Iron replacement therapy dates back to the 17th century, when Sydenham first proposed the use of an oral iron salt to treat “chlorosis”1, a disorder of adolescent girls and young women initially believed to be an hysterical disease, but later recognised as due to iron deficiency anaemia2. The first iron preparations for intravenous (IV) administration3 entered the clinical scenario in the second half of the past century. They were based on a common structure, i.e. a carbohydrate shell surrounding a core containing Fe3+ hydroxide particles. The newer preparations also share this structure, the difference lying in the chemistry of the carbohydrate moieties forming the shell. High molecular weight iron dextran was used initially, but soon raised safety concerns due to an unacceptable rate of severe adverse effects, including death due to anaphylaxis4. This fostered fears that strongly limited physicians’ use of parenteral iron and created emotional barriers which still partially remain5. The high molecular weight iron dextran moiety was subsequently found to be unique in its capacity to elicit an IgE-mediated response6, and it was definitively withdrawn from the market in 19917. In fact, like any longstanding treatment, iron replacement therapy entered clinical practice largely without undergoing rigorous modern randomised clinical trials. Despite the undisputed effectiveness of iron replacement therapy for correcting iron deficiency anaemia, this relative lack of a solid and evidence-based foundation favoured a number of misconceptions that even now continue to pervade clinical practice. The last two decades have witnessed a revolution in the field of iron therapy. On the one hand, the discovery of the hepcidin/ferroportin axis8 and its complex regulatory network9 has elucidated the molecular basis of cellular and systemic iron homeostasis with unprecedented accuracy. On the other hand, new IV iron preparations have been developed, characterised by reassuring safety profiles and easy administration schedules7. Nowadays, the total dose needed either to correct iron deficiency anaemia or restore tissue iron deposits can often be administered in a single infusion lasting no more than 15–30 minutes7,10. Of note, the pharmacokinetics of IV iron is completely different from that of oral iron. IV iron compounds are first taken up by macrophages and then released into the bloodstream. Oral iron is incorporated into transferrin after release from the basolateral membrane of duodenal cells, providing that no condition leading to malabsorption (i.e. coeliac disease, autoimmune or Helicobacter pylori-related chronic gastritis) is present11. Thus, the two treatments cannot be considered interchangeable. In this issue of Blood Transfusion, Manuel Muñoz and Colleagues provide a critical discussion on a number of common misconceptions regarding iron replacement therapy12. The common thread behind their discussion is that, overall, IV iron is underutilised in clinical practice. While there is no doubt in considering this an actual problem, we must be careful not to exceed in the opposite direction. More importantly, we should maximise the irrefutable advantages of the modern IV iron preparations, by promoting their appropriate use in the light of the recent advances in the knowledge on iron pathophysiology. The clinical spectrum of iron deficiency anaemia varies widely from relatively simple cases, such as those secondary to heavy menstrual bleeding in young women without relevant comorbidities, to very complex conditions, such as elderly patients with multiple comorbidities13. Indeed, one of the most relevant challenges in daily clinical practice is adequate detection of iron deficiency in patients with comorbidities, particularly with diseases characterised by subclinical or overt inflammation. Muñoz and Colleagues correctly point out how often iron deficiency is inappropriately excluded simply because serum ferritin levels are within the normal range (see misconception #1)12. Since ferritin is an acute phase reactant14, its diagnostic accuracy is lost when inflammation is present. The so-called anaemia of chronic disease is the second most frequent cause of anaemia after iron deficiency15 (the first, if we consider the hospitalised population), and the two conditions not rarely coexist. Classical examples are patients with inflammatory bowel diseases, chronic haemodialysis, or other prototypic inflammatory disorders such as rheumatoid arthritis, who are at substantial risk of developing absolute iron deficiency for a number of reasons (Figure 1). A variety of ferritin cut-offs have been proposed, ranging from 100 μg/L in classical anaemia of chronic disease16, to 300 μg/L in heart failure (in which subclinical inflammation contributes to anaemia)17, to 500 μg/L in patients with chronic kidney disease18, and even up to 800 μg/L in cancer patients19. In these conditions, guidelines often require the concomitant presence of reduced transferrin saturation (i.e. TSAT <20%) before considering iron replacement therapy. However, such heterogeneous ferritin cut-offs have been determined largely on the basis of consensus among experts, and solid supporting evidence is lacking. Thus, there is an urgent need for research studies aimed at validating and, possibly, harmonising such cut-offs, which would result in appropriate administration of iron replacement therapy only to patients with true iron deficiency. A complementary approach is the search for new biomarkers able to dissect out iron deficiency from an inflammatory background. In this regard, the measurement of circulating hepcidin appears promising20. The regulation of hepcidin production at the molecular level is quite complex9, and circulating levels represent the integrated sum of a number of opposing stimuli20. Though hepcidin is also stimulated by major pro-inflammatory cytokines (e.g. interleukin-6), negative regulation by iron deficiency tends to prevail when both conditions coexist21. The clinical utility of hepcidin in identifying iron deficiency has been demonstrated in pilot studies on patients with inflammatory bowel diseases22, rheumatoid arthritis23, and in African children with a substantial burden of infectious diseases24. However, larger studies are needed to consolidate these results, and to provide sufficient uniformity of hepcidin assays to allow their implementation in clinical practice25. The laboratory diagnosis of iron deficiency in elderly patients with multiple comorbid conditions is another unmet clinical need for which hepcidin assays could be useful13. In the near future, well-designed research projects focusing on the tremendous advances in understanding of iron pathophysiology are expected to increase our ability to diagnose iron deficiency in complex conditions, ultimately leading to more appropriate prescription of iron replacement therapy.
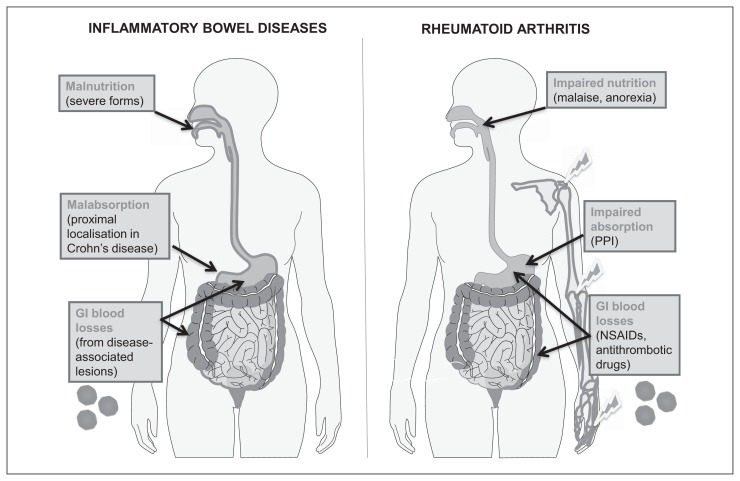
Figure 1.
Factors concurring to determine iron deficiency (ID) in classical inflammatory disorders.
In such conditions, serum ferritin loses its diagnostic power for identifying ID because of stimulation by pro-inflammatory cytokines. Left: inflammatory bowel diseases (IBDs), like Crohn’s disease and ulcerative colitis. Right: rheumatoid arthritis (RA) and related immunological disorders. In RA, gastrointestinal (GI) blood losses are favoured by treatment with non-steroidal anti-inflammatory drugs (NSAIDs) and antithrombotic drugs prescribed for often associated cardiovascular diseases. Proton pump inhibitors (PPI), frequently co-prescribed with NSAIDs, can contribute to decreased iron absorption by increasing gastric pH. In both IBD and RA, frequent blood drawing for laboratory analyses, especially during hospital admissions, can also contribute to causing iron deficiency. RBC: red blood cell.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Stockman R. The treatment of chlorosis by iron and some other drugs. Br Med J. 1893;1:942–4. doi: 10.1136/bmj.1.1688.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guggenheim KY. Chlorosis: the rise and disappearance of a nutritional disease. J Nutr. 1995;125:1822–5. doi: 10.1093/jn/125.7.1822. [DOI] [PubMed] [Google Scholar]
- 3.Nissim JA. Intravenous administration of iron. Lancet. 1947;2:49–51. doi: 10.1016/s0140-6736(47)90053-6. [DOI] [PubMed] [Google Scholar]
- 4.Hamstra RD, Block MH, Schocket AL. Intravenous iron dextran in clinical medicine. JAMA. 1980;243:1726–31. [PubMed] [Google Scholar]
- 5.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338–47. doi: 10.1182/asheducation-2010.1.338. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmen J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–82. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 7.Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program. 2016;2016:57–66. doi: 10.1182/asheducation-2016.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–33. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 10.Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372:1832–43. doi: 10.1056/NEJMra1401038. [DOI] [PubMed] [Google Scholar]
- 11.Hershko C, Camaschella C. How I treat unexplained refractory iron deficiency anemia. Blood. 2014;123:326–33. doi: 10.1182/blood-2013-10-512624. [DOI] [PubMed] [Google Scholar]
- 12.Muñoz M, Gómez-Ramírez S, Besser M, et al. Current misconceptions in diagnosis and management of iron deficiency. Blood Transfus. 2017;15:422–37. doi: 10.2450/2017.0113-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busti F, Campostrini N, Martinelli N, Girelli D. Iron deficiency in the elderly population, revisited in the hepcidin era. Front Pharmacol. 2014;5:83. doi: 10.3389/fphar.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- 15.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 18.Drueke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s) Kidney Int. 2012;82:952–60. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 19.Gilreath JA, Stenehjem DD, Rodgers GM. Diagnosis and treatment of cancer-related anemia. Am J Hematol. 2014;89:203–12. doi: 10.1002/ajh.23628. [DOI] [PubMed] [Google Scholar]
- 20.Girelli D, Nemeth E, Swinkels DW. Hepcidin in the diagnosis of iron disorders. Blood. 2016;127:2809–13. doi: 10.1182/blood-2015-12-639112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darshan D, Frazer DM, Wilkins SJ, Anderson GJ. Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide. Haematologica. 2010;95:1660–7. doi: 10.3324/haematol.2010.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergamaschi G, Di Sabatino A, Albertini R, et al. Serum hepcidin in inflammatory bowel diseases: biological and clinical significance. Inflamm Bowel Dis. 2013;19:2166–72. doi: 10.1097/MIB.0b013e31829a6e43. [DOI] [PubMed] [Google Scholar]
- 23.van Santen S, van Dongen-Lases EC, de Vegt F, et al. Hepcidin and hemoglobin content parameters in the diagnosis of iron deficiency in rheumatoid arthritis patients with anemia. Arthritis Rheum. 2011;63:3672–80. doi: 10.1002/art.30623. [DOI] [PubMed] [Google Scholar]
- 24.Pasricha SR, Atkinson SH, Armitage AE, et al. Expression of the iron hormone hepcidin distinguishes different types of anemia in African children. Sci Transl Med. 2014;6:235re3. doi: 10.1126/scitranslmed.3008249. [DOI] [PubMed] [Google Scholar]
- 25.van der Vorm LN, Hendriks JCM, Laarakkers CM, et al. Toward worldwide hepcidin assay harmonization: identification of a commutable secondary reference material. Clin Chem. 2016;62:993–1001. doi: 10.1373/clinchem.2016.256768. [DOI] [PubMed] [Google Scholar]