Abstract
Flocculation is an important feature for yeast survival in adverse conditions. The natural diversity of flocculating genes in Saccharomyces cerevisiae can also be exploited in several biotechnological applications. Flocculation is mainly regulated by the expression of genes belonging to the FLO family. These genes have a similar function, but their specific contribution to flocculation ability is still unclear. In this study, the distribution of FLO1, FLO5 and FLO8 genes in four S. cerevisiae wine strains was investigated. Subsequently, both FLO1 and FLO5 genes were separately deleted in a flocculent S. cerevisiae wine strain. After gene disruption, flocculation ability and agar adhesion were evaluated. FLO1 and FLO5 genes inheritance was also monitored. All strains presented different lengths for FLO1 and FLO5 genes. Results confirm that in S. cerevisiae strain F6789, the FLO5 gene drives flocculation and influences adhesive properties. Flocculation ability monitoring after a cross with a non-flocculent strain revealed that FLO5 is the gene responsible for flocculation development.
Introduction
The ability to adhere to other cells and substrates is a crucial feature of microorganisms that can be used both for ‘defensive’ or ‘offensive’ purposes. In Saccharomyces cerevisiae, it has the univocal purpose of allowing yeast survival in different biological contexts1. Yeast strains display different adhesion phenotypes such as biofilm, invasive growth, agglutination, chain formation co-flocculation and flocculation. Some wine strains have a specific adhesion phenotype called flocculation. This is an asexual, reversible, calcium-dependent process of yeast cells aggregation into flocs that rapidly sediment to the bottom of the liquid growth substrate2. The propensity to flocculate is a very significant property of wine yeasts, especially for sparkling wine production with traditional methods. Flocculation offers a more cost-effective method for biomass recovery than centrifugation and filtration, which not only requires high capital investment but also consumes more energy3. Indeed, cells that adhere to each other can be conveniently separated from the fermentation product, thus reducing riddling time to only 2 days using automated riddling machines4.
During secondary fermentation, wine yeast cells are usually exposed to different stress conditions such as low pH and temperature, high sulphur dioxide level, high total acidity, lack of nutrients and high ethanol content5. Flocculation is recognized as a way for cells in solution to escape harsh conditions through sedimentation. Compounds produced during wine fermentation such as ethanol, or the aromatic alcohols tryptophol and phenylethanol, are used in S. cerevisiae as quorum sensing molecules6. Based on cell density, these compounds act by modulating FLO genes regulation7. Therefore, flocculation is a cooperative mechanism for protection against multiple chemical stresses6. It is mainly due to a variation of cell wall content in fatty acids, ergosterol and trehalose6, 8. Furthermore, Goossens et al.9 found that flocculation contributes to creating specific micro-environmental conditions that favour mating. As mating is a source of genetic variation, diploid cells can thus sporulate and increase the strain’s survival rate.
Flocculation is a two-stage cell-cell adhesion process based equally on glycan-glycan interaction as well as glycan-lectin binding9. Initially, there is a glycan-glycan interaction (calcium-binding hypothesis of Mill10) reinforced afterwards by specific proteins termed ‘flocculins’ or ‘adhesins’3, ‘lectins’2, 11 or ‘zymolectins’12 (lectin-like theory of Miki et al.11). These proteins protrude from the cell surface and directly bind to mannose residues present on the cell wall of neighbour cells. In any case, the presence of Ca2+ ions is very important because these ions are directly involved in the binding with sugars9 and they allow maintaining the correct conformation of flocculins11. Recently, Goossens et al.9 demonstrated that flocculins are drivers of a homophilic interaction, which ensures species-specific aggregation6. Floc structure is preferentially composed of FLO-expressing cells, probably because of reciprocal interactions. Flo proteins are exclusively responsible for flocculation because they are the most abundant cell wall proteins13, so that binding with glycans from others cell wall proteins is not easily achieved. Furthermore, Flo proteins can only interact with mannans present on adjacent cells: indeed, these adhesins are characterised by the presence of two binding sites that immobilise the N-terminal extremity at the cell surface, and by a low binding affinity ensuring that any occasional binding between flo proteins in the same cell is prevented9.
Flocculins share a common structure that consists of three domains: i) the carboxyl-terminal domain containing a glycosylphosphatidylinositol anchor that allows flocculin binding to the cell surface14, 15 ii) the central domain, rich in serine and threonine residues and heavily glycosylated, is characterized by a large number of tandem repeats that trigger frequent slippage and/or recombination events during DNA replication16 iii) the N-terminal effector region, termed CRD (Carbohydrate-Recognition Domain)17.
Members of the Flo adhesin protein family in S. cerevisiae can be subdivided into two groups18. The FLO1, FLO5, FLO9, and FLO10 genes encode the members of the first group of proteins. These adhesins promote cell-cell adhesion and contribute to the formation of multicellular clumps (flocs). The second group includes Fig2p and Aga1p that are induced during mating19, 20, as well as Flo11p (also known as Muc1p) that is required for diploid pseudohyphal formation and haploid invasive growth21, 22. Besides, FLO8 is a transcriptional activator essential for STA123, FLO1124, FLO125 and FLO93 inducible expression. Most laboratory strains, such as S288C and W303-1A, lack the FLO8 gene, so they hardly flocculate26.
Several studies have nevertheless revealed that there is a high biodiversity of flocculation phenotypes and genotypes among industrial27, 28, wine29, cachaça30 and brewery31 strains of S. cerevisiae. Correspondingly, Verstrepen et al.32 highlighted the role of the number of tandem repeats in the central domain of flocculins in the diversity of phenotypes.
The genes of the first group (FLO1, FLO5, FLO9 and FLO10) share considerable sequence homology, but the evolutionary advantage linked to the existence of a large family of genes involved in the same phenotype is still unclear33. It could be partly explained by the fact that different FLO genes confer quite different flocculation degrees16, 34, or encode proteins that have different responses to proteases or heat treatments35 or still, bind to different sugars9, 34, 36. Furthermore, the subtelomeric position of FLO genes makes them more liable to undergo recombination events32.
In a previous work, flocculation ability has been reported as a not uncommon feature in S. cerevisiae wine strains (i.e. in more than 6.8%)37. Among these, it was possible to identify different degrees of flocculation and also variable developmental behaviours. Out of 1973 strains, 29 flocculent ones were studied for their floc-forming ability using the differential effects of elevated temperature and proteases38. Subsequently, the genetic diversity of FLO1 and FLO5 in flocculent wine strains of S. cerevisiae was studied29. In addition, the same strains were characterised in terms of FLO1, FLO5, FLO8, AMN1 and RGA1 gene expression, growth kinetics and physicochemical properties of the cell surface during a 6-month sparkling wine fermentation period.
The aim of this work was to study the the importance/relevance of FLO1, FLO5 and FLO8 genes for flocculation in haploid strain F6789 that, as reported above, was found to possess higher flocculation ability (resistance to the action of four proteases) and high viability and resistance to sparkling wine fermentation stress. In addition, a strain that had lost its flocculation ability (F7101)29, 38, an autochthonous pulverulent strain isolated from Montepulciano d’Abruzzo wine (RT73)39 and strain 59A, a non-flocculent haploid derivative of Lalvin EC1118, were also used.
Results
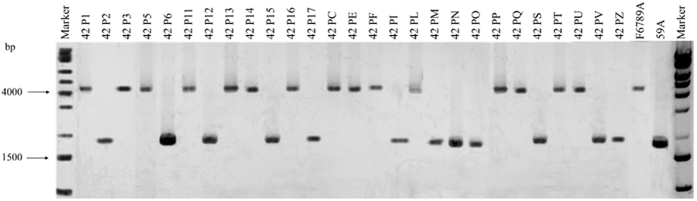
Analysis of FLO genes distribution
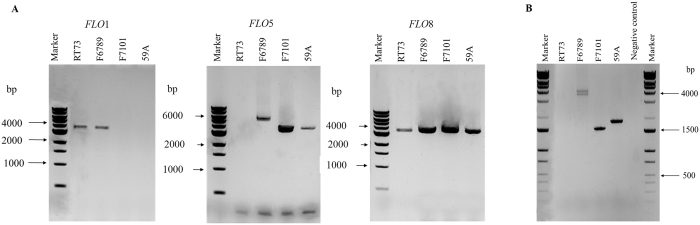
The natural reservoir of FLO genes in S. cerevisiae strains was proposed as a reservoir to improve beverage industry because it allows green biomass separation29, 40, 41. For this reason, this study was first focused on FLO1, FLO5 and FLO8 genes in four S. cerevisiae wine strains (primers’ sequences and PCR conditions are reported in Supplementary Table S1). The whole genes were amplified using primers targeting zones before and after the coding regions. As shown in Fig. 1A, in all strains, the FLO8 gene displayed as expected a band with the same size of 3631 bp, confirming that this gene is present in all the S. cerevisiae strains studied. As regards the FLO1 gene, only two strains (pulverulent strain RT73 and flocculent strain F6789) had the same amplicon (approx. 3500 bp). No PCR products were observed for the pulverulent strains F7101 (yeast that has lost its flocculation ability) and 59A (industrial strain). The failure to amplify FLO1 gene in these two strains may be due to differences in sequences at the primer binding sites as also proposed by other authors or to the absence of the entire or part of the FLO1 gene sequence28, 31. In the same way, strain RT73 showed a short amplicon for gene FLO5, while the flocculent strain F6789 presented a 5000 bp amplicon, which was larger than in the other strains (about 4000 bp). All strains displayed a small band for FLO5 (approx. 150 bp), probably corresponding to a FLO-derived pseudogene. Many studies have demonstrated that the difference in length is due to the presence of tandem repeated sequences in the central region (B domain) of FLO genes32. Therefore, we investigated this domain of the FLO5 gene in our strains. As shown in Fig. 1B, three strains (59A, F6789 and F7101) exhibited at least one band for FLO5, while strain RT73 had no amplicon, because it either harbours a divergent gene in the primer sequence or just lacks gene FLO5. Interestingly, F6789 displayed two amplicons of approx. 4000 bp. Probably, the greater number of tandem repeats displayed by the flocculent strain F6789 hints to a central role of the FLO5 gene in the development of flocculation.
Figure 1.
(A) Specific PCR detection of FLO1, FLO5 and FLO8 genes in S. cerevisiae strains. (B) Intragenic repetitive domains of gene FLO5. PCR mix without DNA was used as negative control.
FLO genes deletion and phenotype investigation
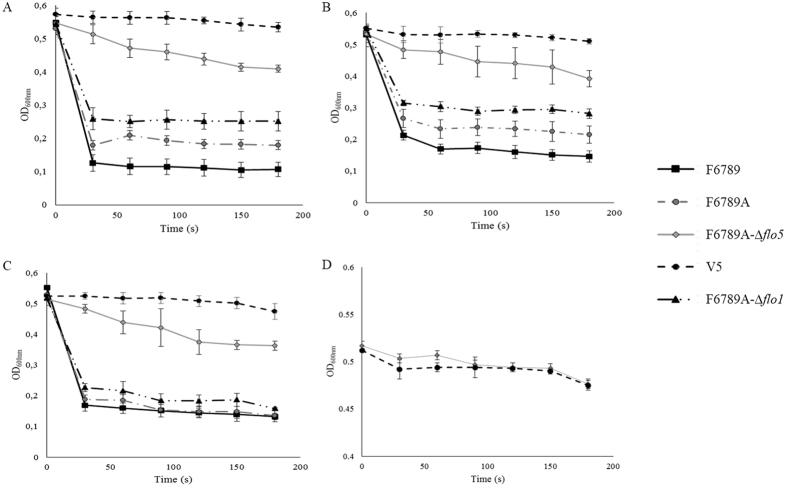
To better understand which one of the FLO genes plays a major role in S. cerevisiae wine strains flocculation, we turned our attention to the F6789 strain. As this is a diploid strain, we obtained haploid clones by sporulation after deletion of the HO gene. This strain showed a sporulation ability of about 12%, with a 55% survival rate. As all the haploid clones obtained were flocculent, we selected the F6789A-Δho clone and both FLO1 and FLO5 genes were separately deleted. The resulting FLO-deleted haploid strains were subjected to a flocculation test in different media (YNB + glucose, YEPD and synthetic must). Results demonstrated that parental strain F6789 and its haploid derivative (F6789A) showed extensive flocculation whatever the medium used (Fig. 2A–C). In fact, these strains showed a decrease in OD600 nm of about 0.4 in YNB + glucose, while the FLO1-deleted F6789A-Δflo1 strain only displayed a weak reduction in OD600 nm (≈0.329). By contrast, strains with a FLO5 deletion (F6789A-Δflo5) exhibited a strong decrease in their flocculation ability and OD600 nm dropped only by 0.14 in 3 min.
Figure 2.
Flocculation level of parental (F6789), haploid (F6789A), transformants (F6789A-Δflo1 F6789A-Δflo5) and V5 strains. V5 is a laboratory haploid yeast derivative from a strain isolated from Champagne. This yeast was previously used as negative control for flocculation (Bidard et al.45) and we are sure that it has no adhesive properties. Flocculation was determined in YNB + glucose (2% w/v) (A), in YEPD (B) and in synthetic must (C). (D) Sedimentation without CaCl2 of non-flocculent strain V5 and F6789A-Δflo5. The data reported are mean values for triplicates. Vertical error bars represent standard deviations.
Interestingly, all flocculent strains and F6789A-Δflo1 presented a hyperbolic curve with a strong OD600 nm reduction already in the first 30 sec. Otherwise, F6789A-Δflo5 strain showed a curve more similar to the non-flocculent strain (V5). In fact, these yeasts presented a slower and more gradual decrease in OD600 nm than the parental, F6789A and F6789A-Δflo1 strains. To investigate whether the residual aggregation was indeed flocculation, the same tests were conducted for strains F6789A-Δflo5 and V5 without CaCl2 addition (Fig. 2D) as it is generally recognized that flocculation can only occur in the presence of Ca2+ 17. As expected, in this case, the strains had the same trend and the curves were superimposed, indicating that this is a typical Ca2+-dependent flocculation. Results demonstrated that in strain F6789, strong flocculation was strongly correlated with the expression of one specific FLO gene, FLO5.
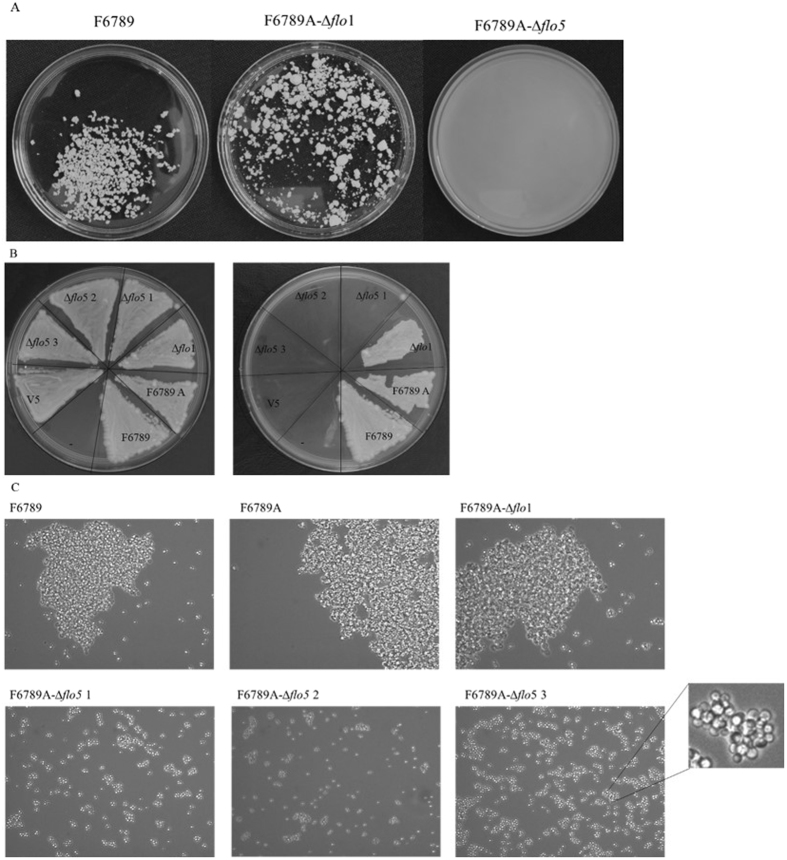
To evaluate the residual flocculation of the transformants, cells were grown in YEPD for 48 h at 28 °C, after which flocs were observed with the naked eye and through an optical microscope (Fig. 3A and C). The parental strain presented, both at macroscopic and microscopic levels, large flocs of about 800000 μm2. The F6789A-Δflo1 strain showed flocs slightly smaller than F6789 (about 650000 μm2). On the other hand, F6789A-Δflo5 transformants were characterized by flocs unobservable to the naked eye. In fact, under the microscope, they presented flocs constituted of 20–30 cells with a 1600 μm2 size.
Figure 3.
(A) Photographs of the parental (F6789), haploid (F6789A) and transformants F6789A-Δflo1 and F6789A-Δflo5 strains. Petri dishes were filled with equivalent volumes of YEPD medium originating from a liquid cell culture in stationary phase. (B) Agar invasion before and after the wash. Strains were spreaded onto YEPD plates and were cultivated for 3 days at 28 °C, followed by 2 days at room temperature. Plates were documented before and after non-adhesive cells were washed off the agar with a gentle stream of water. (C) Optical microscope photos (40X objective) of the parental strain (F6789), haploid strain (F6789A) and transformants.
To evaluate the influence of FLO5 on other adhesion characteristics, an agar surface invasion analysis was conducted. Differences in the intensity of adhesion in F6789 derivatives were highlighted (Fig. 3B). Cells with FLO5 deletion were completely washed off the agar surface as fluffs after a short time of gentle washing. On the contrary, F6789A-Δflo1 cells were not easily washed off the plate and a cell layer remained attached to the agar surface.
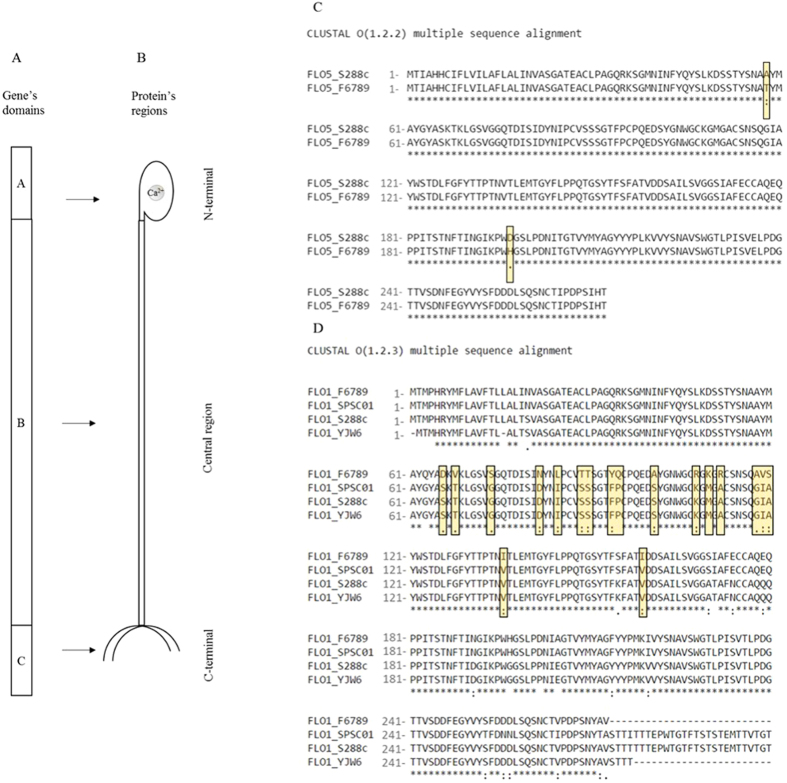
To elucidate whether differences in the flocculation degree were due to a different binding strength of Flop, the N-terminal sequence of FLO1 and FLO5 was investigated. The DNA sequence of the FLO5 N-terminal gene was determined to be similar to the published S288C sequence except for two substitutions. The flocculent strain F6789 presented a guanine to adenine substitution, giving rise to a threonine instead of an alanine at position 58 (Fig. 4C). In position 197, the flocculent strain presented a histidine, where S288C has an aspartic acid residue, this difference being due to a guanine to cytosine substitution. Otherwise, the N-terminal sequence of FLO1 from S. cerevisiae F6789 showed only 83% similarity with the S288C N-terminal of Flo1p. Protein Flo1was aligned with the sequence of other two flocculent strains (Fig. 4D): the first one, SPSC01, is a protoplast fusant between S. cerevisiae and Schizosaccharomyces pombe used for fuel ethanol production42, while YJW6 is a haploid strain of S. cerevisiae with an inserted FLO1S gene obtained by Watari et al.43. F6789 Flo1p displayed 14 differences, showing 11 point mutations, 2 substitutions of 2 bases and 1 substitution of 3 bases.
Figure 4.
(A) Primary-structure of FLO genes. (B) Structure of Flo proteins. (C) Protein alignment of FLO5 protein between S288C and F6789A strains. Alignment with SPSC02 and YJW6 FLO5 sequences was not shown because these were not available in public databases. (D) Protein alignment of FLO1 protein between F6789A, SPSC01 (Accession n. JQ629938/protein id. AFJ20718.1)38, S288C and YJWE6 (Accession n. X78160/protein id. CAA55024.1)40 strains.
Segregation properties of flocculation
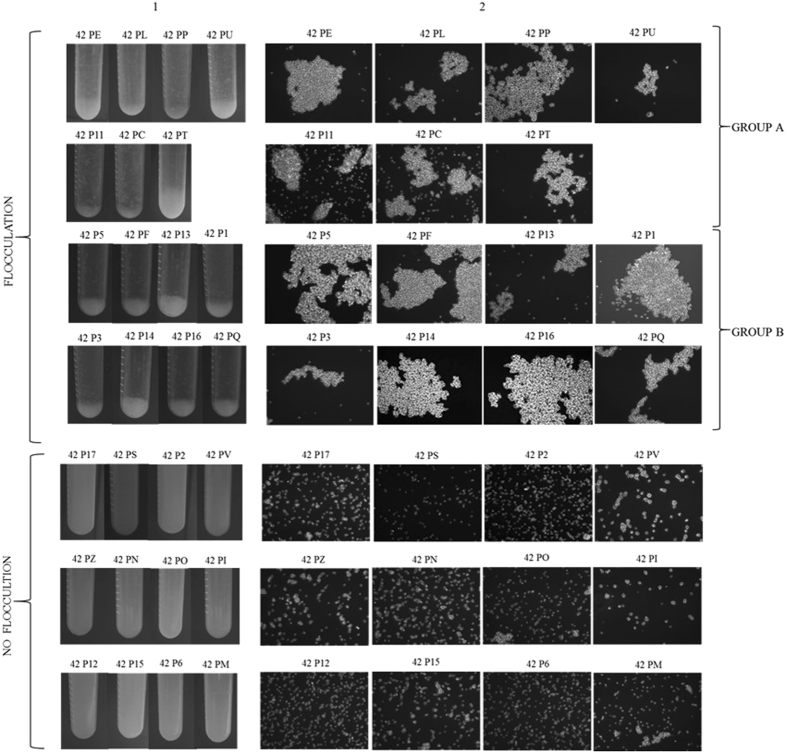
In order to clarify the role of FLO genes in the F6789 strain, the segregation of the flocculation in a cross with a non-flocculent S. cerevisiae strain was monitored. Haploid strain F6789A-Δho was crossed with the non-flocculent S. cerevisiae strain 59A and the resulting hybrid (PDG 42) was induced to sporulate. After 5 days, PDG 42 presented 20% sporulation, with a 72% survival rate. Subsequently, the flocculation ability of segregants in YEPD medium was evaluated (Fig. 5). Sedimentation tests demonstrated that 55.6% of the strains were flocculent at different degrees. In particular, 2 different groups with low and high flocculation degrees were detected (Fig. 5 Column 1). Group A was composed of 7 segregants and group B included 8 strains that presented a high flocculation capacity. These results were confirmed by observations under an optical microscope (Fig. 5 Column 2). Cell size and number in flocs increased from the A to the B group. As described by Silva et al.44, all flocculent segregants presented opaque colonies with a rough aspect, irregular edges, and “castle” cross-section. On the contrary, about 44% of the isolates were pulverulent strains characterised by a dispersed development, a long sedimentation time and white, creamy colonies.
Figure 5.
Phenotypic characterization of hybrids derived from the cross between 59A and F6789A strains. Column (A) sedimentation test: liquid cultures were vigorously mixed and placed in vertical position. Tubes were photographed after 30 s. Column (B) microscope observation (objective 40X).
Then, FLO5-repeated regions in segregants were detected. Moreover, in order to have a more complete picture of FLO genes, FLO1 gene distribution was also investigated (Supplementary Table S2). Figure 6 clearly shows that all flocculation occurrences segregated with the FLO5 form of F6789A indicating that all flocculent strains had inherited their FLO5 gene from F6789A.
Figure 6.
FLO5 gene repeated regions among segregants.
Immunofluorescence
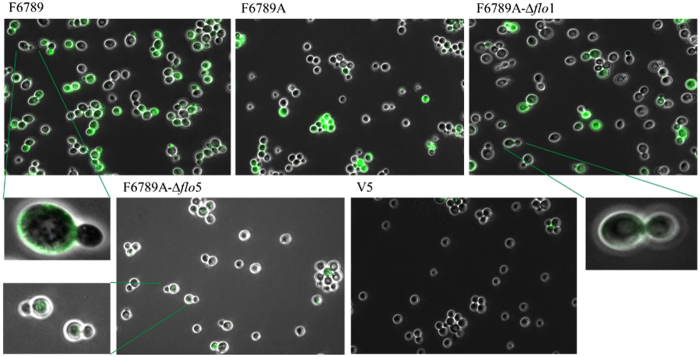
Flo proteins distribution at yeast cell surface was investigated by immunofluorescence staining with polyclonal antibodies. These antibodies, raised against Flo1p and obtained in a previous work45, are able to detect both proteins Flo1 and Flo5. Figure 7 shows that using FITC-labelled Anti-Flop, intact yeast cells of strains F6789 and F6789A could be stained during the stationary phase, thus indicating the presence of Flo proteins on their cell surface. However, flocculin distribution was not homogeneous: F6789 displayed approx. 70% stained cells, while only approx. 65% of the cells were stained in the F6789A strain. The strain harbouring a deleted FLO1 gene (F6789A-Δflo1) displayed only a slight reduction in staining (about 10%). Consistently, the transformants harbouring a deletion of the FLO5 gene (F6789A-Δflo5) presented an important reduction in staining with only 25% fluorescent cells. This strong reduction in Flo proteins detection with the anti-Flop antibodies is in line with a key role of FLO5 in triggering flocculation ability. It is interesting to note that in all studied strains there were cells that were not labelled and cells in which the reacting antigens were present only in specific points on the cell surface.
Figure 7.
Localization of cell wall flocculins by FITC-labelled specific Anti-Flop antibody of parental (F6789), haploid (F6789A), F6789A-Δflo1, F6789A-Δflo5 and V5 strains. The S. cerevisiae cells were grown in YNB+glucose. After 48 h (A600 nm = 1), cells were stained with labelled lectin-specific IgGs and examined under a fluorescence microscope (100X oil objective). No fluorescence was observed in V5 strain nor in cells incubated with TBS only.
Discussion
In S. cerevisiae, cellular aggregation depends on multiple genes controlled by diverse regulatory networks that determine the structure and composition of cellular surfaces46. Flocculation is strongly influenced by the expression of specific genes among which FLO genes play the main role47. In biotechnological applications, the possibility of inducing controlled flocculation in a naturally non-flocculent strain has great potential for improving brewing, winemaking, baking and ethanol-producing yeast strains48, so that studies on functional FLO genes are essential.
Initially, we focused our attention on three dominant flocculation genes, FLO1, FLO5 and FLO836, in four S. cerevisiae wine strains, i.e. three non-flocculent strains (RT73, F7101 and 59A) and one flocculent strain (F6789). FLO family genes are present in all S. cerevisiae strains independently of their flocculation phenotype. Many studies, in fact, demonstrated that FLO genes are extraordinarily diverse in different laboratory, industrial and wine strains of S. cerevisiae 29, 31, 32. In this study, all wine strains presented genes of different lengths. FLO1 gene was similar in the pulverulent strain RT73 and in the flocculent strain F6789. As expected, the regulator gene FLO8 did not show different sizes in all studied strains. On the contrary, FLO5 size was particularly divergent among the tested strains, with repeated regions that ranged from 1500 bp to 4000 bp. Interestingly, FLO5-repeated regions in F6789 flocculent strain presented 2 bands, suggesting this gene is heterozygous in this diploid strain.
The variability in FLO genes length is due to two characteristics: position and structure. FLO genes (FLO1, FLO5, FLO9 and FLO10) are located in subtelomeric positions (∼10 to 40 kb from the telomeres)20 and contain repeated motifs. Genes residing near telomeres undergo frequent recombinations and duplications while, on the other hand, the central region of FLO genes contains several repeated motifs that provide a substrate for recombinations and generation of variation49. In the present study, all tested strains presented two amplicons for the whole FLO5 gene, one corresponding to the entire gene and the other likely to a pseudogene. Previously, strains F7101 and F6789 had just exhibited another pseudogene corresponding to the B domain of FLO129. The presence of genes encoding homologues of one or several of the A, B or C domains is frequent in the FLO gene family7. Because these genes do not encode all three domains, they may have no function in cell surface adhesion7, 49, but they are important in terms of evolution. It was demonstrated for example that a recombination between the S. cerevisiae-type chromosome VIII and chromosome I of bottom-fermenting yeasts gave rise to Lg-FLO150,the most important FLO gene in lager yeasts49.
We have focused our attention on which of the FLO1 and FLO5 genes in F6789 S. cerevisiae wine strain was responsible for the flocculation phenotype. Gene disruption experiments revealed that FLO5 is the most essential gene for flocculation since its deletion eliminated most of the ability to flocculate. FLO5-deleted strains were only able to form little flocs, which consisted in a few dozen cells slowly sinking down to the bottom of the tube. By contrast, deletion of FLO1 had no detectable impact on flocculation ability. A monitoring of flocculation ability after a cross with a non-flocculent strain revealed a clear co-segregation of the flocculation phenotype with the FLO5 gene inheritance. On the contrary, gene FLO1 from F6789A has proved incapable of ensuring flocculation but could increase flocculation degree in flocculating cells (Group B Figure 5, Supplementary Table S2). Therefore, flocculation in F6789 wine strain is controlled by FLO5, while FLO1 has only a marginal role in the determination of the degree of flocculation. These results suggest the possibly crucial role of Flo5p for cell-cell interactions in S. cerevisiae wine strain F6789.
The distribution of Flo proteins at the cell surface gave a picture consistent with a major role of Flo5p; FLO5 deletion induced a strong reduction in the number of fluorescent cells while FLO1 deletion only weakly affected immunofluorescence. These data strengthen the role of FLO5 driving flocculation in the F6789 strain. However, in strain F6789A-Δflo5 only 25% of the cells exhibited some fluorescence. This finding suggests that the residual flocculation is the result of the expression of other flocculin(s), which could be driven at least in part by FLO1.
All considered strains showed non-homogeneous staining with completely unstained cells and cells coloured only in some parts of the cell wall. Our results are in agreement with other studies that demonstrated that flocculins are only expressed in a subpopulation within a community6. The authors highlighted that flocs can be formed by a mixed population of FLO1 and flo1 “cheater” cells that receive the protective benefits. Powell et al.51 suggested that the source of the observed difference in flocculation might be a variation in cell wall composition between young and old cells. They proposed that older cells, that are rougher than young cells and that express FLO genes, might act as nucleation points for floc formation. Other studies demonstrated that flocculins distribution on the cell surface is heterogeneous: El-Kirat-Chatel et al.52 evidenced that Flo1p is distributed in clusters at the cell surface. Furthermore, Bony et al.53 and Javadekar et al.54 observed an intense concentration of flocculins at the neck of the mother–daughter junction and of the buds.
In this study, we highlight the key role of the FLO5 gene in cell-surface adhesion. In fact, strains with a FLO5 deletion exhibited a non-adherent phenotype. It is generally recognized that biofilm formation, pseudohyphal and invasive growth depend of cell wall adhesin gene FLO11 (MUC1). Moreover, Torbensen et al.55 demonstrated that this gene could be replaced by another FLO gene. Furthermore, other studies showed that progressively deleting tandem repeats within FLO1 causes corresponding decreases in different adhesive phenotypes, such as adherence to plastic and flocculation56.
S. cerevisiae strain F6789 showed a high flocculation degree. This led us to investigate the nucleotide sequence of the A domain of Flo5p and Flo1p, i.e. the N-terminal region of the protein responsible for the binding with sugars. The N-terminal alignment of gene FLO5 between F6789 and S288C strains highlighted the presence of only two point mutations. As reported by Veelders et al.17, Flo proteins share high sequence identity; in particular the Flo5 A domain shares high similarity with the N-terminal sequence of Flo1 (94%), Flo9 (89%), and Flo10 (64%). Moreover, point mutations in the N-terminal of Flo5p can increase or decrease substrate affinity determining different binding strengths17. Otherwise, when we compared F6789 Flo1p sequence with other flocculent and laboratory strains, several mutations in the N-terminal region were found. Haploid segregants obtained with the flocculent hybrid PDG 42 that had inherited the FLO1 gene from F6789 were not able to achieve flocculation. It could be due to the mutations in this region or to the different expression of Flo1p29, 40. Further studies are necessary to explain these data.
Except for the FLO genes in model yeast strains such as S288C, our current knowledge of the natural diversity of FLO genes is still very limited28. Even if FLO1 and FLO5 are paralogues57, 58 and even if the molecular basis of flocculation is essentially identical, Watari et al.59 speculated that the flocculation properties of most industrial flocculent strains might derive from FLO5 gene expression. Indeed, all the industrial flocculent strains that they examined were heat-sensitive and chymotrypsin-resistant, indicating that the flocculence phenotype could be determined by the FLO5 gene.
Our study confirms this hypothesis; in fact, we found that in flocculent S. cerevisiae wine strain F6789, the FLO5 gene drives flocculation phenotype. In fact, a FLO5 deletion causes a strong reduction in flocculation ability. Furthermore, this study shows that FLO5 influences the adhesive properties of this flocculent yeast. This specific case confirms that the role of a given flocculin can also be played by another flocculin because the members of this family have partial functional redundancy60. Random events, related to yeast evolution in different environments or in response to specific interspecies association within the microbial ecosystem33, most probably led to the expansion of a specific FLO gene that would become essential for flocculation in a given strain.
Materials and Methods
Saccharomyces cerevisiae strains
In this study 4 S. cerevisiae strains were used: a flocculent strain (F6789), a strain that had lost this capacity (F7101)29, 38, a pulverulent strain isolated from Montepulciano d’Abruzzo wine39, and a non-flocculent strain (59A, a haploid derivative of Lalvin EC1118). The wine strain V5 was used as negative control. Deletion mutants were originated from F6789 (reference parental strain).
To obtain a haploid strain from F6789, the HO gene was deleted using the lithium acetate method61 (sequences primers HO del F and HO del R62 reported in Supplementary Table S1). The G418 resistance marker kanMx4 from the pUG6 plasmid was used. Spores were isolated by micromanipulation (Singer Instruments, Roadwater, United Kingdom). Stable haploid spores disrupted for the HO gene were selected on YEPD plates supplemented with the corresponding antibiotic (G418 at 200 μg/mL).
The mating type of the haploids was determined through crossing experiments with reference strains of known mating type. The F6789A-Δho (MATα) strain was selected for further analysis.
FLO genes deletion
The flocculent S. cerevisiae haploid strain F6789A-Δho was transformed with two different plasmids as reported above. The FLO1-deleted transformant, F6789A-Δflo1, was obtained using a PCR-amplified fragment of the pAG25 plasmid (NatMx4 cassette) carrying the selective marker for nourseothricin, and primers FLO1 del F and FLO1-5 del R (Supplementary Table S1). For FLO5 deletion (F6789A-Δflo5) the pAG32 plasmid (containing the hygromycin resistance) was used and amplification was performed with FLO5 del F and FLO1-5 del R (Supplementary Table S1). The reaction was performed in a total volume of 50 μL using the Ex Taq™ kit (Ex Taq™, TAKARA BIO INC., Otsu, Shiga, Japan). Hygromycin or nourseothricin-resistant colonies were analysed by PCR to confirm the correct genomic integration of the cassette.
Media and culture conditions
Yeast pre-cultures were done by selecting a colony from cultures on fresh yeast extract-peptone-dextrose (YEPD) agar plates (10 g/L Bacto-yeast extract, 20 g/L Bacto-peptone and 20 g/L dextrose). Sporulation was induced on spoMA medium (1 g/L Bacto-yeast extract, 0.5 g/L glucose, 10 g/L potassium acetate and 20 mg/L adenine) at 28 °C. Transformed strains were selected and grown on YEPD medium supplemented with 200 mg/L geneticin (Sigma-Aldrich, Lezennes, France), hygromycin (Sigma-Aldrich) or nourseothricin (Sigma-Aldrich)63. Flocculation degree was quantified in YNB+glucose (YNB 0.67% w/v, glucose 2% w/v), YEPD and synthetic must MS42564. Cells were stored in YEPD broth supplemented with glycerol (Sigma-Aldrich, 20% v/v final concentration) at −80 °C.
PCR amplification of FLO1, FLO5 and FLO8 genes
Primers used in this study are listed in Supplementary Table S1. Based on the S288C genome, primers were designed using the Unipro UGENE software 1.19.0 to selectively amplify the entire sequence of FLO1, FLO5 and FLO8 genes. To develop oligos for FLO1, FLO5 and FLO11, repeated regions in the long intragenic repeats (>40 nt) were identified using the EMBOSS ETANDEM software26 with the threshold score set at 20. Primer specificity was checked with a BLAST analysis of all FLO genes from strain S288C.
Total genomic DNA was extracted using the Wizard® Genomic DNA Purification Kit (Promega Corporation, Charbonnières-les-Bains, Lyon, France), according to the manufacturer’s instructions. PCR amplification reactions were performed in a total volume of 25 μL. Each reaction mixture contained 20 ng template DNA, 10 X Ex Taq™ buffer (Ex Taq™, TAKARA BIO INC.) containing 4.5 mM MgSO4, 4 μL of 10 mM dNTPs, 20 pmol each of the amplification primers and 0.20 μL Takara ex Taq DNA Polymerase. PCR was performed using a TRIO Thermal Cycler (Biometra, Göttingen, Germany). PCR products were visualised on a 1% agarose gel and acquired using the Kodak GL 100 imaging system (Fisher Scientific, Illkirch-Graffenstaden, France). Gel conversion, normalisation and analysis were carried out using Fingerprinting II Informatix™ Software (Bio-Rad, Milan, Italy). PCR products were quantified with a NanoDrop 1000 (Thermo Fisher Scientific, Villebon-sur-Yvette, France), and purified by NucleoSpin Gel and PCR clean-up kit (Macherey Nagel SARL, Sète, France) for sequence analysis.
The N-terminal sequence of FLO1 (primers FLO1 A, FLO1 BF and FLO1 BR) and FLO5 (primers FLO5-AF and FLO5-AR, FLO5-BF and FLO5-BR) genes was amplified using the KAPA HiFi PCR Kits (Kapa Biosystems, Nanterre, France), according to the manufacturer’s instructions. PCR products were sequenced (Eurofins MWG GmbH, Martinsried, Germany) and the resulting sequences translated using the «Expasy Translate Tool» (http://www.expasy.org/tools/dna.html) program. Alignments were made using program Clustal omega (http://www.ebi.ac.uk/Tools/msa/clustalo).
Ploidy level of S. cerevisiae strains
Strain ploidy level was assessed using SYTOX® Green (Invitrogen) fluorescence and flow cytometry according to Delobel and Tesnière65, using an Accuri C6TM flow cytometer. A single colony was selected and grown overnight in liquid YEPD with shaking at 150 rpm. Cells in exponential phase were collected by centrifugation and then re-suspended in 1 mL water. Eight ml of 75% ethanol were added and fixation was performed overnight at 4 °C. Fixed cells were harvested by centrifugation and washed with 1 mL PBS 1X. Cells were re-suspended in 500 μL RNAse A (Qiagen, Paris, France) solution (2 mg/mL RNAse A in 10 mM Tris-HCl, 15 mM NaCl) and incubated at 37 °C for 1 h and finally re-suspended in 200 μL of proteinase K (Roche, Paris, France) solution (1 mg/Ml in PBS 1X). After a 1 h- incubation at 50 °C, cells were collected by centrifugation, re-suspended in 500 μL PBS 1X, sonicated for 15 s, and stored on ice until analysis. Fifty µL of cell suspension were added to 200 μl of SYTOX® Green solution (1.25 µM SYTOX® Green in PBS 1X). Analysis was performed with a Multisizer 3 Particle Counter (Beckman Coulter, Paris, France). The FL1 detector (530/30 nm) was used for the acquisition of SYTOX® Green fluorescence, as this dye is taken up by cells in a manner stoichiometric to the amount of nuclear DNA.
Phenotype investigation
Flocculation trials
Flocculation was quantified by measuring the optical density of yeast suspensions after shaking45. Yeast cells were harvested after 48 h, washed twice in deflocculation buffer (5 mM EDTA; 50 mM sodium citrate pH = 3) and suspended in the same buffer at the final concentration of 5 × 107 cells/mL. Flocculation was induced by addition of CaCl2 (20 mM) to 5 mL of the cell suspension. The tubes were placed on a shaker and agitated at 50 oscillations/min, for 5 min at room temperature. An aliquot of 200 μL of the upper phase was withdrawn and 1 mL of 250 mM EDTA (pH = 8) was added. The optical density at 600 nm was measured immediately and at 30 s intervals for 3 min using a spectrophotometer (Spectronic Model 601, Spectronic Instruments, Rochester, NY, USA). Three individual transformants were used to generate the data with regard to the expression of FLO5 truncations, while only one transformant for FLO1 deletion. All analyses were performed in triplicate.
Invasive growth assay
Invasive growth assay was performed as previously described by Tofalo et al.29. A fresh culture of each strain was isolated on YEPD plates with a toothpick and incubated for 3 days at 28 °C and then for 2 days at room temperature. Deionized water was used to wash all cells off the agar surface, leaving subsurface cells that had actually invaded the agar. Plates were photographed before and after washing. All analyses were performed in triplicate.
Microscopy
Cells were examined using an Axio Imager.A2 epifluorescent microscope (Zeiss, Carl Zeiss Inc., Thornwood, NY, USA).
Cross
Diploid strains were originated by mating 59A (MATa) and F6789A-Δho (MATα) haploid cells and the selection of zygotes was done by micromanipulation (Singer Instruments). Zygote ploidy was tested by flow cytometry as reported above. A diploid hybrid was sporulated and tetrads containing the four haploid meiotic products were dissected. Twenty-seven segregants were considered for further analyses.
Phenotype and genotype of cell aggregation
To test flocculation, cells were cultured in YEPD liquid medium at 28 °C at 240 rpm for 48 h to make the cells enter the stationary phase of growth. Cultures were swirled briefly for 30 s with a vortex shaker before the sediment test. Cells were photographed after 30 s in vertical position. FLO5 repeated regions were investigated as reported above.
Immunofluorescent staining
Immunofluorescent staining was carried out according to Bidard et al.45. The antiserum was pre-adsorbed with V5 strain and was used at a 1:10 dilution. After deflocculation (0.5 M EDTA) stationary-phase yeast cells (107 cells/mL) were centrifugated and suspended in TBS buffer (10 mM Tris-HCl, 140 mM NaCl, 5 mM EDTA, 20 μg/mL cycloheximide). Thirty μL of the antiserum were added and shakenfor 75 min. Cells were harvested by centrifugation and washed three times in TBS buffer. Thirty μL fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (Sigma–Aldrich) diluted (1:160) in the same buffer were added. After 30 min shaking, cells were washed three times in the same buffer and 10 μL water containing 1 mg/mL of p-phenylenediamine were added. Cells were photographed by immunofluorescence microscopy (Zeiss Axio Imager.A2). The light was set at 521 nm and a 100X oil objective was used.
Electronic supplementary material
Acknowledgements
This work is part of the project named “Produzione di Vini spumanti di qualità a partire dai vitigni autoctoni” funded by the Abruzzo Region within the Rural Development Programme 2007/2013. We are grateful to Martine Pradal for providing technical assistance in the development of the experiments. We apologize for the omission of relevant references due to space restrictions. We also thank the reviewers for their suggestions and Philippe Chatelet for his useful suggestions on the text.
Author Contributions
Designed project: B.B., G.S. and R.T. Performed experiments and analysed data: P.D.G., C.T. Wrote manuscript: P.D.G., C.T. and R.T. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09990-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Giovanna Suzzi, Email: gsuzzi@unite.it.
Bruno Blondin, Email: blondin@supagro.inra.fr.
References
- 1.Ielasi FS, Goyal P, Sleutel M, Wohlkonig A, Willaert RG. The mannose-specific lectin domains of Flo1p from Saccharomyces cerevisiae and Lg-Flo1p from S. pastorianus: crystallization and preliminary X-ray diffraction analysis of the adhesin–carbohydrate complexes. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2013;F69:779–782. doi: 10.1107/S1744309113015030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratford M. Lectin-mediated aggregation of yeasts – yeast flocculation. Biotechnol. Genet. Eng. Rev. 1992;10:283–341. doi: 10.1080/02648725.1992.10647891. [DOI] [PubMed] [Google Scholar]
- 3.Verstrepen KJ, Derdelinckx G, Verachtert H, Delvaux FR. Yeast flocculation: what brewers should know. Appl. Microbiol. Biotechnol. 2003;61:197–205. doi: 10.1007/s00253-002-1200-8. [DOI] [PubMed] [Google Scholar]
- 4.Kemp B, Alexandre H, Robillard B, Marchal R. Effect of production phase on bottle-fermented sparkling wine quality. J Agric. Food Chem. 2015;63:19–38. doi: 10.1021/jf504268u. [DOI] [PubMed] [Google Scholar]
- 5.Borrull A, Poblet M, Rozes N. New insights into the capacity of commercial wine yeasts to grow on sparkling wine media. Factor screening for improving wine yeast selection. Food Microbiol. 2015;48:41–48. doi: 10.1016/j.fm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Smukalla S, et al. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell. 2008;135:726–737. doi: 10.1016/j.cell.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bojsen, R. K., Andersen, K. S. & Regenberg, B. Saccharomyces cerevisiae – a model to uncover molecular mechanisms for yeast biofilm biology. FEMS Immunol. Med. Mic.65, 169–182 (2012). [DOI] [PubMed]
- 8.Lei J, Zhao X, Ge X, Bai F. Ethanol tolerance and the variation of plasma membrane composition of yeast floc populations with different size distribution. J biotechnnol. 2007;131:270–275. doi: 10.1016/j.jbiotec.2007.07.937. [DOI] [PubMed] [Google Scholar]
- 9.Goossens KVY, et al. Molecular mechanism of flocculation self-recognition in yeast and its role in mating and survival. mBio. 2015;6:e00427–15. doi: 10.1128/mBio.00427-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mill PJ. The nature of the interactions between flocculent cells in the flocculation of Saccharomyces cerevisiae. J. Gen. Micro. 1964;35:61–68. doi: 10.1099/00221287-35-1-61. [DOI] [PubMed] [Google Scholar]
- 11.Miki BLA, Poon NH, James AP, Seligy VL. Possible mechanism for flocculation interactions governed by gene FLO1. Saccharomyces cerevisiae. J. Bacteriol. 1982;150:878–889. doi: 10.1128/jb.150.2.878-889.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speers RA, Smart K, Stewart R, Jin Y-L. Zymolectins in Saccharomyces cerevisiae. J. I. Brewing. 1998;104:298. [Google Scholar]
- 13.Bony M, Thines-Sempoux D, Barre P, Blondin B. Localization and cell surface anchoring of the Saccharomyces cerevisiae flocculation protein Flo1p. J. Bacteriol. 1997;179:4929–4936. doi: 10.1128/jb.179.15.4929-4936.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittet M, Conzelmann A. Biosynthesis and function of GPI proteins in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2007;1771:405–420. doi: 10.1016/j.bbalip.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Li E, et al. Deletion of intragenic tandem repeats in unit C of FLO1 of Saccharomyces cerevisiae increases the conformational stability of flocculin under acidic and alkaline conditions. PLoS ONE. 2013;8:e53428. doi: 10.1371/journal.pone.0053428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol. 2006;60:5–15. doi: 10.1111/j.1365-2958.2006.05072.x. [DOI] [PubMed] [Google Scholar]
- 17.Veelders M, et al. Structural basis of flocculin mediated social behavior in yeast. Proc. Natl. Acad. Sci. USA. 2010;107:22511–22516. doi: 10.1073/pnas.1013210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goossens KVY, Willaert RG. Flocculation protein structure and cell-cell adhesion mechanism in Saccharomyces cerevisiae. Biotechnol. Lett. 2010;32:1571–158. doi: 10.1007/s10529-010-0352-3. [DOI] [PubMed] [Google Scholar]
- 19.Erdman S, Lin L, Malczynski M, Snyder M. Pheromone-regulated genes required for yeast mating differentiation. Journal of cell biology. 1998;140:461–483. doi: 10.1083/jcb.140.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robyr D, et al. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell. 2002;109:437–446. doi: 10.1016/S0092-8674(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 21.Lambrechts MG, Bauer FF, Marmur J, Pretorius IS. MI, a mucin-like protein that is regulated by Mss10, is critical for pseudohyphal differentiation in yeast. Proc. Natl. Acad. Sci. USA. 1996;93:8419–8424. doi: 10.1073/pnas.93.16.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo WS, Dranginis AM. The cell surface flocculin Flo11 is required for pseudohyphae formation and invasion by Saccharomyces cerevisiae. Mol. Biol. Cell. 1998;9:161–171. doi: 10.1091/mbc.9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim TS, Ahn JY, Yoon JH, Kang HS. STA10 repression of STA gene expression is caused by a defective activator, flo8. Saccharomyces cerevisiae. Curr. Genet. 2003;44:261–267. doi: 10.1007/s00294-003-0447-7. [DOI] [PubMed] [Google Scholar]
- 24.Rupp S, Summers E, Lo HJ, Madhani H, Fink G. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269. doi: 10.1093/emboj/18.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobayashi O, Yoshimoto H, Sone H. Analysis of the genes activated by the FLO8 gene in Saccharomyces cerevisiae. Curr. Genet. 1999;36:256–261. doi: 10.1007/s002940050498. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Styles CA, Fink GR. Saccharomyces cerevisiae S288c has a mutation in FLO8, a gene required for filamentous growth. Genetics. 1996;144:967–978. doi: 10.1093/genetics/144.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Govender P, Bester M, Bauer FF. FLO gene-dependent phenotypes in industrial wine yeast strains. Appl. Microbiol. Biotechnol. 2010;86:931–945. doi: 10.1007/s00253-009-2381-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhao XQ, et al. Exploration of a natural reservoir of flocculating genes from various Saccharomyces cerevisiae strains and improved ethanol fermentation using stable genetically engineered flocculating yeast strains. Proc. Biochem. 2012;47:1612–1619. doi: 10.1016/j.procbio.2011.06.009. [DOI] [Google Scholar]
- 29.Tofalo R, et al. Genetic diversity of FLO1 and FLO5 genes in wine flocculent Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2014;191:45–52. doi: 10.1016/j.ijfoodmicro.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez F, et al. Variable flocculation profiles of yeast strains isolated from cachaça distilleries. Int. J. Food Microbiol. 2014;190:97–104. doi: 10.1016/j.ijfoodmicro.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 31.Van Mulders SE, et al. Flocculation gene variability in industrial brewer’s yeast strains. Appl. Microbiol. Biotechnol. 2010;88:1321–1331. doi: 10.1007/s00253-010-2843-5. [DOI] [PubMed] [Google Scholar]
- 32.Verstrepen KJ, Jansen A, Lewitter F, Fink GR. Intragenic tandem repeats generate functional variability. Nat. Genet. 2005;37:986–990. doi: 10.1038/ng1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossouw D, Bagheri B, Setati ME, Bauer FF. Co-flocculation of yeast species, a new mechanism to govern population dynamics in microbial ecosystems. PloS one. 2015;10:e0136249. doi: 10.1371/journal.pone.0136249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Mulders SE, et al. Phenotypic diversity of Flo protein family-mediated adhesion in Saccharomyces cerevisiae. FEMS Yeast Res. 2009;9:178–190. doi: 10.1111/j.1567-1364.2008.00462.x. [DOI] [PubMed] [Google Scholar]
- 35.Hodgson JA, Berry DR, Johnston JR. Discrimination by heat and proteinase treatments between flocculent phenotypes conferred on Saccharomyces cerevisiae by the genes FLO1 and FLO5. Microbiol. 1985;131:3219–3227. doi: 10.1099/00221287-131-12-3219. [DOI] [PubMed] [Google Scholar]
- 36.Stratford M, Assinder S. Yeast flocculation: Flo1 and NewFlo phenotypes and receptor structure. Yeast. 1991;7:559–574. doi: 10.1002/yea.320070604. [DOI] [PubMed] [Google Scholar]
- 37.Suzzi G, Romano P, Zambonelli C. Flocculation of wine yeasts: frequency, differences, and stability of the character. Can J Microbiol. 1984;30:36–39. doi: 10.1139/m84-006. [DOI] [Google Scholar]
- 38.Suzzi G, Romano P. Flocculent phenotypes in wine yeasts. Lett. Appl. Microbiol. 1991;13:7–10. doi: 10.1111/j.1472-765X.1991.tb00556.x. [DOI] [Google Scholar]
- 39.Suzzi G, et al. Effect of grape indigenous Saccharomyces cerevisiae strains on Montepulciano d’Abruzzo red wine quality. Food Res. Int. 2012;46:22–29. doi: 10.1016/j.foodres.2011.10.046. [DOI] [Google Scholar]
- 40.Tofalo R, et al. Characterization of specialized flocculent yeasts to improve sparkling wine fermentation. J. Appl. Microbiol. 2016;120:1574–1584. doi: 10.1111/jam.13113. [DOI] [PubMed] [Google Scholar]
- 41.Perpetuini G, et al. Biodiversity of autolytic ability in flocculent Saccharomyces cerevisiae strains suitable for traditional sparkling wine fermentation. Yeast. 2016;33:303–312. doi: 10.1002/yea.3151. [DOI] [PubMed] [Google Scholar]
- 42.He LY, Zhao XQ, Bai FW. Engineering industrial Saccharomyces cerevisiae strain with the FLO1-derivative gene isolated from the flocculating yeast SPSC01 for constitutive flocculation and fuel ethanol production. Appl. Energy. 2012;100:33–40. doi: 10.1016/j.apenergy.2012.03.052. [DOI] [Google Scholar]
- 43.Watari J, Nomura M, Sahara H, Koshino S, Keränen S. Construction of flocculent brewer’s yeast by chromosomal integration of the yeast flocculation gene. FLO1. J. Inst. Brew. 1994;100:73–77. doi: 10.1002/j.2050-0416.1994.tb00809.x. [DOI] [Google Scholar]
- 44.Silva CLC, Rosa CA, Oliveira ES. Studies on the kinetic parameters for alcoholic fermentation by flocculent Saccharomyces cerevisiae strains and non-hydrogen sulphide-producing strains. World J. Microbiol. Biotechnol. 2006;22:857–863. doi: 10.1007/s11274-005-9115-z. [DOI] [Google Scholar]
- 45.Bidard F, Bony M, Blondin B, Dequin S, Barre P. The Saccharomyces cerevisiae FLO1 flocculation gene encodes for a cell surface protein. Yeast. 1995;11:809–822. doi: 10.1002/yea.320110903. [DOI] [PubMed] [Google Scholar]
- 46.Rodriguez ME, Orozco H, Cantoral JM, Matallana E, Aranda A. Acetyltransferase SAS2 and sirtuin SIR2, respectively, control flocculation and biofilm formation in wine yeast. FEMS Yeast Res. 2014;14:845–857. doi: 10.1111/1567-1364.12173. [DOI] [PubMed] [Google Scholar]
- 47.Bester MC, Jacobson D, Bauer FF. Many Saccharomyces cerevisiae cell wall protein encoding genes are coregulated by Mss11, but cellular adhesion phenotypes appear only flo protein dependent. G3:Genes Genome Genet. 2012;2:131–141. doi: 10.1534/g3.111.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dequin S. The potential of genetic engineering for improving brewing, wine-making and baking yeasts. Appl. Microbiol. Biotechnol. 2001;56:577–588. doi: 10.1007/s002530100700. [DOI] [PubMed] [Google Scholar]
- 49.Verstrepen KJ, Reynolds TB, Fink GR. Origins of variation in the fungal cell surface. Nat. Rev. Microbiol. 2004;2:533–540. doi: 10.1038/nrmicro927. [DOI] [PubMed] [Google Scholar]
- 50.Ogata T, Izumikawa M, Kohno K, Shibata K. Chromosomal location of Lg-FLO1 in bottom-fermenting yeast and the FLO5 locus of industrial yeast. J. Appl. Microbiol. 2008;105:1186–1198. doi: 10.1111/j.1365-2672.2008.03852.x. [DOI] [PubMed] [Google Scholar]
- 51.Powell CD, Quain DE, Smart KA. The impact of brewing yeast cell age on fermentation performance, attenuation and flocculation. FEMS Yeast Res. 2003;3:149–157. doi: 10.1016/S1567-1356(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 52.El-Kirat-Chatel, et al. Forces in yeast flocculation. Nanoscale. 2015;7:1760–1767. doi: 10.1039/C4NR06315E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bony M, Barre P, Blondin B. Distribution of the flocculation protein, Flop, at the cell surface during yeast growth: the availability of Flop determines the flocculation level. Yeast. 1998;14:25–35. doi: 10.1002/(SICI)1097-0061(19980115)14:1<25::AID-YEA197>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.Javadekar VS, Sivaraman H, Sainkar SR, Khan MI. A mannose-binding protein from the cell surface of flocculent Saccharomyces cerevisiae (NCIM 3528): its role in flocculation. Yeast. 2000;16:99–110. doi: 10.1002/(SICI)1097-0061(20000130)16:2<99::AID-YEA500>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Torbensen R, et al. Amino Acid Transporter Genes Are Essential for FLO11 - Dependent and FLO11 -independent biofilm formation and invasive growth in Saccharomyces cerevisiae. PlosONE. 2012;7:e41272. doi: 10.1371/journal.pone.0041272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Westman JO, Mapelli V, Taherzadeh MJ, Franzén CJ. Flocculation causes inhibitor tolerance in Saccharomyces cerevisiae for second-generation bioethanol production. Appl. Environ. Microbiol. 2014;80:6908–6918. doi: 10.1128/AEM.01906-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teunissen AWRH, Steensma HY. The dominant flocculation genes of Saccharomyces cerevisiae constitute a new subtelomeric gene family. Yeast. 1995;11:1001–1013. doi: 10.1002/yea.320111102. [DOI] [PubMed] [Google Scholar]
- 58.Cunha AF, Missawa SK, Gomes LH, Reis SF, Pereira GA. Control by sugar of Saccharomyces cerevisiae flocculation for industrial ethanol production. FEMS Yeast Res. 2006;6:280–287. doi: 10.1111/j.1567-1364.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- 59.Watari J, Kudo M, Nishikawa N, Kamimura M. Construction of flocculent yeast cells (Saccharomyces cerevisiae) by mating or protoplast fusion using a yeast cell containing the flocculation gene. FLO5. Agric Biol Chem. 1990;54:1677–1681. [Google Scholar]
- 60.Dranginis AM, Rauceo JM, Coronado JE, Lipke PN. A biochemical guide to yeast adhesins: glycoproteins for social and antisocial occasions. Microbiol. Mol. Biol. R. 2007;71:282–294. doi: 10.1128/MMBR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schiestl RH, Gietz RD. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 62.Brice C, Sanchez I, Bigey F, Legras J-L, Blondin B. A genetic approach of wine yeast fermentation capacity in nitrogen-starvation reveals the key role of nitrogen signalling. BMC Genomics. 2014;15:495. doi: 10.1186/1471-2164-15-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goldstein AL, McCusker JH. Three new dominant drug resistance cassettes for gene disruption in Saccharomyces cerevisiae. Yeast. 1999;15:1541–1553. doi: 10.1002/(SICI)1097-0061(199910)15:14<1541::AID-YEA476>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 64.Bely M, Sablayrolles J-M, Barre P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferm. Bioeng. 1990;70:246–252. doi: 10.1016/0922-338X(90)90057-4. [DOI] [Google Scholar]
- 65.Delobel, P. & Tesnière, C. A simple FCM method to avoid misinterpretation in Saccharomyces cerevisiae cell cycle assessment between G0 and sub-G1. PloS ONE.9, e84645 (2014). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.