Abstract
Background
The incidence of alloimmunisation in myelodysplastic syndromes (MDS) during the era of supportive treatment ranges from 9 to 56%. However, it is unknown if the widespread use of hypomethylating agents has changed the risk of immunisation. The aim of this study is to evaluate the impact of azacitidine (AZA) therapy on red blood cell (RBC) alloimmunisation in transfused patients with MDS, myelodysplastic syndromes/myeloproliferative syndromes (MDS/MPS) and secondary acute myeloid leukaemia (AML).
Material and methods
We have analysed retrospectively all patients with MDS, MDS/MPS and secondary AML from MDS, who received their first transfusion in our hospital between January 1995 and December 2014. We have assessed the impact of age, sex, RBC and platelets units transfused, and AZA treatment on developing alloantibodies.
Results
In our study, the number of RBC units transfused increased the risk of developing alloantibodies. However aging and the treatment with AZA were associated with a lower rate of alloimmunisation.
Discussion
Patients with MDS, MDS/MPS and secondary AML who received treatment with AZA developed RBC antibodies at a lower rate than control group. We suggest that aging and immunosuppression due to AZA therapy could develop an immunological tolerance with a weak response to allotransfusions.
Keywords: alloimmunisation, azacitidine, myelodysplastic syndrome
Introduction
Hypomethylating agents such as 5-azacitidine (AZA) are the standard treatment for patients with high-risk myelodysplastic syndromes (MDS)1 and might be an alternative in older patients with acute myeloid leukaemia (AML) who are unfit for intensive chemotherapy2. Around 50% of patients with low-risk MDS respond to erythropoietic agents. However, red blood cell (RBC) transfusion remains an essential component of the management of patients with MDS. Being transfusion-dependent, these patients are at risk of transfusion-related complications such as alloimmunisation. The incidence of alloimmunisation in MDS patients in the era of supportive treatment, prior to AZA, ranged from 9 to 56%3–6.
Autoimmune diseases are observed in 10–20% of patients with MDS. Furthermore, several immunological abnormalities have been described in myelodysplasia including vasculitis, connective tissue disorders and neuropathy7, as well as immunohaematological aberrations4. Chronic inflammatory autoimmune disorders are a risk factor for RBC alloimmunisation8.
These hypersensitive immune states, together with a heavy transfusion burden, could increase the risk of RBC alloimmunisation in MDS patients not receiving chemotherapy or immunosuppressive treatment. Although the role of AZA as an immunomodulator is still poorly understood, several observations suggest that it could play a role in the management of autoimmune disorders in patients with MDS. Al-Ustwani et al. described a decrease in regulatory T cells after AZA treatment in a patient with MDS and an autoimmune disorder with clinical benefits in both conditions9. Similarly, improvement in autoimmune manifestations associated with MDS after AZA therapy has been observed by others10,11.
The aim of this study was to examine risks factors for RBC alloimmunisation and focus on whether the possible immunomodulatory effect of AZA influences the incidence of RBC alloimmunisation in patients with MDS, myelodysplastic/myeloproliferative syndromes (MDS/MPS) or secondary AML.
Material and methods
We retrospectively analysed all patients with MDS, MDS/MPS or secondary AML evolving from MDS who had received their first transfusion in our hospital between January 1995 and December 2014. Patients were included if antibody screening prior to the first transfusion was negative and the patient was screened for antibodies after at least the first transfusion. We excluded one patient with a non-immune alloantibody (anti-M) and patients with a positive direct antiglobulin test.
Before 1998, ABO/Rh typing and RBC antibody screening were performed using the gel technique with a DiaMed-ID microtyping system (DiaMed GmbH, Cressier, Switzerland). Since 1998, we have used a fully automated gel technique on the Ortho AutoVue Innova System (Ortho Clinical Diagnostics, Raritan, NJ, USA), with a three-cell panel (0.8% Surgiscreen RBC reagents 1, 2, and 3 [Ortho Clinical Diagnostics]) in a low-ionic strength saline-(LISS)-enhanced indirect antiglobulin test (IAT). If the RBC antibody screening showed a positive result in the LISS-IAT, the antibody was identified using a commercially available 11-cell panel in the same gel test system.
Throughout the period of the study, RBC units were matched for ABO and D but not, routinely, for other antigens. Since December 2000 all patients were transfused with pre-storage leucoreduced RBC.
The patients were divided into two groups according to the treatment administered: the AZA group and a control group. Patients in the AZA group were treated with AZA from May 2006 at the standard dose (75 mg/m2/day for 7 days every 4 weeks). Patients with high-risk MDS, MDS/MPS or AML received AZA as frontline therapy, whereas patients with low-risk MDS received AZA after failure of erythropoietic agents.
The control group consisted of patients who were not treated with AZA and received supportive care with RBC transfusions, erythropoietic agents and palliative cytoreduction (through the use of hydroxyurea, mercaptopurine or melphalan) when necessary, usually in cases of MDS/MPS or AML. Patients treated with intensive chemotherapy were excluded.
The data collected included age, sex, overall survival and time to alloimmunisation, diagnosis according to the World Health Organization (WHO) 2008 classification, RBC and platelet transfusions, as well as the detection and identification of alloantibodies.
Statistical analysis
Differences in categorical variables between groups were compared using the χ2-test or Fisher’s exact test whereas the Mann-Whitney U-test was used for quantitative variables.
The cumulative incidence of RBC alloimmunisation in relation to the number of RBC units transfused was estimated using competing risk analysis with death from any cause as a competing event. The difference in the cumulative incidence between groups was assessed using Gray’s test. Univariate and multivariate logistic regression analyses were used to identify clinical risk factors associated with alloimmunisation. Variables were conditionally selected in a forward stepwise procedure (inclusion criterion, p<0.05). We consider two-sided p-values <0.05 as statistically significant. All statistical analyses were performed with the free statistical software EZR version 1.30.
Results
A total of 209 patients were included. Their median age at diagnosis was 75 years (range, 35–92) and 57.4% were male. After a median follow-up of 20 months, 157 patients had died with a median overall survival of 28 months (95% confidence interval [95% CI]: 20.9–35.1).
Forty-three patients received AZA, which was administered in a median of six cycles (range, 1–14), and 166 patients formed the control group. The median follow-up from first transfusion was 13 months in the AZA group and 20 months in the control group. The characteristics of both groups are shown in Table I. The percentage of patients with refractory anaemia with excess blasts-2, AML or MDS/MPS was higher in the AZA group. This fact could explain the greater use of RBC transfusions in patients treated with AZA, although the difference observed in numbers of RBC units transfused between the AZA group and control group was not statistically significant. The median age of the patients in the control group was significantly higher than that in the AZA group, but no differences were observed in sex.
Table I.
Patients’ baseline characteristics.
| Patients | Control group | Aza group | p |
|---|---|---|---|
| Age, median (range), years | 75.5 (35–92) | 73 (44–89) | 0.033 |
|
| |||
| Sex | 0.49 | ||
| Male, n (%) | 93 (56%) | 27 (62.8%) | |
| Female, n (%) | 73 (44%) | 16 (37.2%) | |
|
| |||
| MDS WHO subtype, n (%) | <0.0001 | ||
| RCUD | 16 (9.7%) | 0 | |
| RARS | 31 (18.7%) | 1 (2.3%) | |
| RCMD | 47 (28.3%) | 8 (18.6%) | |
| RAEB-1 | 16 (9.7%) | 3 (7%) | |
| RAEB-2 | 17 (10.2%) | 8 (18.6%) | |
| 5q- | 4 (2.4%) | 0 | |
| AML | 18 (10.8%) | 14 (32.6%) | |
| MDS/MPS | 17 (10.2%) | 9 (20.9%) | |
|
| |||
| N. of transfused RBC units, median (range) | 23.50 (4–307) | 34 (2–212) | 0.463 |
|
| |||
| N. of transfused platelet units, mean (95% CI) | 3.72 (2.50–4.92) | 10.21 (5.03–15.39) | 0.004 |
RCUD: refractory cytopenia with unilineage dysplasia; RARS: refractory anaemia with ringed sideroblasts; RCMD: refractory cytopenia with multilineage dysplasia; RAEB: refractory anaemia with excess blasts; AML: acute myeloid leukaemia; MDS/MPS: myelodysplastic/myeloproliferative syndromes; RBC: red blood cell; CI: confidence interval.
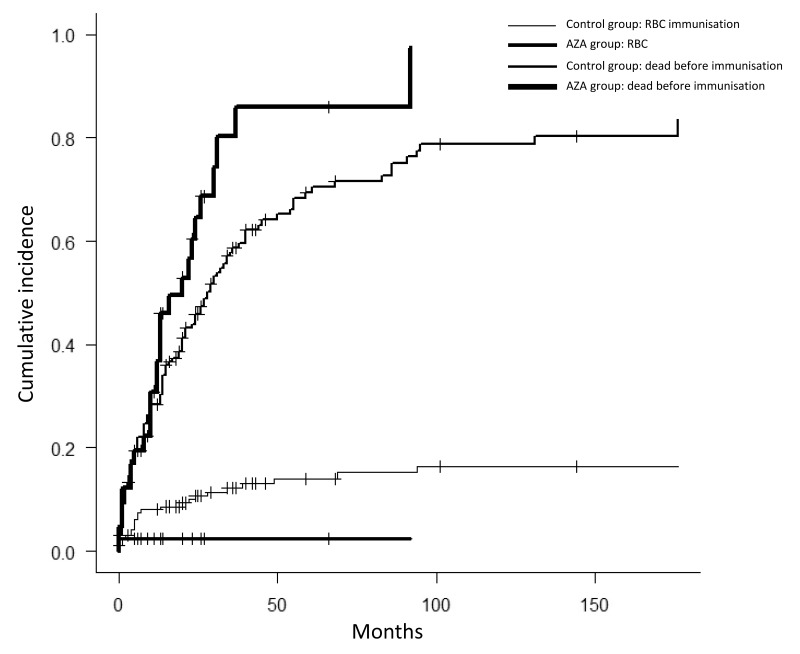
Alloimmunisation was detected in 23 cases (13.9%) in the control group and in one case (2.3%) in the AZA group p=0.033). The median time from first transfusion until detection of alloantibody was 5 months (95% CI: 3.1–6.8). The cumulative incidence of alloimmunisation after 25 RBC units was 8.3% (95% CI: 4.9–12.7): 2.7% (95% CI: 0.2–12.2) in the AZA group and 9.7% (95 CI: 5.7–15.1) in the control group. Figure 1 shows the cumulative incidence of alloimmunisation in the AZA and control groups, with death as a competing risk, according to number of RBC units transfused, as well as the plateau of 12.3% reached after 59 units of RBC had been transfused in the control group.
Figure 1.
Cumulative incidence of RBC alloimmunisation and the competing risk of death, according to the number of RBC units transfused in the AZA group and the control group.
RBC: red blood cell; AZA: 5-azacitidine.
The specificities of the RBC antibodies are shown in Table II. In the control group we found three antibodies whose specificity could not be determined and three patients had more than one alloantibody. Only one alloantibody was observed in the AZA group and it was specific for the K antigen.
Table II.
Red blood cell antibody specificities.
| Antibody | Number (%) |
|---|---|
| Anti-K | 8 (33.3%) |
| Anti-D | 4 (16.7%) |
| Anti-E | 5 (20.8%) |
| Anti-Lua | 1 (4.2%) |
| Anti-C - Anti-D | 1 (4.2%) |
| Anti-E - Anti-K | 2 (8.3%) |
| Unidentified | 3 (12.5%) |
|
| |
| Total | 24 (100%) |
The associations between patients’ characteristics and alloimmunisation are shown in Table III. The number of RBC units transfused was significantly higher in alloimmunised patients (median 53; range, 9–307) than in patients who did not develop alloantibodies (median 23; range, 2–250) (p<0.001). The median age of patients who developed alloantibodies was significantly lower than that of patients who did not. The incidence of alloimmunisation in patients older than 75 years (the median age of participants in our study) was 4.7% whereas it was 18.4% in younger patients (p=0.002). However, no significant differences in sex or number of platelet transfusions were observed between alloimmunised and not alloimmunised patients. Although there was a slightly lower rate of alloimmunisation in patients with AML, no statistically significant differences were observed between the three WHO diagnostic groups: AML (6.2%), MDS (12.6%) and MDS/MPS (11.5%). Alloimmunisation rates in the control group were similar: AML (11.1%), MDS (13.7%) and MDS/MPS (17.6%) (p=0.85).
Table III.
Patients’ characteristics and alloimmunisation.
| Patients | Not alloimmunised | Alloimmunised | p |
|---|---|---|---|
| Age, median (range), years | 75 (44–92) | 70 (35–82) | 0.001 |
|
| |||
| Sex, n (%) | 0.125 | ||
| Male | 110 (59.5%) | 10 (41.7%) | |
| Female | 75 (40.5%) | 14 (58.3%) | |
|
| |||
| WHO diagnostic group, n (%) | 0.594 | ||
| MDS | 132 (71.4%) | 19 (79.2%) | |
| AML | 30 (16.2%) | 2 (8.3%) | |
| MDS/MPS | 23 (12.4%) | 3 (12.5%) | |
|
| |||
| N. of RBC units transfused, median (range) | 23 (2–250) | 53 (9–307) | <0.0001 |
|
| |||
| N. of platelet units transfused, mean (95% CI) | 4.86 (3.35–6.38) | 6.46 (1.21–11.71) | 0.226 |
|
| |||
| AZA treatment, n (%) | 0.033 | ||
| Yes | 42 (22.7%) | 1 (4.2%) | |
| No | 143 (77.3%) | 23 (95.8%) | |
MDS: myelodysplastic syndrome; AML: acute myeloid leukaemia; MDS/MPS: myelodysplastic/myeloproliferative syndromes; RBC: red blood cell; CI: confidence interval; AZA: 5-azacitidine.
Gray’s test revealed significant differences in cumulative incidence of RBC alloimmunisation by age (cut-off 75 years; p=0.002), whereas treatment with AZA was at the limit of statistical significance (p=0.051). No significant differences were observed for sex (p=0.08), platelet transfusions (p=0.63) or the three WHO diagnostic groups (p=0.43).
The multivariate logistic regression showed that the number of RBC units (hazard ratio [HR] 1.013; 95% CI: 1.006–1.021; p<0.001), age (HR 0.922; 95% CI: 0.880–0.966; p=0.001) and AZA therapy (HR 0.118; 95% CI: 0.013–0.877; p=0.016) were prognostic factors significantly associated with the risk of developing alloantibodies. Sex, WHO diagnostic group, and number of platelet units transfused did not have a prognostic value.
Discussion
Several factors may increase the risk of alloimmunisation, including sex, diabetes mellitus, solid malignancy, peripheral blood progenitor cell transplantation12, inflammatory disorders8,13 and the number of RBC transfused3,14,15. Conversely, patients with a haematological malignancy who receive intensive chemotherapy16 or undergo haematopoietic stem cell transplantation, and patients treated with immunosuppressive therapy17 have a lower rate of immunisation to RBC antigens18. It is, however, unknown whether the widespread use of hypomethylating agents in patients with MDS changes the risk of alloimmunisation.
The rate and types of alloantibodies detected in our patients in the control group are consistent with previous reports3–6. We found that anti-K and anti-E were the most prevalent alloantibodies.
In our study, in accordance with the literature, the number of RBC units transfused increased the risk of developing alloantibodies, while aging and treatment with AZA were associated with lower rates of alloimmunisation.
In several studies the risk of developing RBC alloantibodies rose as the cumulative number of RBC transfused units increased. The incidence of alloimmunisation observed by Zalpuri et al. was 3.4% after 20 RBC units and 6.5% after 40 RBC units14. Among patients with haematological diseases, Fluit et al. found that the alloimmunisation rate rose from 7.2% in patients transfused with fewer than 50 RBC units to 32.4% in patients who received more than 50 RBC units19. Sanz et al. reported a cumulative incidence of alloimmunisation of 12.4% after 25 RBC units and a plateau of 19.4% after 130 RBC units among patients with MDS and chronic myelomonocytic leukaemia3. These results are similar to ours, although we found a plateau of 12.3% after the transfusion of 59 RBC units.
The influence of sex on RBC alloimmunisation remains controversial15. Some studies suggest that female sex could be a risk factor12,20, but this finding was not confirmed in others studies19. We did not find any influence of sex on the risk of alloimmunisation in our series.
The role of platelet transfusion on the risk of alloimmunisation has also not been clearly established yet. The frequency of RBC antibody formation in haematological and oncological diseases was lower in patients who received platelet support16. A low frequency of D immunisation (1.44%) was observed in a recent study of patients who received D-incompatible platelet transfusions21. In contrast to these findings, in a multicentre, randomised trial, extended matching of RBC for transfusion decreased rates of alloimmunisation, but concomitant platelet transfusion reduced the utility of this strategy23. The risk of alloimmunisation was not influenced by platelet transfusions in our study. Besides, the logistic regression analysis showed no prognostic impact of platelet transfusions on alloimmunisation. However, although all patients received ABO- and D-compatible RBC, four patients who were D-negative developed anti-D after transfusion of D-incompatible platelets.
The multivariate logistic regression ruled out a possible influence of WHO diagnostic subtype on the risk of alloimmunisation. Although a decreased response to incompatible transfusions in patients with acute leukaemia undergoing intensive chemotherapy has been described, our patients with AML and MDS/MPS treated only with palliative chemotherapy showed the same rates of alloimmunisation as MDS patients.
Aging has been proposed as a factor that can decrease the risk of alloimmunisation. The percentage of anti-D alloimmunisation in D-negative patients less than 77 years old who received D-positive RBC was higher than that in patients over 77 years old (30.5% versus 15.4%)22. However, the influence of age on the risk of immunisation has not been demonstrated in other studies12,19. In our series, the median age was significantly lower in patients who developed alloantibodies and age showed a significant prognostic influence on the risk of alloimmunisation in the multivariate analysis.
The impact of AZA therapy on RBC immunisation has not been analysed previously. In our series, although the group of patients treated with AZA had a similar transfusion burden as the control group and their median age was significantly lower, their incidence of alloantibody formation was significantly lower. Moreover, the multivariate analysis demonstrated that AZA therapy was an independent prognostic factor for alloimmunisation.
Extended phenotype matching of blood for transfusion has led to reductions of RBC immunisation in patients with haemoglobin disorders, especially sickle cell disease24. It has been suggested that this policy could also be effective in MDS25. However, according to our findings, standard pre-transfusion testing without extended phenotyping would be sufficient as an optimal approach for very old patients with MDS especially if they are receiving AZA therapy, given the low frequency of alloimmunisation in these circumstances.
Conclusions
In conclusion, aging reduces the risk of alloimmunisation, at least in patients with MDS, MDS/MPS or AML. Furthermore, patients receiving treatment with AZA develop RBC antibodies less frequently than do patients who receive only supportive care. We, therefore, suggest that the immunosuppression due to AZA therapy could create an immunological tolerance such that patients have a weak response to incompatible transfusions.
Azacitidine is the standard treatment for patients with high-risk MDS and might be an alternative in older patients with AML. The impact of AZA therapy on RBC immunisation had not been analysed previously. We evaluated the risk factors for alloimmunisation in patients diagnosed with MDS, MDS/MPS or AML, finding that the risk increased with the cumulative number of RBC units transfused. However, aging and, probably, treatment with AZA reduce the risk of developing alloantigens.
Footnotes
Presented in a preliminary form as a poster at the EHA 20th Congress, Vienna, Austria, June 11th to 14th, 2015.
Authorship contributions
All Authors made substantial contributions to the conception and design of the study, and acquisition, analysis and interpretation of data. All Authors participated in drafting the article or revising it critically for important intellectual content, and gave final approval of the version to be submitted and any revised versions.
The Authors declare no conflicts of interest.
References
- 1.Fenaux P, Mufti GJ, Hellström-Lindberg E, et al. International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10:223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562–9. doi: 10.1200/JCO.2009.23.8329. [DOI] [PubMed] [Google Scholar]
- 3.Sanz C, Nomdedeu M, Belkaid M, et al. Red blood cell alloimmunization in transfused patients with myelodysplastic syndrome or chronic myelomonocytic leukemia. Transfusion. 2013;53:710–5. doi: 10.1111/j.1537-2995.2012.03819.x. [DOI] [PubMed] [Google Scholar]
- 4.Novaretti MC, Sopelete CR, Velloso ER, et al. Immunohematological findings in myelodysplastic syndrome. Acta Haematol. 2001;105:1–6. doi: 10.1159/000046525. [DOI] [PubMed] [Google Scholar]
- 5.Stiegler G, Sperr W, Lorber C, et al. Red cell antibodies in frequently transfused patients with myelodysplastic syndrome. Ann Hematol. 2001;80:330–3. doi: 10.1007/s002770100308. [DOI] [PubMed] [Google Scholar]
- 6.Arriaga F, Bonanad S, Larrea L, et al. [Immunohematologic study in 112 patients with myelodysplastic syndromes: 10-year analysis]. Sangre (Barc) 1995;40:177–80. [In Spanish.] [PubMed] [Google Scholar]
- 7.Al Ustwani O, Ford LA, Sait SJ, et al. Myelodysplastic syndromes and autoimmune diseases case series and review of literature. Leuk Res. 2013;37:894–9. doi: 10.1016/j.leukres.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ryder AB, Hendrickson JE, Tormey CA. Chronic inflammatory autoinmmune disorders are a risk factor for red blood cell alloimmunization. Br J Haematol. 2016;174:483–5. doi: 10.1111/bjh.13781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Ustwani O, Francis J, Wallace PK, et al. Treating myelodysplastic syndrome improves an accompanying autoimmune disease along with a reduction in regulatory T-cells. Leuk Res. 2011;35:e35–6. doi: 10.1016/j.leukres.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilorge S, Doleris LM, Dreyfus F, Park S. The autoimmune manifestations associated with myelodysplastic syndrome respond to 5-azacytidine: a report on three cases. Br J Haematol. 2011;153:664–5. doi: 10.1111/j.1365-2141.2010.08557.x. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Shimizu N, Tougasaki E, et al. Successful treatment by azacitidine therapy of intestinal Behçet’s disease associated with myelodysplastic syndrome. Int J Hematol. 2013;97:520–4. doi: 10.1007/s12185-013-1316-x. [DOI] [PubMed] [Google Scholar]
- 12.Bauer MP, Wiersum-Osselton J, Schipperus M, et al. Clinical predictors of alloimmunization after red blood cell transfusion. Transfusion. 2007;47:2066–71. doi: 10.1111/j.1537-2995.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- 13.Fasano RM, Booth GS, Miles M, et al. Red blood cell alloimmunization is influenced by recipient inflammatory state at time of transfusion in patients with sickle cell disease. Br J Haematol. 2015;168:291–300. doi: 10.1111/bjh.13123. [DOI] [PubMed] [Google Scholar]
- 14.Zalpuri S, Zwaginga JJ, le Cessie S, et al. Red-blood-cell alloimmunization and number of red-blood-cell transfusions. Vox Sang. 2012;102:144–9. doi: 10.1111/j.1423-0410.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 15.Gehrie E, Tormey CA. The influence of clinical and biological factors on transfusion-associated non-ABO antigen alloimmunization: responders, hyper-responders, and non-responders. Transfus Med Hemother. 2014;41:420–9. doi: 10.1159/000369109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schonewille H, Haak HL, van Zijl AM. Alloimmunization after blood transfusion in patients with hematologic and oncologic diseases. Transfusion. 1999;39:763–71. doi: 10.1046/j.1537-2995.1999.39070763.x. [DOI] [PubMed] [Google Scholar]
- 17.Zalpuri S, Evers D, Zwaginga JJ, et al. Immunosuppressants and alloimmunization against red blood cell transfusions. Transfusion. 2014;54:1981–7. doi: 10.1111/trf.12639. [DOI] [PubMed] [Google Scholar]
- 18.Perseghin P, Balduzzi A, Galimberti S, et al. Red blood cell support and alloimmunization rate against erythrocyte antigens in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2003;32:231–6. doi: 10.1038/sj.bmt.1704114. [DOI] [PubMed] [Google Scholar]
- 19.Fluit CR, Kunst VA, Drenthe-Schonk AM. Incidence of red cell antibodies after multiple blood transfusion. Transfusion. 1990;30:532–5. doi: 10.1046/j.1537-2995.1990.30690333485.x. [DOI] [PubMed] [Google Scholar]
- 20.Verduin EP, Brand A, Middelburg RA, Schonewille H. Female sex of older patients is an independent risk factor for red blood cell alloimmunization after transfusion. Transfusion. 2015;55:1478–85. doi: 10.1111/trf.13111. [DOI] [PubMed] [Google Scholar]
- 21.Cid J, Lozano M, Ziman A, et al. Low frequency of anti-D alloimmunization following D+ platelet transfusion: the Anti-D alloimmunization after D-incompatible Platelet Transfusions (ADAPT) study. Br J Haematol. 2015;168:598–603. doi: 10.1111/bjh.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Porras JR, Graciani IF, Pérez-Simon JA, et al. Prospective evaluation of a transfusion policy of D+ red blood cells into D− patients. Transfusion. 2008;48:1318–24. doi: 10.1111/j.1537-2995.2008.01700.x. [DOI] [PubMed] [Google Scholar]
- 23.Schonewille H, Honohan Á, van der Watering LM, et al. Incidence of alloantibody formation after ABO-D or extended matched red blood cell transfusions: a randomized trial (MATCH study) Transfusion. 2016;56:311–20. doi: 10.1111/trf.13347. [DOI] [PubMed] [Google Scholar]
- 24.Lasalle-Williams M, Nuss R, Le T, et al. Extended red blood cell antigen matching for transfusions in sickle cell disease: a review of a 14-year experience from a single center (CME) Transfusion. 2011;51:1732–9. doi: 10.1111/j.1537-2995.2010.03045.x. [DOI] [PubMed] [Google Scholar]
- 25.Singhal D, Hague S, Roxby D, et al. RBC alloimmunization burden is high in regularly RBC-transfused myelodysplastic syndrome (MDS) patients: a report from South Australian-MDS registry. Proceedings of the 57th Annual Meeting of the American Society of Hematology; 2015. [Abstract 3562]. [Google Scholar]