Abstract
Background
During hypothermic storage, a substantial fraction of red blood cells (RBCs) transforms from flexible discocytes to rigid sphero-echinocytes and spherocytes. Infusion of these irreversibly-damaged cells into the recipient during transfusion serves no therapeutic purpose and may contribute to adverse outcomes in some patients. In this proof-of-concept study we describe the use of hypotonic washing for selective removal of the irreversibly-damaged cells from stored blood.
Materials and methods
Stored RBCs were mixed with saline of various concentrations to identify optimal concentration for inducing osmotic swelling and selective bursting of spherical cells (sphero-echinocytes, spherocytes), while minimising indiscriminate lysis of other RBCs. Effectiveness of optimal treatment was assessed by measuring morphology, rheological properties, and surface phosphatidylserine (PS) exposure for cells from several RBCs units (n=5, CPD>AS-1, leucoreduced, 6 weeks storage duration) washed in hypotonic vs isotonic saline.
Results
Washing in mildly hypotonic saline (0.585 g/dL, osmolality: 221.7±2.3 mmol/kg) reduced the fraction of spherical cells 3-fold from 9.5±3.4% to 3.2±2.8%, while cutting PS exposure in half from 1.48±0.86% to 0.59±0.29%. Isotonic washing had no effect on PS exposure or the fraction of spherical cells. Both isotonic and hypotonic washing increased the fraction of well-preserved cells (discocytes, echinocytes 1) substantially, and improved the ability of stored RBCs to perfuse an artificial microvascular network by approximately 25%, as compared with the initial sample.
Discussion
This study demonstrated that washing in hypotonic saline could selectively remove a significant fraction of the spherical and PS-exposing cells from stored blood, while significantly improving the rheological properties of remaining well-preserved RBCs. Further studies are needed to access the potential effect from hypotonic washing on transfusion outcomes.
Keywords: red blood cells, hypothermic storage, washing, hypotonic saline
Introduction
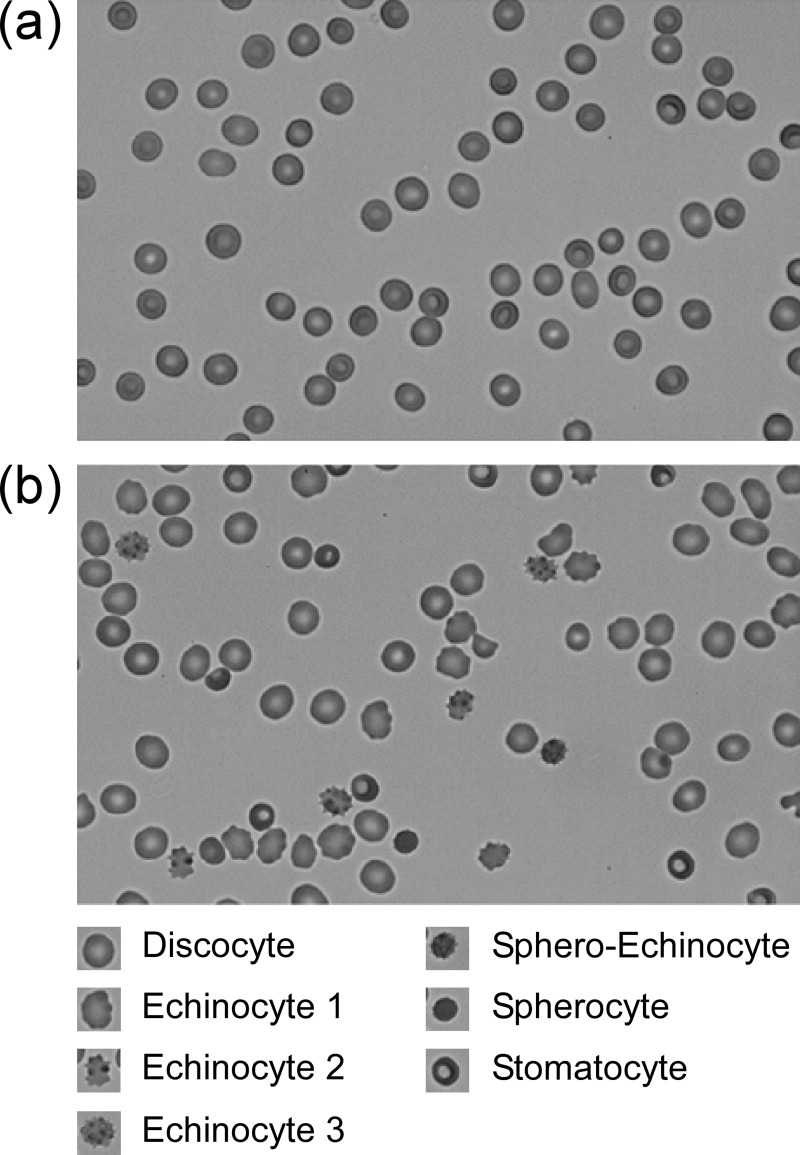
Red blood cells (RBCs) deteriorate progressively during hypothermic storage by accumulating oxidative damage and gradually losing their membrane surface area, volume, and physiologically-relevant deformability1–3. During storage, membranes of stored RBCs will typically develop protrusions that become increasingly spicular and ultimately leave the cell body as microvesicles. This process results in a disproportionately greater loss of membrane surface area than cell volume, leading to a substantial increase in the sphericity of the cell. Affected cells transform from biconcave discocytes through intermediate stages of echinocytosis (echinocyte 1, 2, and 3) to sphero-echinocytes, and finally spherocytes4–11. RBCs proceed through this transformation at different rates producing a morphologically heterogeneous population during storage (Figure 1). While the initial stages of echinocytosis can be partially reversed9,12, the transformation into a sphero-echinocyte or spherocyte is likely irreversible because of the extensive membrane loss through microvesiculation13,14.
Figure 1.
Typical morphological appearance of (a) fresh red blood cells (RBCs) containing primarily discocytes (D), and (b) RBCs that have been stored hypothermically for 42 days showing cells in various stages of morphological deterioration, including echinocyte 1 (E1), echinocyte 2 (E2), echinocyte 3 (E3), sphero-echinocyte (SE), spherocyte (S) and stomatocyte (ST).
Scale bar: 10 μm.
Spherocytes are rigid and therefore capable of obstructing capillaries15,16. They are also prone to lysis and are quickly removed from the circulation by the phagocytic cells of the recipient’s spleen17,18. Infusing spherocytes serves no therapeutic purpose and may produce a host of negative complications, including organ failure and impairment of the body’s defences against infection, particularly in susceptible patients19–22. Sequestering a large number of these cells may overwhelm the reticuloendothelial system in chronically-transfused patients, contributing to iron overload23,24.
In this proof-of-concept study, we investigated a simple technique for removing spherocytes from stored blood via washing in hypotonic saline. Biochemical, biophysical and morphological assays showed that this technique substantially improved the overall quality of stored RBCs. This simple and cost-effective approach could potentially be integrated with conventional centrifugation-based washing techniques to improve the quality of transfusion therapy for patients, particularly those frequently receiving older and/or multiple units of stored blood.
Materials and methods
Blood samples
Red blood cell units (CPD>AS-1, leucoreduced) were purchased from the Gulf Coast Regional Blood Center (Houston, TX, USA), stored in a blood bank refrigerator (Helmer iB111, Helmer Scientific, Noblesville, IN, USA) at 2–6 °C, and sampled upon expiration. Prior to sampling, RBC units were placed on a rocking platform (model 100; VWR, West Chester, PA, USA) for 15 minutes. RBC samples were withdrawn using a sterile syringe (BD, Franklin Lakes, NJ, USA) through a sampling site coupler (Fenwal Inc., Lake Zurich, IL, USA).
Design and fabrication of the microfluidic device for studying response of individual RBCs to hypotonic treatment
The microfluidic device was fabricated using conventional soft lithography from polydimethylsiloxane (PDMS)25. The device consisted of two layers: 1) a first layer comprising a network of parallel distribution channels (105 μm in width) connecting the inlet and the outlet of the device; and 2) a second layer comprising an array of circular wells (20 μm in diameter). All microchannels were approximately 5 μm deep. The device was assembled by reversibly sealing the two layers with their patterned sides facing each other. To load cells into the device, a pulse of a dilute suspension of stored RBCs (1% haematocrit in isotonic saline) was injected via the inlet, causing the reversible seal between the two device layers to be broken momentarily, before reforming and trapping RBCs within the microfluidic wells. The flow of hypotonic or isotonic saline through the feeding channels was used to adjust osmolality in the wells; it took approximately 120 seconds for any osmolality adjustment at the inlet to affect the RBCs within the wells.
RBC washing procedure
The washing procedure was performed by pipette mixing 1 mL of stored RBCs with hypotonic or isotonic saline at a 1:4 ratio (by volume) in a 50 mL centrifuge tube, incubating the mixture for a specified time, and then bringing the osmolality of the sample back to within the isotonic range (290±15 mmol/kg) by adding isotonic saline (0.9 g/dL) to the mixture at a 1:9 ratio (by volume). Washed samples were then centrifuged gently (100 g for 5 min) to remove the excess saline and adjust the haematocrit to approximately 60%, matching the haematocrit of the initial sample from the unit. All blood samples were processed at room temperature. RBCs from both the saline-washed and initial sample (which served as the negative control) were then assayed for free haemoglobin (Hb) concentration, surface phosphatidylserine (PS) exposure, RBC morphology, and the ability of RBCs to perfuse an artificial microvascular network (AMVN). To adjust the haematocrit of the initial sample, a 50 mL sample was centrifuged at 3,000 g for 5 min, and the resulting supernatant was removed and used in sample preparation for the morphological and rheological analyses described below. Hypotonic saline was prepared by diluting isotonic blood bank saline (Thermo Fisher Scientific, Waltham, WA, USA) with ultrapure water. Osmolality of all mixtures and saline solutions were measured using a vapour pressure osmometer (Vapro 5520; Wescor Inc., Logan, UT, USA).
Measurement of Hb concentrations
The concentration of free Hb in the supernatant was measured using modified Drabkin’s method26,27. Briefly, each sample was centrifuged at 1,500 g for 15 min. Forty μL of supernatant from each sample was added to 160 μL of Drabkin’s reagent (RICCA Chemical Company, Arlington, TX, USA), incubated for 20 min, and the absorbance was measured at 550 nm using a plate reader (SpectraMax M5, Molecular Devices, Inc., Sunnyvale, CA, USA). Human Hb standard (Pointe Scientific Inc., Canton, MI, USA) was diluted in ultrapure water to yield a standard calibration curve for concentrations ranging from 0 to 500 mg/dL. The measurement of free Hb was performed in triplicate for each sample.
Measurement of PS exposure
Each sample was diluted in annexin binding buffer (10 mM HEPES, 0.14 M NaCl, 2.5 mM CaCl2, pH 7.4) to yield a concentration of approximately 1 million RBC/100 μL. The cells were fluorescently labelled with 2 μL annexin-V and AlexaFluor® 488 conjugate (Thermo Fisher Scientific Inc., Waltham, MA, USA), and counted using a BD FACS Aria™ III Cell Sorter (BD Biosciences, San Jose, CA, USA). A protocol using a non-calcium-containing buffer (i.e. RBC samples diluted with PBS and then labelled as above) was used as the negative threshold. Scatter plots (FSC-A vs SSC-A, FSC-H vs FSC-W, SSC-H vs SSC-W) were used to differentiate RBC singlets. In the FITC channel, any individual RBC event that was as bright as, or brighter than, the brightest 0.1% RBC singlets in the negative threshold are considered positive for PS exposure. For each sample and control, 20,000 total individual RBC events were collected. The FC data was analysed using FACS Diva v.6.1.2 software (BD Biosciences). The measurement of PS exposure was made in duplicate for each sample.
Morphological analysis
Samples were diluted to a haematocrit of 1% using either isotonic saline (washed samples) or storage medium supernatant (initial samples). A 4 μL drop of each sample was placed on a microscope glass slide coated with PDMS and covered with thin pieces of PDMS to create a film. For each sample, images of at least 2,000 cells were acquired with an inverted microscope (IX73, Olympus America Inc., Center Valley, PA, USA) equipped with a CMOS digital camera (MC1362, Mikrotron GmbH, Germany). A band-pass blue filter (394±50 nm, B-390; Hoya Corp USA, Fremont, CA, USA) was used to improve the image contrast. The morphology of RBCs was classified based on Bessis’ standard8,28.
Measurement of the AMVN perfusion rate
The rheological properties of all RBC samples were assayed by measuring their ability to perfuse an artificial microvascular network (AMVN) following the technique previously described in detail9,29,30. Briefly, washed samples were diluted with isotonic saline to a haematocrit of 40±0.5%; initial samples were diluted to 40±0.5% haematocrit using the storage medium supernatant. A 30 μL sample was driven through the AMVN device using a pressure differential created using a 20 cm water column. The AMVN perfusion rate was measured by acquiring and analysing images of the post-capillary venule section of the AMVN device using a custom image analysis algorithm implemented in MATLAB® (The Math Works Inc., Natick, MA, USA). The measurement of the AMVN perfusion was performed in triplicate for each sample.
Statistical analysis
The results are presented as mean values ± standard deviation, or mean values±standard deviation (minimum-maximum). Statistical significance was determined using a paired two-tailed Student t-test; p<0.05 was considered significant in data between samples from differet treatment methods.
Results
Selective lysis of sphero-echinocytes and spherocytes in hypotonic saline
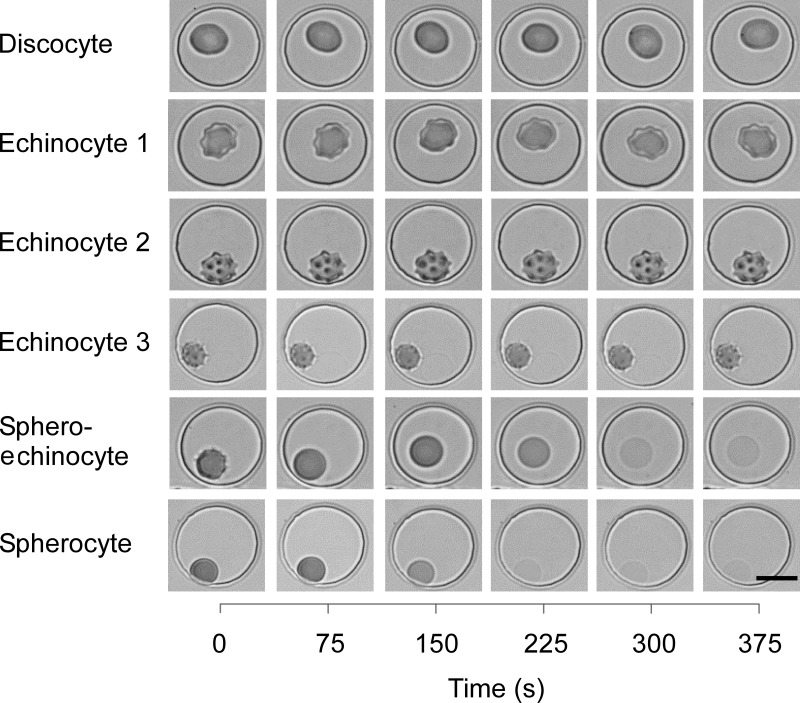
To verify the hypothesis that the elevated susceptibility of sphero-echinocytes and spherocytes to osmotic lysis could be used for eliminating them from stored blood, we trapped individual RBCs in wells of the microfluidic device and observed their response to changes in the osmolality of the suspending medium (from 290 mmol/kg down to 225 mmol/kg, and back to 290 mmol/kg). There was no significant change in the morphology of discocytes and echinocytes during the hypotonic treatment (Figure 2). In contrast, sphero-echinocytes and spherocytes swelled and burst in the hypotonic environment. These preliminary experiments at the single-cell level suggested that an optimal hypotonic treatment that would selectively lyse sphero-echinocytes and spherocytes while preserving all other cells was possible.
Figure 2.
Effect of hypotonic treatment on fresh red blood cells (RBCs) of different morphologies.
Individual RBCs were trapped in circular microwells of 20 μm diameter and 5 μm depth. The osmolality of the suspending medium was adjusted to 225 mmol/kg at t=20 sec, and back to 290 mmol/kg at t=250 sec. There was an approximately 120 sec delay for the osmolality within the microwells to equilibrate with that of the medium flowing over them through the main channel of the device. Scale bar: 10 μm.
To determine the optimal concentration of saline for the hypotonic treatment, we mixed 20 μL RBC samples with 0.225, 0.315, 0.405, 0.45, 0.495, 0.54, 0.585, 0.63, 0.675, 0.765, 0.855, or 0.9 g/dL saline at 1:4 ratio (by volume), and incubated for 0.17 (10 sec), 0.5 (30 sec), 1, 2, 5, 10, 20 or 30 min. Table I shows the resulting osmolality of RBC samples mixed with hypotonic saline at these concentrations. After incubation, the osmolality of the mixture was brought back to within the isotonic range (290±15 mmol/kg) by adding isotonic saline (0.9 g/dL) at a 1:9 ratio (by volume). We used overall percentage of haemolysis as the metric for optimisation of the hypotonic treatment. The initial sample of stored RBCs we used for these experiments contained 12.6±3.1% of sphero-echinocytes and spherocytes combined. Selective lysis of all the spherical cells in the sample would, therefore, increase the overall percentage of haemolysis in the sample by 12.6±3.1%. Our preliminary microfluidic experiments showed that exposure to mildly hypotonic saline (e.g. 225 mmol/kg) (Figure 2) which was roughly equivalent to washing in 0.585 g/dL saline (Table I) induced lysis of sphero-echinocytes and spherocytes, but kept other cells intact. We reasoned, therefore, that the concentration of hypotonic saline for the optimal treatment should be low enough to induce lysis of sphero-echinocytes and spherocytes (e.g. 0.585 g/dL), yet high enough to keep the overall percentage of haemolysis less than the fraction of sphero-echinocytes and spherocytes in the sample (i.e. 12.6±3.1%).
Table I.
Osmolalities of stored red blood cell samples mixed with saline of various concentrations in a 1:4 ratio.
| Saline concentration (g/dL) | Sample osmolality (mmol/kg) |
|---|---|
| 0.225 | 129.8±4.3 |
| 0.315 | 150.8±1.0 |
| 0.405 | 177.8±2.5 |
| 0.45 | 187.7±1.0 |
| 0.495 | 198.7±2.3 |
| 0.54 | 209.5±1.8 |
| 0.585 | 221.7±2.3 |
| 0.63 | 234.0±1.5 |
| 0.675 | 246.5±3.3 |
| 0.765 | 270.5±2.6 |
| 0.855 | 291.2±1.8 |
| 0.9 | 303.7±2.3 |
Values shown are mean±standard deviation (n=3).
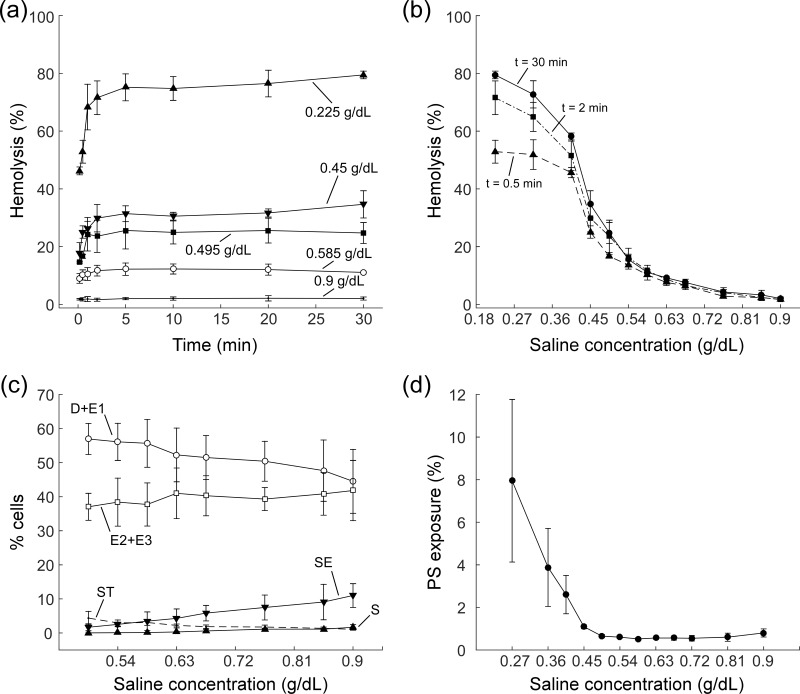
Figure 3 shows the dependence of percentage of haemolysis, RBC morphology, and PS exposure on the concentration of saline and treatment duration. Percentage of haemolysis followed a similar pattern for all concentrations of hypotonic saline, rising rapidly and reaching a plateau after the initial 5 min of treatment. The percentage of haemolysis remained stable for up to 30 min for saline concentrations over 0.585 g/dL, and rose slightly over time for more hypotonic saline (Figure 3a). As saline tonicity decreased, percentage of haemolysis increased from 2.0±0.5% for 0.9 g/dL saline, to 10.7±0.2% for 0.585 g/dL saline, and finally to 76.6±1.3% for the least concentrated saline (0.225 g/dL) used in this study (t=30 min) (Figure 3b). The percentage of haemolysis for saline solutions that were more hypotonic than 0.585 g/dL was significantly higher than the percent fraction of sphero-echinocytes and spherocytes in the initial sample, suggesting an indiscriminate lysis of all RBCs. As the concentration of saline decreased from 0.9 g/dL to 0.585 g/dL, the fraction of sphero-echinocytes decreased from 11.0±3.5% to 3.5±2.7% and the fraction of spherocytes decreased from 1.7±0.8% to 0.1±0.1%, while the fraction of well-preserved cells (discocyte and echinocyte 1) increased from 44.5±9.4% to 55.7±7.0% and the fraction of stomatocytes increased from 1.1±0.1% to 3.1±0.8% (Figure 3c). Finally, reducing saline concentration decreased PS exposure from 0.80±0.19% for isotonic saline down to a minimum of 0.52±0.04% for 0.585 g/dL hypotonic saline. A further reduction of saline concentration, however, produced a sharp increase in PS exposure, also suggesting indiscriminate lysis for concentrations of saline below 0.585 g/dL (Figure 3d). We, therefore, chose 0.585 g/dL as the optimum concentration of saline for the hypotonic treatment because it appeared to greatly reduce the population of sphero-echinocytes and spherocytes in the sample, while minimising indiscriminate lysis of well-preserved RBCs.
Figure 3.
Optimisation of the hypotonic treatment.
(a) Dependence of percentage of haemolysis on duration of treatment with 0.225, 0.45, 0.495, 0.585, and 0.9 g/dL saline. (b) Dependence percentage of haemolysis on concentration of saline (range 0.225–0.9 g/dL) for different treatment durations (t=0.5, 2, and 30 min). (c) Dependence of red blood cell RBC morphology on the concentration of saline for t=30 min treatment duration. (d) Dependence of surface phosphatidylserine (PS) exposure on concentration of saline for t=30 min treatment duration. All values shown as mean ± standard deviation (n=3) for all studies.
The effect of hypotonic washing on properties of stored RBCs
Table II illustrates the impact of washing with 0.585 g/dL hypotonic saline on the morphology of stored RBCs from several RBC units (n=5), and compares the hypotonic treatment with washing in isotonic saline. Hypotonic washing significantly increased the fraction of discocytes and echinocytes 1 from the initial 38.1±19.5% (range 24.4–72.4%) to 61.0±10.7% (range 53.3–79.9%) after the treatment, and significantly reduced the fraction of echinocytes 2 and 3 from the initial 52.0±18.7% (18.9–64.0%) to 32.2±11.4% (12.9–42.0%). The effect of isotonic washing on these types of RBCs was about the same as hypotonic washing. The effect of hypotonic washing on the sphero-echinocytes and spherocytes, however, was drastically different from that of washing in isotonic saline. The number of these cells was reduced by hypotonic treatment nearly 3-fold from the initial 9.5±3.4% (7.0–15.2%) down to 3.2±2.8% (1.4–8.0%), but remained effectively unchanged at 9.2±3.6% (6.6–15.3%) by washing in isotonic saline. Both types of washing increased the fraction of observed stomatocytes significantly: from the initial 0.4±0.2% (0.2–0.6%) to 3.6±0.9% (2.1–4.2%) for hypotonic washing, and to 2.4±1.4% (1.0–4.1%) for isotonic washing. Table II also compares the effect of hypotonic and isotonic washing on PS exposure and the ability of stored RBCs to perfuse an AMVN. Hypotonic washing reduced PS exposure more than 2-fold, from 1.48±0.86% (0.49–2.51%) in the initial sample down to 0.59±0.29% (0.30–1.05%), while isotonic washing kept PS exposure relatively unchanged at 1.40±0.84% (0.49–2.64%). Hypotonic washing increased the AMVN perfusion rate from 0.16±0.01 (0.14–0.18) nL/s in the initial sample to 0.20±0.02 (0.18–0.22) nL/s, which was only marginally (although statistically significantly) higher than the 0.19±0.01 (0.17–0.21) nL/s measured for isotonic washing. Finally, Table II compares the number of RBCs recovered after each type of washing as a percentage of all RBCs that were present in the initial sample. The RBC recovery for hypotonic washing was 82.0±9.7% (65.6–90.2%), which was significantly lower than the 85.2±8.5% (70.4–91.3%) we measured for the isotonic washing.
Table II.
The effect of hypotonic treatment (washing in 0.585 g/dL saline) on red blood cell (RBC) morphology, phosphatidylserine (PS) exposure, the ability of RBCs to perfuse an artificial microvascular network (AMVN), and percentage of RBC recovery for RBCs from 6-week old RBC units (n=5).
| Parameter | Initial sample | Washed sample | ||
|---|---|---|---|---|
|
| ||||
| Isotonic saline | Hypotonic saline | |||
| Morphological class | D + E1 | 38.1±19.5% | 56.8±11.9%* | 61.0±10.7%* |
| E2 + E3 | 52.0±18.7% | 31.6±13.4%* | 32.2±11.4%* | |
| SE + S | 9.5±3.4% | 9.2±3.6% | 3.2±2.8%*† | |
| ST | 0.4±0.2% | 2.4±1.4%* | 3.6±0.9%* | |
|
| ||||
| PS exposure (%) | 1.48±0.86 | 1.40±0.84 | 0.59±0.29*† | |
|
| ||||
| AMVN perfusion rate (nL/s) | 0.16±0.01 | 0.19±0.01* | 0.20±0.02*† | |
|
| ||||
| RBC recovery (%) | 100% | 85.2±8.5%* | 82.0±9.7%*† | |
Values shown are mean±standard deviation;
p<0.05 compared with initial sample;
p<0.05 compared with washing in isotonic saline.
Discussion
Red blood cells respond to decreasing osmolality by swelling, i.e. by utilising their excess surface area to change shape, become more spherical and contain the increasing cell volume31. Spherical cells (sphero-echinocytes and spherocytes) have very little excess surface area and therefore cannot contain the osmotically-driven increase in cell volume by simply changing shape. They also cannot expand their surface area significantly because the RBC membrane can only accommodate 2–4% area expansion before losing integrity32. Our experiments on stored RBCs that were individually confined in microfluidic wells have shown that sphero-echinocytes and spherocytes typically burst even in mildly hypotonic environments, whereas non-spherical types of RBCs remain largely intact under the same conditions (Figure 2). At the larger scale (millilitres of blood), hypotonic washing reduced the fraction of sphero-echinocytes and spherocytes nearly 3-fold (down to 3.2±2.8% from 9.5±3.4% in the initial sample), while isotonic washing kept the fraction of spherical cells at about the same level (9.2±3.6%). Both hypotonic and isotonic washing had approximately the same effect on other types of RBCs (Table II).
Interestingly, in this study, the fraction of stomatocytes increased substantially after both types of washing (more so for hypotonic washing, although the difference was not statistically significant) (Table II). RBCs progress through the echinocytic transformation by continually shedding vesicles and losing more membrane surface area than volume to finally become spherical. Others had previously noticed that echinocytes washed in albumin-Tris buffer could reverse their shape to discocytes or stomatocytes5,33. It is, therefore, likely that the increase in stomatocytes observed for both types of washing was due to late-stage echinocytes, which lost so much surface area that upon shape recovery they appear stomatocytic rather than discocytic. The larger increase in the fraction of stomatocytes after hypotonic washing could also be explained by the well-known stomatocytogenic properties of hypotonic saline34,35.
Hypotonic washing reduced PS exposure nearly 2-fold (down to 0.5±0.29% from 1.48±0.86% in the original sample) (Table II), bringing it to the level usually seen in RBCs stored for only 1–3 weeks36,37. PS (phosphatidylserine) is a membrane phospholipid that is normally found in the inner leaflet of the membrane. Extracellular exposure of PS is an established marker of RBC senescence in vivo, and it has been shown to increase during hypothermic storage15,24,38–40. Because isotonic washing had no significant effect on either the level of PS exposure (1.48±0.86% initially, 1.40±0.84% after isotonic washing) or the fraction of sphero-echinocytes and spherocytes (9.5±3.4% initially, 9.2±3.6% after isotonic washing), it is tempting to speculate that the dramatic reduction of PS exposure after hypotonic washing was due to the selective lysis of sphero-echinocytes and spherocytes. A direct study capable of measuring the level of PS exposure for sphero-echinocytes and spherocytes separately from other types of stored RBCs is needed to test the causality of this correlation.
Washing in either isotonic or hypotonic saline improved the ability of stored RBCs to perfuse an artificial microvascular network (AMVN) by approximately 25%, which agrees well with our earlier work9,29. Recently, we demonstrated the effect of RBC shape on microvascular perfusion in the AMVN41, and therefore such a substantial improvement observed in this study could be explained by overall normalisation of RBC shape produced by both types of washing (Table II). In our recent study on the differences in mechanical properties of RBCs stored conventionally vs anaerobically, we showed that the presence of a small fraction of poorly deformable cells (e.g. sphero-echinocytes and spherocytes) capable of plugging capillaries could affect network perfusion42. Selective removal of sphero-echinocytes and spherocytes could, therefore, explain the somewhat higher AMVN perfusion rates measured for hypotonic washing (Table II).
Hypotonic saline with a concentration as low as 0.45 g/dL (osmolality 154 mmol/kg) is routinely used as a maintenance intravenous fluid43, although recent studies strongly suggest that the use of isotonic saline for maintenance hydration carries a significantly lower risk of adverse events44. The osmolality of hypotonic saline used in our washing procedure (221.7±2.3 mmol/kg) (Table I) is, therefore, well within the range of osmolality of intravenous fluids currently used in routine medical practice. We observed that for the hypotonic saline concentrations ranging 0.495–0.675 g/dL, the difference in PS exposure and the fraction of sphero-echinocytes and spherocytes was relatively small, and therefore a wide range of hypotonic saline concentrations could be used to accomplish the selective removal of sphero-echinocytes and spherocytes from stored blood. Although the RBC recovery for hypotonic washing was lower than that for isotonic washing (because of the selective lysis of sphero-echinocytes and spherocytes), it was still higher than 80% (Table II), which is comparable to the cell recoveries observed for conventional cell washers45,46. We chose 30 min as the maximum period of time for which RBCs were exposed to the hypotonic saline in this study, and used this time period to complete the experiment in which we evaluated the effectiveness of hypotonic washing with saline of optimum concentration (Table II). For a saline concentration of 0.585 g/dL, however, equilibrium of the sample occurred significantly faster and incubations longer than 2–3 min did not produce a significant change in haemolysis (Figure 3a and b). This relatively rapid timescale of the hypotonic washing procedure matches the speed of conventional automated cell washers which often require a minimum of 5–7 min to process an RBC unit47,48. All of these considerations taken together suggest that hypotonic washing of stored RBC units could potentially be implemented within existing practices.
Conclusions
In summary, we have demonstrated that washing stored RBCs in mildly hypotonic saline (but not in isotonic saline) could substantially reduce the fraction of spherical and PS-exposing cells in the overall population of stored RBCs. Both hypotonic and isotonic washing, however, induced a similar degree of overall RBC shape normalisation and caused a significant improvement in the ability of stored RBCs to perfuse an artificial microvascular network with respect to the initial sample. Collectively, our data show that washing in hypotonic saline is capable of selectively removing spherical and PS-exposing cells while maintaining the established benefits of RBC washing.
Footnotes
Funding
This work was supported, in part, by a 2012 NIH Director’s Transformative Research Award from the National Heart, Lung, and Blood Institute of the National Institutes of Health (R01HL117329, PI: SSS).
Authorship contributions
SSS, SCG, GK and HX designed the study. HX, GK, BCS, EV and NZP performed the experiments. SSS, HX and NZP designed and fabricated the microfluidic device master wafers. HX, GK, BCS and SSS analysed and interpreted the data, and wrote the manuscript. All Authors have critically reviewed, edited and approved the manuscript.
The Authors declare no conflicts of interest.
References
- 1.Kim-Shapiro DB, Lee J, Gladwin MT. Storage lesion: role of red blood cell breakdown. Transfusion. 2011;51:844–51. doi: 10.1111/j.1537-2995.2011.03100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess JR. Red cell changes during storage. Transfus Apher Sci. 2010;43:51–9. doi: 10.1016/j.transci.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 3.Hess JR. Measures of stored red blood cell quality. Vox Sang. 2014;107:1–9. doi: 10.1111/vox.12130. [DOI] [PubMed] [Google Scholar]
- 4.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion. 2015;55:2659–71. doi: 10.1111/trf.13211. [DOI] [PubMed] [Google Scholar]
- 5.Reinhart WH, Chien S. Red cell rheology in stomatocyte-echinocyte transformation: roles of cell geometry and cell shape. Blood. 1986;67:1110–8. [PubMed] [Google Scholar]
- 6.Berezina TL, Zaets SB, Morgan C, et al. Influence of storage on red blood cell rheological properties. J Surg Res. 2002;102:6–12. doi: 10.1006/jsre.2001.6306. [DOI] [PubMed] [Google Scholar]
- 7.Bain BJ. Blood Cells: A Practical Guide. 5th ed. Hoboken, NJ: John Wiley & Sons; 2015. [Google Scholar]
- 8.Piety NZ, Gifford SC, Yang X, Shevkoplyas SS. Quantifying morphological heterogeneity: a study of more than 1 000 000 individual stored red blood cells. Vox Sang. 2015;109:221–30. doi: 10.1111/vox.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinhart WH, Piety NZ, Deuel JW, et al. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion. 2015;55:1872–81. doi: 10.1111/trf.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blasi B, D’Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–6. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 11.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-storedoleucocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reinhart S, Schulzki T, Bonetti P, Reinhart W. Studies on metabolically depleted erythrocytes. Clin Hem Microcircul. 2013;56:161–73. doi: 10.3233/CH-131682. [DOI] [PubMed] [Google Scholar]
- 13.Hägerstrand H, Isomaa B. Vesiculation induced by amphiphiles in erythrocytes. BBA-Biomembranes. 1989;982:179–86. doi: 10.1016/0005-2736(89)90053-9. [DOI] [PubMed] [Google Scholar]
- 14.Rumsby MG, Trotter J, Allan D, Michell R. Recovery of membrane micro-vesicles from human erythrocytes stored for transfusion: a mechanism for the erythrocyte discocyte-to-spherocyte shape transformation. Biochem Soc Trans. 1977;5:126–8. doi: 10.1042/bst0050126. [DOI] [PubMed] [Google Scholar]
- 15.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfus Sci. 1997;18:447–58. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- 16.Tinmouth A, Chin-Yee I. The clinical consequences of the red cell storage lesion. Transfus Med Rev. 2001;15:91–107. doi: 10.1053/tmrv.2001.22613. [DOI] [PubMed] [Google Scholar]
- 17.Deplaine G, Safeukui I, Jeddi F, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood. 2011;117:e88–95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schloesser LL, Korst DR, Clatanoff DV, Schilling RF. Radioactivity over the spleen and liver following the transfusion of chromium51-labelled erythrocytes in hemolytic anemia. J Clin Invest. 1957;36:1470. doi: 10.1172/JCI103544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donadee C, Raat NJ, Kanias T, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hod EA, Brittenham GM, Billote GB, et al. Transfusion of human volunteers with older, stored red blood cells produces extravascular hemolysis and circulating non-transferrin-bound iron. Blood. 2011;118:6675–82. doi: 10.1182/blood-2011-08-371849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hod EA, Spitalnik SL. Harmful effects of transfusion of older stored red blood cells: iron and inflammation. Transfusion. 2011;51:881–5. doi: 10.1111/j.1537-2995.2011.03096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brissot P, Ropert M, Le Lan C, Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. BBA-Gen Subjects. 2012;1820:403–10. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Hess JR. Conventional blood banking and blood component storage regulation: opportunities for improvement. Blood Transfus. 2010;8:s9. doi: 10.2450/2010.003S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 25.McDonald JC, Duffy DC, Anderson JR, et al. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Drabkin DL, Austin JH. Spectrophotometric studies I. Spectrophotometric constants for common hemoglobin derivatives in human, dog, and rabbit blood. J Biol Chem. 1932;98:719–33. [Google Scholar]
- 27.Moore GL, Ledford ME, Merydith A. A micromodification of the Drabkin hemoglobin assay for measuring plasma hemoglobin in the range of 5 to 2000 mg/dl. Biochem Med. 1981;26:167–73. doi: 10.1016/0006-2944(81)90043-0. [DOI] [PubMed] [Google Scholar]
- 28.Bessis M. Red cell shapes. An illustrated classification and its rationale. In: Bessis M, Weed RI, Leblond PF, editors. Red Cell Shape. Berlin, Heidelberg: Springer; 1973. pp. 1–25. [Google Scholar]
- 29.Burns JM, Yang X, Forouzan O, et al. Artificial microvascular network: a new tool for measuring rheologic properties of stored red blood cells. Transfusion. 2012;52:1010–23. doi: 10.1111/j.1537-2995.2011.03418.x. [DOI] [PubMed] [Google Scholar]
- 30.Sosa JM, Nielsen ND, Vignes SM, et al. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin Hemorheol Microcirc. 2014;57:275–89. doi: 10.3233/CH-131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gifford SC, Frank MG, Derganc J, et al. Parallel microchannel-based measurements of individual erythrocyte areas and volumes. Biophys J. 2003;84:623–33. doi: 10.1016/S0006-3495(03)74882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans EA, Waugh R, Melnik L. Elastic area compressibility modulus of red cell membrane. Biophysical J. 1976;16:585. doi: 10.1016/S0006-3495(76)85713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrell JE, Huestis WH. Calcium does not mediate the shape change that follows ATP depletion in human erythrocytes. BBA-Biomembranes. 1982;687:321–8. doi: 10.1016/0005-2736(82)90562-4. [DOI] [PubMed] [Google Scholar]
- 34.Canham PB. The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J Theor Biol. 1970;26:61–81. doi: 10.1016/s0022-5193(70)80032-7. [DOI] [PubMed] [Google Scholar]
- 35.Stasiuk M, Kijanka G, Kozubek A. [Transformations of erythrocytes shape and its regulation]. Postepy Biochem. 2009;55:425–33. [In Polish.] [PubMed] [Google Scholar]
- 36.Bennett-Guerrero E, Veldman TH, Doctor A, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cardo LJ, Hmel P, Wilder D. Stored packed red blood cells contain a procoagulant phospholipid reducible byoleucodepletion filters and washing. Transfus Apher Sci. 2008;38:141–7. doi: 10.1016/j.transci.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Sophocleous RA, Mullany PR, Winter KM, et al. Propensity of red blood cells to undergo P2X7 receptor-mediated phosphatidylserine exposure does not alter during in vivo or ex vivo aging. Transfusion. 2015;55:1946–54. doi: 10.1111/trf.13101. [DOI] [PubMed] [Google Scholar]
- 39.Boas FE, Forman L, Beutler E. Phosphatidylserine exposure and red cell viability in red cell aging and in hemolytic anemia. Proc Natl Acad Sci USA. 1998;95:3077–81. doi: 10.1073/pnas.95.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolfs JL, Comfurius P, Bevers EM, Zwaal RF. Influence of erythrocyte shape on the rate of Ca2+-induced scrambling of phosphatidylserine. Mol Membr Biol. 2003;20:83–91. [PubMed] [Google Scholar]
- 41.Piety NZ, Reinhart WH, Pourreau PH, et al. Shape matters: the effect of red blood cell shape on perfusion of an artificial microvascular network. Transfusion. 2016;56:844–51. doi: 10.1111/trf.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burns JM, Yoshida T, Dumont LJ, et al. Deterioration of red blood cell mechanical properties is reduced in anaerobic storage. Blood Transfus. 2016;14:80–8. doi: 10.2450/2015.0241-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015;373:1350–60. doi: 10.1056/NEJMra1412877. [DOI] [PubMed] [Google Scholar]
- 44.McNab S, Duke T, South M, et al. 140 mmol/L of sodium versus 77 mmol/L of sodium in maintenance intravenous fluid therapy for children in hospital (PIMS): a randomised controlled double-blind trial. Lancet. 2015;385:1190–7. doi: 10.1016/S0140-6736(14)61459-8. [DOI] [PubMed] [Google Scholar]
- 45.De Vroege R, Wildevuur W, Muradin J, et al. Washing of stored red blood cells by an autotransfusion device before transfusion. Vox Sang. 2007;92:130–5. doi: 10.1111/j.1423-0410.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 46.Bennett-Guerrero E, Kirby BS, Zhu H, et al. Randomized study of washing 40- to 42-day-stored red blood cells. Transfusion. 2014;54:2544–52. doi: 10.1111/trf.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Vroege R, Wildevuur WR, Muradin JAG, et al. Washing of stored red blood cells by an autotransfusion device before transfusion. Vox Sang. 2007;92:130–5. doi: 10.1111/j.1423-0410.2006.00852.x. [DOI] [PubMed] [Google Scholar]
- 48.Gruber M, Breu A, Frauendorf M, et al. Washing of banked blood by three different blood salvage devices. Transfusion. 2013;53:1001–9. doi: 10.1111/j.1537-2995.2012.03853.x. [DOI] [PubMed] [Google Scholar]