Abstract
Non-small cell lung cancer (NSCLC) accounts for about 85–90% of lung cancer cases, which represents the leading cause of cancer-related death in the world. The majority of lung cancer patients doesn't respond well to conventional chemo-/radio-therapeutic regimens and have a poor prognosis. The recent introduction of targeted therapy and immunotherapy gives new hopes to NSCLC patients, but their outcome/prognosis is far from satisfactory. The translation initiation EIF4F complex has been shown to play important roles in cancer progression, but its functional role and therapeutic effect in lung cancers especially NSCLC remain largely unknown. In this current review, we summarize recent findings regarding the role of EIF4F complex in NSCLC progression and targeted therapy potentials. We also discuss the unanswered questions and future directions in this field.
Keywords: EIF4F, EIF4A, EIF4E, EIF4G, eukaryotic initiation factors
INTRODUCTION
Lung cancer is the leading cause of cancer-related death in the world, which can be classified as small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) [1]. NSCLCs account for about 85–90% of lung cancer cases, which encompassing lung adenocarcinomas, lung squamous cell carcinomas, and large cell carcinomas [1]. Clinically, the majority of NSCLC patients doesn't respond well to current chemo-/radio-therapeutic regimens and have a dismal 5-year survival rate of ˜15% [2]. Most recently, introduction of targeted therapy and immunotherapy gives new hopes to NSCLC patients, but the outcome/prognosis is far from satisfactory. For instance, targeted therapeutic drugs such as gefitinib inhibiting mutated epidermal growth factor receptor (EGFR) exhibit good initial effects, but the drug resistance inevitably develops after 10-month of treatment and patients will succumb to this disease [3]. Meanwhile, inhibition of immune checkpoint factors such as PD-1 and PD-L1 has yielded good clinical responses and improved overall survival in certain NSCLC patients, however, only 15–20% of NSCLC patients respond to such therapy and affordability is another serious issue, since a single-course (7-month) treatment will cost more than $100,000 [4, 5]. Thus, there is an urgent need to better understand the mechanisms of lung carcinogenesis and to identify new therapeutic targets for improving the treatment.
The protein synthesis or mRNA translation is tightly controlled in the eukaryotic cells. Dysregulating this process can contribute to the carcinogenesis by affecting key cancer pathways [6–8]. The eukaryotic initiation factor 4F (EIF4F) complex is a heterotrimeric complex composed of a 5′ mRNA cap-binding subunit EIF4E, the large scaffolding protein EIF4G, and the ATP-dependent RNA helicase EIF4A [9]. Meanwhile, the heterogeneity of the EIF4F complex is further increased by the isoforms of individual EIF4 protein (at least 3 isoforms for each EIF4 protein) [9]. The EIF4F complex recruits mRNA to the ribosome then helps the ribosome to scan the 5′ untranslated region (5′ UTR) in search of an initiation codon. Over the last two decades, the EIF4F complex has been shown to play important role in oncogenesis [10, 11]. There are several strategies have been developed for directly targeting EIF4F complex, which include the down-regulation of EIF4E with antisense oligonucleotides (ASOs), disrupting EIF4F complex formation, impeding EIF4E–cap interaction and targeting EIF4A [9]. However, there is limited data about its functions and therapeutic effects in lung cancer especially NSCLC. The current review will summarize recent findings regarding the role of EIF4F complex in NSCLC progression as well as its potential therapeutic value.
The expression of EIF4F complex components in NSCLC and its prognostic value
In NSCLC, EIF4E is arguably the most studied component of EIF4F complex and serves as a rate-limiting factor of cap-dependent translation initiation. Emerging evidence has shown that EIF4E, especially its phosphorylated form is up-regulated in NSCLC tumor tissues, which is correlated with a shorter survival and increased lymph node metastasis [12–14]. Moreover, EIF4E is also co-regulated with some other tumor-associated proteins in NSCLC. For example, one study shows that EIF4E(+)/cyclin D1(−) NSCLC patients have poorer clinical outcome, while EIF4E(−)/cyclin D1(+) patients have a more favorable outcome, and patients with EIF4E(+)/cyclin D1(+) have an intermediate outcome [15].
Compared to EIF4E, the expression and clinical implication of other components of the EIF4F complex (such as EIF4G and EIF4A) in NSCLC are far less understood. Our group recently reports that the expressional level of EIF4G1 is much higher in NSCLC cell lines and primary tumor tissues, than their normal controls [16]. Meanwhile, another study shows that NSCLC patients with low EIF4A2 expression have worse overall survival and disease-free survival [17]. Thus, varying EIF4F components may play different roles in lung carcinogenesis and more research needs to be engaged to determine the correlation between these EIF4F complex components expression and NSCLC prognosis in the future.
The EIF4F complex and NSCLC cell survival and proliferation
We recently reports that stably silencing EIF4G1 by shRNA significantly reduces NSCLC cell proliferation and anchorage-independent growth in vitro, through inducing apoptosis as well as G0/G1 cell cycle arrest [16]. Treatment with EIF4E/EIF4G interaction inhibitor, 4EGI-1, can dramatically inhibit the cell growth and induce apoptosis in NSCLC cell cultures [18]. Moreover, 4EGI-1 can enhance the apoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on NSCLC cells, by inducing CCAAT/enhancer-binding protein homologous protein-dependent DR5 and ubiquitin/proteasome-mediated degradation of cellular FLICE-inhibitory protein (c-FLIP) [18]. Meanwhile, EIF4E can be directly targeted by the tumor suppressor miR-34c-3p and knock-down of EIF4E inhibits NSCLC cell proliferation [19]. In accord with this, miR-34c-3p expression is significantly decreased in NSCLC patient biopsies as well as cancer cell lines, which thus allows overexpressed EIF4E to promote lung carcinogenesis [19]. Along this line, a recent study shows that antisense oligonucleotide targeting EIF4E (4EASO) significantly inhibits NSCLC cell proliferation as well as the expression of several oncogenic proteins such as VEGF, c-MYC and Osteopontin [20].
The EIF4F complex and NSCLC metastasis
Metastasis reflects the invasiveness of tumor cells and serves as a key prognosis factor for NSCLC. Our recent data show that stably silencing EIF4G1 reduces NSCLC cell invasion and migration [16], indicating an important role of EIF4 factors in NSCLC metastasis. Consistently, Li et al. have reported that elevated expression of EIF4E in NSCLC is associated with a stronger tumor invasion [21]. Another study confirms that knock-down of EIF4E or EIF4G1 prevents NSCLC cell migration and epithelial-to-mesenchymal transition (EMT), which represent essential steps for tumor metastasis [22]. Interestingly, secretome of human bone marrow mesenchymal stem cells can simultaneously decrease the levels of EIF4E and EIF4G1 and attenuate the invasiveness of NSCLC cells [23], which further supports the notion that EIF4E/G1 likely play important roles in NSCLC metastasis. In accord with this, miR-34c-3p mediated knock-down of EIF4E also inhibits NSCLC cell migration and invasion [19].
The EIF4F complex and NSCLC chemoresistance
The acquisition of multidrug resistance is a major reason to cause a failure of chemotherapy in NSCLC patients. Accumulating evidence has shown that the EIF4F complex components are involved in NSCLC multidrug resistance. For example, elevated expression of EIF4E is tightly linked to NSCLC resistance to erlotinib, gefitinib, cisplatin, and gemcitabine [20, 21, 24, 25]. Interestingly, several EIF4-targeting miRNAs are likely to be involved in the chemoresistance in NSCLC. Hao et al. recently reported that the suppression of EIF4G2 by miR-379 sensitizes NSCLC cells to cisplatin [26]. However, Wang et al. reported that suppression of EIF4E by miR-141 increases NSCLC cell resistance to docetaxel and the expression of EIF4E in docetaxel chemoresistant NSCLC patients is markedly lower than those of docetaxel sensitive NSCLC patients [27]. Therefore, it is unclear whether the individual EIF4F complex components may play different roles in the resistance of varying therapeutic agents in NSCLC.
The crosstalk between the EIF4F complex and intracellular signaling and partners in NSCLC
The formation and function of the EIF4F complex need to be tightly regulated to maintain the cellular homeostasis. Some major signal transduction pathways (e.g., PI3K/Akt/mTOR or MAPK) [28–30] and transcription factors (e.g., MYC or C/EBPα) have been shown to regulate the EIF4F complex [31, 32]. In NSCLC, the rapamycin mediated inhibition of mTOR signaling can increase the phosphorylation of both Akt and EIF4E through a negative feedback mechanism, while the PI3K inhibitors such as LY294002 can reverse such undesirable effects [33]. Other studies also demonstrate that simultaneous inhibition of PI3K/Akt and mTOR signaling exerts synergistic anticancer activity against NSCLC in vitro and in vivo [34, 35]. Another study reveals that the c-jun N-terminal kinase (JNK) can activate cap-dependent translation in NSCLC cells as an alternative pathway, which is independent of PI3K/Akt/mTOR signaling [36].
Using tandem affinity purification combined with mass spectrometry (TAP-MS) screening approach, we have identified that ubiquitin-specific protease 10 (USP10) can directly interact with EIF4G1 and inhibit EIF4G1 activities in NSCLC cells [16], although the underlying mechanism remains largely unknown and further investigations are warrant to solve this puzzle.
CONCLUSIONS
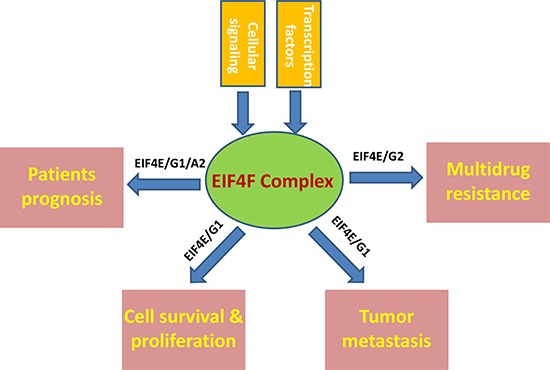
Even though the recent targeted therapy and immunotherapy improve the average survival of a small group of lung cancer patients, the initial non-response, acquired resistance, recurrence are still serious problems faced by the majority of NSCLC patients. Furthermore, given the high heterogeneity of lung cancer, the monotherapy usually does not work for most patients. As the components of the translation machinery integrate almost all oncogenic signals [9], targeting the EIF4F complex components holds the promise for overcoming a major hurdle associated with intra-tumor heterogeneity. Moreover, because malignant cells exhibit augmented activity of most of the EIF4F complex components, it is hypothesized that the tumor cells become ‘addicted’ to elevated protein synthesis and more sensitive to translation machinery targeted therapy [37, 38]. Unlike other solid tumors, there are much fewer data about the expression, regulation, and biological roles of the EIF4F complex components in NSCLC, which have been summarized in Figure 1. Several key questions remain unanswered and need to be addressed in the future: 1) there is still lacking of large-scale clinical studies to investigate the expression of EIF4F complex components especially different isoforms in NSCLC biopsies, as well as their correlation with tumor stages, histology, metastasis and prognosis. 2) are the expression and/or functions of EIF4F complex components affected by genetic landscape of the NSCLCs such as mutation of the EGFR, HER2, and MET genes as well as fusion oncogenes involving anaplastic lymphoma kinase (ALK), ROS1 and RET [39, 40]? 3) A more detailed regulatory/interaction network for EIF4F complex in NSCLC is still missing. 4) more effective EIF4F complex inhibitors or other targeted therapeutic strategies need to be developed to accelerate the “bench to bedside” switch.
Figure 1. Schematic of known and putative contributions of EIF4F complex to NSCLC pathogenesis.
ACKNOWLEDGMENTS AND FUNDING
This work was partially supported by grants from a DOD Career Development Award (CA140437), the Leukemia Research Foundation, the Louisiana Clinical and Translational Science Center Pilot grants (U54GM104940 from NIH), as well as the funds from the National Natural Science Foundation of China (81472547, 81672924 and 81400164), the Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai (PWRL2014-01), Shanghai Science and Technology Committee funding (16411964800) and the Health Science and Technology Development Fund of Pudong New Area of Shanghai (PKJ2015-Y16). Funding sources had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST
All the authors declare no conflicts of interest.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware KE, Hinz TK, Kleczko E, Singleton KR, Marek LA, Helfrich BA, Cummings CT, Graham DK, Astling D, Tan AC, Heasley LE. A mechanism of resistance to gefitinib mediated by cellular reprogramming and the acquisition of an FGF2-FGFR1 autocrine growth loop. Oncogenesis. 2013;2:e39. doi: 10.1038/oncsis.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundar R, Cho BC, Brahmer JR, Soo RA. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol. 2015;7:85–96. doi: 10.1177/1758834014567470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. Am Health Drug Benefits. 2015;8:9. [PMC free article] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Topisirovic I, Sonenberg N. mRNA translation and energy metabolism in cancer: the role of the MAPK and mTORC1 pathways. Cold Spring Harb Symp Quant Biol. 2011;76:355–367. doi: 10.1101/sqb.2011.76.010785. [DOI] [PubMed] [Google Scholar]
- 9.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14:261–278. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 10.Hagner PR, Schneider A, Gartenhaus RB. Targeting the translational machinery as a novel treatment strategy for hematologic malignancies. Blood. 2010;115:2127–2135. doi: 10.1182/blood-2009-09-220020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E–from translation to transformation. Oncogene. 2004;23:3172–3179. doi: 10.1038/sj.onc.1207549. [DOI] [PubMed] [Google Scholar]
- 12.Fan S, Ramalingam SS, Kauh J, Xu Z, Khuri FR, Sun SY. Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Biol Ther. 2009;8:1463–1469. doi: 10.4161/cbt.8.15.8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshizawa A, Fukuoka J, Shimizu S, Shilo K, Franks TJ, Hewitt SM, Fujii T, Cordon-Cardo C, Jen J, Travis WD. Overexpression of phospho-eIF4E is associated with survival through AKT pathway in non-small cell lung cancer. Clin Cancer Res. 2010;16:240–248. doi: 10.1158/1078-0432.CCR-09-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Zhu C, Chen B, Zhang X, Ye M, Lin A. [Expression and its clinical significance of eIF4E in non-small cell lung cancer]. [Article in Chinese] Zhongguo Fei Ai Za Zhi. 2010;13:1132–1135. doi: 10.3779/j.issn.1009-3419.2010.12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury T, Alrawi S, Ramnath N, Li Q, Grimm M, Black J, Tan D. Eukaryotic initiation factor-4E and cyclin D1 expression associated with patient survival in lung cancer. Clin Lung Cancer. 2009;10:58–66. doi: 10.3816/CLC.2009.n.009. [DOI] [PubMed] [Google Scholar]
- 16.Cao Y, Wei M, Li B, Liu Y, Lu Y, Tang Z, Lu T, Yin Y, Qin Z, Xu Z. Functional role of eukaryotic translation initiation factor 4 gamma 1 (EIF4G1) in NSCLC. Oncotarget. 2016;7:24242–24251. doi: 10.18632/oncotarget.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaoyan X, Juanjuan Y, Yalan T, Ping H, Jianzhong L, Qinian W. Downregulation of EIF4A2 in non-small-cell lung cancer associates with poor prognosis. Clin Lung Cancer. 2013;14:658–665. doi: 10.1016/j.cllc.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Fan S, Li Y, Yue P, Khuri FR, Sun SY. The eIF4E/eIF4G interaction inhibitor 4EGI-1 augments TRAIL-mediated apoptosis through c-FLIP Down-regulation and DR5 induction independent of inhibition of cap-dependent protein translation. Neoplasia. 2010;12:346–356. doi: 10.1593/neo.10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Wang X, Li J, Gu K, Lv L, Zhang S, Che D, Cao J, Jin S, Yu Y. miR-34c-3p functions as a tumour suppressor by inhibiting eIF4E expression in non-small cell lung cancer. Cell Prolif. 2015;48:582–592. doi: 10.1111/cpr.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thumma SC, Jacobson BA, Patel MR, Konicek BW, Franklin MJ, Jay-Dixon J, Sadiq A, JR De A Graff, Kratzke RA. Antisense oligonucleotide targeting eukaryotic translation initiation factor 4E reduces growth and enhances chemosensitivity of non-small-cell lung cancer cells. Cancer Gene Ther. 2015;22:396–401. doi: 10.1038/cgt.2015.34. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Fan S, Koo J, Yue P, Chen ZG, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. Elevated expression of eukaryotic translation initiation factor 4E is associated with proliferation, invasion and acquired resistance to erlotinib in lung cancer. Cancer Biol Ther. 2012;13:272–280. doi: 10.4161/cbt.18923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Attar-Schneider O, Drucker L, Gottfried M. Migration and epithelial-to-mesenchymal transition of lung cancer can be targeted via translation initiation factors eIF4E and eIF4GI. Lab Invest. 2016;96:1004–1015. doi: 10.1038/labinvest.2016.77. [DOI] [PubMed] [Google Scholar]
- 23.Attar-Schneider O, Zismanov V, Drucker L, Gottfried M. Secretome of human bone marrow mesenchymal stem cells: an emerging player in lung cancer progression and mechanisms of translation initiation. Tumour Biol. 2016;37:4755–4765. doi: 10.1007/s13277-015-4304-3. [DOI] [PubMed] [Google Scholar]
- 24.Patel MR, Jay-Dixon J, Sadiq AA, Jacobson BA, Kratzke RA. Resistance to EGFR-TKI can be mediated through multiple signaling pathways converging upon cap-dependent translation in EGFR-wild type NSCLC. J Thorac Oncol. 2013;8:1142–1147. doi: 10.1097/JTO.0b013e31829ce963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang W, Tian X, Bai F, Han R, Wang J, Shen H, Zhang X, Liu Y, Yan X, Jiang F, Xing L. Pim-1 kinase is a target of miR-486–5p and eukaryotic translation initiation factor 4E, and plays a critical role in lung cancer. Mol Cancer. 2014;13:240. doi: 10.1186/1476-4598-13-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao GJ, Hao HJ, Ding YH, Wen H, Li XF, Wang QR, Zhang BB. Suppression of EIF4G2 by miR-379 potentiates the cisplatin chemosensitivity in nonsmall cell lung cancer cells. FEBS Lett. 2017;591:636–645. doi: 10.1002/1873-3468.12566. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Ma J, Ji X, Xu F, Wei Y. miR-141 regulation of EIF4E expression affects docetaxel chemoresistance of non-small cell lung cancer. Oncol Rep. 2017;37:608–616. doi: 10.3892/or.2016.5214. [DOI] [PubMed] [Google Scholar]
- 28.Roux PP, Topisirovic I. Regulation of mRNA translation by signaling pathways. Cold Spring Harb Perspect Biol. 2012. p. 4. [DOI] [PMC free article] [PubMed]
- 29.Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenwald IB, Lazaris-Karatzas A, Sonenberg N, Schmidt EV. Elevated levels of cyclin D1 protein in response to increased expression of eukaryotic initiation factor 4E. Mol Cell Biol. 1993;13:7358–7363. doi: 10.1128/mcb.13.12.7358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khanna-Gupta A, Abayasekara N, Levine M, Sun H, Virgilio M, Nia N, Halene S, Sportoletti P, Jeong JY, Pandolfi PP, Berliner N. Up-regulation of translation eukaryotic initiation factor 4E in nucleophosmin 1 haploinsufficient cells results in changes in CCAAT enhancer-binding protein alpha activity: implications in myelodysplastic syndrome and acute myeloid leukemia. J Biol Chem. 2012;287:32728–32737. doi: 10.1074/jbc.M112.373274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR. Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res. 2005;65:7052–7058. doi: 10.1158/0008-5472.CAN-05-0917. [DOI] [PubMed] [Google Scholar]
- 34.Xu CX, Li Y, Yue P, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. The combination of RAD001 and NVP-BEZ235 exerts synergistic anticancer activity against non-small cell lung cancer in vitro and in vivo. PLoS One. 2011;6:e20899. doi: 10.1371/journal.pone.0020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan N, Afaq F, Khusro FH, Mustafa Adhami V, Suh Y, Mukhtar H. Dual inhibition of phosphatidylinositol 3-kinase/Akt and mammalian target of rapamycin signaling in human nonsmall cell lung cancer cells by a dietary flavonoid fisetin. Int J Cancer. 2012;130:1695–1705. doi: 10.1002/ijc.26178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel MR, Sadiq AA, Jay-Dixon J, Jirakulaporn T, Jacobson BA, Farassati F, Bitterman PB, Kratzke RA. Novel role of c-jun N-terminal kinase in regulating the initiation of cap-dependent translation. Int J Oncol. 2012;40:577–582. doi: 10.3892/ijo.2011.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggero D. Translational control in cancer etiology. Cold Spring Harb Perspect Biol. 2013. p. 5. [DOI] [PMC free article] [PubMed]
- 38.Silvera D, Formenti SC, Schneider RJ. Translational control in cancer. Nat Rev Cancer. 2010;10:254–266. doi: 10.1038/nrc2824. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14:535–546. doi: 10.1038/nrc3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosell R, Karachaliou N. Lung cancer: Maintenance therapy and precision medicine in NSCLC. Nat Rev Clin Oncol. 2013;10:549–550. doi: 10.1038/nrclinonc.2013.152. [DOI] [PubMed] [Google Scholar]


