Abstract
Background
In Europe, red cell concentrates (RCC) are usually stored in SAGM (saline-adenine-glucose-mannitol). During storage, in vitro red cell quality declines, including lowered energy status and increased cell lysis. Recently, several additive solutions (ASs), designed to diminish the decline in in vitro quality during storage, have been developed. These new solutions have mainly been developed to better maintain red blood cell (RBC) 2,3-biphosphoglycerate (2,3 BPG) levels and energy status during storage. High levels of 2,3 BPG allow for better oxygen release while high energy status is necessary for function and survival of RBC in vivo. In a paired study design, RBC ASs were compared for their ability to provide improved in vitro quality during hypothermic storage.
Materials and methods
For each experiment, 5 whole blood units held overnight were pooled and split. The whole blood units were processed according to the buffy coat method. RBCs were resuspended in either SAGM, PAGGSM, PAG3M, E-Sol 5 or AS-7 and leucoreduced by filtration. RCCs were stored for eight weeks at 2–6 °C and sampled weekly for analysis of in vitro quality parameters.
Results
Red cell concentrates stored in PAG3M, E-Sol 5 and AS-7 showed significantly higher lactate production and higher levels of intracellular adenosine triphosphate (ATP) and total adenylate. 2,3 BPG levels rapidly declined during storage in SAGM and PAGGSM. The decline in 2,3 BPG was inhibited during storage in E-Sol 5 and AS-7, while in PAG3M, 2,3 BPG level increased above the initial level till day 35 and remained detectable till day 56. Haemolysis was comparable for all ASs until day 35, upon prolonged storage, haemolysis in SAGM was higher than with the other ASs. As compared to SAGM, storage in PAGGSM, PAG3M, E-Sol 5 and AS-7 better maintained morphological properties.
Discussion
Storage of RBCs in the new generation ASs yield RBCs with more stable metabolite levels and improved overall quality during storage as compared with RBCs stored in SAGM.
Keywords: red blood cells; additive solution; glycolysis; ATP; 2,3 BPG
Introduction
During refrigerated storage, the red blood cell (RBC) undergoes biophysical and biochemical changes, known as the storage lesion. The alterations include, amongst others, decreased RBC stability, as measured by the release of haemoglobin, and alterations in various metabolites and the metabolic status of the cell, including decrease of intracellular adenosine triphosphate (ATP) and 2,3-biphosphoglycerate (2,3 BPG)1–3. The 2,3 BPG levels are important for the haemoglobin (Hb) affinity for oxygen and thus for the oxygen delivery to tissues. ATP as energy source is important for the overall functioning of RBCs, for example, for maintenance of cytoskeleton flexibility and phospholipid asymmetry4. Data published by Högman5 and Heaton6, showed a correlation between energy status of the RBC and post-transfusion recovery and survival.
The storage lesion depends on blood processing and storage conditions. In Europe, whole blood can be held for up to 24 hours at room temperature before processing, while in the US this is limited to 8 hours7. Overnight room temperature hold of WB has obvious logistical advantages, but can potentially have a negative effect on component quality, including reduced RBC ATP and 2,3 BPG levels8,9.
In addition to whole blood holding time, also the additive solution (AS) used to store the RBC influences RBC properties. Even during cold storage, RBC metabolism generates lactic acid which lowers the extracellular pH resulting in decreased glycolytic activity. Therefore, storage in the conventional ASs, such as saline-adenine-glucose-mannitol (SAGM), results in a decline in 2,3 BPG and ATP levels7. New generation AS solutions have been shown to limit the effect of overnight RT hold on RBC biochemical parameters10–12. These new RBC ASs, including AS-7 (commercially available as SOLX; Haemonetics Corporation, Braintree, MA, USA)11,13,14, phosphate-adenine-glucose-guanosine-gluconate-mannitol (PAG3M)10,15 and Erythro-Sol (E-Sol)16,17 may also reduce the decline in in vitro RBC quality parameters and could lead to improved transfusion safety and efficacy14,18. In contrast to the traditional unbuffered, hypertonic and slightly acidic ASs, such as SAGM, the newer solutions, such as AS-7, E-Sol and PAG3M, are iso- or hypotonic, chloride-free, phosphate-buffered alkaline solutions. The absence of extracellular chloride results in the so-called “chloride shift”19 in which intracellular chloride is replaced by hydroxyl anions resulting in increased intracellular pH (pHin). The hypotonic nature of the modern ASs results in slightly swollen RBC compared to storage in SAGM which may prevent loss of cell membrane fractions during storage20. Since the higher cell volume could negatively influence the passage of the RBC through in line leucoreduction filters, the new ASs, like AS-7 and E-Sol 5, are mainly used in combination with whole blood leucoreduction11,17.
The objective of this study was to compare the ASs SAGM, phosphate-adenine-glucose-guanosine-saline-mannitol (PAGGSM), PAG3M, E-Sol 5 and AS-7 for 56-day cold storage of buffy coat removed, leucoreduced RBC, prepared from whole blood (WB) held overnight. For this, 5 whole blood units were used pooled and split. RBC were assayed weekly for in vitro markers for red cell quality.
Materials and methods
The composition of the various additive solutions used is shown in Table I. SAGM, PAGGSM and E-Sol 5 were obtained from Fresenius Kabi (Emmer Compascuum, The Netherlands), PAG3M and AS-7 were prepared in house and sterilised by microfiltration (0.2 μm filter). The osmolality of the solutions was measured using an Osmomat 030-D osmometer (Gonotec, Berlin, Germany).
Table I.
Composition of red blood cell additive solutions.
| Constituents (mmol/L) | SAGM | PAGGSM | PAG3M | E-Sol 5 | AS-7 |
|---|---|---|---|---|---|
| NaCl | 150 | 72 | - | - | - |
| NaHCO3 | - | - | - | - | 26 |
| Na2HPO4 | - | 16 | 8 | 20 | 12 |
| NaH2PO4 | - | 8 | 8 | - | - |
| Gluconate | - | - | 40 | - | - |
| Citrate | - | - | - | 25 | - |
| Adenine | 1.25 | 1.4 | 1.4 | 2 | 2 |
| Guanosine | - | 1.4 | 1.4 | - | - |
| Glucose | 45 | 47 | 47 | 111 | 80 |
| Mannitol | 30 | 55 | 55 | 41 | 55 |
| pH (at 37 °C) | 5.7 | 6.0 | 8.2 | 8.4 | 8.5 |
| Osmolality (mOsmol/kg) | 376 | 287 | 278 | 301 | 228 |
SAGM: saline-adenine-glucose-mannitol; PAGGSM: phosphate-adenine-glucose-guanosine-saline-mannitol; PAG3M: phosphate-adenine-glucose-guanosine-gluconate-mannitol; E-Sol: Erythro-Sol; AS: additive solution.
Blood collection and processing
All volunteer blood donors were non-remunerated. They all met standard donation criteria and gave their written, informed consent to the study, in accordance with the institution’s guidelines and practices. This study was approved by the institutional medical ethical committee, in accordance with the standards laid down in the 1964 Declaration of Helsinki.
A total of 20 whole blood (WB) units, 500 mL ±2%, were collected in quadruple, bottom-and-top collection systems containing 70 mL of citrate-phosphate-dextrose (CPD; Fresenius Kabi, Emmer Compascuum, The Netherlands). The WB units were placed on butane-1,4-diol cooling plates (Compocool, Fresenius Kabi) to allow their temperatures to adjust to 20–24 °C21. After overnight hold, 5 ABO compatible whole blood units were pooled and split (n=4). For the units destined to be stored in alternative additive solution, the SAGM from the collection system was replaced by an equal volume of the AS of choice. WB units were processed according to the routine buffy coat procedure as previously described22. Briefly, after a hard spin (Sorvall RC12BP; Sysmex, Etten-Leur, The Netherlands), the WB units were separated in components, using an automated blood component separator (Compomat G5; Fresenius Kabi). After separation, 110 mL of additive solution was added to the RBC via the filter. After careful mixing, the units were stored for 1 hour at room temperature and then WBC reduced using the inline leucoreduction filter (BioR; Fresenius Kabi). Based on pilot studies, this 1-hour rest period was added to reduce the effect on filtration time for some of the tested ASs. Leucoreduced red cell concentrates (RCC) were stored at 2–6 °C in a standard blood bank refrigerator for 56 days and sampled weekly.
Haematologic parameters were determined on a haematology analyser (XT2000i; Sysmex). Haemolysis was determined as described previously10. Briefly, cell-free supernatants were obtained by centrifugation of the RCC at 2,000 g for 10 min followed by an additional centrifugation of the supernatant at 12,000 g for 5 min. Free haemoglobin (Hb) was determined by absorbance measurement of supernatant at 415 or 514 nm by a spectrophotometer (Eon plate reader; Bio Tek, Bad Friedrichshall, Germany), with correction for plasma absorption if necessary. Haemolysis was expressed as a percentage of total Hb present in the RBC after correction for haematocrit.
Extracellular potassium, glucose, lactate and pH were measured with a blood gas analyser (ABL90 Flex; Radiometer Benelux BV, Zoetermeer, The Netherlands).
Intracellular pH (pHin) and intracellular chloride concentration (Clin) were measured as described previously1. Briefly, RBC were pelleted by centrifugation and the supernatant was removed. RBCs were frozen in liquid nitrogen and thawed. pH and chloride concentration of the lysate was measured using a blood gas analyser. Adenine and guanine nucleotides and 2,3 BPG levels were determined in neutralised perchloric acid extracts. Adenine and guanine nucleotides were assayed, using an HPLC method23. Total adenylate content was calculated as the sum of ATP, ADP and AMP levels. 2,3 BPG was analysed with the 2,3 BPG kit of Roche (Mannheim, Germany).
Morphology was determined after fixation of the cells with methanol and was expressed as percentage of echinocytes. To quantify the amount of erythrocytes exposing phosphatidyl serine (PS) on their cell surface, cells were stained with FITC-labelled AnnexinV, essentially as previously described4. Briefly, samples of red cells were incubated with AnnexinV-FITC (VPS-Diagnostics, Hoeven, The Netherlands) and analysed on a flow cytometer (FACSCalibur; Becton Dickinson, Breda, The Netherlands). The percentage of Annexin V-positive cells was determined by comparison with a negative control that was incubated with EGTA.
Statistical analysis
Results obtained during storage in the alternative additive solutions were compared with those obtained in SAGM. Results were analysed with repeated-measures analysis of variance, followed by a Dunnett’s test to compare the values during storage in SAGM (Instat, v.3.06; GraphPad, San Diego, CA, USA). Differences compared to SAGM were considered significant when p-values were less than 0.05.
Results
Cellular composition was comparable for all RCCs, indicating an efficient pool and split procedure. Leucoreduction filtration times for RCC resuspended in SAGM were approximately 25 minutes (Table II). With the exception of PAG3M, filtration times were significantly increased with the alternative ASs. With filtration times exceeding 1 hour, filtration times were longest for RCC in AS-7.
Table II.
Characteristics of red blood cells in additive solution.
| Parameter | SAGM | PAGGSM | PAG3M | E-Sol 5 | AS-7 |
|---|---|---|---|---|---|
| Filtration times (min) | 25±4 | 35±4# | 33±4 | 41±6# | 72±26# |
|
| |||||
| Day 1 | |||||
| MCV (fL) | 88±0.7 | 87±0.7 | 83±0.8 | 85±0.9 | 85±1.4 |
| Ht (L/L) | 0.57±0.02 | 0.56±0.01 | 0.54±0.01 | 0.55±0.01 | 0.56±0.02 |
| Echinocytes (%) | 9±5 | 7±5 | 6±4 | 6±4 | 4±2 |
| Annexin V+ cells (%) | 1.3±0.8 | 1.6±0.4 | 1.7±0.1 | 1.6±0.2 | 1.9±0.5 |
|
| |||||
| Day 56 | |||||
| MCV (fL) | 92±2.6 | 84±2.3# | 80±1.9# | 81±1.7# | 82±1.4# |
| Ht (L/L) | 0.60±0.02 | 0.55±0.01# | 0.54±0.01# | 0.54±0.01# | 0.54±0.01# |
| Echinocytes (%) | 44±5 | 35±4# | 37±2# | 36±3# | 32±4# |
| Annexin V+ cells (%) | 6.8±0.9 | 7.2±0.3 | 7.5±0.5 | 7.0±0.5 | 6.6±0.9 |
| Lactate production (mmol/1012 cells/day) | 0.07±0.01 | 0.07±0.01 | 0.11±0.01# | 0.08±0.01# | 0.09±0.01# |
Results shown represent mean±SD (n=4).
p<0.05 as compared to storage in SAGM.
SAGM: saline-adenine-glucose-mannitol; PAGGSM: phosphate-adenine-glucose-guanosine-saline-mannitol; PAG3M: phosphate-adenine-glucose-guanosine-gluconate-mannitol; E-Sol: Erythro-Sol; AS: additive solution; MCV: mean corpuscular volume; Ht: haematocrit.
Metabolic parameters during storage
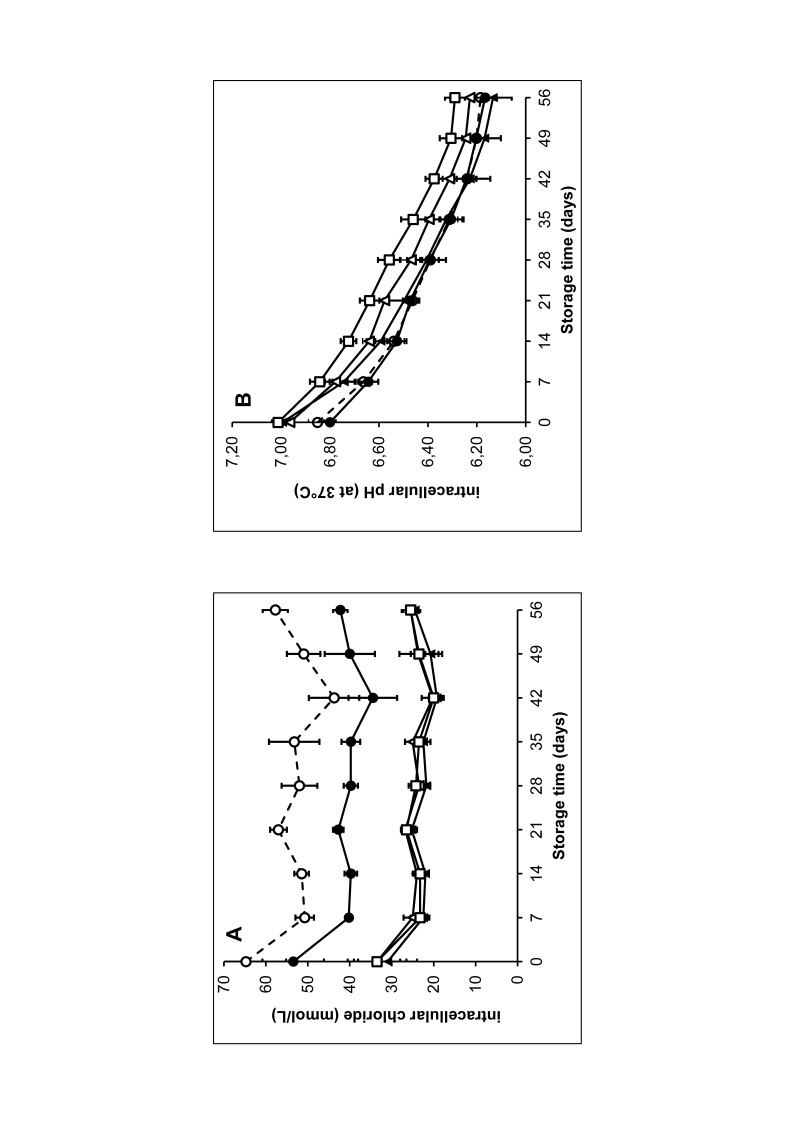
Resuspension of RBC in the chloride-free additive solutions PAG3M, E-Sol 5 and AS-7 resulted in a decreased intracellular chloride concentration (Clin) as compared to SAGM (Figure 1A). Clin remained lower during the entire storage period. PAGGSM, which has a lower chloride concentration than SAGM, showed intermediate Clin values. The lower Clin as observed with the chloride-free media was accompanied by increased pHin as compared to SAGM and PAGGSM (Figure 1B). pHin remained higher during the entire storage period for RBC in E-Sol 5 and AS-7, while in PAG3M, pHin was higher for the first two weeks of storage only.
Figure 1.
Intracellular chloride (A) and intracellular pH of red blood cells (RBC) stored at 2–6 °C in saline, adenine, glucose, mannitol (SAGM) (○), PAGSM (●), PAG3M (▲), E-Sol 5 (△) and AS-7 (□).
Results shown represent mean±SD (n=4). SAGM: saline-adenine-glucose-mannitol; PAGGSM: phosphate-adenine-glucose-guanosine-saline-mannitol; PAG3M: phosphate-adenine-glucose-guanosine-gluconate-mannitol; E-Sol: Erythro-Sol; AS: additive solution.
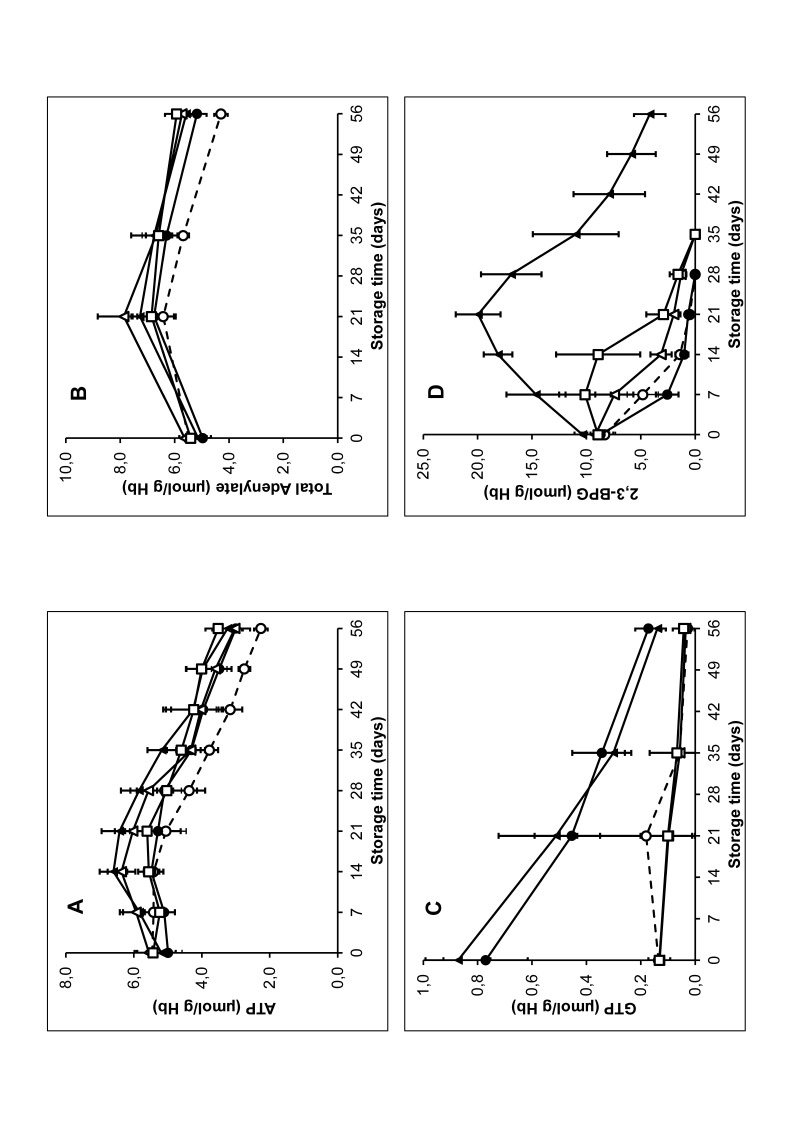
Glycolytic activity, measured as lactate production was increased in the chloride-free additive solutions as compared to SAGM and PAGGSM (see Table II). The increase in lactate was accompanied by a concomitant decrease in glucose levels (data not shown). Initial ATP levels were comparable for all ASs (Figure 2A). During the first three weeks of storage, the ATP content remained relatively constant with SAGM and PAGGSM, and was slightly increased with PAG3M, E-Sol 5 and AS-7, the increase being most pronounced with PAG3M. Upon prolonged storage, ATP levels gradually decreased in all RCCs. From day 21 on, ATP levels of RCC in SAGM were significantly lower as compared to the other ASs. From day 42 on, no differences in ATP content were found between the other ASs. After an initial increase, total adenylate levels gradually decreased during storage (Figure 2B). After 56 days of storage in SAGM, total adenylate level was decreased to 78±4% of the initial value. With the alternative ASs, total adenylate levels remained above 100% of the initial values during the whole storage period, without significant differences between the different ASs. Guanosine-5′-triphosphate (GTP) levels, which also add to the RBC metabolic pool, were strongly increased when stored in the guanosine-containing solutions PAGGSM and PAG3M as compared to storage in the other ASs (Figure 2C).
Figure 2.
Red blood cell (RBC) metabolite concentrations during storage at 2°–6°C in saline, adenine, glucose, mannitol (SAGM) (○), PAGSM (●), PAG3M (▲), E-Sol 5 (△) and AS-7 (□).
(A) ATP. (B) Total adenylate. (C) GTP. (D) 2,3 BPG. Results shown represent mean±SD (n=4). SAGM: saline-adenine-glucose-mannitol; PAGGSM: phosphate-adenine-glucose-guanosine-saline-mannitol; PAG3M: phosphate-adenine-glucose-guanosine-gluconate-mannitol; E-Sol: Erythro-Sol; AS: additive solution.
Large differences were found for 2,3 BPG levels during storage. 2,3 BPG levels rapidly declined during storage in SAGM and PAGGSM (Figure 2D). The decline in 2,3 BPG levels was somewhat inhibited during storage in E-Sol 5 while in AS-7, 2,3 BPG levels were maintained at the initial level till day 14 of storage. When stored in PAG3M, 2,3 BPG levels were increased above the initial level till day 35 and were above the detection limit till day 56.
Haematologic and morphological parameters
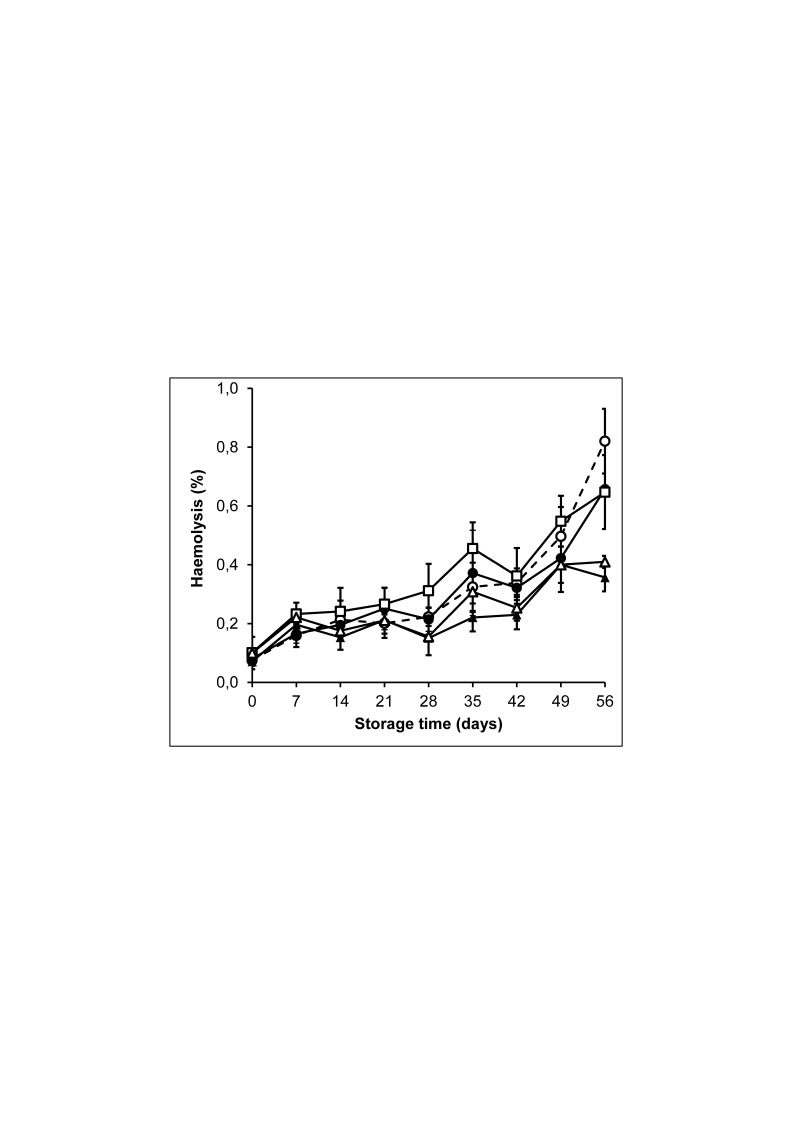
Storage in SAGM resulted in slight swelling of the cells as measured by an increased mean corpuscular volume (MCV) and haematocrit (Table II). With the other additive solutions, this swelling was not observed. Haemolysis gradually increased during storage. Up to 35 days of storage, haemolysis was comparable for all ASs and well below our current requirement of 0.8% (Figure 3). Upon prolonged storage, differences in haemolysis became apparent, being the highest for SAGM (0.8% at day 56), intermediate for AS-7 and PAGGSM (0.65% haemolysis), and lowest for PAG3M and E-Sol 5 (0.4% haemolysis). The number of echinocytes gradually increased during storage, being most pronounced in SAGM (Table II), and no differences were observed between the other ASs. At day 56 of storage, the percentage of Annexin V positive cells was below 10% for all RCCs without any significant difference between the different additive solutions (Table II).
Figure 3.
Haemolysis of red blood cells (RBC) stored at 2–6 °C in saline, adenine, glucose, mannitol (SAGM) (○), PAGSM (●), PAG3M (▲), E-Sol 5 (△) and AS-7 (□).
Results shown represent mean±SD (n=4). SAGM: saline-adenine-glucose-mannitol; PAGGSM: phosphate-adenine-glucose-guanosine-saline-mannitol; PAG3M: phosphate-adenine-glucose-guanosine-gluconate-mannitol; E-Sol: Erythro-Sol; AS: additive solution.
Discussion
This study was performed to determine whether the alternative additive solutions, PAGGSM, PAG3M, E-Sol 5 and AS-7, better maintain in vitro quality of RCC components prepared from whole blood after overnight storage at room temperature before processing, compared to storage in the conventional SAGM solution. In this study, we performed leucoreduction of RCC in additive solution in contrast to previous studies with E-Sol 5 and AS-7, in which RCCs prepared from leucoreduced whole blood were used11,17. Leucoreduction filtration times were increased with the new generation ASs, most likely because of the lower osmolality of the solutions and subsequent swelling of the RBC. Although there are differences in composition between PAG3M, E-Sol 5 and AS-7, the solutions are designed to support higher rates of RBC metabolism by keeping the pHin at higher levels during storage. Collection of whole blood in the anti-coagulant CPD (pH 5.6) and the use of current additive solutions with a pH of around 6, results in a relatively low initial pHin. During storage, RBC produce lactate due to the metabolism of glucose, resulting in a further decrease of pHin. To better maintain the pH during RBC storage, the new generation ASs not only are alkaline, but also have a higher buffer capacity due to the addition of phosphate (PAGGSM, PAG3M, E-Sol 5, AS-7) and bicarbonate (AS-7). In addition, the new ASs are chloride-free, resulting in a higher pHin because of the chloride shift. Low chloride concentration in the AS and passive diffusion of chloride out of the cells result in a net loss of intracellular chloride. To maintain electrical neutrality, hydroxyl anions will diffuse into the cell proportional to the chloride loss where they increase the intracellular pH10,19.
Preservation of the energy status of RBC is crucial for the RBC function and in vivo survival. ATP content is indirectly related to post transfusion survival, because normal ATP levels are necessary to prevent membrane loss by microvesiculation, and also to maintain active, outward transport of phospholipids like phosphatidylserine (PS), thus preventing premature clearance from the recipient’s circulation by macrophages4,24. During the hypothermic storage, the ATP levels gradually declined, accompanied by an increase in the levels of ADP and AMP. In the glycolytic pathway, both AMP and ADP can be converted back to ATP, so both these substances can still contribute to the cell’s energy status, expressed as the total adenylate content. US and European guidelines dictate an in vivo 24-hour post-transfusion survival of at least 75%25–27. As a surrogate marker for in vivo survival, total adenylate content can be used. Högman et al.5 not only found a good correlation between total adenylate content and post transfusion recovery, but also that the minimum total adenylate content needed to meet this requirement was 82% of the original levels. While RBC stored in SAGM failed to meet this requirement beyond 35 days of storage, the chloride-free solutions and PAGGSM were able to maintain this level up to day 56 of storage. It has recently been postulated for mice red cells that lipid peroxidation might be associated with poor 24-hour recovery28. It would, therefore, be of interest to study the effect of the different additive solutions on oxidation of lipids during storage.
During RBC storage in the conventional additive solution SAGM, 2,3 BPG rapidly declines and is depleted within two weeks of storage. The chloride-free additive solutions E-Sol 5 and AS-7 inhibited the decline in 2,3 BPG levels for approximately two weeks, while in PAG3M the 2,3 BPG levels increase during the first three weeks of storage above the initial values followed by a gradual decline upon further storage. The slower decline of 2,3 BPG in the chloride-free solutions could be ascribed to the higher pHin, resulting in a higher glycolytic activity10,15. The additional effect of PAG3M on 2,3 BPG production might be explained by the described effect of guanosine on the activity of the key allosteric regulatory enzyme phosphofructokinase (PFK) in the glycolytic pathway thus raising the levels of both ATP and 2,3 BPG1,29,30.
In all chloride-free additive solutions (both most pronounced in PAG3M), ATP and 2,3 BPG levels show a non-linear trend during storage and seem to follow a multiple stage trend: an initial increase (production) is followed by a plateau stage and a subsequent decrease (consumption). This phenotype is consistent with previous findings from metabolomic studies which indicated that during refrigerated storage three distinct metabolic phases, occurring on days 0–10, days 10–18 and after 18 days, could be identified31,32. PAG3M seems to extend the initial metabolic phase (production) to at least day 21, implicating improved quality and a lower metabolic age of cells stored in PAG3M as compared to the other additive solutions31.
In the present study, di-ethylhexyl-phthalate (DEHP)-plasticised PVC systems were used for whole blood collection and red cell storage. The use of DEHP in blood bags is under discussion due to toxicity concerns33,34. Literature seems to indicate that the current red cell additive solutions may not be the best to use in combination with non-DEHP plastic. A recent study, using collection systems made entirely of di(isononyl) cyclohexane-1,2-dicarboxylate (Hexamoll® DINCH; BASF SE, Ludwigshafen, Germany) plasticised PVC, showed that if the red cell storage solution SAGM was replaced by PAGGSM or PAG3M, haemolysis rates were better preserved35. It would be of interest to investigate whether E-Sol 5 and AS-7 also show this positive effect on red cell integrity during storage in DEHP-free systems.
Conclusions
Red blood cells prepared from WB held overnight at room temperature and stored in the recently developed chloride-free additive solutions PAG3M, E-Sol-5 and AS-7 had significantly elevated levels of the key metabolites ATP, total adenylate and 2,3 BPG, compared with the paired control RBCs in SAGM. In particular, PAG3M was efficient in preventing the depletion of 2,3 BPG. These results indicate that storage of RBCs in the new generation additive solutions yield RBCs with more stable metabolite levels and improved overall quality during storage as compared with RBCs stored in SAGM.
Footnotes
Authorship contributions
All of the Authors contributed to the conception and design of the study, and analysed and interpreted the data. All of the Authors critically revised the manuscript for important intellectual content and approved the final version submitted for publication. HK collected data and carried out statistical analyses. JL wrote the manuscript. PvdM and DdK helped to interpret the results and reviewed the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Burger P, Korsten H, de Korte, et al. An improved red blood cell additive solution maintains 2,3-diphosphoglycerate and adenosine triphosphate levels by an enhancing effect on phosphofructokinase activity during cold storage. Transfusion. 2010;50:2386–92. doi: 10.1111/j.1537-2995.2010.02700.x. [DOI] [PubMed] [Google Scholar]
- 2.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion. 2016;56:852–62. doi: 10.1111/trf.13460. [DOI] [PubMed] [Google Scholar]
- 4.Verhoeven AJ, Hilarius PM, Dekkers DW, et al. Prolonged storage of red blood cells affects aminophospholipid translocase activity. Vox Sang. 2006;91:244–51. doi: 10.1111/j.1423-0410.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 5.Hogman CF, de Verdier CH, Ericson A, et al. Studies on the mechanism of human red cell loss of viability during storage at +4 degrees C in vitro. I. Cell shape and total adenylate concentration as determinant factors for posttransfusion survival. Vox Sang. 1985;48:257–68. doi: 10.1111/j.1423-0410.1985.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 6.Heaton WA. Evaluation of posttransfusion recovery and survival of transfused cells. Transfus Med Rev. 1992;6:153–69. doi: 10.1016/s0887-7963(92)70166-7. [DOI] [PubMed] [Google Scholar]
- 7.Roback JD, Combs MR, Grossman BJ, et al., editors. Technical manual. 16th ed. Bethesda, MD: AABB; 2008. [Google Scholar]
- 8.Hughes JD, Macdonald VW, Hess JR. Warm storage of whole blood for 72 hours. Transfusion. 2007;47:2050–6. doi: 10.1111/j.1537-2995.2007.01429.x. [DOI] [PubMed] [Google Scholar]
- 9.Van der Meer PF, Cancelas JA, Cardigan R, et al. Evaluation of overnight hold of whole blood at room temperature before component processing: effect of red blood cell (RBC) additive solutions on in vitro RBC measures. Transfusion. 2011;51:15S–24S. doi: 10.1111/j.1537-2995.2010.02959.x. [DOI] [PubMed] [Google Scholar]
- 10.de Korte D, Kleine M, Korsten HG, et al. Prolonged maintenance of 2,3-diphosphoglycerate acid and adenosine triphosphate in red blood cells during storage. Transfusion. 2008;48:1081–9. doi: 10.1111/j.1537-2995.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 11.Dumont LJ, Cancelas JA, Maes LA, et al. Overnight, room temperature hold of whole blood followed by 42-day storage of red blood cells in additive solution-7. Transfusion. 2015;55:485–90. doi: 10.1111/trf.12868. [DOI] [PubMed] [Google Scholar]
- 12.Veale MF, Healey G, Sran A, et al. AS-7 improved in vitro quality of red blood cells prepared from whole blood held overnight at room temperature. Transfusion. 2015;55:108–14. doi: 10.1111/trf.12779. [DOI] [PubMed] [Google Scholar]
- 13.Hess J. An update on solutions for red cell storage. Vox Sang. 2006;91:13–9. doi: 10.1111/j.1423-0410.2006.00778.x. [DOI] [PubMed] [Google Scholar]
- 14.D’Alessandro A, Nemkov T, Hansen KC, et al. Red blood cell storage in additive solution-7 preserves energy and redox metabolism: a metabolomics approach. Transfusion. 2015;55:2955–66. doi: 10.1111/trf.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger P, Korsten H, Verhoeven AJ, et al. Collection and storage of red blood cells with anticoagulant and additive solution with a physiologic pH. Transfusion. 2012;52:1245–52. doi: 10.1111/j.1537-2995.2011.03472.x. [DOI] [PubMed] [Google Scholar]
- 16.Högman CF, Knutson F, Lööf H, et al. Improved maintenance of 2,3-DPG and ATP in RBCs stored in a modified additive solution. Transfusion. 2002;42:824–9. doi: 10.1046/j.1537-2995.2002.00148.x. [DOI] [PubMed] [Google Scholar]
- 17.Radwanski K, Thill M, Min K. Red cell storage in E-Sol 5 and Adsol additive solutions: paired comparison using mixed and non-mixed study design. Vox Sang. 2014;106:322–9. doi: 10.1111/vox.12108. [DOI] [PubMed] [Google Scholar]
- 18.Cancelas JA, Dumont LJ, Maes LA, et al. Additive solution-7 reduces the red blood cell storage lesion. Transfusion. 2015;55:491–8. doi: 10.1111/trf.12867. [DOI] [PubMed] [Google Scholar]
- 19.Meryman HT, Hornblower M. Manipulating red cell intra- and extracellular pH by washing. Vox Sang. 1991;60:99–104. doi: 10.1111/j.1423-0410.1991.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 20.Veale MF, Healey G, Sparrow RL. Effect of additive solutions on red blood cell (RBC) membrane properties of stored RBCs prepared from whole blood held for 24 hours at room temperature. Transfusion. 2011;51:25S–33S. doi: 10.1111/j.1537-2995.2010.02960.x. [DOI] [PubMed] [Google Scholar]
- 21.Pietersz R, de Korte D, Reesink HW, et al. Storage of whole blood for up to 24 hours at ambient temperature prior to component preparation. Vox Sang. 1989;56:145–50. doi: 10.1111/j.1423-0410.1989.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 22.Bontekoe IJ, van der Meer PF, Mast G, de Korte D. Separation of centrifuged whole blood and pooled buffy coats using the new CompoMat G5: 3 years experience. Vox Sang. 2014;107:140–7. doi: 10.1111/vox.12140. [DOI] [PubMed] [Google Scholar]
- 23.de Korte D, Haverkort WA, van Gennip AH, et al. Nucleotide profiles of normal human blood cells determined by high-performance liquid chromatography. Anal Biochem. 1985;147:197–209. doi: 10.1016/0003-2697(85)90028-4. [DOI] [PubMed] [Google Scholar]
- 24.Kamp D, Sieberg T, Haest CW. Inhibition and stimulation of phospholipid scrambling activity. Consequences for lipid asymmetry, echinocytosis, and microvesiculation of erythrocytes. Biochemistry. 2001;40:9438–46. doi: 10.1021/bi0107492. [DOI] [PubMed] [Google Scholar]
- 25.AABB. Standards for Blood Banks and Transfusion Services. 30th ed. Bethesda, MD: AABB; 2016. [Google Scholar]
- 26.Council of Europe. Guide to the preparation, use and quality assurance of blood components. 18th ed. Strasbourg: Council of Europe Publishing; 2015. European Directorate for the Quality of Medicine and Health Care. [Google Scholar]
- 27.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 28.De Wolski K, Fu X, Dumont LJ, et al. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica. 2016;101:578–86. doi: 10.3324/haematol.2015.139139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino T, Yachie-Kinoshita A, Hirayama A, et al. Dynamic simulation and metabolome analysis of long-term erythrocyte storage in adenine-guanosine solution. PLoS ONE. 2013;8:e71060. doi: 10.1371/journal.pone.0071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56:980–93. doi: 10.1111/trf.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paglia G, D’Alessandro A, Rolfsson O, et al. Biomarkers defining the metabolic age of red blood cells during cold storage. Blood. 2016;128:e43–50. doi: 10.1182/blood-2016-06-721688. [DOI] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sampson J, de Korte D. DEHP-plasticised PVC: relevance to blood services. Transfus Med. 2011;21:73–83. doi: 10.1111/j.1365-3148.2010.01056.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Meer PF, Reesink HW, Panzer S, et al. Should DEHP be eliminated in blood bags? Vox Sang. 2014;106:176–95. doi: 10.1111/vox.12099. [DOI] [PubMed] [Google Scholar]
- 35.Lagerberg JW, Gouwerok E, Vlaar R, et al. In vitro evaluation of the quality of blood products collected and stored in systems completely free of di(2-ethylhexyl)phthalate-plasticized materials. Transfusion. 2015;55:522–31. doi: 10.1111/trf.12870. [DOI] [PubMed] [Google Scholar]