 |
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 2. Major Characteristics of Fifteen Controlled Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) versus Surgery alone with Systemic Chemotherapy (SC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
| Author/ Year/ Country | Participating Institutions |
Study Period | Design | Level of Evidence | Number of CRC PC | Treatment strategy | |
|---|---|---|---|---|---|---|---|
| HIPEC group | Control group | ||||||
| Elias D/ 2001/ France [35] | 1 | 1993-1999 | prospective | IIa | 55 (55/64) | HIPEC 27 pts HIPEC: 1. MMC (5, 8, or 10 mg/L) for 1 h between 41 °C and 44 °C using the Coliseum technique. 2. MMC (20 mg/m2) + DDP (200 mg/m2) for 1 h between 41 °C and 44 °C using the Coliseum technique. EPIC: MMC (10 g/m2) on Day 1 + 5-FU (500 mg/m2) form Day 2 to Day 6 lasted 23 h No SC |
EPIC37 pts EPIC: MMC (10 g/m2) on Day 1 + 5-FU (500 mg/m2) form Day 2 to Day 6 lasted 23 h No HIPEC No SC |
| Elias D/ 2007/ France [36] | 1 | 1999-2002 1994-2000 |
prospective | IIa | 46 (46/46) | CRS+HIPEC 23 pts HIPEC: L-OHP (460 mg/m2) for 35 min between 42-44°C using the Coliseum technique; Before starting HIEPC, 5-FU (400 mg/m2) and LV (20 mg/m2) by intravenous perfusion. EPIC: MMC (10 mg/m2) at day 0, then 5-FU (650 mg/m2) for the next 4days SC (not reported) |
EPIC 23 pts EPIC: MMC (10 mg/m2) at day 0, then 5-FU (650 mg/m2) for the next 4days SC (not reported) No HIPEC |
| Elias D/ 2009/ France [28] | 6 (Only one centre conducted HIPEC, the rest of 5 as a control) |
1998-2003 | retrospective | IIa | 96 (96/96) | Neoadjuvant IPC+CRS+HIPEC+SC 48 pts Neoadjuvant IPC: L-OHP or CPT-11 (not reported the detailed regimen) HIPEC: L-OHP (460 mg/m2) over 30 min at 43°C using the Coliseum technique. Before starting HIEPC, 5-FU 400 mg/m2 and LV 20 mg/m2 by intravenous perfusion. SC: 1. FU Plus CPT-11 or L-OHP, LV; 2. Cap Plus L-OHP; 3. CPT-11 plus C225 and CPT |
Surgery and/or SC 48 pts SC: 1. FU Plus CPT-11 or L-OHP, LV; 2. Cap Plus L-OHP; 3. CPT-11 plus C225 and CPT No HIPEC No EPIC |
Note: CRC: colorectal cancer; PC: peritoneal carcinomatosis; Pts: patients; MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; Cap: capecitabine; C225: cetuximab; CPT: camptothecin; BEV: bevacizumab; DXL: docetaxel; CBP: carboplatin; PAN: panitumumab;
Table 3. Major Characteristics of Fifteen Controlled Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) versus Surgery alone with Systemic Chemotherapy (SC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
| Author/ Year/ Country | Participating Institutions |
Study Period | Design | Level of Evidence | Number of CRC PC | Treatment strategy | |
|---|---|---|---|---|---|---|---|
| HIPEC group | Control group | ||||||
| Elias D/ 2010/ France [14] | 25 (a central database) |
1990-2007 | retrospective | IIa | 523 (523/523) | CRS+HIPEC with/without SC 443 pts CRS+HIPEC+EPIC with/without SC 9 pts HIPEC: 1. MMC (30-50 mg/m2) ± DDP (50-100 mg/m2) during 60 to 120min at 41°C using Coliseum or closed abdomen technique; 2. L-OHP (360-460 mg/m2)±CPT-11 (200 mg/m2) +intravenous 5-FU and LV during 30 minutes at 43°C using Coliseum or closed abdomen technique. EPIC: MMC (10 g/m2) on Day 1+5-FU (600 mg/m2) form Day 2 to Day 6 lasted 23 h SC: not reported the detailed regimen |
CRS+EPIC with/without SC 84 pts EPIC: MMC (10 g/m2) on Day 1+5-FU (600 mg/m2) form Day 2 to Day 6 lasted 23 h SC: not reported the detailed regimen No HIPEC |
| Esquivel J/ 2014 / America [29] |
21 (The American Society of Peritoneal Surface Malignancies (ASPSM)) |
1985-2012 | retrospective | IIa | 1,013 (1,013/1,013) | CRS+HIPEC 705 pts HIPEC: The chemotherapy drugs L-OHP or MMC or others but not reported the remaining details. SC (not detailed reported) No EPIC |
SC alone 308 pts SC (not detailed reported) No EPIC No HIPEC |
| Franko J/ 2010/ America [37] |
3 (one centre conducted HIPEC, two centre as a control) |
2001-2007 | retrospective | IIa | 105 (105/105) | CRS+HIPEC+SC 67 pts HIPEC: MMC 30mg for the first hour, followed by an additional 10 mg for 40 more minutes using the closed abdomen technique. (Perfusion fluid temperature not reported) No EPIC SC: 1. 5-FU and CPT-11; 2. L-OHP or biological agents (BEV and/or C225) |
Surgery + SC 38 pts SC: 1. 5-FU and CPT-11; 2. L-OHP or biological agents (BEV and/or C225) No EPIC No HIPEC |
Note: CRC: colorectal cancer; PC: peritoneal carcinomatosis; Pts: patients; MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; Cap: capecitabine; C225: cetuximab; CPT: camptothecin; BEV: bevacizumab; DXL: docetaxel; CBP: carboplatin; PAN: panitumumab;
Table 4. Major Characteristics of Fifteen Controlled Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC) versus Surgery alone with Systemic Chemotherapy (SC) and/or Early Postoperative Intraperitoneal Chemotherapy (EPIC).
| Author/ Year/ Country | Participating Institutions |
Study Period | Design | Level of Evidence | Number of CRC PC | Treatment strategy | |
|---|---|---|---|---|---|---|---|
| HIPEC group | Control group | ||||||
| Gervais MK/ 2013/ Canada [38] | 1 | 2004-2011 | retrospective | IIa | 40 (40/40) | Neoadjuvant SC with/without neoadjuvant radiotherapy+ CRS+HIPEC+SC 25 pts Neoadjuvant SC: BEV HIPEC: L-OHP (460 mg/m2) for 30 min between 42-44°C using the Coliseum technique; Before starting HIEPC, 5-FU (400 mg/m2) and LV (20 mg/m2) by intravenous perfusion. No EPIC SC: 5-FU, LV, L-OHP, and/or CPT-11, with or without BEV |
Neoadjuvant SC with/without neoadjuvant radiotherapy + surgery + SC 15 pts Neoadjuvant SC: BEV SC: 5-FU, LV, L-OHP, and/or CPT-11, with or without BEV No EPIC No HIPEC |
| Glehen O/ 2004/ France [13] | 28 (a central database) |
1987-2002 | retrospective | IIa | 506 (506/506) | CRS+HIPEC with/without SC 383 pts CRS+HIPEC with/without EPIC/SC 112 pts HIPEC: MMC/MMC+DDP, L-OHP, MMC+CPT-11, 5-FU, others during 30 to 90 min at 40-43°C using Coliseum or closed abdomen technique. EPIC: 5-FU (15 mg/kg/d) on Day 1-5 after surgery SC: 1. 5-FU + LV with/without DDP/L-OHP; 2. 5-FU alone; 3. 5-FU + LV+ L-OHP+ CPT-11; 4. Others and unknown |
CRS+EPIC with/without SC 235 pts EPIC: 5-FU (15 mg/kg/d) on Day 1-5 after surgery SC: 1. 5-FU + LV with/without DDP/L-OHP; 2. 5-FU alone; 3. 5-FU + LV+ L-OHP+ CPT-11; 4. Others and unknown No HIPEC |
| Goéré D/ 2015/ France [99] | 1 | 2000-2010 | retrospective | IIa | 139 (139/180) | Neoadjuvant SC +CRS+HIPEC+SC with/without EPIC 139 pts HIPEC: L-OHP+CPT-11 (72%), CPT-11 alone (15%), other items not reported. SC: 1. 5-FU + L-OHP; 2. 5-FU + CPT-11; 3. 5-FU alone EPIC: MMC/5-FU |
Neoadjuvant SC +Surgery+SC 41 pts SC: 1. 5-FU + L-OHP; 2. 5-FU + CPT-11; 3. 5-FU alone |
Note: CRC: colorectal cancer; PC: peritoneal carcinomatosis; Pts: patients; MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; Cap: capecitabine; C225: cetuximab; CPT: camptothecin; BEV: bevacizumab; DXL: docetaxel; CBP: carboplatin; PAN: panitumumab;
Table 7. Major Characteristics of Sixty-one Single Arm Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
| Author/ Years/ Country | Participating Institutions |
Study Period | Design | Level of evidence | Number of CRC PC | HIPEC |
|---|---|---|---|---|---|---|
| Evers/ 2011/ Netherlands [51] | 1 | 2001-2009 | retrospective | III | 108 (108/194) | MMC (35 mg/m2) for 90 min at 40-41 °C, perfusion mode not reported. |
| Faron M/ 2016/ France [100] | 1 | 2003-2012 | retrospective | III | 173 (173/173) | Before HIPEC, LV (20 mg/m2) and 5-FU (400 mg/m2) were delivered by systemic i.v. HIPEC: L-OHP (300 mg/m2) and CPT-11 (200 mg/m2) for 30 min between 43 °C using closed abdomen technique. |
| Franko/ 2008/ America [52] | 1 | 2001-2007 | retrospective | III | 65 (65/65) | MMC (40 mg/m2) for 90 min using closed abdomen technique. (have not reported the liquid perfusion temperature) |
| Frøysnes/ 2016/ Norway[103] | 1 | 2004-2013 | retrospective | III | 119 (119/144) | MMC (35 mg/m2) for 90 min between 39.5 °C and 41.2 °C using closed abdomen technique until 2008, and thereafter a closed technique with open abdomen |
| Glehen/ 2003/ France [53] | 1 | 1998-2001 | prospective | IIb | 26 (26/56) | MMC (0.7 mg/kg) for 90 min at 46-48 °C using closed abdomen technique. |
| Glehen/ 2004/ France [16] | 1 | 1989-2002 | retrospective | III | 53 (53/53) | MMC (total dose 40-60 mg) for 90 min at 46-48 °C using closed abdomen technique. |
| Glehen/ 2010/ France [54] | 25 | 1989-2007 | retrospective | III | 523 (523/1290) | MMC (30-50 mg/m2) with or without DDP (50-100 mg/m2) for 60-120 min at 41-42.5 °C using the coliseum or closed abdomen technique. L-OHP (360-460 mg/m2) with or without CPT-11 (100-200 mg/m2) with or without intravenous 5-FU and LV delivered over 30 min at 43°C using the coliseum or closed abdomen technique. |
| Gomes da Silva/ 2005/ America [55] | 1 | 1981-2004 | retrospective | III | 11 (11/11) | MMC (10 mg/m2 in females and 12.5 mg/m2 in males) for 90 min at 41-42 °C using closed abdomen technique. |
| Gusani/ 2008/ America [56] | 1 | 2002-2005 | retrospective | III | 28 (25/122) | MMC (30 mg) for 60 min at 40-42 °C using closed abdomen technique, after 60 min, additional MMC (10 mg) was added for 40 more min. |
| Hamilton/ 2011/ Canada [57] | 1 | 2000-2008 | retrospective | III | 31 (31/101) | MMC (12-15 mg) for 90 min at 40-42 °C using coliseum technique. |
| Hompes/ 2012/ Belgium [58] | 6 | 2004-2008 | prospective | IIb | 39 (39/48) | L-OHP (460 mg/m2) for 30 min at 41-42 °C using coliseum technique. Before HIPEC, systemic LV (20 mg/m2) and 5-FU (400 mg/m2) were administered. |
Note: MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; NR: not reported
Table 8. Major Characteristics of Sixty-one Single Arm Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
| Author/ Years/ Country | Participating Institutions |
Study Period | Design | Level of evidence | Number of CRC PC | HIPEC |
|---|---|---|---|---|---|---|
| Hompes/ 2014/ Belgium [59] | 2 | 2004-2006 2006-2010 |
retrospective | IIb | 95 (95/95) | MMC (35 mg/m2) for 90 min at 41-42 °C using coliseum or closed abdomen technique. Before HIPEC, LV (20 mg/m2) and 5-FU (400 mg/m2) were delivered by systemic i.v. L-OHP (460 mg/m2) for 30 min at 41-42 °C using coliseum or closed abdomen technique. |
| Iversen/ 2013/ Denmark [60] | 1 | 2006-2012 | retrospective | III | 34 (34/80) | MMC (35 mg/m2) for 90 min at 41.0-42.5 °C using coliseum technique. |
| Kecmanovic/ 2005/ Serbia and Montenegro [61] | 1 | 1996-2003 | retrospective | III | 18 (18/18) | MMC (12.5 mg/m2, max. 25 mg for males; 10.0 mg/m2, max. 20 mg for females) for 120 min at 42 °C using closed abdomen technique |
| Kianmanesh/ 2007/ France [62] | 1 | 1992-2005 | retrospective | III | 43 (43/43) | MMC (120 mg) + DDP (200 mg/m2) for 90-120 min at 47-50 °C using coliseum or closed abdomen technique. |
| Klaver/ 2011/ Netherlands [63] | 1 | 1997-2008 | retrospective | III | 21 (21/21) | MMC (35 mg/m2) for 90 min at 41°C using coliseum technique. |
| Klaver/ 2012/ Netherlands [64] | 2 | 1996-2010 | retrospective | III | 17 (17/24) | MMC or L-OHP for 90 min at 42°C using coliseum technique. |
| Kuijpers/ 2013/ Netherlands [65] | 6 | 1995-2012 | retrospective | III | 660 (660/960) | MMC (35 mg/m2) (in three fractions (one half, one fourth, and one fourth of the total dose)) for 90 min at 41-42 °C using coliseum technique. |
| Kuijpers/ 2014/ Netherlands [66] | 1 | 2004-2012 | retrospective | III | 73 (73/73) | MMC (35 mg/m2) for 90 min at 41-42 °C using coliseum technique. |
| Lanuke/ 2009/ Canada [67] |
1 | 2000-2008 | prospective | IIb | 31 (31/101) | MMC (12-15 mg) for 60 min at 40-42 °C using coliseum technique. |
| Levine/ 2014/ America [68] |
1 | 1991-2013 | retrospective | III | 232 (232/1000) | MMC (30 mg) for 60-90 min at 38.5-43 °C using coliseum technique; L-OHP (200 mg/m2) for selected patients. |
| Maillet M/ 2016/ France [101] | 4 | 2004-2012 | retrospective | III | 231 (231/231) | NR |
| McConnell/ 2013/ Canada [69] | 1 | 2000-2011 | retrospective | III | 245 (245/245) | MMC (12-15 mg) for 60 min at 40-42 °C using coliseum or closed abdomen technique. L-OHP (400 mg/m2) for 60 min at 40-42 °C using coliseum or closed abdomen technique with a simultaneous dose of intravenous 5-FU (800 mg).. |
| Nikolic/ 2014/ Serbia [70] |
1 | 2005-2012 | retrospective | III | 61 (61/61) | L-OHP (410 mg/m2) for 30-60 min at 41 °C using closed abdomen technique. |
Note: MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; NR: not reported
Table 9. Major Characteristics of Sixty-one Single Arm Researches on Peritoneal Carcinomatosis (PC) from Colorectal Cancer (CRC) Treated with Cytoreductive Surgery (CRS) plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC).
| Author/ Years/ Country | Participating Institutions |
Study Period | Design | Level of evidence | Number of CRC PC | HIPEC |
|---|---|---|---|---|---|---|
| Passot/ 2012/ France [21] |
1 | 1991-2010 | retrospective | III | 120 (120/120) | MMC (10 mg/ml, total dose 40-60mg) for 90 min at 46-48 °C using closed abdomen technique. MMC (0.7 mg/kg) + CPT-11 (100 mg/m2) for 90 min at 44-46 °C using closed abdomen technique. MMC (30-50 mg/m2) with or without DDP (50-100 mg/m2) for 60-120 min at 41-42.5 °C using coliseum technique or closed abdomen technique. L-OHP (360-460 mg/m2) with or without CPT-11 (100-200 mg/m2) with or without intravenous 5-FU and LV for 30 min at 43°C using coliseum technique or closed abdomen technique. |
| Passot/ 2016/ France [104] |
1 | 1989-2015 | retrospective | III | 342 (342/1,125) | Idem (Passot/ 2012/ France [21]) |
| Pilati/ 2003/ Italy [71] |
1 | 1995-2001 | retrospective | III | 46 (46/46) | MMC (3.3 mg/m2/L) with or without DDP (25 mg/m2/L) for 90 min at 41.2-42.1 °C using coliseum technique or closed abdomen technique. |
| Prada-Villaverde/ 2014/ Spain [72] | 15 | 2000-2011 | retrospective | III | 539 (539/539) | MMC or L-OHP for 30-120 min at 40-43°C using coliseum or closed abdomen technique. |
| Quenet/ 2011/ France [73] | 2 | 1998-2007 2002-2007 |
prospective | IIb | 146 (146/146) | L-OHP (460 mg/m2) with intravenous 5-FU (400 mg/m2) and LV (20 mg/m2) for 30 min at 42-45 °C using coliseum technique. L-OHP (300 mg/m2) with CPT-11 (200 mg/m2) with intravenous 5-FU (400 mg/m2) and LV (20 mg/m2) for 30 min at 42-45 °C using coliseum technique. |
| Rivard/ 2014/ Canada [74] | 1 | 2003-2011 | retrospective | III | 68 (68/68) | NR |
| Rodt/ 2013/ Denmark [75] | 1 | 2006-2011 | retrospective | III | 19 (19/35) | NR |
| Shen/ 2004/ America [20] |
1 | 1991-2002 | retrospective | III | 77 (77/77) | MMC (total dose 30 mg) for 60-120 min at 38.5-43 °C using closed abdomen technique. |
| Shen/ 2008/ America [76] |
1 | 1992-2005 | retrospective | III | 55 (55/150) | MMC (total dose 30 mg) for 60-120 min at 38.5-43 °C using closed abdomen technique. |
| Simkens GA/ 2015/ Netherlands [102] | 1 | 2007-2013 | retrospective | III | 133 (133/133) | MMC (35 mg/m2) for 90 min at 41.1 °C using open-coliseum technique. |
| Swellengrebel/ 2009/ Netherlands [77] | 1 | 1999-2005 | retrospective | III | 92 (92/92) | MMC (35 mg/m2) for 90 min at 41-42 °C using coliseum technique. |
Note: MMC: mitomycin C; DDP: cisplatin; FU: fluorouracil; LV: leucovorin; L-OHP: oxaliplatin; CPT-11: irinotecan; NR: not reported
Table 12. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| USA, 17 | St. John Hospital [29, 72] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Tufts Medical Center [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| University of Illinois [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| University of Miami [29, 72] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| American Society of Peritoneal Surface Malignancies (ASPSM) [29] | ? | NR | Y | Y | others | NR | NR | ||
| Mount Sinai Medical Center [78] | 51 | C | 40 mg | 41-43 | 90 | ||||
| Sharp Health Care [13] | ? | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Subtotal | >1061 | C | 30/40 mg 10-20 mg/m2 10 or 12.5 mg/m2 |
Y, 200 mg/m2 | MMC+CPT-11, 5-FU | 42 (40-43) | 90 (60-90) /30 | ||
| France, 14 | Centre Hospitalo-Universitaire Lyon Sud [14, 16, 21, 29, 13, 40, 42, 53, 72, 101] | ||||||||
| Subtotal/Median/Range | >500 | C | 10 mg/L 0.7 mg/kg 40-60 mg 30-50 mg/m2 |
30-50 mg/m2 + 50-100 mg/m2 | 360 mg/m2 360-460 mg/m2 |
MMC+CPT-11, 5-FU MMC (0.7 mg/kg) + CPT-11 (100 mg/m2) L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) |
44 (46-48) /43 | 90 (60-90) /30 | |
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 13. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| France, 14 | Gustave Roussy Institute [13, 14, 28, 35, 36, 49, 50, 54, 73, 99-101] | ||||||||
| Subtotal/Median/Range | >700 | O | 5, 8, or 10 mg/L 20 mg/m2 12.9+/-3.8 mg/m2 30-50 mg/m2 |
20 mg/m2 + 200 mg/m2 30-50 mg/m2 + 50-100 mg/m2 |
460 mg/m2 360-460 mg/m2 |
MMC+CPT-11, 5-FU L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) L-OHP (300 mg/m2) + CPT-11 (200 mg/m2) |
43 (41-44) /43 | 60 (60-90) /30 | |
| Val d’Aurelle Center [13, 14, 54, 73] | |||||||||
| Subtotal/Median/Range | >66 | O | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 460 mg/m2 360-460 mg/m2 |
L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) L-OHP (300 mg/m2) + CPT-11 (200 mg/m2) |
43.5 (40-43) /43 | 60 (60-90) /30 | |
| Centre Hospitalo-Universitaire l’Archet [13, 14, 54] | |||||||||
| Subtotal/Median/Range | >25 | O/C | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41.5 (41-43) /43 | 60 (60-90) /30 or 60 | |
| Paul Papin Institute [13, 14, 54] | |||||||||
| Subtotal/Median/Range | >25 | O/C | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41.5 (41-43) /43 | 60 (60-90) /30 or 60 | |
| French Association of Surgery [14, 54] | |||||||||
| Subtotal/Median/Range | ? | O/C | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41 (41-43) /43 | 90 (60-120) /30 or 60 | |
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 14. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| France, 14 | Hospital Lariboisiere [29, 72] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Louis-Mourier University Hospital [62, 62, 101] | |||||||||
| Subtotal/Median/Range | >250 | O/C | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 201 mg + 200 mg/m2 |
360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 42 (41-42.5) /48.5 (47-50) /30-43 |
90 (90-120) /60 | |
| Centre Hospitalier de Bellevue [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Centre Hospitalo-Universitaire Dijon [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Centre Jean Perrin [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| CHU of Nice [48] | 74 | O/C | 10 or 12.5 mg/m2 | 43 | 90 | ||||
| Lyon Civil Hospices, South Lyon University Hospital Center [54] | ? | O/C | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41-42.5, 30-43 | 90/60 | |
| Université Claude Bernard Lyon [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Subtotal | >1038 | O | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | MMC+CPT-11, 5-FU L-OHP + CPT-11 MMC + CPT-11 |
41.5 (40-43) /43 | 60 (60-90) /30 or 60 | |
| Italy, 8 | National Cancer Institute of Milan [29, 46, 72] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | 42 (41.5-43) /43 | 60 (60-90) /30 | ||
| San Giuseppe Hospital [13, 46, 82] | |||||||||
| Subtotal/Median/Range | >65 | O/C | Y | 3.3 mg/m2/L + 25 mg/m2/L 16 mg/m2 + 100 mg/m2 |
460 mg/m2 | MMC+CPT-11, 5-FU | 42 (41.5-43) /43 | 60 (60-90) /30 | |
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 15. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| Italy, 8 | Regina Elena National Cancer Institute [13, 46] | ||||||||
| Subtotal/Median/Range | >25 | O/C | Y | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | MMC+CPT-11, 5-FU | 42 (41.5-43) /43 | 60 (60-90) /30 | |
| University of Padua [46, 71] | |||||||||
| Subtotal/Median/Range | >46 | O/C | 3.3 mg/m2/L | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | 42 (41.5-43) /43 | 90 (60-90) /33 | ||
| Istituto Nazional Tumori [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Ospedale di Bentivoglio [46] | ? | O/C | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | 41.5-43/43 | 60-90/30 | |||
| San Camillo-Forlanini Hospital [46] | ? | O/C | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | 41.5-43/43 | 60-90/30 | |||
| San Giovanni Battista Antica Sede Hospital [92] | 25 | C | 15 mg/m2 | 42 | 60 | ||||
| Subtotal | >186 | C | Y | 3.3 mg/m2/L + 25 mg/m2/L | 460 mg/m2 | MMC+CPT-11, 5-FU | 42 (41.5-43) /43 | 60 (60-90) /30 | |
| Belgium, 6 | Jolimont Hospital [14,54, 58] | ||||||||
| Subtotal/Median/Range | ? | O | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 460 mg/m2 |
L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41.5 (41-42.5) /43 | 90 (60-120) /30 or 60 | |
| Ghent University Hospital [47, 58] | |||||||||
| Subtotal/Median/Range | >152 | O | 35 mg/m2 | 460 mg/m2 | 41 (41-42) | 60 (60-90) /30 | |||
| University Hospitals Gasthuisberg [58, 59] | |||||||||
| Subtotal/Median/Range | >39 | O | 460 mg/m2 | 41-42 | 30 | ||||
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 16. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| Belgium, 6 | I-Biostat, Katholieke Universiteit Leuven and Universiteit Hasselt [58] | ? | O | 460 mg/m2 | 41-42 | 30 | |||
| UCL Mont-Godinne [58] | ? | O | 460 mg/m2 | 41-42 | 30 | ||||
| Ziekenhuis Oost-Limburg [58] | ? | O | 460 mg/m2 | 41-42 | 30 | ||||
| Subtotal | >191 | O | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 460 mg/m2 | L-OHP + CPT-11 | 41 (41-42) /41-42 | 90 (60-90) /30 or 60 | |
| Netherlands, 6 | Netherlands Cancer Institute [12, 19,51, 59, 65, 66, 77, 89] | ||||||||
| Subtotal/Median/Range | 863 | O | 35 mg/m2 | 41.5 (41-42) | 90 | ||||
| Catharina Hospital Eindhoven [44, 63-65, 84, 85, 102] | |||||||||
| Subtotal/Median/Range | >300 | O | 35 mg/m2 | 41.5 (41-42) | 90 | ||||
| Sint Antonius Hospital Nieuwegein [44, 65, 84, 85] | |||||||||
| Subtotal/Median/Range | >121 | O | 35 mg/m2 | 41.5 (41-42) | 90 | ||||
| Radboud University Nijmegen Medical Center [64] | 12 | O | 35 mg/m2 | 41-42 | 90 | ||||
| University Medical Center Groningen [64] | 48 | O | 35 mg/m2 | 41-42 | 90 | ||||
| VU Medical Centre Amsterdam [64] |
17 | O | 35 mg/m2 | 41-42 | 90 | ||||
| Subtotal | >1432 | O | 35 mg/m2 | 41.5 (41-42) | 90 | ||||
| Spain 6 |
Hospital San Jaime [29, 71] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Hospital Torrecardenas [29, 71] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 17. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| Spain, 6 | M. D. Anderson Cancer Center [29, 71] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| San Jose Hospital [29, 71] | |||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Hospital Infanta Cristina [71] |
? | O/C | Y | Y | others | 40-43 | 30-120 | ||
| Hospital Santiago Apostol [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Subtotal | >25 | O/C | Y | Y | MMC+CPT-11, 5-FU | 41.5 (40-43) | 90 (30-120) /30 | ||
| Canada, 2 | University of Calgary [56, 66, 68, 73] | ||||||||
| Subtotal/Median/Range | 375 | O | 12-15 mg | 400 mg/m2 | 41.5 (40-42) | 60 | |||
| Maisonneuve-Rosemont Hospital, University of Montreal [14, 38, 53] | |||||||||
| Subtotal/Median/Range | >40 | O | 30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP (360-460 mg/m2) + CPT-11 (100-200 mg/m2) | 41.5 (41-42.5) /43 (42-43) | 90 (60-120) /30 or 60 | |
| Subtotal | >415 | O | 12-15 mg /30-50 mg/m2 | 30-50 mg/m2 + 50-100 mg/m2 | 360-460 mg/m2 | L-OHP + CPT-11 | 41.5 (41-42.5) /43 (42-43) | 60 or 90 (60-120) /30 or 60 | |
| Greece, 2 | Metaxa Cancer Memorial Hospital [29, 71] | ||||||||
| Subtotal/Median/Range | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Didimotichon General Hospital [13] | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Subtotal | >25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 41.5 (40-43) | 30-90 | |
| Australia, 1 | St. George Hospital [15, 26, 29, 34, 41, 45, 64, 72, 81, 90, 91] | ||||||||
| Subtotal | >618 | O | 10-12.5 mg/m2 | 350 mg/m2 | 42 | 90 or 30 | |||
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Table 18. Summary of HIEPC-related procedures in different PC institutions or countries (published researches).
| Country /No. Institutions |
Major Institutions | No. patients | Mode | HIPEC-MMC alone | HIPEC-MMC+DDP | HIPEC-L-OHP alone | HIPEC-other | Temperature (°C) |
Duration (min) |
|---|---|---|---|---|---|---|---|---|---|
| China, 1 | Zhongnan Hospital of Wuhan University [39] | ||||||||
| Subtotal | 62 | O | MMC (30 mg) + DDP (120 mg) | 43.0±0.5 | 90 | ||||
| Norway, 1 | Norwegian Radium Hospital [103] | ||||||||
| Subtotal | 109 | O/C | 35 mg/m2 | 41.4 (39.5-42.1) | 90 | ||||
| Denmark, 1 | Aarhus University Hospital [60, 75] | ||||||||
| Subtotal | 53 | O | 35 mg/m2 | 41-42.5 | 90 | ||||
| Germany, 1 | University of Wuerzburg Medical Centre [15, 29, 72] | ||||||||
| Subtotal | >11 | O | 10-20 mg/m2 | Y | 42 /40-43 | 90 /30 | |||
| Israel. 1 | Tel Aviv Medical Center [13] | ||||||||
| Subtotal | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Japan, 1 | Shizuoka Cancer Centre [13] | ||||||||
| Subtotal | 25 | O/C | Y | Y | Y | MMC+CPT-11, 5-FU | 40-43 | 30-90 | |
| Mexico, 1 | Instituto Jalisciense de Cancerologia [29, 72] | ||||||||
| Subtotal | ? | O/C | Y | Y | 40-43 | 30-120 | |||
| Serbia and Montenegro, 1 | First Surgical University Hospital, Clinical Center of Serbia [61] | ||||||||
| Subtotal | 18 | C | 10 or 12.5 mg/m2 | 42 | 120 | ||||
Note: C: closed abdomen technique for HIPEC; O: open abdomen technique for HIPEC; Y: yes; MMC: mitomycin C; DDP: cisplatin; 5-FU: fluorouracil; L-OHP: oxaliplatin; CPT-11: irinotecan
Patients characteristics
In this meta-analysis, the median complete cytoreduction (CC0-1) rate was 72.2% (range, 32.4% - 100%), including 4 studies with 100% CC0 [28, 35, 36, 40], 7 studies with 50% - 99% CC0 [14, 15, 26, 29, 34, 37, 99], and 4 studies with <50% CC0 [12, 13, 38, 39]. Major clinico-pathologic characteristics of the 6,857 CRC PC patients (sample size ranging from 11 to 660) in 61 non-controlled studies are listed by Table 6-10.
HIPEC characteristics
Major technical features of HIPEC procedures in each institution are summarized in Table 11-19. HIPEC was performed using only open technique in 22 institutions and only closed techniques 10 institutions, with 41 institutions used both open and closed techniques. The commonly used chemotherapy agents were mitomycin C (MMC) alone (n = 63, dosage of 30-50 mg/m2 in 88% of institutions, median temperature 41.5°C, ranging from 40 - 43°C, and median duration 90 min, ranging from 60 - 90 min), oxaliplatin (L-OHP) alone (n = 43, dosage of 460 mg/m2 in 60% of institutions, median temperature 43°C, ranging from 40 - 43°C; and median duration 60 min), and a combination of MMC and cisplatin (CDDP) (n = 24, dosage of 30-50 mg/m2 + 50-100 mg/m2 in 33% of institutions).
Primary results for meta-analysis
Meta-analysis outcomes
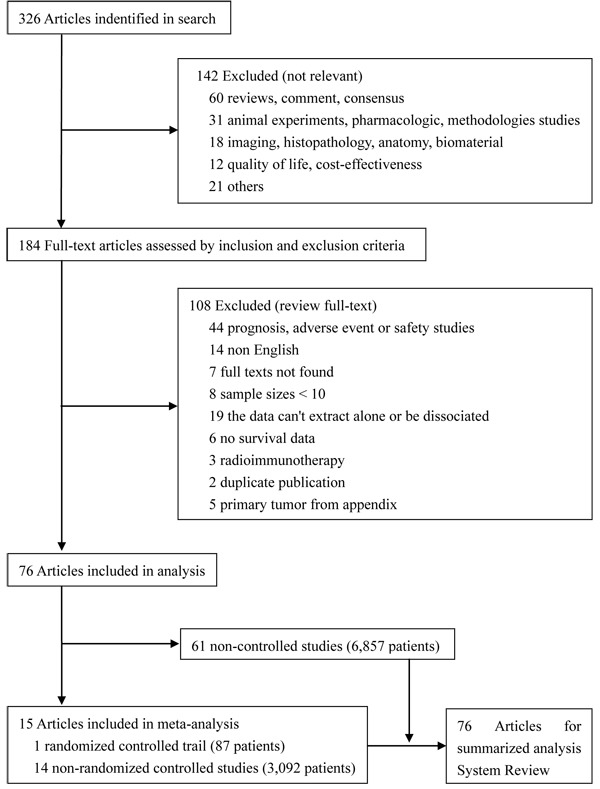
The summarized HRs for OS in the 15 controlled researches was 2.67 (95% CI, 2.21-3.23, I2 = 0%, P < 0.00001) (Figure 2a), suggesting that CRC PC patients could obtain more benefits from CRS plus HIPEC than traditional therapy, without apparent heterogeneity among the studies (P = 0.81, I2 = 0%).
Figure 2.
Forest plots of 15 studies displaying the results of the meta-analysis on hazard ratios (HR) for overall survival (OS) (a); Sensitivity analysis of sample size difference (b), published-time difference (c), and geographic-distribution difference (d).
Sensitivity analysis of summarized HR and 95% CI showed no difference after choosing random effects model and fixed effects model. In terms of sample size difference, 15 researches were divided into three subgroups (sample size <50, 50-100, >100) by a sensitivity study for a stratified meta-analysis. The summarized HR and 95% CI showed no difference, with no between-subgroup heterogeneity (P = 0.48, I2 = 0%) (Figure 2b). In a sensitivity analysis, four studies with potential heterogeneity was removed due to small sample size [34] or asymmetrical sample size between two groups [14, 26, 40, 99], but the summary HR was 2.81 (95%CI, 2.28-3.48, I2 = 0%, P heterogeneity = 0.56).
There was no statistically significant heterogeneity of HRs for published-time pertinence (P = 0.52) (Figure 2c) and geographic-distribution pertinence (P = 0.43) (Figure 2d).
Analysis of chemotherapy regimens
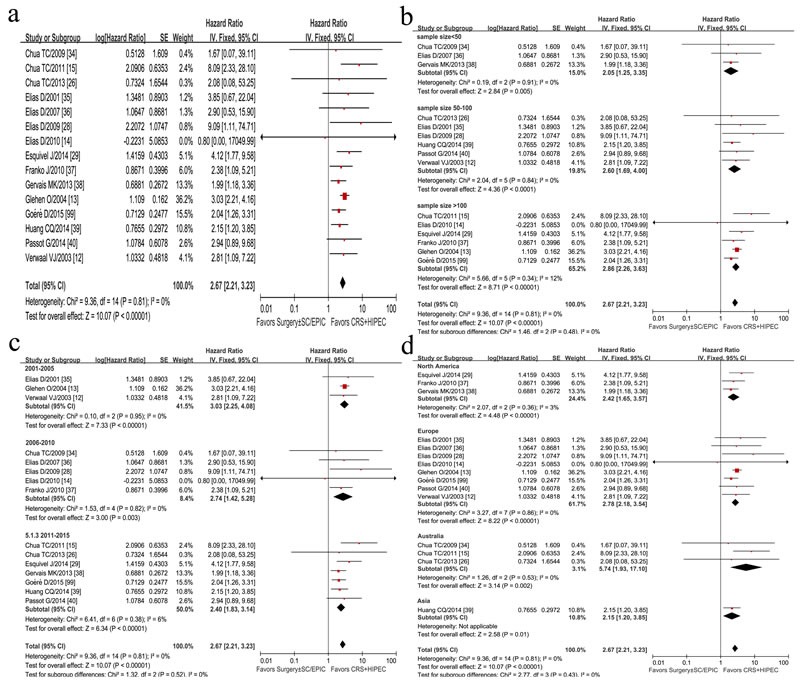
Regarding the effect of different chemotherapy regimens in HIPEC procedure on the efficacy on OS or DFS, 15 researches were divided into 3 subgroups: group of MMC based chemotherapy, group of L-OHP based chemotherapy, and group of other regimens. The heterogeneity showed no significant difference (P = 0.27, I2 = 24.1%), which revealed that difference of chemotherapy regimens of HIPEC was not associated with OS and DFS after CRS and HIPEC in this meta-analysis (Figure 3a). A further analysis of difference in median year survival rate between group of CRS plus HIPEC and group of traditional treatment was conducted by independent-samples T test stratified by MMC and L-OHP subgroups (Figure 3b and Figure 3c).
Figure 3.
Forest plots of 15 studies evaluating heterogeneity test of chemotherapy regimens difference (MMC based chemotherapy; L-OHP based chemotherapy; others) in HIPEC procedure (a); The difference of mean year survival rate between CRS+HIPEC group and traditional group for MMC-basic (Mitomycin C, MMC) HIPEC procedure (b), for L-OHP-basic (Oxaliplatin, L-OHP) HIPEC procedure (c); Forest plots of 15 studies evaluating heterogeneity test of the proportion of CC0 difference (d).
MMC-based HIPEC procedure
OS data by MMC-based HIPEC procedure were available in 7 studies with 614 patients [12, 13, 15, 34, 35, 37, 39]. Due to more patients received MMC regimen in studies by Elias et al. [35] (21 patients for MMC regimen, while 6 patients for L-OHP regimen) and Glehen et al. [13] (322 patients for MMC regimen, while 32 patients for L-OHP regimen and 29 patients for others), these two studies were included in MMC subgroup. The stratification analysis showed that OS of patients receiving HIPEC by MMC was significantly improved (HR = 2.88, 95% CI, 2.26-3.68, I2 = 0%, P < 0.00001) (Figure 3a), with 1-, 3-, and 5-year survival rates of 79.5%, 38.8%, and 34%, respectively (Figure 3b). In comparison, the corresponding survival rates in the traditional group were 54.9%, 18.3%, and 9.7%, respectively (Figure 3b).
L-OHP-based chemotherapy in HIPEC procedure
Four studies using L-OHP based chemotherapy in HIPEC procedures of 283 patients [28, 36, 38, 40, 99]. A statistically significant benefit for OS was revealed in HIPEC group (HR = 2.18, 95% CI, 1.57-3.04, I2 = 0%, P < 0.00001) (Figure 3a), with the 1-, 3-, and 5-year survival rates of 93%, 59%, and 43%, respectively in HIPEC group vs. 63%, 25%, and 14%, respectively in traditional group (Figure 3c).
Other chemotherapy regimes in HIPEC procedure
Three trials [14, 26, 29] were identified as the subgroup of other regimen due to difficulties in identifying them as MMC subgroup or L-OHP subgroup since mixed chemotherapy regimens were used in HIPEC during the whole disease course. A significant survival benefit in HIPEC group vs. traditional group (HR = 3.90, 95% CI, 1.73-8.81, I2 = 0%, P < 0.00001) was demonstrated (Figure 3a).
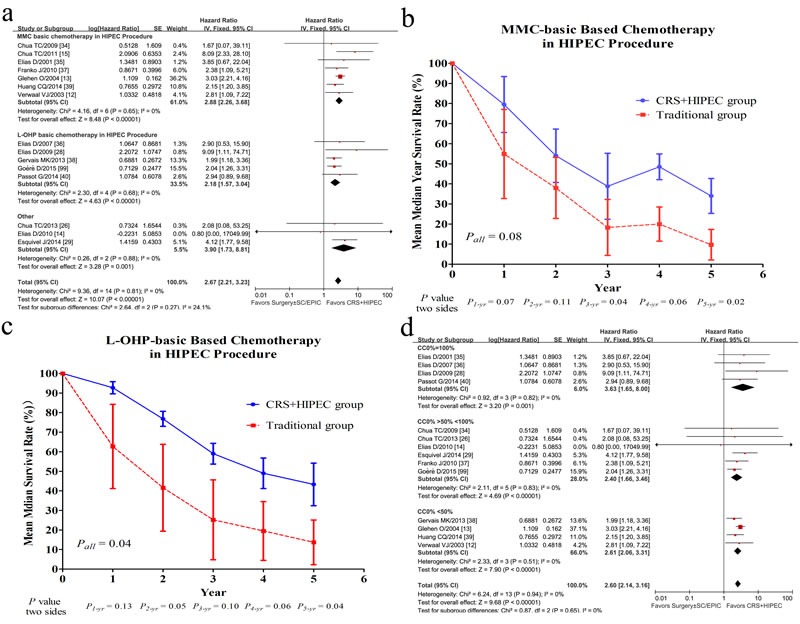
Publication bias
Publication bias was evaluated with funnel plot analyses, as shown in Figure 4, and the funnel plot was symmetric. No apparent publication bias was found in our OS meta-analysis with Begg's test (z continuity corrected = 0.99, Pr >|z|continuity corrected = 0.32) (Figure 4a), or with Egger's test (t = 0.82, P >|t|= 0.427, 95%CI of bias: -0.49˜1.1) (Figure 4b).
Figure 4.
Funnel plots of this meta-analysis by Begg's test (a), and by Egger's test (b).
Summary of HIPEC-related data
In 15 controlled studies and 59 single-arm studies, HIPEC-related outcomes including survival rates, median OS and 95% CI, DFS/RFS, PFS, follow-up time, morbidity, and mortality, are summarized in Table 20-25 and Figure 5
Table 20. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Controlled Studies | ||||||||||||
| Chua TC/ 2009/ Australia [34] | ≈84 | ≈50 | ≈26 | NA | NA | NR | NR | 13 | NR | NR | NR | 18 (9-59) |
| Chua TC/ 2011/ Australia [15] | 92 | NR | 55 | NR | 30 | NR | NR | 38 | 30.2 - 45.2 | NR | 17 (1-216) (two groups) |
17 (1-126) |
| Chua TC/ 2013/ Australia [26] | NR | NR | NR | NR | 41 | NR | NR | 38 | 21.1 - 54.9 | NR | 33 (22.4-43.8) (RFS) |
22 (5-88) |
| Elias D/ 2001/ France [35] | NR | ≈70 | ≈53 | ≈53 | ≈44 | 8.1 | Overall: 54.6 | ≈54 | NR | NR | ≈26 2-,3-,5-yr 54.7%, 39.4% and 18.4% (two groups) |
51.7 (8.1-89.3) |
| Elias D/ 2007/ France [36] | ≈96 | ≈78 | ≈63 | ≈54 | 54 | 0 | 4 | NA | NR | NR | NR | 113 (70-188) |
| Elias D/ 2009/ France [28] | NR | 81 | NR | NR | 51 | NR | NR | 62.7 | NR | NR | NR | 95.7 vs. 63 |
| Elias D/ 2010/ France [14] | NR | NR | 40 | NR | 25.5 | NR | NR | 31 | NR | NR | ≈9 1-,3-,5-yr 47%, 15% and 10% (two groups) |
NR |
| Esquivel J/ 2014/ America [29] | NR | NR | 66 | NR | 58 | NR | NR | 41 | 38.0-46.3 | NR | NR | 25 vs. 8 (0.1-128) |
| Franko J/ 2010/ America [37] | ≈92 | ≈66 | ≈51 | ≈44 | ≈28 | NR | NR | 34.7 | NR | NR | NR | NR |
| Gervais MK/ 2013/ Canada [38] | ≈92 | ≈76 | 61 | ≈53 | 36 | 4 | 20 | ≈54 | NR | NR | ≈8 | 22.8 (2-81) |
| Glehen O/ 2004/ France [13] | NR | NR | NR | NR | NR | NR | NR | 21.6/19.2 | NR | NR | NR | 53 (5-192) |
| Goéré D/ 2015/ France [99] | ≈90 | ≈72 | 52 | ≈40 | ≈32 | 5.8 | 29.5 | ≈35 | NR | NR | NR | 60 (47-74) |
| Huang CQ/ 2014/ China [39] | 63.6 | 20.0 | 16.0 | NR | NR | 0 | 28.6 | 13.7 | 10.0-16.5 | NR | NR | 41.5 (11.5-70.9) |
| Passot G/ 2014/ France [40] | NR | NR | NR | NR | NR | NR | NR | 36 | NR | NR | NR | NR |
| Verwaal VJ/ 2003/ Netherlands [12] | ≈66 | ≈42 | ≈32 | NR | NR | 8 | 19 | 22.4 | NR | NR | NR | 21.6 |
| Subtotal of 15 studies (Mean ± SD; Median/Range) |
84.5 ± 12.6 vs. 58.1 ± 20.6 91 (63.6-96) vs. 54 (27.5-87) |
61.7 ± 20.3 vs. 38.8 ± 18.7 70 (20-81) vs. 42 (12-65) |
46.8 ± 16.2 vs. 23.6 ± 15.2 52 (16-66) vs. 18 (0-47) |
48.8 ± 6.4 vs. 20.4 ± 10.1 53 (44-54) vs. 22 (14-33) |
40.0 ± 11.5 vs. 18.1 ± 14.1 38 (25.5-58) vs. 18 (0-44) |
4.3 ± 3.7 vs. 6.2 ± 4.2 5 (0-8.1) vs. 6.3 (0-11.1) |
19.8 ± 9.2 vs. 20.5 ± 12.3 19.5 (4-29.5) vs. 23 (3.1-31.6) |
34.3 ± 14.8 vs. 18.8 ± 8.8 35 (13-62.7) vs. 17 (8.5-34) |
43.8 ± 32.8 vs. 29.7 ± 29.3 25 (17-113) vs. 18 (8-63) |
|||
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Table 25. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC single arm studies | ||||||||||||
| Winer/ 2014/ America [88] |
53 | 22 | 22 | ≈13 | ≈13 | 6.7 | 22.2 | 12.2 | 7.5-17.2 | 9.3 (3.3-17.8) 1-,3-yr, 47%, 16% |
NR | 52.8 (12.5-138) |
| Witkamp/ 2001/ Netherlands [89] | NR | 45 | 23 | NR | NR | 3 | 38 | NR | NR | NR | 11 (3-29) (RFS) | 38 (26-52) |
| Yan/ 2006/ Australia [90] |
72 | 64 | NR | NR | NR | 0 | NR | 29 | 2-39 | NR | NR | 12 (2-39) |
| Yan/ 2008/ Australia [91] |
79 | 67 | 39 | NR | NR | NR | NR | 29 | 1-56 | NR | NR | 14 (1-56) |
| Zanon/ 2006/ Italy [92] | ≈75 | ≈60 | ≈28 | NR | NR | 4 | 24 | 30.3 | 17.0-52.2 | 17.3 (5.72-28.9) | NR | NR |
| Total of 76 studies (Mean±SD; Median/Range) |
79.7 ± 14.5; 83 (12-100) |
56.5 ± 17.3; 60 (17-89) |
42.3 ± 17.1; 42 (9-89.4) |
33.8 ± 15.4; 34.5 (0-85) | 27.5 ± 14.1; 31 (0-58) |
2.8 ± 2.9 2.5 (0-12) |
33.0 ± 13.4 32.9 (4-60) |
29.2 ± 11.3 29 (12-62.7) |
13.1 ± 3.2 13.6 (7-17.3) |
15.9 ± 7.7 12.6 (8-36.9) |
33.1 ± 22.5 | |
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Figure 5.
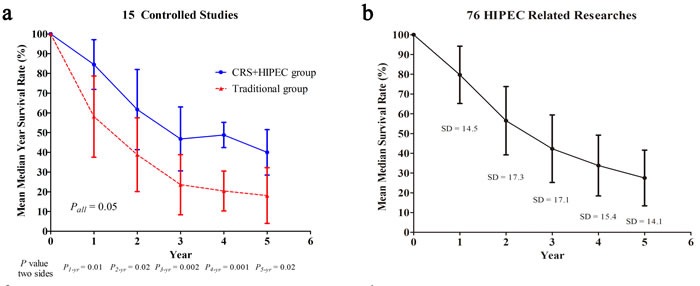
The summarized median year survival rates between CRS+HIPEC group and traditional group for 15 controlled studies (a); The summarized median year survival rates on 76 HIPEC related studies (b).
Table 21. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC single arm studies | ||||||||||||
| Alzahrani/ 2015/ Australia [41] | ≈84 | 56 | ≈40 | ≈26 | 24 | 1.2 | 23.3 | 28 | NR | NR | NR | NR |
| Beaujard/ 2000/ France [42] | NR | NR | NR | NR | NR | NR | NR | 12 | NR | NR | NR | NR |
| Bijelic/ 2008/ Australia [43] | ≈94 | ≈56 | ≈44 | ≈23 | 17 | NR | NR | 30 | NR | 15 | NR | Mean: 40.8 Median: 29.5 |
| Braam/ 2014/ Australia [44] | NR | NR | NR | NR | 6 | NR | NR | 14.9 | NR | NR | 11.4 | 26.2 |
| Cao/ 2009/ Australia [45] | 83.6 | 65.4 | 51.4 | 32.1 | 32.1 | NR | NR | 37.0 | 1-72 | NR | NR | 19 (1-72) |
| Cavaliere/ 2006/ Italy [46] | NR | NR | 25.8 | NR | NR | 3.3 | 22.5 | 19 | NR | NR | 16 | 16 |
| Ceelen/ 2014/ Belgium [47] | ≈75 (NNT) ≈75 (NCA) ≈96 (NCB) |
≈57 (NNT) ≈47 (NCA) ≈89 (NCB) |
≈39 (NNT) ≈30 (NCA) ≈71 (NCB) |
≈32 (NNT) ≈19 (NCA) NA (NCB) |
≈25 (NNT) ≈13 (NCA) |
NR | NR | 27 (included APP) 24 (Right colon) 27 (Left colon) 35 (Rectal) 25 (NNT) 22 (NCA) 39 (NCB) 30 (AC) 22 (NAC) |
20.8-33.2 (included APP) 10.3-37.7 (Right colon) 22.8-31.2 (Left colon) 4.9-65 (Rectum) 19.1-30.9 (NNT) 12.9-31.1 (NCA) 17.6-60.4 (NCB) 20.7-39.3 (AC) 14.2-29.8 (NAC) |
NR | NR | 18 |
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Table 22. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC single arm studies | ||||||||||||
| Desantis/ 2014/ France [48] | ≈88 | ≈72 | 60.3 | ≈47 | 37 | 1 (all) | 12.5 (all) | 45.9 | NR | NR | 16.8 1-,3-,5-yr 61.3%, 30.4% and 22.8% |
NR |
| Elias/ 2004/ France [49] | 83 | 74 | 65 | NR | NR | 8.3 | 41.6 | NR | NR | NR | 18 1-,2-,3-yr 61%, 50% and 50% |
27.4 (18.3-49.6) |
| Elias/ 2014/ France [50] | 91.4 | ≈74 | 54 | ≈47 | 36.5 | 4.2 | 17 | ≈41 | NR | NR | NR | 62.4 (55.6-77.6) |
| Evers/ 2011/ Netherlands [51] | NR | NR | NR | NR | 36 | NR | NR | 49.2 vs. 41.3 (Ovarian metastases vs. without ovarian metastases) |
NR | NR | 36.9 vs. 32.5 (Ovarian metastases vs. without ovarian metastases) |
22 (1 week – 97 mo) |
| Faron / 2016/ France [100] | NR | NR | NR | NR | 42 | 4.6 | 47 | 41 | 32-50 | NR | 17.7 (12-19) 5-yr: 14% |
48.5 (41.0-56.3) |
| Franko/ 2008/ America [52] | ≈79 (MVR) ≈12 (NVR) |
≈46 (MVR) ≈30 (NVR) |
≈31 (MVR) ≈30 (NVR) |
≈16 (MVR) ≈30 (NVR) |
0 (MVR) ≈15 (NVR) |
1.4 | 60 | 20.2 (MVR) 14.3 (NVR) |
NR | NR | NR | NR |
| Frøysnes/ 2016/ Norway [103] | ≈93 | ≈78 | 65 | ≈45 | 36 | 0 | 15.1 | 47 | 42-52 | NR | 10 (7-12) | 45 (35-55) |
| Glehen/ 2003/ France [53] | NR | NR | NR | NR | NR | 1.8 (all) | 28.6 (all) | 17.5 | 4.4-53.6 | NR | NR | 18.1 (4.4-56) (all) |
| Glehen/ 2004/ France [16] | 55 | 32 | NR | NR | 11 | 4 | 23 | 12.8 | NR | NR | NR | 59.5 (2-148) |
| Glehen/ 2010/ France [54] | ≈80 | ≈56 | 41 | ≈33 | 26 | 4.1 (all) | 33.6 (all) | 30 | NR | NR | 1-,3-,5-yr 77%, 49% and 37% | 45.3 (20.3-90.9) (all) |
| Gomes / 2005/ America [55] | ≈60 | ≈30 | ≈20 | ≈20 | 0 | NR | NR | 20 | NR | NR | NR | 15.7 (1-51) |
| Gusani/ 2008/ America [56] | ≈74 | ≈49 | ≈49 | ≈39 | NR | 0 | 29.8 (all) | ≈23.6 | NR | NR | NR | 35.9 (19.0-57.7) (all) |
| Hamilton/ 2011/ Canada [57] | ≈79 | ≈62 | 38 | ≈34 | 34 | NR | NR | 27 | 0-87 | NR | 9 (0-87) 3-,5-yr 34%,26% |
28 (0-119) (all) |
| Hompes/ 2012/ Belgium [58] | 97.9 | 88.7 | ≈84 | NA | NA | 0 | 52.1 | NA | NA | NR | 19.8 (12–upper limit not defined) (RFS) | 22.7 (3.2-55.7) |
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Table 23. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC single arm studies | ||||||||||||
| Hompes/ 2014/ Belgium [59] | ≈91 (L-OHP) ≈88 (MMC) |
≈68 (L-OHP) ≈59 (MMC) |
≈53 (L-OHP) ≈42 (MMC) |
≈45 (L-OHP) ≈33 (MMC) |
NA | 0 | 41.1 | 37.1 (L-OHP) 26.5 (MMC) |
22.4-52.8 (L-OHP) 16.9-64.8 (MMC) |
NR | 12.2 (7.2-undefined) (L-OHP) 13.8(7.0-25.8) (MMC) (RFS) |
33.6 (L-OHP) 61.2 (MMC) |
| Iversen/ 2013/ Denmark [60] | ≈97 | 60 | 47 | 38 | 38 | 2.9 | 32.4 | ≈31 | NR | NR | NR | 16.0 (0.9–71.3) |
| Kecmanovic/ 2005/ Serbia and Montenegro [61] | ≈85 | ≈85 | ≈85 | ≈85 | NA | 0 | 44.4 | 15 | 1-57 | NR | NR | 21 (1-56) |
| Kianmanesh/ 2007/ France [62] | ≈95 | 72 | ≈57 | 44 | 44 | 2.3 | 39 | 38.4 | 32.8-43.9 | NR | NR | NR |
| Klaver/ 2011/ Netherlands [63] | 71 | ≈56 | ≈43 | ≈35 | ≈18 | NR | NR | 28 | 3-100 | NR | NR | NR |
| Klaver/ 2012/ Netherlands [64] | 83 | ≈52 | ≈26 | ≈26 | NA | 0 | 33.3 | 35 | 20.0-49.9 | NR | 12 (7.7-16.3) | 10.5 (1-52) |
| Kuijpers/ 2013/ Netherlands [65] | ≈84 | ≈62 | 46 | ≈37 | 31 | 3 included PMP |
34 included PMP |
33 | 28-38 | 15 (13–17) | NR | 41 (35-46) included PMP |
| Kuijpers/ 2014/ Netherlands [66] | ≈87 | ≈62 | 45 | ≈37 | ≈32 | 0 | 30 | 30 | 19-41 | 15 (14-16) | NR | 47 (43-51) |
| Lanuke/ 2009/ Canada [67] | ≈85 | ≈58 | ≈46 | NA | NA | 4 (all) | 39 (all) | 26 | 1-48 | NR | 8 (1-31) | 12 (1-48) |
| Levine/ 2014/ America [68] | ≈69 | ≈38 | ≈27 | ≈19 | ≈17 | 3.8 (all) | 42 (all) | ≈19 | NR | NR | NR | NR |
| Maillet/ 2016/ France [101] | NR | NR | 58 | NR | 34 | 4 | NR | 43.3 | NR | 12.4 | NR | NR |
| McConnell/ 2013/ Canada [69] | NR | NR | NR | NR | NR | 0 | 36.9 | NR | NR | NR | NR | NR |
| Nikolic/ 2014/ Serbia [70] | 78.6 | 58.7 | ≈53 | ≈50 | ≈42 | NR | NR | 51 | >22 | NR | 23 (>16) 1-,2-,6-yr 68.3%, 46.7% and 38.1% |
22 (1-83) |
| Passot/ 2012/ France [21] | 77 | 51 | NR | NR | 33 | NR | NR | 36.2 | NR | NR | NR | 58.5 (1-183) |
| Passot/ 2016/ France [104] | ≈83 | ≈65 | ≈51 | ≈38 | 31 | NR | 30 | 36 | NR | NR | 11 | NR |
| Pilati/ 2003/ Italy [71] | ≈68 | 31 | NR | NR | NR | 0 | 35 | 18 | NR | 13 | NR | 14.5 |
| Prada-Villaverde/ 2014/ Spaini [72] | ≈85 | ≈63 | ≈45 | ≈38 | ≈35 | NR | NR | 31.4 | NR | NR | NR | NR |
| Quenet/ 2011/ France [73] | ≈92 | ≈72 | ≈36 | ≈47 | ≈44 | 4.1 | 47.2 | 41 | 32–60 | NR | 15.7 (12–18) (RFS) | 48.5 (41.0–56.3) |
| Rivard/ 2014/ Canada [74] | ≈88 (Colon) ≈80 (Rectal) |
≈68 (Colon) ≈24 (Rectal) |
≈46 (Colon) ≈30 (Rectal) |
NA | NA | NR | NR | ≈31 (Colon) ≈18 (Rectal) |
NR | NR | 10.9 3-yr, 15% |
30.3 (2-88) |
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Table 24. Survival of Patients with CRC PC Treated by CRS and HIPEC and/or EPIC and/or SC: Summary of 76 Researches.
| Author/ Years/ Country | 1-yr SR (%) | 2-yr SR (%) | 3-yr SR (%) | 4-yr SR (%) | 5-yr SR (%) | Mortality Rate (%) |
Morbidity Rate (%) |
Median OS (mo) |
OS 95% CI (mo) |
PFS(95% CI) (mo) | DFS/RFS (95% CI) (mo) |
Follow-up times (range) (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HIPEC single arm studies | ||||||||||||
| Rodt/ 2013/ Denmark [75] | ≈52 | ≈36 | ≈12 | 0 | 0 | 0 | 9.4 (all) | 12.7 | 4.0-21.4 | NR | NR | 13 (1-44) |
| Shen/ 2004/ America [20] | NR | NR | 25 | NR | 17 | 12 | 30 | 16 | 10-26 | 7 (3-31) | NR | 15 |
| Shen/ 2008/ America [76] | 91 | ≈60 | 48 | ≈32 | 26 | 5.5 | 41.8 | 34 | 23-45 | NR | NR | 86 |
| Simkens/ 2015/ Netherlands [102] | NR | NR | 42 | NR | NR | 3 | 24.8 | 27 | 18.8-35.3 | NR | 1-yr: 35% | 22.9 (0.4-75.3) |
| Swellengrebel/ 2009/ Netherlands [77] |
NR | NR | NR | NR | NR | NR | NR | 25.6 | 20.9-29.4 | 13.6 (11.2-16.4) | NR | NR |
| Tabrizian/ 2014/ America [78] | 74.0 | ≈47 | 89.4 | NA | NA | NR | NR | NR | NR | NR | 12.4±1.8 (RFS) 1-,3-yr, 46.9%, 73.9% |
15.7±1.2 |
| Teo/ 2013/ Singapore [79] | ≈87 | ≈58 | ≈36 | ≈18 | ≈18 | 0 | 56 (all) | ≈28 | NR | NR | ≈10 | 21 (13.9-31.3) (all) |
| Teo/ 2014/ Singapore [80] | 83.7 | ≈53 | 38.2 | 19.1 | 19.1 | 0 | 40 | 27.1 | 15.3-39.1 | NR | 9.4 (5.5-18.7) 1-,3-,5-yr, 43.8%, 22.3%, 22.3% |
24.7 (0.6-81.8) |
| Ung/ 2013/ Australia [81] | ≈84 (Colon) | ≈63 (Colon) | ≈53 (Colon) | ≈37 (Colon) | 33 (Colon) | NR | NR | 37.1 (Colon) 29.6 (Rectal) |
NR | NR | 12.6 (Colon) 19.0 (Rectal) |
23.3 (1-156) (all) |
| Vaira/ 2010/ Italy [82] |
100 (L-OHP) ≈61 (MMC) |
≈60 (L-OHP) ≈17 (MMC) |
≈18 (L-OHP) ≈9 (MMC) |
≈18 (L-OHP) ≈4 (MMC) |
NA (L-OHP) 0 (MMC) |
2.5 | 55 | 24.6 (L-OHP) 16.6 (MMC) |
NR | NR | NR | NR |
| van Leeuwen / 2008/ Sweden [83] | ≈82 | ≈65 | NA | NA | NA | >1 (all) | 56.3 (all) | NA | NA | NR | 2-yr, 33.5% (all) | 13 (2-37) (all) |
| van Oudheusden/ 2014/ Netherlands [84] |
≈86 | ≈70 | ≈43 | ≈30 | ≈22 | 1.8 | 22.1 | 36.1 | NR | NR | NR | 16.2 (0.13-90) |
| van Oudheusden / 2015/ Netherlands [85] |
≈87 | ≈68 | 44 | ≈38 | ≈27 | NR | 13.5 | 35.1 | NR | NR | NR | 12.7(0.10-90.2) |
| Varban/ 2009/ America [86] | ≈63 | 36.8 | ≈25 | 17.4 | ≈16 | 7.7 | 40.1 | 15.8 | 13.5-20.2 | NR | NR | 13.4 |
| Verwaal/ 2005/ Netherlands [19] | 75 | NR | 28 | NR | 19 | NR | NR | 21.8 | 19.0-25.5 | NR | NR | 46 |
| Votanopoulos/ 2013/ America [87] | ≈63 (Colon) ≈83 (Rectal) |
≈31 (Colon) ≈36 (Rectal) |
25.1 (Colon) 28.2 (Rectal) |
NR | NR | 5.7 (Colon) 0 (Rectal) |
57 (Colon) 46 (Rectal) |
17.3 (Colon) 14.6 (Rectal) |
NR | NR | NR | 88.1 (Colon) 40.1 (Rectal) |
Note: yr: year; SR: survival rate; mo: months; OS: overall survival; PFS: progression-free survival; RFS: recurrence-free survival; DFS: disease-free survival; NA: not achieved; NR: not reported; PMP: pseudomyxoma peritonei; L-OHP: oxaliplatin; MMC: mitomycin; all: all tumors in researches; MVR: multivisceral resection group; NVR: No visceral resection group; APP: appendix; NNT: non-neoadjuvant therapy; NCA: neoadjuvant chemotherapy alone; NCB: neoadjuvant chemotherapy + bevacizumab; AC: adjuvant chemotherapy; NAC: non- adjuvant chemotherapy
Adverse events
In controlled studies, the mean (± SD) mortality and morbidity rates were 4.3% (± 3.7%) and 19.8% (± 9.2%) in HIPEC groups, 6.2% (± 4.2%) and 20.5% (± 12.3%) in traditional groups, respectively (Table 20-25). No significant difference in mortality (P = 0.423) or morbidity (P = 0.815) was detected between HIPEC group and traditional group by T test. In the integrated HIPEC-related data of 76 studies, mean mortality and morbidity was 2.8% (± 2.9%) and 33.0 (± 13.4%), respectively.
DISCUSSION
Due to the tumor biologic characteristics of colorectal cancer, about 10-13% patients have already progressed to PC when CRC is diagnosed [3, 7], which has a poor prognosis. In order to improve the efficacy, a comprehensive treatment strategy with combination of CRS plus HIPEC had been developed. With wide application of this treatment, CRS plus HIPEC has been proved capable to achieve better survival in selected patients with PC from colorectal cancer.
This meta-analysis of 15 controlled studies demonstrated that CRS+HIPEC comprehensive therapeutic strategy could bring significant survival benefit for selected CRC PC patients than traditional treatment of palliative surgery alone or systemic chemotherapy (HR = 2.67, 95% CI 2.21-3.23, P < 0.00001). In addition, the summarizing analysis of these 76 studies showed that the median OS was about 29 months in HIPEC group, which is significant longer compared with median OS of 17.9 months for CRC PC patients receiving contemporary chemotherapy reported by Kerscher et al (n = 2,406) [7]. These results provide further supporting evidence that CRS+HIPEC as the principal comprehensive treatment can bring more survival benefit to selected patients with CRC PC than traditional therapy.
The different regimens used in chemotherapy may be one potential confounding factor for survival outcomes. In order to investigate the influence of chemotherapy regimens on postoperative survival, a stratification analysis between MMC based regimens and L-OHP based regimens was conducted. The results of heterogeneity showed no significant difference (P = 0.50). These results are inconsistent with the reports by Elias et al [14], which showed that OS advantage for L-OHP regimens over non-L-OHP regimens (32 vs. 25 months, P = 0.02). However, L-OHP used in HIPEC was not an independent prognostic factor for survival in the study of Elias and colleagues. A multi-center retrospective controlled study reported by Prada-Villaverde et al. [72] showed that of 539 patients undergoing CRS plus HIPEC, L-OHP based HIPEC and MMC based HIPEC achieved similar median OS (31.4 vs. 32.7 months, P = 0.925). Similarly, the study of Hompes et al. [59] yielded the same conclusion that there was not obvious benefit in OS for HIPEC with L-OHP (37.1 months) or MMC (26.5 months) (P = 0.45). Although different chemotherapy regimens in HIPEC may have an effect on stability and reliability of this meta-analysis, the result of heterogeneity analysis was in accordance with above studies. As a result, both MMC and L-OHP were the feasible chemotherapy drugs in HIPEC for CRC PC patients to achieve similar efficacy.
Moreover, there are also some doubts that different chemotherapy in intravenous or postoperative intraperitoneal therapy regimens, even targeted therapy, had interference on the survival outcomes in meta-analysis. The doubts were removed by the report of Kerscher et al [7]. In 2,406 CRC patients of no-PC and PC, the survival outcomes for contemporary chemotherapy regimens (oxaliplatin or irinotecan) were compared with 5-FU regimens. For the CRC patients (without PC), survival outcomes for contemporary regimens were increased over 5-FU regimens (5-year survival rate 71.6% vs. 63.3%, P = 0.001). On the contrary, for patients with PC from CRC, the survival of L-OHP or irinotecan agent was similar to 5-FU regimens (P > 0.05), regardless of synchronous PC (2-year survival rate 31.1% vs. 19.1%, P = 0.092, and 5-year survival rate 20.8% vs. 5.8%, P = 0.081) or metachronous PC (2-year survival rate 71.5% vs. 58.5%, P = 0.329, and 5-year survival rate 28.1% vs. 24.4%, P = 0.411).
There were a few statistical flaws in this meta-analysis. For example, only one RCT [12] was included. It may be due to the difficulty of performing RCT. Therefore, we had to select meticulously current studies of best evidence level besides the only RCT. However, this meta-analysis showed acceptable outcomes of low heterogeneity and sensitivity. Regrettably, a patient-level (based on single patient data) meta-analysis as the gold standard for meta-analysis was not performed because of the difficulty in obtaining vast data from each database or institution. In addition to meta-analysis, this report provided a summary of 76 clinical studies published until today about CRS and HIPEC, which can get a review of published studies. In order to get the best evidence level results, more RCTs and prospective, multicenter, large-scale clinical trials need to be performed in future studies.
Observing available data from 6 controlled studies (a total of 470patients) [12, 35, 36, 38, 39, 99], mortality or morbidity were found similar in both groups of HIPEC and traditional surgery, which was 4.3% vs. 5.0% and 19.8% vs. 19.5%, respectively. The summarized HIPEC-related mortality and morbidity in 48 articles (the total number of patients, n = 4,809) [12, 16, 20, 35, 36, 38, 39, 46, 48-50, 52-54, 56, 58-62, 64-69, 71, 73, 75, 76, 79, 80, 82-90, 92, 100-104] were 2.8% (SD, ± 2.9%; range, 0-12%) and 33.0% (SD, ± 13.4%; range, 4-60%), respectively. Some large-sample retrospective studies and population-based analysis found a series of approximate results that the range of mortality was 2%-5.6% and morbidity was 25%-34% [93-97]. Furthermore, a systematic review of morbidity and mortality for CRS+HIPEC by Chua et al. [98] showed that the mortality and morbidity range from 0.9% to 5.8% and 12% to 52%, respectively. Though evidence proved that safety for CRS+HIPEC was acceptable, a meta-analysis on mortality and mortality for CRS+HIPEC may be able to provide more convincing results on the mortality and morbidity.
With the summary of 76 studies, it is found that although HIPEC is now widely accepted and performed in most institutions, details of performing HIPEC varies among different institutions. As we noted, there are several mainly different techniques concerning HIPEC including 1) “open” or “closed” technique, 2) using MMC and/or L-OHP, 3) mono-chemotherapy or combination of chemotherapy regimens, 4) temperature and duration of HIPEC. These can be further studied in future studies.
In conclusion, this meta-analysis showed that CRS+HIPEC comprehensive therapeutic strategy was associated with improvement of OS in CRC PC patients, and the results of the meta-analysis were proved of good reliability by low heterogeneous and robust sensitivity. Meanwhile, CRS and HIPEC can be performed with acceptable safety according to summary results of all 76 studies.
MATERIALS AND METHODS
Search strategy
The following databases were systematically searched up to July 31, 2016 including PubMed, Science Citation Index, EMBASE, and MEDLINE. The Cochrane Central Register of Controlled Trials, the National Institutes of Health trial registry, and conference proceeding articles from major oncologic and gastrointestinal cancer meetings were also sought for published results. The key words included “colon”, “rectum”, “colorectal”, “cancer”, “peritoneal carcinomatosis”, “hyperthermic intraperitoneal chemotherapy”, and synonyms and related terms for these words. The MeSH terms included “colon cancer”, “rectal cancer”, “colorectal cancer”, “peritoneal carcinomatosis”, “hyperthermic chemotherapy”, “hyperthermic intraperitoneal chemotherapy”, “HIPEC”, “intraperitoneal chemohyperthermia”, and “IPCH”. The combined application of “key words terms” and “MeSH terms” were conducted to improve the efficiency and accuracy of literature search.
Selection criteria
For inclusion in the meta-analysis and summarized HIPEC-related data analysis, a study had to fulfill the following criteria: (1) According to the North-England evidence-based guidelines [105, 106], excluded from IV levels evidence of literatures were included; (2) All patients were diagnosed CRC PC; (3) For assessing CRS+HIPEC±SC/EPIC, the intervening measure group was CRS+HIPEC±SC/EPIC, while the control group was traditional therapy of surgery and/or SC; For systematic review of CRS+HIPEC to treat CRC PC, HIPEC-related literatures involving clinical efficacy evaluation were included; (4) The key outcome measures should be included in literatures, such as OS, disease-free survival (DFS), recurrence-free survival (RFS), progression-free survival (PFS), year survival rate, morbidity and mortality [107], multivariate analysis, follow-up times; (5) English language; (6) To reduce the effect of publication bias, both fully published articles and abstracts were eligible for inclusion.
Exclusion criteria: (1) Animal studies, pathological research, imageology research, pharmacokinetics research, quality of life assessment, literature review, commentary, letter, book, etc; (2) Duplicate publication or overlapping data (chose the largest and latest sample size); (3) The sample size is less than 10; (4) Multiple cancer; (5) Unresectable liver metastases or others distant metastasis; (6) missing rate of follow-up > 5%.
Data extraction
Three authors analyzed data from a meta-analysis of 15 controlled researches of CRS plus HIPEC group vs. surgery and/or SC group and a summarized analysis of 76 researches of HIPEC group. The following data were extracted from each article: (1) Major clinico-pathologic characteristics and detail HIPEC regimens; (2) Survival and advent events. All relevant text, tables, and figures were reviewed for data extraction. For equivocal literatures or discrepancies between two independently assessed reviewers, these were resolved by discussion and consensus with a third author.
Statistical methods
All meta-analysis were performed using Review Manager 5. Overall survival (OS) or disease-free survival (DFS) in all studies were extracted from original literature. If not achieved accurate data in original text, hazard ratios (HRs) for time-to-event outcomes with 95% confidence intervals (95% CI) in two groups were estimated by Tierney's methods [108]. The heterogeneity in the meta-analysis was evaluated by I2 statistics [109] and T test [110] was calculated for each result in summarizing analysis of all HIPEC-related data from the included 76 articles. If I2 >50%, it was defined as the unacceptable heterogeneity. If I2 <50%, fixed effect model was used to get pooled HR and 95% CI; otherwise, random effects model was used if moderate heterogeneity. For a sensitivity analysis, we investigated the different research features of eligible trials, which included statistical methods, methodological quality, sample sizes, and clinical factors on HIPEC-related effect, after that, summarizing each subgroup data in term of Mental-Haenszel stratification analysis. According to Egger's test [111] and Begg's test [112], publication bias was considered to be inevitable when P < 0.10. The funnel plot analyses using ‘STATA: Data Analysis and Statistical Software version 12.0’, was to observe the results of meta-analysis whether any publication bias.
ACKNOWLEDGEMENTS AND GRANT SUPPORT
This study was supported by the grants for the Young Teacher Foundation of the Fundamental Research Fund for the Central Universities (No. 413000009), the Wuhan Clinical Research Center for Peritoneal Carcinomatosis (No. 2015060911020462), the Hubei Province's Outstanding Medical Academic Leader Program (No. [2013]4) and the Science Fund for Doctorate Mentors by China's Ministry of Education (No. 20120141110042).
Footnotes
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–67. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–63. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 3.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–50. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 4.de Gramont A, Figer A, Seymour M, Homerin M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer G, Papamichael D, Le Bail N, Louvet C, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938–47. doi: 10.1200/JCO.2000.18.16.2938. [DOI] [PubMed] [Google Scholar]
- 5.Adachi T, Hinoi T, Egi H, Shimomura M, Ohdan H. Oxaliplatin and molecular-targeted drug therapies improved the overall survival in colorectal cancer patients with synchronous peritoneal carcinomatosis undergoing incomplete cytoreductive surgery. Surg Today. 2015;45:986–92. doi: 10.1007/s00595-014-1017-y. [DOI] [PubMed] [Google Scholar]
- 6.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–67. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerscher AG, Chua TC, Gasser M, Maeder U, Kunzmann V, Isbert C, Germer CT, Pelz JO. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: a longitudinal experience of 2406 patients over two decades. Br J Cancer. 2013;108:1432–39. doi: 10.1038/bjc.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugarbaker PH, Gianola FJ, Speyer JC, Wesley R, Barofsky I, Meyers CE. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery. 1985;98:414–22. [PubMed] [Google Scholar]
- 9.Sugarbaker PH. Peritonectomy procedures. Ann Surg. 1995;221:29–42. doi: 10.1097/00000658-199501000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacquet P, Averbach AM, Stephens AD, Sugarbaker PH. Cancer recurrence following laparoscopic colectomy. Report of two patients treated with heated intraperitoneal chemotherapy. Dis Colon Rectum. 1995;38:1110–14. doi: 10.1007/BF02133989. [DOI] [PubMed] [Google Scholar]
- 11.Sugarbaker PH, Cunliffe WJ, Belliveau J, de Bruijn EA, Graves T, Mullins RE, Schlag P. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol. 1989;16:83–97. [PubMed] [Google Scholar]
- 12.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–43. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 13.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, Gutman M, Tentes AA, Lorimier G, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–92. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 15.Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, Doerfer J, Germer CT, Kerscher AG, Pelz JO. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18:1560–67. doi: 10.1245/s10434-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 16.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747–54. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 17.Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, Giovannini M, Lasser P. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11:518–21. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Raynard B, Farkhondeh F, Goéré D, Rouquie D, Ciuchendea R, Pocard M, Ducreux M. Peritoneal carcinomatosis of colorectal origin. Gastroenterol Clin Biol. 2006;30:1200–04. doi: 10.1016/s0399-8320(06)73512-6. [DOI] [PubMed] [Google Scholar]
- 19.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12:65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 20.Shen P, Hawksworth J, Lovato J, Loggie BW, Geisinger KR, Fleming RA, Levine EA. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol. 2004;11:178–86. doi: 10.1245/aso.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Passot G, Vaudoyer D, Cotte E, You B, Isaac S, Noël Gilly F, Mohamed F, Glehen O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann Surg. 2012;256:125–29. doi: 10.1097/SLA.0b013e318255486a. [DOI] [PubMed] [Google Scholar]
- 22.Haslinger M, Francescutti V, Attwood K, McCart JA, Fakih M, Kane JM, 3rd, Skitzki JJ. A contemporary analysis of morbidity and outcomes in cytoreduction/hyperthermic intraperitoneal chemoperfusion. Cancer Med. 2013;2:334–42. doi: 10.1002/cam4.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turrini O, Lambaudie E, Faucher M, Viret F, Blache JL, Houvenaeghel G, Delpero JR. Initial experience with hyperthermic intraperitoneal chemotherapy. Arch Surg. 2012;147:919–23. doi: 10.1001/archsurg.2012.988. [DOI] [PubMed] [Google Scholar]
- 24.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–32. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 25.Chua TC, Liauw W, Saxena A, Al-Mohaimeed K, Fransi S, Zhao J, Morris DL. Evolution of locoregional treatment for peritoneal carcinomatosis: single-center experience of 308 procedures of cytoreductive surgery and perioperative intraperitoneal chemotherapy. Am J Surg. 2011;201:149–56. doi: 10.1016/j.amjsurg.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Chua TC, Liauw W, Zhao J, Morris DL. Comparative analysis of perioperative intraperitoneal chemotherapy regimen in appendiceal and colorectal peritoneal carcinomatosis. Int J Clin Oncol. 2013;18:439–46. doi: 10.1007/s10147-012-0397-5. [DOI] [PubMed] [Google Scholar]
- 27.Cavaliere F, De Simone M, Virzì S, Deraco M, Rossi CR, Garofalo A, Di Filippo F, Giannarelli D, Vaira M, Valle M, Pilati P, Perri P, La Pinta M, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol. 2011;37:148–54. doi: 10.1016/j.ejso.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, Bonastre J. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–85. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 29.Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, Gomez-Portilla A, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann Surg Oncol. 2014;21:4195–201. doi: 10.1245/s10434-014-3798-z. [DOI] [PubMed] [Google Scholar]
- 30.Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2009;16:2152–65. doi: 10.1245/s10434-009-0487-4. [DOI] [PubMed] [Google Scholar]
- 31.Esquivel J, Sticca R, Sugarbaker P, Levine E, Yan TD, Alexander R, Baratti D, Bartlett D, Barone R, Barrios P, Bieligk S, Bretcha-Boix P, Chang CK, et al. Society of Surgical Oncology Annual Meeting Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Ann Surg Oncol. 2007;14:128–33. doi: 10.1245/s10434-006-9185-7. [DOI] [PubMed] [Google Scholar]
- 32.Yonemura Y, Canbay E, Ishibashi H. Prognostic factors of peritoneal metastasis from colorectal cancer following cytoreductive surgery and perioperative chemotherapy. Scien-tificWorldJournal. 2013;2013:978394. doi: 10.1155/2013/978394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Yu Y, Liu Y. Report on the 9th international congress on peritoneal surface malig-nancies. Cancer Biol Med. 2014;11:281–84. doi: 10.7497/j.issn.2095-3941.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chua TC, Pelz JO, Kerscher A, Morris DL, Esquivel J. Critical analysis of 33 patients with peritoneal carcinomatosis secondary to colorectal and appendiceal signet ring cell carcinoma. Ann Surg Oncol. 2009;16:2765–70. doi: 10.1245/s10434-009-0536-z. [DOI] [PubMed] [Google Scholar]
- 35.Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 36.Elias D, Benizri E, Di Pietrantonio D, Menegon P, Malka D, Raynard B. Comparison of two kinds of intraperitoneal chemotherapy following complete cytoreductive surgery of colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:509–14. doi: 10.1245/s10434-006-9167-9. [DOI] [PubMed] [Google Scholar]
- 37.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL. Zeh HJ 3rd. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–62. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 38.Gervais MK, Dubé P, McConnell Y, Drolet P, Mitchell A, Sideris L. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from colorectal cancer. J Surg Oncol. 2013;108:438–43. doi: 10.1002/jso.23431. [DOI] [PubMed] [Google Scholar]
- 39.Huang CQ, Feng JP, Yang XJ, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol. 2014;109:730–39. doi: 10.1002/jso.23545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passot G, You B, Boschetti G, Fontaine J, Isaac S, Decullier E, Maurice C, Vaudoyer D, Gilly FN, Cotte E, Glehen O. Pathological response to neoadjuvant chemotherapy: a new prognosis tool for the curative management of peritoneal colorectal carcinomatosis. Ann Surg Oncol. 2014;21:2608–14. doi: 10.1245/s10434-014-3647-0. [DOI] [PubMed] [Google Scholar]
- 41.Alzahrani N, Ferguson JS, Valle SJ, Liauw W, Chua T, Morris DL. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: long-term results at St George Hospital, Aus-tralia. ANZ J Surg. Epub ahead of print. https://doi.org/10.1111/ans.13152. Published July 14, 2015. [DOI] [PubMed]
- 42.Beaujard AC, Glehen O, Caillot JL, Francois Y, Bienvenu J, Panteix G, Garbit F, Grandclément E, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia with mitomycin C for digestive tract cancer patients with peritoneal carcinomatosis. Cancer. 2000;88:2512–19. doi: 10.1002/1097-0142(20000601)88:11<2512::aid-cncr12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 43.Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol. 2008;98:295–99. doi: 10.1002/jso.21084. [DOI] [PubMed] [Google Scholar]
- 44.Braam HJ, van Oudheusden TR, de Hingh IH, Nienhuijs SW, Boerma D, Wiezer MJ, van Ramshorst B. Patterns of recurrence following complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. J Surg Oncol. 2014;109:841–47. doi: 10.1002/jso.23597. [DOI] [PubMed] [Google Scholar]
- 45.Cao CQ, Yan TD, Liauw W, Morris DL. Comparison of optimally resected hepatectomy and peritonectomy patients with colorectal cancer metastasis. J Surg Oncol. 2009;100:529–33. doi: 10.1002/jso.21369. [DOI] [PubMed] [Google Scholar]
- 46.Cavaliere F, Valle M, De Simone M, Deraco M, Rossi CR, Di Filippo F, Verzi S, Giannarelli D, Perri P, Pilati PL, Vaira M, Di Filippo S, Garofalo A. 120 peritoneal carcinomatoses from colorectal cancer treated with peritonectomy and intra-abdominal chemohyperthermia: a S.I.T.I.L.O. multicentric study. In Vivo. 2006;20:747–50. [PubMed] [Google Scholar]
- 47.Ceelen W, Van Nieuwenhove Y, Putte DV, Pattyn P. Neoadjuvant chemotherapy with bevacizumab may improve outcome after cytoreduction and hyperthermic intraperitoneal chemoperfusion (HIPEC) for colorectal carcinomatosis. Ann Surg Oncol. 2014;21:3023–28. doi: 10.1245/s10434-014-3713-7. [DOI] [PubMed] [Google Scholar]
- 48.Desantis M, Bernard JL, Casanova V, Cegarra-Escolano M, Benizri E, Rahili AM, Benchimol D, Bereder JM. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC) Langenbecks Arch Surg. 2015;400:37–48. doi: 10.1007/s00423-014-1253-z. [DOI] [PubMed] [Google Scholar]
- 49.Elias D, Pocard M, Sideris L, Edè C, Ducreux M, Boige V, Lasser P. Preliminary results of intraperitoneal chemohyperthermia with oxaliplatin in peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:455–56. doi: 10.1002/bjs.4399. [DOI] [PubMed] [Google Scholar]
- 50.Elias D, Faron M, Goéré D, Dumont F, Honoré C, Boige V, Malka D, Ducreux M. A simple tumor load-based nomogram for surgery in patients with colorectal liver and peritoneal metastases. Ann Surg Oncol. 2014;21:2052–58. doi: 10.1245/s10434-014-3506-z. [DOI] [PubMed] [Google Scholar]
- 51.Evers DJ, Verwaal VJ. Indication for oophorectomy during cytoreduction for intraperitoneal metastatic spread of colorectal or appendiceal origin. Br J Surg. 2011;98:287–92. doi: 10.1002/bjs.7303. [DOI] [PubMed] [Google Scholar]
- 52.Franko J, Gusani NJ, Holtzman MP, Ahrendt SA, Jones HL, Zeh HJ, 3rd, Bartlett DL. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol. 2008;15:3065–72. doi: 10.1245/s10434-008-0105-x. [DOI] [PubMed] [Google Scholar]
- 53.Glehen O, Mithieux F, Osinsky D, Beaujard AC, Freyer G, Guertsch P, Francois Y, Peyrat P, Panteix G, Vignal J, Gilly FN. Surgery combined with peritonectomy procedures and intraperitoneal chemohyperthermia in abdominal cancers with peritoneal carcinomatosis: a phase II study. J Clin Oncol. 2003;21:799–806. doi: 10.1200/JCO.2003.06.139. [DOI] [PubMed] [Google Scholar]
- 54.Glehen O, Gilly FN, Boutitie F, Bereder JM, Quenet F, Sideris L, Mansvelt B, Lorimier G, Msika S, Elias D, French Surgical Association Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer. 2010;116:5608–18. doi: 10.1002/cncr.25356. [DOI] [PubMed] [Google Scholar]
- 55.Gomes da Silva R, Cabanas J, Sugarbaker PH. Limited survival in the treatment of carcinomatosis from rectal cancer. Dis Colon Rectum. 2005;48:2258–63. doi: 10.1007/s10350-005-0189-3. [DOI] [PubMed] [Google Scholar]
- 56.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, Edwards RP, Brown CK, Holtzman MP, Zeh HJ, Bartlett DL. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–63. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 57.Hamilton T, Lanuke K, Mack LA, Temple WJ. Long-term follow-up in the treatment of peritoneal carcinomatosis. Am J Surg. 2011;201:650–54. doi: 10.1016/j.amjsurg.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Hompes D, D’Hoore A, Van Cutsem E, Fieuws S, Ceelen W, Peeters M, Van der Speeten K, Bertrand C, Legendre H, Kerger J. The treatment of peritoneal carcinomatosis of colorectal cancer with complete cytoreductive surgery and hyperthermic intraperitoneal peroperative chemotherapy (HIPEC) with oxaliplatin: a Belgian multicentre prospective phase II clinical study. Ann Surg Oncol. 2012;19:2186–94. doi: 10.1245/s10434-012-2264-z. [DOI] [PubMed] [Google Scholar]
- 59.Hompes D, D’Hoore A, Wolthuis A, Fieuws S, Mirck B, Bruin S, Verwaal V. The use of Oxaliplatin or Mitomycin C in HIPEC treatment for peritoneal carcinomatosis from colorectal cancer: a comparative study. J Surg Oncol. 2014;109:527–32. doi: 10.1002/jso.23546. [DOI] [PubMed] [Google Scholar]
- 60.Iversen LH, Rasmussen PC, Hagemann-Madsen R, Laurberg S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: the Danish experience. Colorectal Dis. 2013;15:e365–72. doi: 10.1111/codi.12185. [DOI] [PubMed] [Google Scholar]
- 61.Kecmanovic DM, Pavlov MJ, Ceranic MS, Sepetkovski AV, Kovacevic PA, Stamenkovic AB. Treatment of peritoneal carcinomatosis from colorectal cancer by cytoreductive surgery and hyperthermic perioperative intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:147–52. doi: 10.1016/j.ejso.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, Hay JM, Flamant Y, Msika S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klaver YL, de Hingh IH, Boot H, Verwaal VJ. Results of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy after early failure of adjuvant systemic chemotherapy. J Surg Oncol. 2011;103:431–34. doi: 10.1002/jso.21836. [DOI] [PubMed] [Google Scholar]
- 64.Klaver YL, Chua TC, de Hingh IH, Morris DL. Outcomes of elderly patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for colorectal cancer peritoneal carcinomatosis. J Surg Oncol. 2012;105:113–18. doi: 10.1002/jso.22019. [DOI] [PubMed] [Google Scholar]
- 65.Kuijpers AM, Mirck B, Aalbers AG, Nienhuijs SW, de Hingh IH, Wiezer MJ, van Ramshorst B, van Ginkel RJ, Havenga K, Bremers AJ, de Wilt JH, EA Te Velde, Verwaal VJ. Cytoreduction and HIPEC in the Netherlands: nationwide long-term outcome following the Dutch protocol. Ann Surg Oncol. 2013;20:4224–30. doi: 10.1245/s10434-013-3145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuijpers AM, Mehta AM, Boot H, van Leerdam ME, Hauptmann M, Aalbers AG, Verwaal VJ. Perioperative systemic chemotherapy in peritoneal carcinomatosis of lymph node positive colorectal cancer treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Oncol. 2014;25:864–69. doi: 10.1093/annonc/mdu031. [DOI] [PubMed] [Google Scholar]
- 67.Lanuke K, Mack LA, Temple WJ. Phase II study of regional treatment for peritoneal carcinomatosis. Am J Surg. 2009;197:614–18. doi: 10.1016/j.amjsurg.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 68.Levine EA, Stewart JH, 4th, Shen P, Russell GB, Loggie BL, Votanopoulos KI. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg. 2014;218:573–85. doi: 10.1016/j.jamcollsurg.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107:591–96. doi: 10.1002/jso.23276. [DOI] [PubMed] [Google Scholar]
- 70.Nikolic S, Dzodic R, Zegarac M, Djurisic I, Gavrilovic D, Vojinovic V, Kocic M, Santrac N, Radlovic P, Radosavljevic D, Pupic G, Martinovic A. Survival prognostic factors in patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy: a single institution experience. J BUON. 2014;19:66–74. [PubMed] [Google Scholar]
- 71.Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508–13. doi: 10.1245/aso.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Prada-Villaverde A, Esquivel J, Lowy AM, Markman M, Chua T, Pelz J, Baratti D, Baumgartner JM, Berri R, Bretcha-Boix P, Deraco M, Flores-Ayala G, Glehen O, et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J Surg Oncol. 2014;110:779–85. doi: 10.1002/jso.23728. [DOI] [PubMed] [Google Scholar]
- 73.Quenet F, Goéré D, Mehta SS, Roca L, Dumont F, Hessissen M, Saint-Aubert B, Elias D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294–301. doi: 10.1097/SLA.0b013e3182263933. [DOI] [PubMed] [Google Scholar]
- 74.Rivard JD, McConnell YJ, Temple WJ, Mack LA. Cytoreduction and heated intraperitoneal chemotherapy for colorectal cancer: are we excluding patients who may benefit? J Surg Oncol. 2014;109:104–09. doi: 10.1002/jso.23446. [DOI] [PubMed] [Google Scholar]
- 75.Rodt AP, Svarrer RO, Iversen LH. Clinical course for patients with peritoneal carcinomatosis excluded from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2013;11:232. doi: 10.1186/1477-7819-11-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shen P, Thai K, Stewart JH, Howerton R, Loggie BW, Russell GB, Levine EA. Peritoneal surface disease from colorectal cancer: comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann Surg Oncol. 2008;15:3422–32. doi: 10.1245/s10434-008-0127-4. [DOI] [PubMed] [Google Scholar]
- 77.Swellengrebel HA, Zoetmulder FA, Smeenk RM, Antonini N, Verwaal VJ. Quantitative intra-operative assessment of peritoneal carcinomatosis - a comparison of three prognostic tools. Eur J Surg Oncol. 2009;35:1078–84. doi: 10.1016/j.ejso.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 78.Tabrizian P, Shrager B, Jibara G, Yang MJ, Romanoff A, Hiotis S, Sarpel U, Labow DM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg. 2014;18:1024–31. doi: 10.1007/s11605-014-2477-5. [DOI] [PubMed] [Google Scholar]
- 79.Teo MC, Tan GH, Tham CK, Lim C, Soo KC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol. 2013;20:2968–74. doi: 10.1245/s10434-013-2947-0. [DOI] [PubMed] [Google Scholar]
- 80.Teo MC, GH Ching Tan, Lim C, Chia CS, Tham CK, Soo KC. Colorectal peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: the experience of a tertiary Asian center. Asian J Surg. 2015;38:65–73. doi: 10.1016/j.asjsur.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Ung L, Chua TC, David L M. Peritoneal metastases of lower gastrointestinal tract origin: a comparative study of patient outcomes following cytoreduction and intraperitoneal chemotherapy. J Cancer Res Clin Oncol. 2013;139:1899–908. doi: 10.1007/s00432-013-1507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vaira M, Cioppa T, D’Amico S, de Marco G, D’Alessandro M, Fiorentini G, De Simone M. Treatment of peritoneal carcinomatosis from colonic cancer by cytoreduction, peritonectomy and hyperthermic intraperitoneal chemotherapy (HIPEC). Experience of ten years. In Vivo. 2010;24:79–84. [PubMed] [Google Scholar]
- 83.van Leeuwen BL, Graf W, Pahlman L, Mahteme H. Swedish experience with peritonectomy and HIPEC. HIPEC in peritoneal carcinomatosis. Ann Surg Oncol. 2008;15:745–53. doi: 10.1245/s10434-007-9700-5. [DOI] [PubMed] [Google Scholar]
- 84.van Oudheusden TR, Braam HJ, Nienhuijs SW, Wiezer MJ, van Ramshorst B, Luyer MD, Lemmens VE, de Hingh IH. Cytoreduction and hyperthermic intraperitoneal chemotherapy: a feasible and effective option for colorectal cancer patients after emergency surgery in the presence of peritoneal carcinomatosis. Ann Surg Oncol. 2014;21:2621–26. doi: 10.1245/s10434-014-3655-0. [DOI] [PubMed] [Google Scholar]
- 85.van Oudheusden TR, Braam HJ, Nienhuijs SW, Wiezer MJ, van Ramshorst B, Luyer P, de Hingh IH. Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol. 2015;111:237–42. doi: 10.1002/jso.23784. [DOI] [PubMed] [Google Scholar]
- 86.Varban O, Levine EA, Stewart JH, McCoy TP, Shen P. Outcomes associated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy in colorectal cancer patients with peritoneal surface disease and hepatic metastases. Cancer. 2009;115:3427–36. doi: 10.1002/cncr.24385. [DOI] [PubMed] [Google Scholar]
- 87.Votanopoulos KI, Swett K, Blackham AU, Ihemelandu C, Shen P, Stewart JH, Levine EA. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal carcinomatosis from rectal cancer. Ann Surg Oncol. 2013;20:1088–92. doi: 10.1245/s10434-012-2787-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Winer J, Zenati M, Ramalingam L, Jones H, Zureikat A, Holtzman M, Lee K, Ahrendt S, Pingpank J, Zeh HJ, Bartlett DL, Choudry HA. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2014;21:1456–62. doi: 10.1245/s10434-013-3328-4. [DOI] [PubMed] [Google Scholar]
- 89.Witkamp AJ, de Bree E, Kaag MM, Boot H, Beijnen JH, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive cytoreductive surgery followed by intra-operative hyperthermic intraperitoneal chemotherapy with mitomycin-C in patients with peritoneal carcinomatosis of colorectal origin. Eur J Cancer. 2001;37:979–84. doi: 10.1016/s0959-8049(01)00058-2. [DOI] [PubMed] [Google Scholar]
- 90.Yan TD, Chu F, Links M, Kam PC, Glenn D, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma: non-mucinous tumour associated with an improved survival. Eur J Surg Oncol. 2006;32:1119–24. doi: 10.1016/j.ejso.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 91.Yan TD, Morris DL. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for isolated colorectal peritoneal carcinomatosis: experimental therapy or standard of care? Ann Surg. 2008;248:829–35. doi: 10.1097/SLA.0b013e31818a15b5. [DOI] [PubMed] [Google Scholar]
- 92.Zanon C, Bortolini M, Chiappino I, Simone P, Bruno F, Gaglia P, Airoldi M, Deriu L, Mashiah A. Cytoreductive surgery combined with intraperitoneal chemohyperthermia for the treatment of advanced colon cancer. World J Surg. 2006;30:2025–32. doi: 10.1007/s00268-005-0486-y. [DOI] [PubMed] [Google Scholar]
- 93.Dimick JB, Cowan JA, Jr, Upchurch GR, Jr, Colletti LM. Hospital volume and surgical outcomes for elderly patients with colorectal cancer in the United States. J Surg Res. 2003;114:50–56. doi: 10.1016/s0022-4804(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 94.Pal N, Axisa B, Yusof S, Newcombe RG, Wemyss-Holden S, Rhodes M, Lewis MP. Volume and outcome for major upper GI surgery in England. J Gastrointest Surg. 2008;12:353–57. doi: 10.1007/s11605-007-0288-7. [DOI] [PubMed] [Google Scholar]
- 95.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 96.Noordzij PG, Poldermans D, Schouten O, Bax JJ, Schreiner FA, Boersma E. Postoperative mortality in The Netherlands: a population-based analysis of surgery-specific risk in adults. Anesthesiology. 2010;112:1105–15. doi: 10.1097/ALN.0b013e3181d5f95c. [DOI] [PubMed] [Google Scholar]
- 97.Jakobson T, Karjagin J, Vipp L, Padar M, Parik AH, Starkopf L, Kern H, Tammik O, Starkopf J. Postoperative complications and mortality after major gastrointestinal surgery. Medicina (Kaunas) 2014;50:111–17. doi: 10.1016/j.medici.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 98.Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg. 2009;249:900–07. doi: 10.1097/SLA.0b013e3181a45d86. [DOI] [PubMed] [Google Scholar]
- 99.Goéré D, Souadka A, Faron M, Cloutier AS, Viana B, Honoré C, Dumont F, Elias D. Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol. 2015;22:2958–64. doi: 10.1245/s10434-015-4387-5. [DOI] [PubMed] [Google Scholar]
- 100.Faron M, Macovei R, Goéré D, Honoré C, Benhaim L, Elias D. Linear Relationship of Peritoneal Cancer Index and Survival in Patients with Peritoneal Metastases from Colorectal Cancer. Ann Surg Oncol. 2016;23:114–19. doi: 10.1245/s10434-015-4627-8. [DOI] [PubMed] [Google Scholar]
- 101.Maillet M, Glehen O, Lambert J, Goere D, Pocard M, Msika S, Passot G, Elias D, Eveno C, Sabaté JM, Lourenco N, André T, Gornet JM, BIG-RENAPE Working Group Early Postoperative Chemotherapy After Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Isolated Peritoneal Carcinomatosis of Colon Cancer: A Multicenter Study. Ann Surg Oncol. 2016;23:863–69. doi: 10.1245/s10434-015-4914-4. [DOI] [PubMed] [Google Scholar]
- 102.Simkens GA, van Oudheusden TR, Luyer MD, Nienhuijs SW, Nieuwenhuijzen GA, Rutten HJ, de Hingh IH. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann Surg Oncol. 2015;22:2656–62. doi: 10.1245/s10434-014-4297-y. [DOI] [PubMed] [Google Scholar]
- 103.Frøysnes IS, Larsen SG, Spasojevic M, Dueland S, Flatmark K. Complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastasis in Norway: prognostic factors and oncologic outcome in a national patient cohort. J Surg Oncol. 2016;114:222–27. doi: 10.1002/jso.24290. [DOI] [PubMed] [Google Scholar]
- 104.Passot G, Vaudoyer D, Villeneuve L, Kepenekian V, Beaujard AC, Bakrin N, Cotte E, Gilly FN, Glehen O. What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: A 25-year experience with 1,125 procedures. J Surg Oncol. 2016;113:796–803. doi: 10.1002/jso.24248. [DOI] [PubMed] [Google Scholar]
- 105.North of England Antidepressant Guideline Development Group Evidence Based Guideline for the choice of antidepressants for depression in primary care. Newcastle upon Tyne: Centre for Health Services Research. 1997.
- 106.Eccles M, Rousseau N, Freemantle N. Updating evidence-based clinical guidelines. J Health Serv Res Policy. 2002;7:98–103. doi: 10.1258/1355819021927746. [DOI] [PubMed] [Google Scholar]
- 107.National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Available: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [Accessed January 11, 2016]
- 108.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 111.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]


