Abstract
In COPD, pulmonary hyperinflation causes decreased stroke volume thereby decreased oxygen pulse (O2P). While O2P flattening is related to myocardial ischemia in cardiac patients, O2P patterns have seldom been explored in COPD. The aims of the study were to investigate O2P-curve patterns and associated factors in COPD. Seventy-five patients with stable COPD were enrolled. The demographics, cardiac size, physiological measurements and stress EKG were compared among O2P-curve pattern groups. An algorithm to identify O2P-curve patterns was developed in 28 patients. In the remaining 45 patients after excluding two with poor effort, this algorithm revealed 20 (44%) flattening, 16 (36%) increasing, and nine (20%) decreasing patterns. The flattening-type group had lower body mass, cardiac size, and diffusing capacity, and larger lung volumes (p = 0.05–<0.0001) compared to the increasing-type group. During exercise, the flattening-type group had a lower operable O2P and more hyperventilation and dyspnea (p = 0.02–<0.01). None had ST-T changes. Most differences were related to body mass and mildly to inspiratory fraction. The decreasing-type group performed higher effort than the increasing-type group (p < 0.05). In conclusion, O2P flattening was common and was associated with reduced body mass and pulmonary hyperinflation rather than with myocardial ischemia. The decreasing-type may be caused by motivation to exercise.
Introduction
In non-invasive cardiopulmonary exercise testing (CPET), oxygen pulse (O2P) is defined as oxygen uptake (O2) divided by heart rate. It indicates the capability of oxygen consumption in all body tissues per heart beat, and is a function of stroke volume and oxygen extraction by cells. O2P reflects stroke volume, and is approximately five times the slope of the normal oxygen consumption-heart rate relationship1, as oxygen extraction by muscle cells is expected to increase in a predicted manner when exercising2, 3. Hence, O2P can be continuously monitored non-invasively to reflect stroke volume during CPET.
Reduced O2P with a plateau, or decreasing pattern4, 5 when approaching peak exercise has been attributed to cardiac dysfunction or myocardial ischemia in patients with cardiac impairment. Although the relationship between a flattened O2P curve and severity of myocardial ischemia is not linear6, O2P remains an indicator of training effect7, and a prognosticator of heart failure8–11 and primary pulmonary hypertension12. Recently, three patterns of O2P curve have been reported, two of which include plateaus or decreases which are thought to be related to cardiac dysfunction4. Moreover, an O2P value ≤80% of the predicted value has been reported to be non-specific for low stroke volume, because anemia, carboxyhemoglobinemia, poor blood oxygenation in the lung, right to left shunt, and low peripheral oxygen extraction have been associated with low O2P3.
A reduced O2P has been reported in patients with chronic obstructive pulmonary disease (COPD), and this has been associated with swings in intrathoracic pressure resulting from deranged ventilatory mechanics13 or dynamic hyperinflation14. This reduction in O2P has been shown to be partly reversed in patients with emphysema after pharmacological15 or surgical lung volume reduction16–18. To the best of our knowledge, the O2P curve patterns in patients with COPD performing CPET has yet to be investigated, given COPD frequently coexists with ischemic heart disease19. We hypothesized that O2P curve patterns are not related to myocardial ischemia in patients with COPD. The aims of the study, therefore, were to investigate: (1) the types of O2P patterns and (2) their associated factors in patients with COPD. These findings may affect the currently-used algorithm for reporting CPET and provide insight into the mechanisms that determine O2P curve patterns.
Methods
Study Design
In this observational cross-sectional comparative study, we grouped patients based on O2P curve patterns obtained from CPET. Due to the variability in O2P curves, each curve was smoothed using computer software. Demographics, cardiac size measured with chest radiography and two-dimensional echocardiography, lung function, CPET, and arterial blood gas were compared among groups. The Institutional Review Board of Chung Shan Medical University Hospital (CS11144) and Chang Gung Memorial Hospital (CMRP 443) approved this study and all participants provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki.
Subjects
The COPD was diagnosed according to the GOLD criteria20. The enrollment criteria were patients with COPD who underwent lung function tests and the ratio of their forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) was <70%. They were invited to perform the symptom-limited incremental CPET with arterial blood gas and lactate measurements. All patients must be clinically stable, and their medications must be not significantly changed one month before undergoing the tests. The exclusion criteria were if the patients had significant co-morbidities such as left ventricular failure (ejection fraction <50%), atrial fibrillation, renal failure (creatinine >2 mg/dL), cancer, hemoglobin ≤10 g/dL, peripheral artery occlusive disease, and uncontrolled diabetes mellitus or hypertension. Peripheral artery occlusive disease was diagnosed by history, medical record, and symptomatology. Participating in any physical training program during the study period was not allowed.
Protocols and Measurements
Anthropometric and biochemical measurements
Body mass index, triceps skinfold thickness, and mid-upper arm circumference were measured. All measurements were made in triplicate by an experienced nutritionist, and the middle value was recorded for analysis. Complete blood cell analysis, carboxyhemoglobin, and selected biochemical tests were performed.
Oxygen–cost diagram (OCD) A 10-cm long vertical line marked with everyday activities was used by the patients to assess daily activities21. The distance from point zero was measured and scored.
Pulmonary function testing
Air flows and lung volumes were measured by spirometry and plethysmography (6200 Autobox DL, Yorba Linda, CA, USA or MasterScreen™ Body, Carefusion, Wuerzburg, Germany) at body temperature, ambient atmospheric pressure, and fully saturated, using the best of three readings22–24. The single-breath technique was used to measure the diffusing capacity for carbon monoxide (DLCO). A 12-second maneuver of rapid and deep breathing was used to calculate the maximum voluntary ventilation (MVV). All lung volume data were obtained before inhaling 400 μg of fenoterol HCl and spirometry data were obtained before and after inhaling fenoterol. Maximal inspiratory/expiratory pressures were measured at residual volume and total lung capacity, respectively, before and 7 minutes after exercising (RPM, Micro Medical, Rochester, UK) three times, with a one-minute recovery period between efforts, with the best results being used for analysis.
Maximum cardiopulmonary exercise testing
After acclimating to a computer-controlled brake cycle ergometer and revealing stable exercise gas exchange (Medical Graphics, St. Paul, MN, USA), each subject completed a 2 minutes of rest and 2 minutes of unloaded cycling followed by a ramp-pattern exercise test to the symptom limited. Work rate was selected at a rate of 5–20 watts/minute based on a derived protocol formula according to the OCD scores25. The O2 (ml/min), CO2 output (CO2) (ml/min), minute ventilation ( E), pulse rate and oxyhemoglobin saturation (SPO2), and 12-lead electrocardiography were continuously measured. Blood pressure was measured at the end of each minute and at the point where the patients expressed peak exercise. Dyspnea was scored using the modified Borg scale every minute when the patients were performing the exercise. Calibrations of pneumotachograph and O2 and CO2 analyzers, anaerobic threshold (AT) measurement, and O2peak predictions were performed as reported previously25. O2peak was symptom-limited, and defined as the highest recorded value averaged over the last 15 seconds of loaded exercise.
A pre-requisite for final analysis of the data was the subjects must achieve the maximum exercise effort26, 27, including heart rate ≥85% of predicted maximum, respiratory exchange ratio ≥1.09, pH ≤7.35, bicarbonate concentration ([HCO3 −]) ≤21 meq/L, changes (Δ) in [HCO3 −] or [lactate] between at rest and peak exercise ≥4 meq/L. Each criterion represented one point. Each maximum effort level point was scored from 1–6, with the total score representing the effort level of exercise.
| 1 |
where predicted maximum heart rate = 220 − age
| 2 |
where O2Ppeak = measured O2P at peak exercise and O2Pmax predicted = O2 predicted maximum/predicted maximum heart rate
The definition of ventilatory limitation was breathing reserve (BR) either <30% or <11–15 L/min, and was calculated as27:
| 3 |
where E peak/direct MVV expressing E demand/capacity ratio
| 4 |
| 5 |
| 6 |
Development of smoothing techniques for O2P curve
To avoid breath-by-breath noise, all O2P data from unloading to peak exercise were obtained after averaging every 15 seconds with smoothing (Supplement file) using the computer software (Microcal Origin v 4.1, Microcal Software Inc., Northampton, MA, USA). By demonstrating residuals to the model fits, the process of curves smoothing using computer software was attested not causing impact on results (Supplement file: Appendix Figure 3). After developing the smoothing technique, the investigators used the algorithm, which showed intra- and inter-rater agreements using κ statistics of 0.7 (95%CI 0.52–0.89) and 0.7 (95%CI 0.52–0.88), respectively.
Chest radiography
Chest radiographs with posteroanterior view were obtained within 1 month from enrollment and evaluated by two pulmonologists blinded to the clinical information. The hila-thoracic ratio, cardiothoracic ratio, and diameter of the anterior descending pulmonary artery on upright posteroanterior chest radiographs were measured28 using the DICOM viewing software (Infinitt PACS, v3.0.11.3, VN3, Infinitt, Korea). The inter-rater agreement using Pearson’s correlations were 0.66, 0.87, and 0.58 for the hila-thoracic ratio, cardiothoracic ratio, and diameter of the anterior descending pulmonary artery, respectively (p < 0.01–0.0001). Average values were recorded for analysis.
Two-dimensional echocardiography
Two-dimensional echocardiography (iE33, Philips, Seattle, USA) was performed with parasternal, apical and subcostal views29–31 within 4 weeks before or after CPET. If there were acute exacerbations of COPD in the time between the two tests, one of the tests was postponed. The echocardiography was conducted by an experienced technician or cardiologist who was blinded to the clinical data, lung function and CPET reports. The stored data were reviewed by two experienced cardiologists who were not blinded to the measurements.
Arterial blood sampling and lactate determination
Blood samples were drawn from the brachial artery via an arterial catheter connected to a pressure transducer within the last 15 seconds of each minute after the start of exercise to peak exercise. Plasma lactate was also analyzed (YSI, Yellow Springs, Ohio, USA). The VD/VT was calculated as follows:
| 7 |
where VD/VT indicates dead space volume and tidal volume ratio. The breathing valve dead space was so small (approximately 30 mL) that it was ignored.
Statistical Analysis
Data were summarized as mean ± standard deviation or frequency and percentage. All of the data were shown to be normally distributed by the Kolmogorov-Smirnov test and therefore p values were calculated by ANOVA with Tukey’s correction for multiple comparisons to compare means between the three groups. Fisher’s exact method with Holm’s correction for multiple comparisons was used in contingency table analysis for categorical variables. A p < 0.05 was considered to be significant, and 0.05–0.1 as marginally significant32. Statistical analyses were performed using SAS software v9.4 (SAS Institute Inc., Cary, NC, USA) and Microcal Origin v4.1.
Results
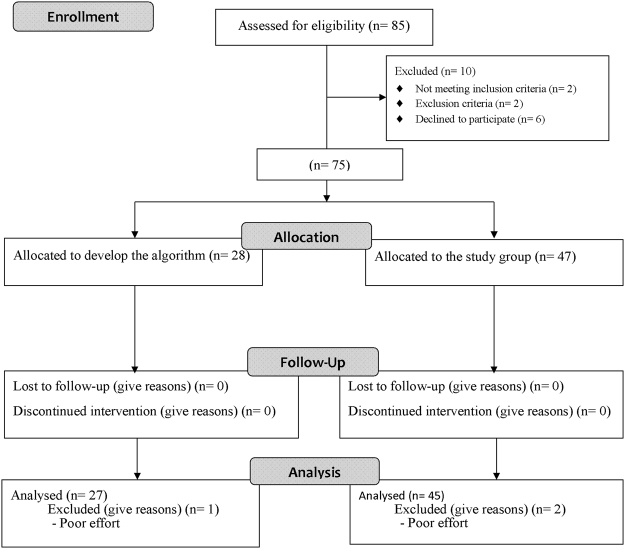
Seventy-five patients were enrolled and completed the study (Fig. 1), of whom 28 were used to develop the algorithm to identify O2P-curve patterns (Supplement file). The remaining 47 patients were used as the study group, two of whom were excluded from analysis due to poor effort (exercise duration of 2 minutes) leaving 45 patients for the final analysis. Twenty patients (44%) with an increasing O2P pattern initially followed by a flattening pattern were classified in the plateau-type group, 16 patients (36%) with an increasing pattern during loaded exercise were classified in the increasing-type group, and nine patients (20%) with a decreasing pattern during the last few minutes were classified in the decreasing-type group.
Figure 1.
Flow diagram. A total of 85 patients with chronic obstructive pulmonary disease were screened. 75 patients were enrolled and 28 patients of them were used for developing the algorithm of identifying O2P-curve patterns. The remaining 47 patients completed the observational study.
The plateau-type group had lower body weight, body mass index, and triceps skin-fold compared to the increasing-type group (Table 1, p = 0.03–<0.0001) and also lower creatinine levels compared to the decreasing-type group (p = 0.05). Differences in other anthropometric and biochemical data were insignificant between the groups. The cardiothoracic ratio in chest radiography and end-diastolic right ventricle area measured in sub-costal 4-chamber view were or tended to be smaller in the plateau-type group (Table 2, p = 0.004 and 0.06, respectively).
Table 1.
Demographic and biochemistry data of patients with chronic obstructive pulmonary disease grouped by the patterns of oxygen pulse (n = 47*, mean ± SD).
| N= | Increasing (Inc) | Plateau (Plat) | Decreasing (Dec) | P | ||
|---|---|---|---|---|---|---|
| 16 | 20 | 9 | Inc vs Plat | Plat vs Dec | Inc vs Dec | |
| Age, years | 65.7 ± 6.3 | 65.3 ± 6.7 | 64.2 ± 2.3 | 0.98 | 0.89 | 0.82 |
| Height, cm | 164.6 ± 6.2 | 165.5 ± 7.2 | 164.4 ± 5.8 | 0.90 | 0.92 | 1.0 |
| Weight, kg | 67.2 ± 9.9 | 55.3 ± 8.3 | 60.3 ± 13.8 | 0.003 | 0.44 | 0.24 |
| Body mass index, kg/m2 | 24.8 ± 3.2 | 20.1 ± 2.0 | 22.2 ± 3.9 | <0.0001 | 0.20 | 0.09 |
| Smoke, pack·year | 46.6 ± 24.0 | 39.7 ± 11.6 | 40.7 ± 24.9 | 0.54 | 0.99 | 0.75 |
| Oxygen-cost diagram, cm. | 7.0 ± 1.7 | 7.1 ± 1.3 | 6.9 ± 1.1 | 0.98 | 0.96 | 0.99 |
| Triceps skin-fold thickness, mm | 7.6 ± 2.9 | 5.4 ± 1.8 | 6.1 ± 3.0 | 0.03 | 0.79 | 0.33 |
| Mid-arm circumference, cm | 28.1 ± 4.5 | 26.3 ± 2.2 | 28.0 ± 3.3 | 0.28 | 0.44 | 1.0 |
| Hemoglobin, gm | 15.0 ± 1.5 | 14.6 ± 1.7 | 15.1 ± 1.0 | 0.68 | 0.76 | 1.0 |
| Carboxy-hemoglobin, % | 1.3 ± 1.1 | 1.5 ± 0.8 | 1.1 ± 0.6 | 0.75 | 0.50 | 0.88 |
| Albumin, g/dL | 4.2 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.3 | 0.48 | 0.72 | 0.98 |
| Creatinine, mg/dL | 1.1 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 0.79 | 0.05 | 0.17 |
*2 patients excluded from the analysis due to poor exercise performance. Bolded numbers indicating statistical significance or the largest number of each variable across the three groups.
Table 2.
Chest radiographic and 2-dimensional echocardiographic data of patients with chronic obstructive pulmonary disease grouped by the patterns of oxygen pulse (mean ± SD).
| N= | Increasing (Inc) | Plateau (Plat) | Decreasing (Dec) | P | ||
|---|---|---|---|---|---|---|
| 16 | 20 | 9 | Inc vs Plat | Plat vs Dec | Inc vs Dec | |
| Chest radiography | ||||||
| Hilum-thorax ratio | 0.36 ± 0.03 | 0.36 ± 0.04 | 0.37 ± 0.02 | 0.91 | 0.81 | 0.64 |
| Cardiothoracic ratio | 0.49 ± 0.05 | 0.42 ± 0.06 | 0.42 ± 0.06 | 0.004 | 0.95 | 0.06 |
| Anterior descending PA | 1.7 ± 0.4 | 1.6 ± 0.3 | 1.6 ± 0.4 | 0.96 | 0.95 | 0.86 |
| 2- dimensional echocardiography | ||||||
| Apical 4-chamber view | ||||||
| End-diastolic RV, cm2 | 14.8 ± 4.3 | 12.5 ± 2.9 | 13.0 ± 3.8 | 0.18 | 0.94 | 0.47 |
| End-systolic RV, cm2 | 8.3 ± 1.9 | 7.8 ± 1.8 | 6.9 ± 1.7 | 0.73 | 0.44 | 0.16 |
| Subcostal 4-chamber view, yes/no | ||||||
| End-diastolic RV, cm2 | 15.6 ± 3.8 | 13.0 ± 2.8 | 14.1 ± 3.0 | 0.06 | 0.68 | 0.50 |
| End-systolic RV, cm2 | 8.3 ± 2.4 | 7.3 ± 1.8 | 7.0 ± 2.1 | 0.36 | 0.96 | 0.30 |
| Long and short axes view | ||||||
| RV wall thickness, mm | 6.1 ±2.3 | 6.1 ±1.7 | 5.9 ± 2.3 | 1.0 | 0.98 | 1.0 |
PA: pulmonary artery; RV, right ventricular; IVS: intraventricular septum. Bolded numbers indicating statistical significance or the largest number of each variable across the three groups.
The plateau-type group had a larger TLC and higher rate of inspiratory capacity/TLC ratio (inspiratory fraction) ≤25% (Table 3, all p = 0.05–0.01), lower DLCO (p < 0.0001) and poorer recovery of maximum inspiratory pressure after exercise (p = 0.05).
Table 3.
Lung function of the patients with chronic obstructive pulmonary disease grouped by the patterns of oxygen pulse (mean ± SD).
| N= | Increasing (Inc) | Plateau (Plat) | Decreasing (Dec) | P | ||
|---|---|---|---|---|---|---|
| 16 | 20 | 9 | Inc vs. Plat | Plat vs. Dec | Inc vs. Dec | |
| Total lung capacity (TLC), L | 6.27 ± 0.90 | 6.94 ± 1.1 | 6.00 ± 0.80 | 0.11 | 0.05 | 0.79 |
| TLC, pred % | 128 ± 20 | 145 ± 18 | 123 ± 18 | 0.02 | 0.01 | 0.82 |
| Functional residual capacity (FRC), L | 4.49 ± 0.75 | 5.22 ± 1.19 | 4.36 ± 0.62 | 0.07 | 0.08 | 0.94 |
| Residual volume (RV), L | 3.52 ± 0.62 | 4.11 ± 1.10 | 3.40 ± 0.54 | 0.11 | 0.10 | 0.94 |
| Inspiratory capacity/TLC < 25%,n/subtotal n | 3/16 | 12/20 | 2/9 | 0.01 | 0.06 | 0.83 |
| Diffusing capacity for carbon monoxide, pred % | 85 ± 19 | 57 ± 17 | 68 ± 5 | <0.0001 | 0.27 | 0.06 |
| Forced vital capacity (FVC), pred % | 76 ± 22 | 88 ± 17 | 75 ± 23 | 0.19 | 0.24 | 0.98 |
| Forced expired volume in one second (FEV1), pred% | 49 ± 14 | 54 ± 22 | 46 ± 18 | 0.63 | 0.56 | 0.96 |
| Stage 1, n= | 0 | 3 | 0 | |||
| Stage 2, n= | 7 | 7 | 4 | 0.73F | 0.73F | |
| Stage 3, n= | 7 | 8 | 3 | |||
| Stage 4, n= | 2 | 2 | 2 | |||
| FEV1/FVC | 0.52 ± 0.12 | 0.48 ± 0.16 | 0.47 ± 0.07 | 0.66 | 0.98 | 0.66 |
| Maximum inspiratory pressure (MIP), cm H2O | 73 ± 21 | 67 ± 16 | 65 ± 18 | 0.64 | 0.96 | 0.58 |
| Maximum expiratory pressure (MEP), cm H2O | 113 ± 29 | 95 ± 13 | 103 ± 21 | 0.07 | 0.69 | 0.51 |
| ΔMIP post-pre exercise, cm H2O | 14 ± 19 | 0 ± 9 | 5 ± 18 | 0.05 | 0.74 | 0.33 |
| ΔMEP post-pre exercise, cm H2O | 5 ± 21 | 3 ± 16 | −3 ± 11 | 0.92 | 0.67 | 0.49 |
DLCO: diffusing capacity for carbon monoxide; pred: predicted; Δ: difference. Bolded numbers indicating statistical significance or the largest number of each variable across the three groups. The p value with a superscript F indicating a Fisher’s test for testing the association between Stages of COPD and the patterns.
The frequency of dyspnea limiting exercise was higher in the plateau-type group compared to the increasing-type and decreasing-type groups (Table 4, both p < 0.05). Only one patient had chest pain (at the right side). During exercise, none of the patients had ST changes or T wave inversion on EKG. At peak exercise, the plateau-type group had lower O2, O2P, operable O2P (Table 4, all p = 0.01–0.02), and higher inspiratory duty cycle, mean inspiratory flow normalized by O2peak, E/O2 and E/CO2 (all p = 0.03–0.0004). The decreasing-type group was similar to the plateau-type group regarding all exercise variables. However, compared to the increasing-type group, the decreasing-type group had a larger decrease in pH (p = 0.05), increase in plasma lactate level, maximum effort score, breathing frequency, and rapid shallow breathing index between at rest and peak exercise (all p = 0.01–0.04). All of these differences were more related to BMI than to predicted TLC% or the frequency of inspiratory fraction ≤25% (Tables 5 and 6, Fig. 2).
Table 4.
Cardiopulmonary exercise test and arterial blood gas and lactate data at peak exercise in the patients with chronic obstructive pulmonary disease grouped by the oxygen pulse (O2P)-curve patterns (mean ± SD).
| Type | Increasing (Inc) | Plateau (Plat) | Decreasing (Dec) | P | ||
|---|---|---|---|---|---|---|
| N= | 16 | 20 | 9 | Inc vs Plat | Plat vs Dec | Inc vs Dec |
| Limiting symptoms | ||||||
| Dyspnea, n = 34 | 10(62.5%) | 19(95%) | 5(55%) | 0.02 M | 0.03 M | 0.99 |
| Fatigue, n = 12 | 2(12.5%) | 6(30%) | 4(44%) | 0.26 | 0.67 | 0.14 |
| Chest pain on the right side, n=1 | 0 | 0 | 1 | |||
| Exercise power, watt | 108 ± 46 | 82 ± 27 | 95 ± 54 | 0.16 | 0.73 | 0.73 |
| O2, mL/min | 1253 ± 369 | 948 ± 252 | 1093 ± 403 | 0.02 | 0.52 | 0.48 |
| O2% pred max, % | 74 ± 17 | 68 ± 23 | 69 ± 23 | 0.67 | 0.97 | 0.88 |
| CO2, mL/min | 1306 ± 456 | 1028 ± 316 | 1204 ± 511 | 0.12 | 0.54 | 0.82 |
| Systolic/diastolic blood pressure, mmHg | 226 ± 22/101 ± 13 | 212 ± 33/98 ± 14 | 229 ± 45/105 ± 9 | 0.42/0.70 | 0.43/0.38 | 0.98/0.81 |
| Heart rate, beat/min | 131.4 ± 22.5 | 130.8 ± 18.2 | 144.6 ± 19.0 | 0.99 | 0.21 | 0.27 |
| Heart rate %pred, % | 81 ± 12 | 80 ± 12 | 88 ± 11 | 0.97 | 0.17 | 0.26 |
| O2P, mL/min/beat | 9.5 ± 2.2 | 7.3 ± 1.9 | 7.5 ± 2.6 | 0.01 | 0.96 | 0.08 |
| O2P %pred, % | 92 ± 18 | 85 ± 26 | 79 ± 27 | 0.66 | 0.80 | 0.40 |
| O2P < 80%pred, n/subtotal n | 4/16 | 9/20 | 6/9 | 0.21 | 0.28 | 0.04 |
| ΔO2P, peak-rest, mL/min/beat | 6.2 ± 1.9 | 4.4 ± 1.8 | 4.4 ± 2.2 | 0.02 | 0.99 | 0.08 |
| Minute ventilation, L/min | 39.4 ± 12.3 | 38.8 ± 12.2 | 38.7 ± 13.1 | 0.99 | 1.0 | 0.99 |
| Breathing frequency, b/min | 31 ± 4 | 32 ± 5 | 37 ± 8 | 0.67 | 0.12 | 0.04 |
| Rapid shallow breathing index, b/L | 26.3 ± 8.6 | 29.3 ± 9.6 | 40.0 ± 24.2 | 0.78 | 0.11 | 0.04 |
| Inspiratroy duty cycle | 0.4 ± 0.03 | 0.43 ± 0.05 | 0.40 ± 0.03 | 0.03 | 0.23 | 0.88 |
| VT/TI/O2 | 1.3 ± 0.2 | 1.6 ± 0.3 | 1.5 ± 0.3 | 0.03 | 0.75 | 0.36 |
| Minute ventilation/O2 | 31.6 ± 4.7 | 41.2 ± 8.5 | 36.6 ± 6.5 | 0.0004 | 0.24 | 0.21 |
| Minute ventilation/CO2 | 30.9 ± 5.1 | 38.2 ± 6.4 | 34.3 ± 7.7 | 0.003 | 0.27 | 0.40 |
| Dead space and tidal volume ratio | 0.40 ± 0.10 | 0.45 ± 0.09 | 0.44 ± 0.13 | 0.35 | 0.97 | 0.63 |
| PaCO2, mm Hg | 48.9 ± 8.1 | 43.0 ± 7.6 | 47.3 ± 6.8 | 0.08 | 0.36 | 0.87 |
| PaO2, mm Hg | 69.1 ± 16.5 | 73.4 ± 18.6 | 68.3 ± 14.5 | 0.75 | 0.75 | 0.99 |
| ΔpH, rest-peak | 0.07 ± 0.04 | 0.08 ± 0.02 | 0.1 ± 0.03 | 0.43 | 0.23 | 0.03 |
| Lactate, meq/L | 4.3 ± 1.6 | 5.5 ± 1.9 | 6.5 ± 2.2 | 0.18 | 0.38 | 0.03 |
| ΔLactate, peak-rest, meq/L | 3.2 ± 1.5 | 4.2 ± 1.8 | 5.6 ± 2.3 | 0.17 | 0.22 | 0.01 |
| Maximum points | 2 ± 2 | 3 ± 2 | 4 ± 2 | 0.22 | 0.43 | 0.04 |
| ΔBorg/ΔO2, A.U./mL/min | 10.0 ± 4.7 | 14.0 ± 6.5 | 12.3 ± 9.5 | 0.18 | 0.80 | 0.68 |
O2: oxygen uptake, VT/TI/O2: tidal volume/inspiratory time ratio normalized with O2, A.U.: absolute unit. Δ: difference. Maximum points: respiratory exchange ratio ≥1.09, heart rate ≥85% of predicted maximum, pH ≤ 7.35, bicarbonate concentration ([HCO3 −]) ≤21 meq/L, the change in [HCO3 −] between at rest and at peak exercise ≥4 meq/L, and the change in lactate concentration between at rest and at peak exercise ≥4 meq/L. Each criterion represented one point for maximum exercise. The points of maximum effort level were scored from 1–6 points. The accumulated points represented the effort level of exercise. Please refer to text. Bolded numbers indicating statistical significance or the largest number of each variable across the three groups.The p values with a superscript M indicating a Holm’s adjusted p value 0.06–0.1 by a Fisher’s test. Other symptoms limited also occurred in the increasing-type group, one being too hot, two having dry mouth, one oxyhemoglobin desaturation; one having foot sliding out on biking in the plateau-type group. Two patients having multiple limiting symptoms in the plateau-type group, one patient having multiple limiting symptoms in the decreasing-type group.
Table 5.
Body mass index (BMI), inspiratory fraction (IC/TLC), and total lung capacity (TLC)% correlated with the variables significant differences across the three O2P patterns (N = 45).
| Correlation Coefficients | |||
|---|---|---|---|
| BMI | IC/TLC | TLC% | |
| Cardiothoracic ratio | 0.641‡ | 0.395* | −0.497** |
| EDRV, cm2 | 0.399† | 0.168 | −0.216 |
| IC/TLC | 0.284¶ | — | −0.486† |
| TLC% | −0.462** | — | — |
| Diffusing capacity for CO% pred. | 0.507† | 0.364* | −0.253¶ |
| Maximum expiratory pressure pre, cmH2O | 0.434** | 0.063 | −0.358* |
| Maximum inspiratory pressure post-pre, cmH2O | 0.133 | −0.17 | 0.009 |
| O2Ppeak, mL/beat | 0.418** | 0.357* | −0.138 |
| O2Ppeak, operable, mL/beat | 0.427** | 0.348* | −0.149 |
| VT/TI/O2 peak | −0.325* | 0.001 | 0.13 |
| Inspiratory duty cycle peak | −0.399** | 0.139 | −0.216 |
| Minute ventilation/O2 peak | −0.418** | 0.082 | 0.21 |
| Minute ventilation/ CO2 peak | −0.486† | −0.16 | 0.339* |
| Breathing frequency peak, breath/min | −0.119 | 0.214 | −0.075 |
| Rapid shallow breathing index peak, breath/min/L | −0.231 | −0.19 | −0.054 |
EDRV: subcostal 4-chamber view end-diastolic right ventricle area, CO: carbon monoxide, pre: before exercise, post-pre: difference between after and before exercise, VT/TI/O2peak: tidal volume and inspiratory time ratio normalized by oxygen uptake at peak exercise, CO2: CO2 output. * p < 0.05, **<0.01, †<0.001, ‡<0.0001, ¶<0.1.
Table 6.
Multiple linear regression with body mass index (BMI) and inspiratory fraction (inspiratory capacity and total lung capacity ratio) on the variables significant different across three oxygen pulse (O2P) patterns (N = 45).
| BMI | IC/TLC | |||
|---|---|---|---|---|
| coefficient | SE | coefficient | SE | |
| Cardiothoracic ratio | 0.011† | 0.003 | 0.213* | 0.101 |
| EDRV, cm2 | 0.395* | 0.16 | 2.479 | 6.54 |
| Diffusing capacity for carbon monoxide % pred | 0.028** | 0.008 | 0.287 | 0.368 |
| Maximum expiratory pressure pre¸cm H2O | 2.962** | 0.979 | −19.8 | 42.5 |
| O2Ppeak, mL/beat | 0.228* | 0.093 | 7.67¶ | 4.17 |
| O2Ppeak, operable, mL/beat | 0.207* | 0.081 | 6.4¶ | 3.65 |
| O2Ppeak% pred | −0.011 | 0.01 | 0.836¶ | 0.455 |
| VT/TI/O2 peak | −0.029* | 0.012 | 0.378 | 0.563 |
| Inspiratory duty cycle peak | −0.004* | 0.002 | 0.125 | 0.083 |
| Minute ventilation/O2 peak | −1.093** | 0.324 | 22.3 | 14.5 |
| Minute ventilation/ CO2 peak | −0.941** | 0.275 | −1.762 | 12.3 |
EDRV: sub-costal 4-chamber view end-diastolic right ventricle area, CO: carbon monoxide, pre: before exercise, post-pre: difference between after and before exercise, VT/TI/O2 peak: tidal volume and inspiratory time ratio normalized by oxygen uptake at peak exercise, CO2: CO2 output. All variables were standardized. To avoid co-linearity problems, all variables were centered. SE is the standard error of the parameter estimates. * p < 0.05, **<0.01, †<0.0001, ¶<0.1.
Figure 2.
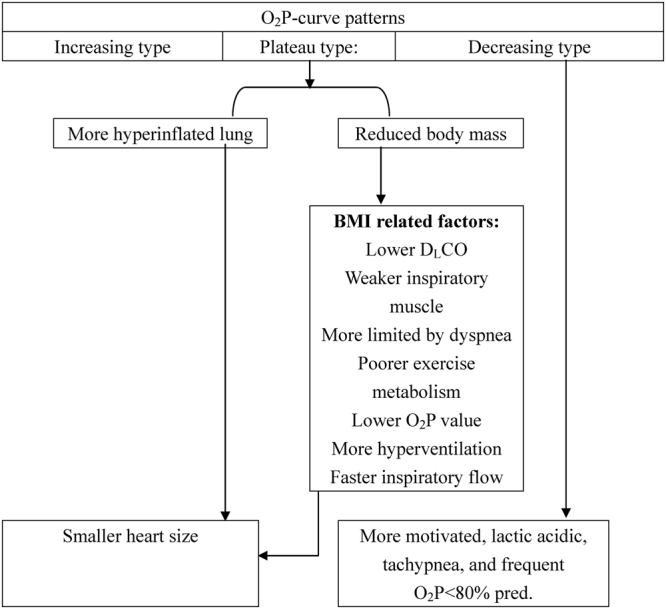
Summary of oxygen pulse (O2P)-curve patterns and its relation to demographics and physiology in patients with chronic obstructive pulmonary disease. BMI: body mass index, DLCO: diffusing capacity for carbon monoxide.
Discussion
In this study, we identified three O2P curve patterns in response to symptom-limited incremental exercise in patients with COPD: increasing, plateau, and decreasing. To the best of our knowledge, this is the first study to investigate O2P curve patterns in patients with COPD.
O2P curve flattening and stress EKG
Detection of exercise-induced myocardial ischemia can be improved by integrating the duration of O2P flattening and O2 work rate slope with stress EKG in patients with documented coronary artery disease33. Adding CPET and gas exchange measurements such as O2P curve flattening has been reported to be of value in diagnosing and quantifying both overt and occult myocardial ischemia5. However, another study reported that flattening of the O2P curve during exercise can only be used to detect extensive but not mild myocardial ischemia6. Moreover, a study on multivariate criteria in diagnosing cardiac causes of exercise limitation found that the O2P curve flattening pattern was not superior to predicted O2P%4. Despite these inconsistent clinical implications of O2P curve flattening, 16.6% of patients with COPD have ischemic heart disease34, which enhances the importance of this pattern. However, we cannot definitively conclude that this pattern was associated with myocardial ischemia in patients with COPD, as none of the patients had significant ST segment or T wave changes in EKG or significant chest pain or oppression during exercise.
O2P curve flattening and its associated factors
The patients with a plateau-type O2P curve had a reduced body mass, smaller heart size, weaker inspiratory muscles, lower diffusing capacity, greater lung volume, and higher rate of inspiratory fraction ≤25% (Tables 1–3 and Fig. 2). This group also had a lower metabolism and O2P, and a higher frequency of dyspnea and hyperventilation at peak exercise (Table 4). These aforementioned differences among the three groups were most correlated with BMI and less with inspiratory fraction or predicted TLC% (Table 5). O2P and operable O2P were more significantly associated with BMI than with inspiratory fraction, but insignificantly with predicted TLC% (Tables 5 and 6). There were significant differences in the frequency of inspiratory fraction ≤25% among the three groups, however inspiratory fraction was modestly correlated with O2P% peak predicted (Tables 3 and 6). These findings suggest that inspiratory fraction influences the O2P pattern in a threshold manner rather than a linear relationship.
The cardiac size was associated with both BMI and inspiratory fraction, with the latter contributing less (Table 6). This is partly consistent with a previous study that suggested that hyper-inflated lungs or emphysema may compress the heart35 or reduce O2P14. We speculate that both reduced body mass and dynamic hyperinflation contribute to intra-thoracic pressure swings which mechanically constrain blood return to the right heart and/or increase afterload to the right and left ventricles13 in a threshold manner, thereby flattening the O2P curve. The additional importance of inspiratory fraction is that it is associated with mortality36 when the fraction is ≤25% or predicts forecasting peak O2 < 60% when the fraction is <28%37.
Other factors influencing the O2P curve patterns might be also co-linear with BMI (Fig. 2). O2 and O2P at peak exercise were significantly different across the three groups (Table 4, p = 0.02 and 0.01, respectively), however the differences became insignificant when peak O2 or O2P was presented with predicted value, suggesting the differences were co-linear with body mass. This is consistent with previous reports in which O2 was affected by body mass38, ventilation capacity16, dynamic hyperinflation39, and dead-space ventilation, and O2P at peak exercise was affected by BMI, inspiratory fraction ≤25%, predicted FEV1%, and hand grip force14. In contrast, the rapid shallow breathing index differed significantly between the decreasing-type and the increasing-type groups (Table 4), however the index was associated with motivation to perform exercise (see below) and not BMI or inspiratory fraction (Table 5).
Decreasing O2P curve pattern
The decreasing-type group was similar to the plateau-type group in demographics, cardiac size, lung function, and exercise physiology except for having higher creatinine levels and lower lung volumes (Tables 1–4, all p ≤ 0.05). This was because the plateau-type had smaller muscle mass but larger lung volumes. Compared to the increasing-type, the decreasing-type group had higher maximum effort scores, more acidity, and more rapid breathing pattern at peak exercise, suggesting that this group was more motivated to perform (Table 4).
Factors dissociated with O2P curve patterns
The O2P curve patterns were not related to pack-years of cigarette smoking, OCD or forced spirometry values. OCD mimics exercise capacity expressed in MET normalized with body weight21, which may weaken the association between OCD and O2P curve pattern. The O2P curve patterns were not related to exercise power, dynamic heart rate, blood pressure, ventilation capacity, dead space ventilation, PaO2, or PaCO2 at peak exercise. In addition, dynamic dead space ventilation was significantly correlated with dynamic O2P (with peak O2P, r = −0.65, p < 0.0001, with operable O2P, r = −0.68, p < 0.0001), suggesting that dynamic dead space ventilation cannot explain O2P curve patterns.
Study limitations
First, we did not thoroughly investigate myocardial ischemia by nuclear medicine or coronary angiography, so that the relationship between myocardial ischemia and O2P curve flattening could not be established. However, EKG did not reveal significant ST segment or T wave changes, and no chest pain or oppression occurred during exercise (Table 4) and recovery from exercise. Moreover, no cardiac events developed during the follow-up period (at least 2 years). These findings indicate that myocardial ischemia may not have been the major cause of O2P curve flattening. Second, selection bias may have occurred as patients with obvious coronary artery disease and left heart failure were excluded, so the results cannot be generalized to all subjects who perform CPET. Third, according to a previous report on co-morbidities with ischemic heart disease34, eight of our cohort should have had ischemic heart disease, although we did not find this. Fourth, we did not measure dynamic inspiratory capacity. However, it seems reasonable that dynamic hyperinflation can be deduced from static hyperinflation since both are highly correlated39. Fifth, muscle extraction of oxygen has been assumed to be predictable or the frequency of abnormal muscle extraction of oxygen is low in the general population and patients with COPD. However, the rate of mitochondrial myopathy has been estimated to be 8.5% in the general population40. Sixth, patient’s BMI or body surface area is related to the cardiovascular size41 and may influence the magnification factors of radiography thereby influencing parallax and measurements in chest radiographs42. However, the cardiovascular size measured with cardiothoracic and hila-thoracic ratios reported in the current study is less influenced by body size41. However, the possibility of impact of the magnification factors on measurement of the anterior descending pulmonary artery cannot be excluded. In addition, we did not evaluate the inter-rater agreement in measurement of cardiac size with two-dimensional echocardiography thereby probably introducing measurement bias. Lastly, because high-resolution computed tomography was not performed to evaluate the severity of emphysema, the relationships among O2P curve patterns, emphysema, and inspiratory fraction could not be determined.
Clinical implications
By categorization of patients with COPD into the three groups according to the O2P curve patterns, the clinicians may acknowledge that O2P curve flattening is much less associated with myocardial ischemia thereby preventing performing unnecessary investigations. Accordingly, providing the optimal management to improve the O2P curve flattening can be reached by ameliorating body mass and inspiratory fraction. Whether these measurements can be used in a prognostic manner is to be shown. Finally, further research on the O2P curve patterns in patients with heart failure and with COPD concurrently and direct measure of hemodynamics of both patient groups is recommended.
Conclusions
By analyzing O2P with a smoothing technique, we identified three patterns of O2P curve, and the flattening pattern was common in patients with COPD when performing CPET. This pattern was further related to exercise-limiting dyspnea and reduced body mass, and modestly related to pulmonary hyperinflation but not to myocardial ischemia. The decreasing-type may be caused by motivation to exercise.
Electronic supplementary material
Developing an algorithm to identify patterns of oxygen pulse
Author Contributions
M.L.C.: initiated and designed the study, analyzed and interpreted the data, wrote the manuscript, and approved the version to be published. I.F.L.: reviewed and statistically analyzed the data, revised the manuscript, and approved the version to be published. S.F.H.: reviewed the data, revised the manuscript, and approved the version to be published. M.J.H.: reviewed the data, revised the manuscript, and approved the version to be published.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11189-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whipp BJ, Higgenbotham MB, Cobb FC. Estimating exercise stroke volume from asymptotic oxygen pulse in humans. J. Appl. Physiol. 1996;81:2674–9. doi: 10.1152/jappl.1996.81.6.2674. [DOI] [PubMed] [Google Scholar]
- 2.Stringer W, Hansen JE, Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J. Appl. Physiol. 1997;82:908–12. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 3.Wasserman, K., Hansen, J. E., Sue, D. Y., Stringer, W. W. & Whipp, B. J. Measurements during integrative cardiopulmonary exercise testing in Principles of exercise testing and interpretation (ed. Wasserman, K.) (Lippicott Williams & Wilkins, Philadelphia, 2005).
- 4.Ahmad MN, et al. Multivariate Criteria Most Accurately Distinguish Cardiac from Noncardiac Causes of Dyspnea. Tex. Heart. Inst. J. 2015;42:514–21. doi: 10.14503/THIJ-14-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhry S, et al. Exercise-induced myocardial ischemia detected by cardiopulmonary exercise testing. Am. J. Cardiol. 2009;103:615–619. doi: 10.1016/j.amjcard.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munhoz EC, et al. Flattening of oxygen pulse during exercise may detect extensive myocardial ischemia. Med. Sci. Sports Exerc. 2007;39:1221–6. doi: 10.1249/mss.0b013e3180601136. [DOI] [PubMed] [Google Scholar]
- 7.Warburton DER, et al. Effectiveness of high-intensity interval training for the rehabilitation of patients with coronary artery disease. Am. J. Cardiol. 2005;95:1080–4. doi: 10.1016/j.amjcard.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 8.Cohen-Solala A, et al. Comparison of the long term prognostic value of peak exercise oxygen pulse and peak oxygen uptake in patients with chronic heart failure. Heart. 1997;78:572–6. doi: 10.1136/hrt.78.6.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laukkanen JA, Kurl S, Salonen JT, Lakka TA, Rauramaa R. Peak oxygen pulse during exercise as a predictor for coronary heart disease and all cause death. Heart. 2006;92:1219–24. doi: 10.1136/hrt.2005.077487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira RB, et al. Maximal exercise oxygen pulse as a predictor of mortality among male veterans referred for exercise testing. Eur. J. Cardiol. Prev. Rehab. 2009;16:358–64. doi: 10.1097/HJR.0b013e3283292fe8. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira RB, et al. Does peak oxygen pulse complement peak oxygen uptake in risk stratifying patients with heart failure? Am. J. Cardiol. 2009;104:554–8. doi: 10.1016/j.amjcard.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groepenhoff H, et al. Exercise testing to estimate survival in pulmonary hypertension. Med. Sci. Sports Exerc. 2008;40:1725–32. doi: 10.1249/MSS.0b013e31817c92c0. [DOI] [PubMed] [Google Scholar]
- 13.Montes de Oca M, Rassulo J, Celli BR. Respiratory muscle and cardiopulmonary function during exercise in very severe COPD. Am. J. Respir. Crit. Care Med. 1996;154:1284–9. doi: 10.1164/ajrccm.154.5.8912737. [DOI] [PubMed] [Google Scholar]
- 14.Vassaux C, et al. Effects of hyperinflation on the oxygen pulse as a marker of cardiac performance in COPD. Eur. Respir. J. 2008;32:1275–82. doi: 10.1183/09031936.00151707. [DOI] [PubMed] [Google Scholar]
- 15.Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996;153:967–75. doi: 10.1164/ajrccm.153.3.8630581. [DOI] [PubMed] [Google Scholar]
- 16.Benditt JO, Lewis S, Wood DE, Klima L, Albert RK. Lung volume reduction surgery improves maximal O2 consumption, maximal minute ventilation, O2 pulse, and dead space-to-tidal volume ratio during leg cycle ergometry. Am. J. Respir. Crit. Care Med. 1997;156:561–6. doi: 10.1164/ajrccm.156.2.9611032. [DOI] [PubMed] [Google Scholar]
- 17.Come CE, et al. Lung deflation and oxygen pulse in COPD: results from the NETT randomized trial. Respir. Med. 2012;106:109–19. doi: 10.1016/j.rmed.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lammi MR, Ciccolella D, Marchetti N, Kohler M, Criner GJ. Increased oxygen pulse after lung volume reduction surgery is associated with reduced dynamic hyperinflation. Eur. Respir. J. 2012;40:837–843. doi: 10.1183/09031936.00169311. [DOI] [PubMed] [Google Scholar]
- 19.de Lucas-Ramos P, et al. Chronic obstructive pulmonary disease as a cardiovascular risk factor. Results of a case-control study (CONSISTE study) Int. J. Chron. Obstruct. Pulmon. Dis. 2012;7:679–86. doi: 10.2147/COPD.S36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.GOLD Committees. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (revised 2011). Disclosure forms for GOLD Committees are posted on the GOLD Website, www.goldcopd.org (2011).
- 21.McGavin CR, Artvinli M, Naoe H, McHardy GJ. Dyspnoea, disability, and distance walked: comparison of estimates of exercise performance in respiratory disease. Br. Med. J. 1978;2:241–3. doi: 10.1136/bmj.2.6132.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ATS/ERS Statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 166, 518–624 (2002). [DOI] [PubMed]
- 23.Miller MR, et al. General considerations for lung function testing. Eur. Respir. J. 2005;26:153–61. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, et al. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Chuang ML, Lee CH, Lin IF. Using the oxygen-cost diagram in ramp-slope selection for dyspneic patients. Intern. Med. 2010;49:1325–32. doi: 10.2169/internalmedicine.49.3094. [DOI] [PubMed] [Google Scholar]
- 26.Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels/the Criteria Committee of the New York Heart Association. (ed. Dolgin, M.) 253–256. (Little Brown & Co Inc, 1994).
- 27.McNicholl DM, Megarry J, McGarvey LP, Riley MS, Heaney LG. The utility of cardiopulmonary exercise testing in difficult asthma. Chest. 2011;139:1117–23. doi: 10.1378/chest.10-2321. [DOI] [PubMed] [Google Scholar]
- 28.Chetty KG, Brown SE, Light RW. Identification of pulmonary hypertension in chronic obstructive pulmonary disease from routine chest radiographs. Am. Rev. Respir. Dis. 1982;126:338–41. doi: 10.1164/arrd.1982.126.2.338. [DOI] [PubMed] [Google Scholar]
- 29.Bertoli, L. et al. Usefulness of two-dimentional echocardiography in the assessment of right heart in chronic obstructive lung disease. Progress in Respiration Research. Basel: Karger. 91–100 (1985).
- 30.Danchin N, et al. Two-dimensional echocardiographic assessment of the right ventricle in patients with chronic obstructive lung disease. Chest. 1987;92:229–33. doi: 10.1378/chest.92.2.229. [DOI] [PubMed] [Google Scholar]
- 31.Lang RM, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. 2015;16:233–71. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 32.Rosner, B. Hypothesis testing: one-sample inference in Fundamentals of Biostatisitcs. 7th ed. 204–258 (Brooks/Cole, Cengage Learning 2011).
- 33.Belardinelli R, et al. Exercise-induced myocardial ischaemia detected by cardiopulmonary exercise testing. Eur. Heart J. 2003;24:1304–1313. doi: 10.1016/S0195-668X(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 34.Patel ARC, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141:851–857. doi: 10.1378/chest.11-0853. [DOI] [PubMed] [Google Scholar]
- 35.Jörgensen K, et al. Reduced intrathoracic blood volume and left and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007;131:1050–1057. doi: 10.1378/chest.06-2245. [DOI] [PubMed] [Google Scholar]
- 36.Casanova C, et al. Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005;171:591–7. doi: 10.1164/rccm.200407-867OC. [DOI] [PubMed] [Google Scholar]
- 37.Albuquerque AL, et al. Inspiratory fraction and exercise impairment in COPD patients GOLD stages II-III. Eur. Respir. J. 2006;28:939–44. doi: 10.1183/09031936.06.00040506. [DOI] [PubMed] [Google Scholar]
- 38.Palange P, et al. Effect of reduced body weight on muscle aerobic capacity in patients with COPD. Chest. 1998;114:12–8. doi: 10.1378/chest.114.1.12. [DOI] [PubMed] [Google Scholar]
- 39.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001;164:770–7. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 40.Flaherty KR, et al. Unexplained exertional limitation characterization of patients with a mitochondrial myopathy. Am. J. Respir. Crit. Care Med. 2001;164:425–432. doi: 10.1164/ajrccm.164.3.2005110. [DOI] [PubMed] [Google Scholar]
- 41.Pirro, F. et al. Chest X-ray cardiac anatomy and pathology: correlation with Angiocardiography, CT, and MR imaging. Electronic presentation online system, 1–48, doi:10.1594/ecr2013/C-1945 (2013).
- 42.Bush A, Gray H, Denison D. M. Diagnosis of pulmonary hypertension from radiographic estimates of pulmonary arterial size. Thorax. 1988;43:127–131. doi: 10.1136/thx.43.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Developing an algorithm to identify patterns of oxygen pulse