Abstract
Background
A fast-track anaemia clinic (FTAC) for the management of moderate-to-severe iron-deficiency anaemia (IDA) was established in our Emergency Department in 2010. In this FTAC, the replacement of packed red cell transfusion by ferric carboxymaltose administration was proven to be safe and effective. The aim of this study was a cost-analysis of IDA management in the FTAC, comparing this management with the previous standard care pathway consisting of packed red cell transfusion, if needed, and referral to outpatient specialised care.
Materials and methods
A cost study was performed for patients with IDA who were at risk of requiring transfusion (haemoglobin <9 g/dL) but did not require hospitalisation. Total IDA treatment costs in the FTAC were compared to those theoretically incurred if these patients had been managed using the standard care pathway. In addition, a sensitivity analysis considering variations of up to ±30% in ferric carboxymaltose and packed red cell acquisition costs was performed (49 possible scenarios).
Results
Between 2012 and 2015, 238 IDA patients were treated in the FTAC. The average treatment cost was € 594±337/patient in the FTAC group and € 672±301/patient in the standard care pathway group, with a saving of € 78±28/patient (95% CI, 22–133; p<0.001). The sensitivity analysis showed that IDA treatment costs in the FTAC (€ 480–722/patient), compared with those of the standard care pathway (€ 550–794/patient), resulted in significant cost-savings for all studied scenarios (€ 51–104/patient; p<0.005).
Discussion
The administration of ferric carboxymaltose for IDA management in a FTAC may be cost-saving compared with the standard care pathway.
Keywords: Emergency Department, iron deficiency anaemia, intravenous iron, transfusion, costs
Introduction
Anaemia is defined by a concentration of haemoglobin (Hb) <13 g/dL in adult males and <12 g/dL in non-pregnant women1. A recent global estimate suggested that more than two billion people were anaemic in 2010, with iron deficiency being the most common cause since it is responsible for approximately 50% of all cases of anaemia2. In our environment, iron-deficiency anaemia (IDA) is most commonly caused by gastrointestinal pathology and excessive menstrual blood loss. Its prevalence among the adult population is 1.0 to 2.5%, a figure that rises to up to 10% in women of childbearing age3. However, robust data on the prevalence of anaemia in the population cared for in the hospital Emergency Department (ED) are lacking4.
Ideally, anaemic patients should be diagnosed and treated in primary care centres, but primary care doctors may not order iron parameters. Other diagnostic studies (e.g., endoscopy) are performed at reference hospitals. This can delay treatment initiation for several weeks, even in patients with a suspected malignancy. For this reason, many patients call the ED to gain more rapid access to a particular specialist or a specific diagnostic test, resulting in ED overflow and increased hospital admissions5. Moderate-to-severe anaemia is one of the most frequent causes of hospitalisation6.
Another important aspect of anaemia in the ED, besides its severity, is its acuteness. Patients with acute anaemia, which is generally caused by haemorrhage or haemolysis, may present with haemodynamic instability, frequently requiring transfusion even at relatively high haemoglobin levels (Hb 9–10 g/dL), although a restrictive transfusion policy is preferred whenever possible7. In contrast, sub-acute anaemia may have developed over time, allowing physiological compensatory mechanisms to act. Thus, the clinical picture will depend on a patient’s overall health status. Adaptation to sub-acute anaemia may be hampered in patients with cardiac, circulatory or respiratory comorbidity, who may also need transfusion. Patients with compensated anaemia should undergo investigations to classify the type of anaemia and pharmacological treatment should be attempted; however, such patients often receive ill-considered packed red cell (PRC) transfusion. Transfusions in the ED account for a significant proportion of hospital PRC use (10–30%)8,9.
In 2010, a unit for fast anaemia care was established in our hospital ED (the “fast-track anaemia clinic”, FTAC). In this clinic, we prioritised the use of intravenous (IV) iron over PRC transfusion in IDA patients. Over 80% of patients treated in the FTAC had a Hb increment ≥2 g/dL and/or achieved a Hb ≥12 g/dL, and their red blood cell indices and iron parameters normalised. Seventeen per cent of these anaemic patients required PRC transfusion during follow-up (2 units/patient), and nine mild adverse drug events were witnessed10. These data support the effectiveness and safety of IV iron administration.
There is currently a lack of data on the relative cost-effectiveness of the two therapeutic options. The aim of this study was to perform an evaluation, from the perspective of the health care provider (National Health System), of IDA treatment costs in the FTAC for patients who were at risk of requiring PRC transfusion but did not require hospitalisation. Costs of IDA management in the FTAC (FTAC group) were compared to those theoretically derived from the previous standard care pathway (SCP) for moderate-to-severe anaemia, consisting of PRC transfusion, if needed, and referral to outpatient specialised care for classification of their anaemia and its treatment (SCP group).
Materials and methods
Study design
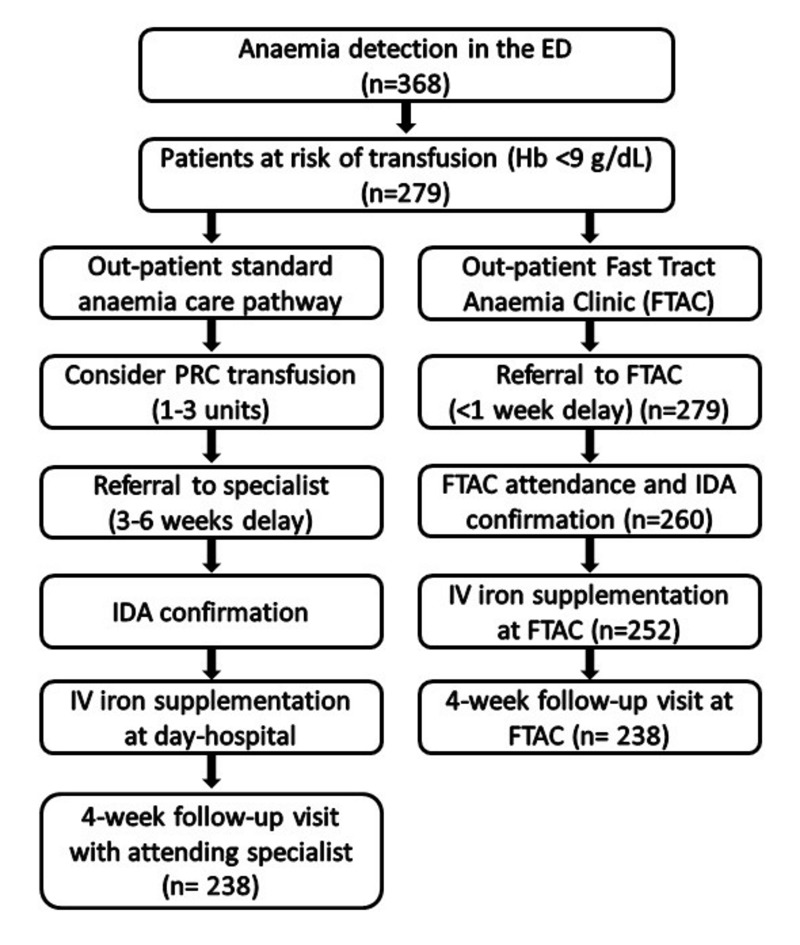
We performed a cost study of IDA management for patients presenting at our ED between January 2012 and December 2015 with sub-acute IDA, at risk of requiring transfusion (Hb <9 g/dL) but not requiring immediate hospitalisation. Anaemia management costs in the FTAC were compared to those that would have been incurred had these patients entered the previous SCP (Figure 1). The time horizon of the analysis in both groups was the 4-week follow-up visit. Our Institutional Ethics Committee waived the need to obtain informed consent from the patients for this analysis and approved the study.
Figure 1.
Study design and patients’ disposition.
ED: Emergency Department, Hb: haemoglobin, IDA: iron-deficiency anaemia, IV: intravenous, PRC: packed red cells.
Anaemia management
Management of moderate-to-severe anaemia in the FTAC10
In patients with suspected or known IDA, blood samples were drawn in the ED for assessment of full blood counts, iron profile, creatinine, vitamin B12 and folic acid. Patients were booked for a FTAC appointment in fewer than 7 days (the subsequent Thursday). During this FTAC appointment, we proceeded to confirm the presence of IDA, requested additional tests or investigations (if necessary) to determine its cause, administered IV iron treatment (if there were no contraindications and the patient did not refuse this thearpy) and followed up the patient. Blood counts and iron profile were re-assessed 4 weeks after completing IV iron supplementation.
Standard care pathway for moderate-to-severe anaemia
Until the FTAC had been established, patients with moderate-to-severe anaemia used to be referred from the ED to the relevant specialist (outpatient care). This usually resulted in a delay of several weeks before classification and treatment of their anaemia. To avoid anaemia signs/symptoms while on the waiting list for evaluation, some were given PRC transfusions (1–3 units) in the ED to achieve a Hb level just above their recommended transfusion threshold (Table I). Once the presence and cause of the IDA had been confirmed, IV iron treatment was administered in the day-hospital, and patients were followed up by the attending specialist. The theoretical SCP group was constructed by applying this SCP to the actual patient population completing IDA treatment in the FTAC.
Table I.
Haemoglobin (Hb) thresholds for packed red cell transfusion, according to the patients’ characteristics.
| Patient’s characteristics | Transfusion threshold* |
|---|---|
| Sub-acute anaemia in asymptomatic young patients | Hb <5 g/dL |
| Sub-acute anaemia in young patients with signs/symptoms** and without risk criteria *** | Hb <6 g/dL |
| Sub-acute anaemia in older patients with signs/symptoms and without risk criteria | Hb <7 g/dL |
| Sub-acute anaemia in patients with risk criteria and without signs/symptoms | Hb <8 g/dL |
| Sub-acute anaemia in patients with risk criteria and signs/symptoms | Hb <9 g/dL |
Adapted from the Recommendations from the Spanish Society for Blood Transfusion and Cellular Therapy13;
Clinical signs/symptoms: hypotension, tachycardia, tachypnoea, dizziness or fatigue;
Risk criteria: coronary or valvular disease, heart failure, vascular cerebral disease chronic pulmonary obstructive disease, etc.
Intravenous iron supplementation
In the FTAC group, all consenting patients with moderate-to-severe IDA received IV iron, if they had no contraindications (1st trimester of pregnancy, active infection, iron overload, hypersensitivity to an IV iron formulation or any of its inactive components). The total iron dose (TID) required was calculated using a simplified formula that takes into account the patient’s Hb and body weight11. Ferric carboxymaltose (FCM; Ferinject, Vifor, Saint Galene, Switzerland) was administered by IV infusion at doses of 500–1,000 mg in 100–200 mL saline over 15 minutes and the patients were monitored for 30 minutes after the infusion. FCM was given once a week, at a maximum dose of 1,000 mg/week. Patients were scheduled for a follow-up visit 4 weeks after completion of iron supplementation to check the haematological response. Non-responders were scheduled for subsequent follow-up visits or referred to another department.
In the previous SCP for moderate-to-severe anaemia, total iron dose was calculated using the same simplified formula. For patients not requiring PRC transfusion, total iron dose was calculated using Hb level at presentation. For those who would have received a PRC transfusion in the ED, the estimated post-transfusion Hb level was used instead (considering that 1 PRC unit would increase the Hb by 1 g/dL). IV iron infusion would have been performed in our day-hospital12.
Packed red cell transfusion
According to national recommendations of the Spanish Society of Blood Transfusion and Cellular Therapy13, PRC transfusion in the FTAC group during follow-up was considered for patients who had a Hb level below the pre-established transfusion threshold, presented with clinical signs/symptoms of anaemia/hypoxaemia and/or had risk criteria (Table I).
In the SCP group, patients would have received the minimum quantity of PRC (1–3 units in a single session) to attain a Hb level of 0.5–1.0 g/dL above their corresponding transfusion threshold (Table I), to avoid anaemia signs/symptoms while on the waiting list for specialist evaluation.
Demographic and clinical data
A set of demographic and clinical data was collected for all patients. These data included age, gender, patient’s precedence and referral, cause of the IDA, laboratory parameters, results of investigations (e.g., colonoscopy, radiology), transfusion rate, Hb concentration, haematological response (anaemia correction and/or a Hb increase of at least 2 g/dL), total IV iron dose, number of treatment sessions, and FCM-related adverse drug effects.
Cost calculations
For the purpose of this study, we only considered fixed and variable costs related to patients’ blood management (IV iron administration and PRC transfusion). To simplify the model, we did not include the costs of: (i) laboratory tests and investigations (necessary to ascertain the presence and cause of the IDA); (ii) follow-up appointments (assumed to be the same in both study arms); (iii) possible adverse reactions to IV iron or PRC (given their low incidence and the small number of patients included); (iv) additional PRC transfusions in the SCP group during follow-up; or (v) societal costs (e.g., loss of working hours).
For the calculation of IV iron treatment costs in both groups (Table II), we considered: (i) acquisition costs for FCM, using the manufacturer’s free sale price14; (ii) costs of FCM administration (15 minutes/session) and (iii) costs of post-administration observation (30 minutes/session), according to official prices for health care services in the Autonomous Community of Madrid15. We considered administration and observation costs to be equal in both the FTAC and the day-hospital.
Table II.
Cost elements.
| Intravenous iron | |
| Ferric carboxymaltose (FCM) infusion (500–1,000 mg/session) | |
| FCM acquisition cost1 | € 19.24/100 mg |
| FCM administration cost (15 minutes)2 | € 11.5/infusion |
| Post-FCM observation cost (30 minutes)2 | € 26/infusion |
| Packed red cell (PRC) transfusion | |
| PRC transfusion (1–3 units/session) | |
| PRC acquisition cost3 | € 140/unit |
| PRC pre-transfusion tests costs4 | € 92/session |
| PRC administration cost (120 minutes)4,5 | € 92/unit CH |
| Post-PRC observation costs (30+60 minutes)2,6 | € 69/session |
Manufacturer’s free selling price14;
day-hospital cost: € 277/6 hours (€ 0.77/min) (official prices for healthcare services in the Autonomous Community of Madrid)15;
Hospital Transfusion Service; includes re-assessment of blood group for each PRC unit (official prices for healthcare services in the Autonomous Community of Madrid)15;
Hospital Transfusion Service; includes two patient’s blood group assessments and one screening for irregular antibodies (official prices for healthcare services in the Autonomous Community of Madrid)15;
Recommendations of the Spanish Society of Blood Transfusion and Cellular Therapy13;
includes the time waiting for pre-transfusion tests (30 minutes/session), and post-transfusion observation time (60 minutes/session), in accordance with the Recommendations of the Spanish Society of Blood Transfusion and Cellular Therapy 13.
For the calculation of PRC transfusion costs (Table II), we considered: (i) acquisition and blood group reassessment costs of PRC units in our hospital Transfusion Service; (ii) costs of pre-transfusion tests in our hospital Transfusion Service (including 2 patient’s blood group assessments and screening for irregular antibodies); (iii) costs of the time to wait for pre-transfusion tests (30 minutes/session), PRC administration (120 minutes/PRC unit) and post-transfusion observation (60 minutes/session) in the FTAC or day-hospital. We considered the recommendations from the Spanish Society of Blood Transfusion13 for PRC transfusion and the official prices for healthcare services in the Autonomous Community of Madrid15.
The estimated saving or incremental cost for management of anaemia in the FTAC compared with the SCP was obtained by comparing the total treatment costs for both alternatives. A sensitivity analysis of estimated saving or incremental cost was also performed taking into account variations in acquisition costs for FCM and PRC (up to ±30%).
Statistical analysis
Data were expressed as percentage (%), mean±standard deviation (SD) or mean and 95% confidence interval (95% CI). Pearson’s chi-square test or Fisher’s exact test was used for the comparison of qualitative variables. A paired Student’s t test was used to compare quantitative variables. All statistical computations were performed with IBM-SPSS statistics 22 (Licensed to the University of Málaga, Spain) and a p-value <0.05 was considered statistically significant.
Results
Fast-track anaemia clinic group
Between January 2012 and December 2015, 368 patients presented with anaemia at the ED. Of these, 279 presenting with suspected or known IDA were referred to the FTAC, 260 attended the FTAC appointment, 252 received IV FCM supplementation, and 238 attended the 4-week follow-up assessment (Figure 1). Thus, data analysis was restricted to 238 patients (170 women/68 men; age, 65±21 years; weight, 71±12 kg).
Most were referred from the ED (64%) or primary care (29%), and presented with a mean baseline Hb of 7.6±1.0 g/dL (Table III). The most frequent causes of anaemia were gastrointestinal (37%) or gynaecological (32%) blood loss and malignancy (22%). Additional investigations requested were upper/lower digestive tract endoscopy (37%), computerised tomography (16%), and tests for occult blood in the faeces (12%). Only eight patient presented with vitamin B12 deficiency (<200 pmol/L) and received supplementation (1 mg, given intramuscularly) and none presented with folic acid deficiency. The patients were given 1420±630 mg IV FCM (500–4500 mg) in one to five infusions (Table IV). At the 4-week follow-up, the mean Hb increment was 4.4±1.8 g/dL (p<0.001) (Table III), 89% of patients had had a Hb increment ≥2 g/dL, and in 56% the IDA had been corrected. Significant improvements in mean corpuscular volume, serum iron and ferritin levels were observed (p<0.001) (Table III). During the follow-up, PRC transfusion was required in 91 (38.2%) patients who received 189 units (2.1 units/patient) (Table IV). Four mild adverse events were observed in patients given IV FCM (1 headache, 1 urticarial reaction, 1 diarrhoea, 1 superficial phlebitis).
Table III.
Haematological and biochemical parameters before (baseline) and after treatment (4-week follow-up visit).
| Parameter | Baseline | 4 week follow-up | Change | p |
|---|---|---|---|---|
| Haemoglobin (g/dL) | 7.6±1.0 | 12.0±1.7 | 4.4±1.8 | 0.001 |
| MCV (fL) | 73±12 | 87±10 | 14±12 | 0.001 |
| Serum iron (mg/dL) | 23±42 | 71±34 | 48±56 | 0.001 |
| Ferritin (ng/mL) | 19±44 | 308±302 | 296±257 | 0.001 |
| Vitamin B12 (pmol/L)* | 397±188 | NA | NA | NA |
| Folic acid (nmol/L)** | 9±5 | NA | NA | NA |
MCV: mean corpuscular volume; NA: not assessed.
Only 8 patients presented with vitamin B12 deficiency and received supplementation (1 mg, i.m.).
No patient presented with folic acid deficiency.
Table IV.
Comparative cost analysis.
| SCP group | FTAC group | |
|---|---|---|
| Transfusion, n (%) | 149 (62.6) | 91 (38.2)* |
| PRC units (n) | 282 | 189 |
| PRC index (units/patient) | 1.2±1.1 | 0.8±1.1* |
| PRC acquisition costs (€/patient) | 166±154 | 111±160* |
| Pre-transfusion test costs (€/patient) | 58±45 | 44±63* |
| PRC administration costs (€/patient) | 109±101 | 73±105* |
| Post-PRC observation costs (€/patient) | 43±33 | 33±47* |
| PRC transfusion total costs (€/patient) | 376±324 | 262±373* |
| FCM dose (mg) | 1,250±250 | 1,420±630 |
| FCM acquisition costs (€/patient) | 240±49 | 273±122 |
| FCM administration costs (€/patient) | 17±6 | 18±7 |
| Post-FCM observation costs (€/patient) | 39±13 | 41±16 |
| FCM supplementation total cost (€/patient) | 296±68 | 332±144 |
| Treatment total costs (€/patient) | 672±301 | 594±377* |
| Saving (€/patient), mean (95% CI) | 78 (22–133)* | |
FCM: ferric carboxymaltose; FTAC: fast-tract anaemia clinic; PRC: packed red cells; SCP: standard care pathway; 95% CI: 95% confidence interval;
p<0.01, with respect to the SCP group.
Standard care pathway group
If the 238 patients who completed treatment in the FTAC had entered the previous SCP for moderate-to-severe anaemia, 149 (62.6%) would have theoretically required 282 PRC units (1 unit in 53, 2 units in 59, 3 units in 37) to raise their baseline Hb (7.4±1.4 g/dL) to the desired level (9.2±0.7 g/dL) while waiting several weeks for outpatient anaemia classification and treatment. There would have been differences in PRC transfusion rate according to age (17% for patients <65 years; 96% for those ≥65 years). The IV FCM dose (1,250±250 mg) was calculated using baseline Hb in non-transfused patients, and estimated post-transfusion Hb in those transfused. The total IV dose would have been given in one or two sessions. These data were used to estimate the cost of anaemia managed according to the SCP.
Cost analysis
According to the cost elements listed in Table II, in the FTAC group the costs of FCM supplementation were € 332/patient and those of PRC transfusion were € 262/patient; the corresponding figures for the SPC group would have been € 296/patient and € 376/patient, respectively (Table IV). Thus, the average treatment costs were € 594/patient for the FTAC group and € 672/patient for the SCP group, with savings of € 78/patient in the former group (95% CI, 22–133; p<0.001) (Table IV).
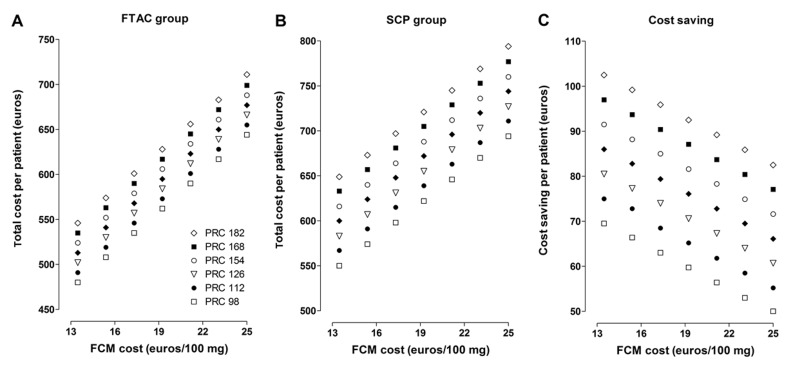
The sensitivity analysis, in which variations in acquisition costs of FCM and PRC were considered (up to ±30%, 49 possible scenarios), showed IDA treatment costs between € 480/patient and € 711/patient for those in the FTAC group (Figure 2A), and between € 550/patient and € 794/patient) for those in the SPC group (Figure 2B). In the most favourable scenario for the FTAC (FCM € 13.5/100 mg, PRC € 182/unit) total costs were € 545 and € 649/patient, for patients in the FTAC and SPC group, respectively (p<0.001), with savings of € 104/patient (95% CI: 42–166). In the least favourable scenario for the FTAC (FCM € 25/100 mg, PRC € 98/unit) total costs were € 644 and € 694/patient, respectively (p<0.005), with savings of € 51/patient (95% CI: 2–100). Therefore, compared with the previous SCP, management of moderate-to-severe anaemia in a FTAC resulted in significant cost savings for all studied scenarios (Figure 2C).
Figure 2.
Sensitivity analysis of management costs for moderate-to-severe iron deficiency anaemia in the Fast-Track Anaemia Clinic in the Emergency Department (FTAC group, A) or with standard outpatient specialised care (SCP group, B), and differences between them (Cost saving, C), considering variations of up to 30% in the acquisition costs (euros) of ferric carboxymaltose (FCM) and packed red cell (PRC) units.
Cost savings were significant in all 49 analysed scenarios (p<0.01).
Discussion
Acute, sub-acute, or chronic anaemia is a common finding among patients attending an ED. If their anaemia was moderate or severe, many of these patients used to receive a PRC transfusion before referral to specialised outpatient care or hospitalisation for investigation and treatment of the anaemia4. In 2010, a FTAC was established in our ED. This clinic is similar to other rapid diagnosis units (RDU) for the management of potentially serious pathologies10,16. The FTAC enables rapid differential diagnosis, treatment and follow-up of patients with moderate-to-severe anaemia. It was shown that the use of IV iron in this FTAC is efficacious at improving Hb levels and replenishing iron stores, at the same time as reducing the risk of PRC transfusion10.
Our cost analysis suggests that IDA management in the FTAC is also cost-effective, (Table IV). This comparative cost analysis relies on three elements that should be discussed: the adequacy of PRC transfusions in the SCP group, the cost of PRC transfusion, and the cost of IV iron supplementation.
Patients with sub-acute or chronic anaemia can tolerate very low Hb levels. However, the available evidence indicates that a Hb <6 g/dL is associated with worse outcomes when compared to higher Hb levels, and that PRC transfusion is also associated with worse outcomes4. It is, therefore, important to decide when to transfuse. Several medical societies have issued recommendations, emphasising the application of “restrictive criteria”13,17–21. It is important to decide the goal of transfusion; that is, what is the minimum, therapeutically effective PRC dose. For asymptomatic anaemia, a “safe” approach might be to transfuse if Hb <6–8 g/dL to attain levels not higher than 9–10 g/dL, depending on the patient’s age, co-morbidity (risk criteria), and clinical needs. In stable, non-bleeding patients, transfusion of a single PRC unit is often a valid option.
In the SCP group, we applied Spanish Society of Blood Transfusion recommendations with respect to the transfusion threshold (Table I)13, and the minimum number of PRC units would have been administered to achieve a Hb level (0.5-1-0 g/dL above the transfusion threshold) while waiting for outpatient classification and treatment of the anaemia (3–6 weeks) (Table III). This practice is common in the ED for patients with severe anaemia (Hb <8 g/dL), especially in those with chronic blood loss. Even if they were referred to a RDU or standard specialised care, or hospitalised for investigation of their anaemia, 65% received PRC transfusion22. A recent multicentre analysis of 908 PRC transfusion episodes in ED showed that 78.6% were appropriate. A post-transfusion Hb assessment was performed in only 72.4% of patients who had an appropriate PRC transfusion, and 45% of these were considered over-transfused. Overall, 41% (584/1,433) of evaluable PRC units were deemed unnecessarily transfused23. In the SCP group, 17% of those <65 years and 96% of those ≥65 years would have needed PRC units (1–3 units/patient) to increase their baseline Hb from 7.4±1.4 g/dL to 9.2±0.7 g/dL, while waiting for outpatient classification and treatment of their anaemia. We consider this estimate to be appropriate and not resulting in over-transfusion.
With respect to PRC transfusion costs, according to data supplied by our Transfusion Service, the PRC acquisition cost was € 140/unit, including re-assessment of the unit’s blood group (Table II). This cost would be within the price range observed in other studies from Spain (€ 155/unit), the USA (€ 150–190/unit), Switzerland (€ 145/unit) and Austria (€ 115/unit)24–26. On the other hand, PRC administration costs varied between € 146/unit and € 253/unit, depending on the number of units transfused in each session (Table II), and were significantly lower than those reported for Austria (€ 300/unit), Switzerland (€ 315/unit) and the USA (€ 390–700/unit)25,26. In our study, even considering a 30% increase in PRC acquisition cost (€ 182/unit), the total cost for two PRC units in a single transfusion session (€ 709) would be lower than those recently estimated from six European studies (€ 878)27. We, therefore, consider that PRC transfusion costs have not been overestimated.
This cost-analysis refers to FCM, the only IV formulation used in the FTAC since 2012. The average costs of FCM supplementation were € 332/patient and € 296/patient for patients in the FTAC and SCP groups, respectively (approximately € 236/1,000 mg). In two cost-minimisation studies, the costs of administering 1,000 mg of FCM were lower, varying between € 235 and € 24928,29. These costs may be reduced by eliminating the post-infusion observation period (€ 26 per session). The European Medicines Agency recommends close monitoring for signs of hypersensitivity during and for at least 30 minutes after every administration of an IV iron product30. The guidelines for risk minimisation and management of hypersensitivity reactions to IV iron, especially for patients at risk (e.g., who have had a previous adverse reaction to IV iron or other drugs, have a history of severe atopy, pre-existing severe respiratory or cardiac disease, or are taking beta-blockers or ACE inhibitors)31. In contrast, since IV iron should not be associated with severe delayed reactions, the Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference considered that there is no physiological basis to recommend that patients should be observed for 30 minutes after an infusion of IV iron is completed32. We, therefore, consider that IV iron supplementation costs have not been under-estimated.
Other IV iron formulations are available in Spain: iron sucrose (€ 11.2/100 mg, maximum 200 mg/30 minutes), low molecular weight iron dextran (€ 10.3/100 mg, 1,000 mg/60–180 minutes), and iron isomaltoside-1000 (€ 19.24/100 mg, 1,000 mg/30 minutes)14. According to data in Table II, the total costs to administer 1,000 mg of each of these formulations would be € 365, € 172–269, and € 238, respectively. Assuming all available IV iron formulations are equally safe and efficacious at correcting IDA33,34, the total cost of IDA management in the FTAC would be similar using any of them, except iron sucrose because of the need for multiple visits to correct the total iron dose with this compound35. These data also indicate that a single dose of IV iron has advantages over lower doses, both for the patient (fewer phlebotomies, fewer hours of work lost, etc.) and for the healthcare system (fewer visits to the FTAC, fewer transfers by ambulance, etc.).
The costs of laboratory tests and investigations were assumed to be the same with both anaemia management strategies and were not considered in this analysis. They should, however, be similar to those in a RDU for potentially serious diseases, including anaemia. In this regard, the results of various studies including thousands of patients show that the average diagnostic cost in a RDU is around € 750/process, which is far from that for hospitalised patients (≈€ 3,300/process)22,36–38.
Costs due to adverse events associated with PRC transfusion or FCM administration were not estimated or considered in this analysis given their low incidence and the small number of patients studied. However, according to data from the Food and Drug Administration (for the years 2001–2003; 30 million doses of IV iron), the incidence of serious adverse drug events (2.2 per million doses), including mortality (0.4 per million doses), associated with IV iron infusions is much lower than the incidence associated with PRC transfusion (10 per million PRC units and 4 per million, respectively)39,40.
To increase the efficiency of the FTAC, we should consider the referral of patients who require immediate transfusion but not hospitalisation. In this regard, at the Pennine Acute Trust (United Kingdom), it was estimated that if IDA patients were stabilised with one PRC unit and then treated with IV iron, the number of PRC units transfused in the ED would decrease by 4.5%, with potential savings of approximately € 60,000/year7.
The use of a theoretical SCP group, instead of an historical or prospective comparator group, is recognised as a study limitation. However, in our opinion, the study maintains its strengths of clinical experience and easy applicability to other institutions, adapting the cost figures presented in Table II to local costs. The design and conduction of randomised trials involving intervention and control groups would help to elucidate the cost-efficacy of IDA management in a FTAC, as an alternative to the conventional practice of referring patients to specialised outpatient care or admitting them to hospital.
Conclusions
Based on a cost-analysis, patients with IDA presenting at an ED and treated in a FTAC incurred average treatment costs of € 594±337/patient compared to € 672±301/patient in a theoretical group managed with the SCP, with a saving of € 78±28/patient. The sensitivity analysis showed that IDA treatment costs in the FTAC resulted in significant cost savings for all studied scenarios. Thus, the administration of ferric carboxymaltose for IDA management in a FTAC may be cost-saving compared with the SCP.
Acknowledgements
The Authors acknowledge invaluable help from the hospital managers and healthcare personnel in implementing this anaemia care pathway in the Emergency Department of La Paz University Hospital, Madrid (Spain).
Footnotes
Disclosure of conflict of interest
MQD has received honoraria for consultancy or lectures and/or travel support from Octapharma (Spain & Switzerland) and Vifor Pharma (Spain) but not for this work. JAGE has received honoraria for consultancy or lectures and/or travel support from Wellspect HealthCare (Sweden), Roche (Spain), Vifor Pharma (Spain & Switzerland), Amgen (Spain), Janssen-Cilag (Spain) and Novartis (Spain) but not for this work. MM has received honoraria for consultancy or lectures and/or travel support from Wellspect HealthCare (Sweden), Roche (Spain), Vifor Pharma (Spain & Switzerland), PharmaCosmos (Denmark) and Zambon (Spain) but not for this work. AMB, JP, RMR and SGR have nothing to declare.
Authorship contribution
MQD: study design, data collection, manuscript review and approval. RMR and AMB: data collection, analysis, and interpretation, manuscript review and approval. SGR, JP and JAGE: data interpretation, manuscript review and approval. MM: study design, data analysis and interpretation, manuscript writing and approval.
References
- 1.World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. WHO/NMH/NHD/MNM/11.1. [Accessed on 20/06/2015]. Available at: http://www.who.int/vmnis/indicators/haemoglobin.pdf.
- 2.Kassebaum NJ, Jasrasaria R, Naghavi M, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. doi: 10.1182/blood-2013-06-508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bilbao J. Anemias carenciales I: anemia ferropénica. Inf Ter Sist Nac Salud. 2006;30:35–41. [Google Scholar]
- 4.Janz TG, Johnson RL, Rubenstein SD. Anemia in the emergency department: evaluation and treatment. Emerg Med Pract. 2013;15:1–15. quiz 15–6. [PubMed] [Google Scholar]
- 5.Zambrana JL, Delgado M, Cruz G. Impact on hospital days of care due to unnecessary emergency admissions. Rev Esp Salud Pública. 2005;79:541–9. doi: 10.1590/s1135-57272005000500004. [DOI] [PubMed] [Google Scholar]
- 6.Villalta J, Sisó A, Cereijo AC, et al. [Appropriateness of hospitalization in a short stay unit of a teaching hospital. A controlled study]. Med Clin (Barc) 2004;122:454–6. doi: 10.1016/s0025-7753(04)74270-0. [In Spanish.] [DOI] [PubMed] [Google Scholar]
- 7.Hogshire L, Carson JL. Red blood cell transfusion: what is the evidence when to transfuse? Curr Opin Hematol. 2013;20:546–51. doi: 10.1097/MOH.0b013e32836508bd. [DOI] [PubMed] [Google Scholar]
- 8.Allameddine A, Heaton M, Jenkins H, et al. Inappropriate use of blood transfusion in emergency department in a tertiary care hospital and potential saving with patient blood management [abstract] Transfus Med. 2014;24( Suppl 1):25. [Google Scholar]
- 9.Tirado-Anglés G, Gangutia-Hernández S, Rodríguez-Chacón L, et al. Influence of a transfusion pocket guide on physicians’ transfusion practices, 2010–2013 [abstract] Transfus Med. 2014;24( Suppl 1):27. [Google Scholar]
- 10.Quintana-Díaz M, Fabra-Cadenas S, Gómez-Ramírez S, et al. A fast-track anaemia clinic in the Emergency Department: feasibility and efficacy of intravenous iron administration for treating sub-acute iron deficiency anaemia. Blood Transfus. 2016;14:126–33. doi: 10.2450/2015.0176-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evstatiev R, Marteau P, Iqbal T, et al. FERGI Study Group. FERGIcor, a randomized controlled trial on ferric carboxymaltose for iron deficiency anemia in inflammatory bowel disease. Gastroenterology. 2011;141:846–853.e1–2. doi: 10.1053/j.gastro.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Vaglio S, Prisco D, Biancofiore G, et al. Recommendations for the implementation of a Patient Blood Management programme. Application to elective major orthopaedic surgery in adults. Blood Transfus. 2016;14:23–65. doi: 10.2450/2015.0172-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sociedad Española de Transfusión Sanguínea y Terapia Celular. Guía sobre la transfusión de componentes sanguíneos y derivados plasmáticos. 4th ed. SETS; Barcelona: 2010. pp. 43–53. [Google Scholar]
- 14.Consejo General de Colegios Oficiales de Farmacéuticos. Bases de datos de medicamentos. [Accessed on 20/06/2016]. Available at: http://www.portalfarma.com.
- 15.ORDEN 731/2013, de 6 de septiembre, del Consejero de Sanidad, por la que se fijan los precios públicos por la prestación de los servicios y actividades de naturaleza sanitaria de la Red de Centros de la Comunidad de Madrid. B.O.C.M. Núm 215. Septiembre. 2013. [Accessed on 20/06/2016]. Available at: http://www.bocm.es/boletin/CM/2013/09/10/21500.PDF.
- 16.Pericás JM, Aibar J, Soler N, et al. Should alternatives to conventional hospitalisation be promoted in an era of financial constraint? Eur J Clin Invest. 2013;43:602–15. doi: 10.1111/eci.12087. [DOI] [PubMed] [Google Scholar]
- 17.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 19.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozek-Langenecker SA, Afshari A, Albadalejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesth. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 21.Practice guidelines for perioperative blood management: an update report from the American Society of Anesthesiologists Task Force on Perioperative Blood Management. Anesthesiology. 2015;122:241–75. doi: 10.1097/ALN.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 22.Bosch X, Palacios F, Inclán-Iríbar G, et al. Quick diagnosis units or conventional hospitalisation for the diagnostic evaluation of severe anaemia: a paradigm shift in public health systems? Eur J Intern Med. 2012;23:159–64. doi: 10.1016/j.ejim.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Quintana-Díaz QM, Borobia AM, García-Erce JA, et al. USEES-URG Research Group. Appropriate use of red blood cell transfusion in emergency departments: a study in five emergency departments. Blood Transfus. 2017;15:199–206. doi: 10.2450/2016.0324-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Erce JA, Bisbe E, Alfonso E, Muñoz M. The ‘true’ costs of blood: The average unit production cost of an erythrocyte concentrate in Spain from a societal perspective. Transf Altern Transf Med. 2010;11( Suppl 1):16. [Google Scholar]
- 25.Shander A, Hofmann A, Ozawa S, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 26.Toner RW, Pizzi L, Leas B, et al. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy. 2011;9:29–37. doi: 10.2165/11530740-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Abraham I, Sun D. The cost of blood transfusion in Western Europe as estimated from six studies. Transfusion. 2012;52:1983–8. doi: 10.1111/j.1537-2995.2011.03532.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhandari S. A hospital-based cost minimization study of the potential financial impact on the UK health care system of introduction of iron isomaltoside 1000. Ther Clin Risk Manag. 2011;7:103–13. doi: 10.2147/TCRM.S17536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvet X, Ruíz MÀ, Dosal A, et al. Cost-minimization analysis favours intravenous ferric carboxymaltose over ferric sucrose for the ambulatory treatment of severe iron deficiency. PLoS One. 2012;7:e45604. doi: 10.1371/journal.pone.0045604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. [Accessed on 22/07/2015]. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2013/06/newsdetail_001833.jsp&mid=WC0b01ac058004d5c1.
- 31.Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99:1671–6. doi: 10.3324/haematol.2014.111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdougall IC, Bircher AJ, Eckardt KU, et al. Conference Participants. Iron management in chronic kidney disease: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016;89:28–39. doi: 10.1016/j.kint.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31–8. doi: 10.1002/ajh.24201. [DOI] [PubMed] [Google Scholar]
- 34.Auerbach M, Adamson J, Bircher A, et al. On the safety of intravenous iron, evidence trumps conjecture. Haematologica. 2015;100:e214–5. doi: 10.3324/haematol.2014.121004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 36.Bosch X, Jordán A, Coca A, López-Soto A. Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7:41–7. doi: 10.1002/jhm.931. [DOI] [PubMed] [Google Scholar]
- 37.Bosch X, Jordán A, López-Soto A. Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31:114–23. doi: 10.1016/j.ajem.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Bosch X, Foix A, Jordan A, et al. Outpatient Quick Diagnosis Units for the evaluation of suspected severe diseases: an observational, descriptive study. Clinics. 2011;66:737–41. doi: 10.1590/S1807-59322011000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chertow GM, Mason PD, Vaage-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–82. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 40.Stainsby D, Jones H, Asher D, et al. SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]