Abstract
Objective:
Recent data suggest stroke incidence is decreasing over time, but it is unknown whether incidence is decreasing in women and men to the same extent.
Methods:
Within our population of 1.3 million, all incident strokes among residents ≥20 years old were ascertained at all hospitals during July 1993–June 1994 and calendar years 1999, 2005, and 2010. A sampling scheme was used to ascertain out-of-hospital cases. Sex-specific incidence rates per 100,000 among black and white participants, age- and race-adjusted, were standardized to the 2000 US Census population. Trends over time by sex were compared; a Bonferroni correction was applied for multiple comparisons.
Results:
Over the 4 study periods, there were 7,710 incident strokes; 57.2% (n = 4,412) were women. Women were older than men (mean ± SE 72.4 ± 0.34 vs 68.2 ± 0.32, p < 0.001). Incidence of all strokes decreased over time in men (263 [confidence interval 246–281] to 192 [179–205], p < 0.001) but not in women (217 [205–230] to 198 [187–210], p = 0.15). Similar sex differences were seen for ischemic stroke (men, 238 [223–257] to 165 [153–177], p < 0.01; women, 193 [181–205] to 173 [162–184], p = 0.09). Incidence of all strokes and of ischemic strokes was similar between women and men in 2010. Incidence of intracerebral hemorrhage and subarachnoid hemorrhage were stable over time in both sexes.
Conclusions:
Decreases in stroke incidence over time are driven by a decrease in ischemic stroke in men. Contrary to previous study periods, stroke incidence rates were similar by sex in 2010. Future research is needed to understand why the decrease in ischemic stroke incidence is more pronounced in men.
To accurately assess the public health effects of stroke and to effectively reduce stroke mortality, it is crucial to understand epidemiologic differences between women and men. Because of an increased lifetime risk of stroke as well as worse functional outcomes following stroke,1–4 women may be affected disproportionately by stroke; for these reasons, epidemiologic differences by sex are especially important to investigate.
Though previous literature has consistently shown that age-adjusted stroke risk is higher in men,5–7 women face a higher lifetime risk of stroke compared with men due to longer life expectancies. Overall stroke incidence has been decreasing over time,8 but whether there are sex differences in temporal trends of stroke incidence is less clear. The fact that stroke has declined to the fifth leading cause of death for men but remains the fourth leading cause of death for women suggests that stroke incidence may be decreasing faster in men compared with women.9,10 In addition, some reports indicate that case-fatality may be decreasing more in men than in women.5,11
Recent decreases in stroke incidence are likely a result of improved control of risk factors, including hypertension, diabetes, dyslipidemia, and smoking.11,12 Given known sex differences in risk factor profiles, understanding temporal patterns in the prevalence of stroke risk factors by sex is necessary to reduce stroke incidence and mortality in both sexes. Recently, more attention has been paid to stroke prevention in women as demonstrated by published guidelines for approaches to managing stroke risk factors that are more prevalent in or unique to women.13 It is unknown whether sex-specific prevention strategies are being implemented routinely or if they are effective.
Our primary study objective was to determine whether stroke incidence has been decreasing over the past 16 years to the same extent in women compared with men. Our second objective was to investigate 30-day stroke case-fatality rates over time by sex. A third objective was to compare the prevalence of stroke risk factors and related medication use between women and men over time as potential contributors to sex differences in stroke incidence.
METHODS
Study setting/population.
We used data from the Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS), a population-based study of a largely biracial population of approximately 1.3 million people who reside in a 5-county region of Southwest Ohio/Northern Kentucky. All first-ever strokes among adult (≥20 years old) residents of the region were ascertained during 4 study periods: July 1993 to June 1994, and calendar years 1999, 2005, and 2010. All cases presenting to the acute care hospitals in the study region (14–17 hospitals depending on the study period) were included and identified using ICD-9 codes 430–436. Cases not identified through hospital records were ascertained through records from the region's public health clinics, hospital-based outpatient clinics, and coroners' offices, as well as through sampling of primary care offices and nursing homes in the study region, according to a predefined scheme. All cases of stroke during the study periods were verified by study physicians. Cases of TIA were excluded. Methods for case screening and verification remained consistent across all 4 time periods. Further details regarding the GCNKSS methodology have been previously published.14 This study was approved by the institutional review board at each participating hospital during each study period.
Stroke incidence.
Sex-specific incidence rates per 100,000, adjusted for age and race, and standardized to the 2000 US census population were calculated for overall stroke and for the individual stroke subtypes ischemic stroke, intracerebral hemorrhage (ICH), and subarachnoid hemorrhage (SAH) during each study period. For our main study outcome, trends in incidence rates by sex were compared over time and specifically between 1993/1994 and 2010.
Case-fatality.
Case-fatality was defined as death from any cause within 30 days of incident stroke. Deaths were verified using a combination of death certificate data from the Ohio and Kentucky Departments of Vital Statistics and the national Social Security Death Index. If death was reported during the index hospitalization, the state mortality sources or Social Security Death Index was used for corroboration. Sex-specific case-fatality rates, adjusted for age and race, were calculated for all stroke subtypes and for specific stroke subtypes (ischemic stroke, ICH, SAH) for each of the 4 study periods.
Stroke risk factors.
Data on the prevalence of stroke risk factors and medication use were taken from our general population survey (a random-digit dial survey) conducted during each of 4 study periods: 1995, 2000, 2005, and 2011. The survey sampling design, using predefined quotas, is such that survey participants' characteristics (sex, race, age) are representative of the GCNKSS ischemic stroke study population. Detailed methodology regarding the survey has been published previously.15,16 Survey respondents were asked to report whether they were ever diagnosed with common stroke risk factors and whether they were currently taking medications to control certain conditions. We examined the proportion of women vs men who reported having the risk factors hypertension, hypercholesterolemia, diabetes, heart disease, prior stroke/TIA, and current smoking and who reported using antihypertensive medications, lipid-lowering agents, and aspirin. Data on warfarin use were available only in 2011.
Statistical approach.
Demographics.
Demographic characteristics by sex were compared over time in years, using a general linear model. A Tukey-Kramer adjustment was used to control for multiple comparisons across years.
Stroke incidence.
Incidence rates with 95% confidence intervals were estimated using the age-, race-, and sex-specific raw counts from the GCNKSS as the numerator. The denominator was the population age-, race-, and sex-specific estimate for the appropriate study year for the 5-county area. This rate was adjusted to the 2000 US census.17 The primary comparison was the change in incidence between 1993/1994 and 2010. We also compared sex-specific incidence rates between adjacent study periods (1993/1994 vs 1999, 1999 vs 2005, and 2005 vs 2010). Due to these 4 multiple comparisons, p values were adjusted using a Bonferroni correction. To compare the trend over time between women and men, a general linear model was used with an offset of the log of the population size.
Case-fatality.
General linear models adjusted for age, race, and year were used to examine changes over time and the difference between women and men; the model assumed a Poisson distribution with a log link. A Tukey-Kramer adjustment for multiple comparisons was used when examining differences between women and men for case-fatality at each timepoint.
Risk factors.
Generalized linear models were used to assess overall trends over the 4 study periods by sex for each risk factor, medication, or demographic variable, and to compare women vs men at each timepoint; Tukey-Kramer adjustments were used to control for the multiple comparisons. Interaction terms of sex by year were included.
For all analyses, testing was 2-sided, and p < 0.05 was considered significant (an adjusted p value when noted due to multiple testing). Data analyses were performed using SAS, Version 9.4 (SAS Institute, Cary, NC). Further details regarding statistical methods are in the supplemental data at Neurology.org.
RESULTS
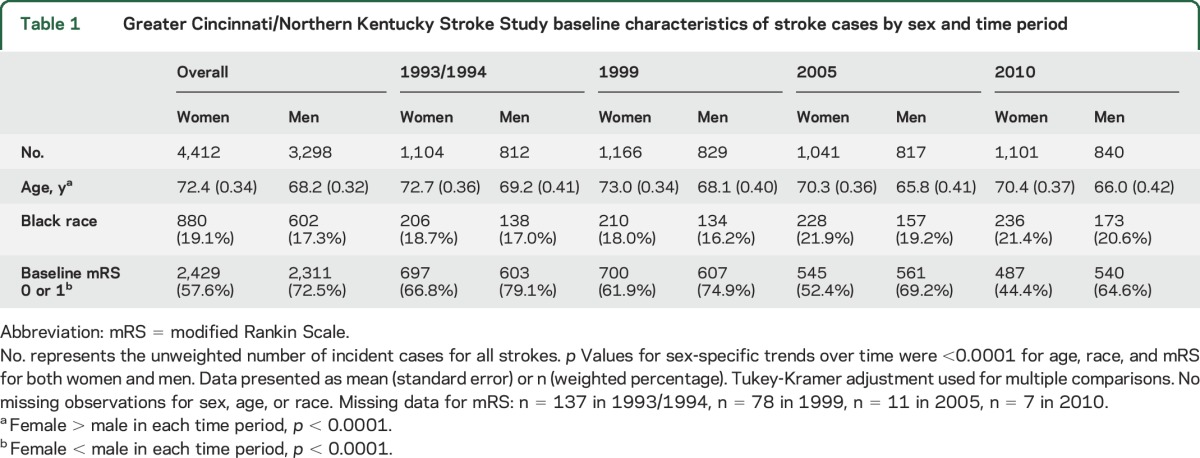
Table 1 shows baseline characteristics by sex. Across the 4 study periods, there were 7,710 incident strokes, 4,412 (57.2%) of which occurred in women. On average, women were older in each study period; the overall mean age for women was 71.6 (SD 15.1) vs 67.3 (SD 14.1) for men (p < 0.001). Overall, the proportion of black patients was similar by sex (women, n = 880, 19.9% vs men, n = 602, 18.3%, p = 0.91). Overall, a smaller proportion of women had prestroke modified Rankin Scale (mRS) scores of 0 or 1 than men (n = 2,429, 55.1% vs n = 2,311, 70.1%, p < 0.0001).
Table 1.
Greater Cincinnati/Northern Kentucky Stroke Study baseline characteristics of stroke cases by sex and time period
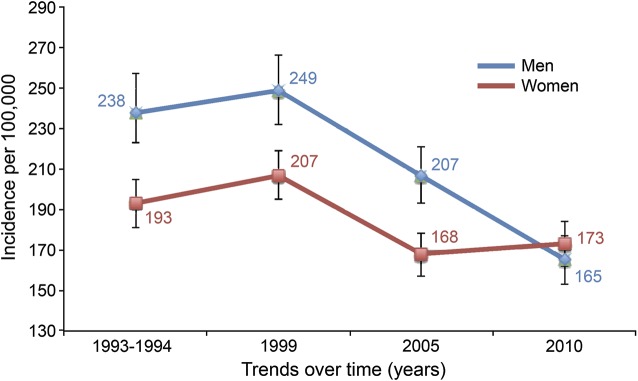
The decrease in incidence of all strokes (table 2, figure) over time was significant only in men. There was a decrease in incidence between 1993/1994 and 2010 for men (263 per 100,000 in 1993/1994 to 192 per 100,000 in 2010, p < 0.001) but not for women (217 per 100,000 in 1993/1994 to 198 per 100,000 in 2010, p = 0.15). The year by sex interaction had a p value of 0.10. In 2010, incidence of all strokes was not different between women and men (p = 0.50). The decrease in incidence of ischemic stroke over time was significant only in men. There was a decrease in incidence between 1993/1994 and 2010 in men (238 per 100,000 in 1993/1994 to 165 per 100,000 in 2010, p < 0.001) but not in women (193 per 100,000 in 1993/1994 to 173 per 100,000 in 2010, p = 0.09). The p value was 0.07 for the year by sex interaction. There was no difference in incidence of ischemic stroke between women and men in 2010 (p = 0.31). ICH and SAH incidence did not decrease over time between first and last study periods in women (p = 0.99 and p = 0.70, respectively) or men (p = 0.46 and p = 0.16, respectively).
Table 2.
Greater Cincinnati/Northern Kentucky Stroke Study stroke incidence rates per 100,000 (95% confidence interval) over time by sex, adjusted to the 2000 US population
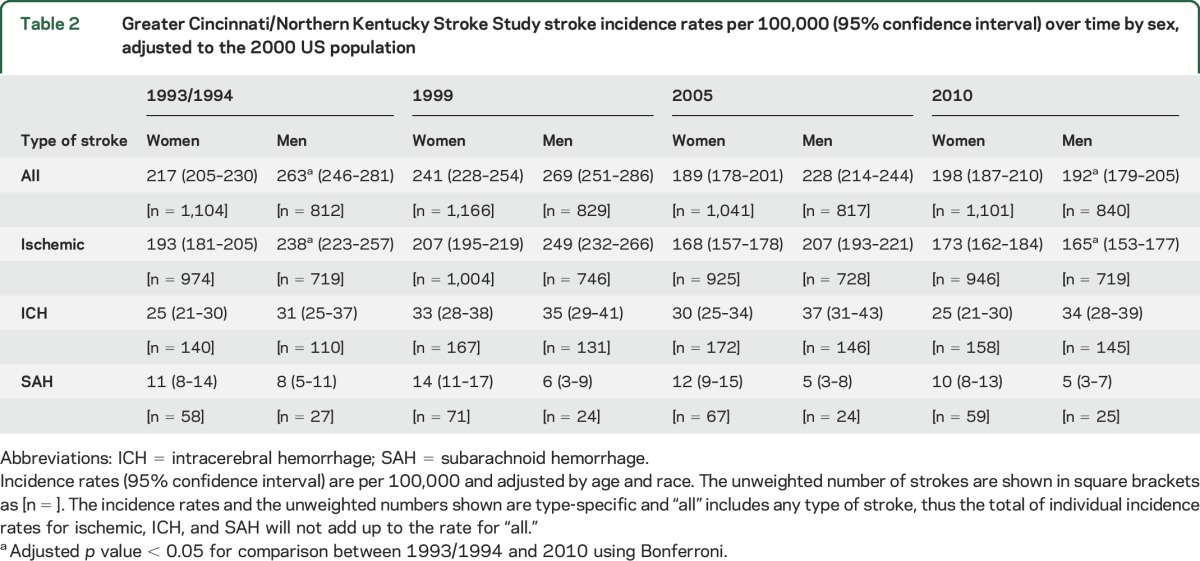
Figure. Ischemic stroke incidence rates, trends over time by sex.
Incidence rates and 95% confidence intervals. Incidence rates were adjusted by age and race. p Value for comparisons made between 1993/1994 and 2010 was <0.001 in men and 0.09 in women. Bonferroni corrections were made for multiple comparisons.
When adjacent study periods were compared, rates of all stroke for 1993/1994 vs 1999 were similar for both women (p = 0.06) and men (p = 0.64). Although rates for women decreased between 1999 and 2005 (p < 0.0001), they were not different between 2005 and 2010 (p = 0.27). Rates for men, however, decreased between 1999 and 2005 (p = 0.003) and between 2005 and 2010 (p = 0.002). Similar patterns were observed with ischemic stroke. Rates for women did not change for 1993/1994 vs 1999 (p = 0.57), decreased for 1999 vs 2005 (p < 0.0001), and did not change for 2005 vs 2010 (p = 0.48), whereas the rates for men did not change for 1993/1994 vs 1999 (p = 0.35) but decreased for both 1999 vs 2005 (p = 0.001) and 2005 vs 2010 (p < 0.0001). When trends over time were compared between women and men, sex by time interaction terms had p values of 0.11, 0.07, 0.79, and 0.59 for all stroke, ischemic stroke, ICH, and SAH, respectively.
There were no significant differences in case-fatality over time (table 3) in either sex or between women and men within each study period for all strokes, for ischemic strokes, or for SAH.
Table 3.
Greater Cincinnati/Northern Kentucky Stroke Study 30-day case-fatality rates (adjusted for age and race) after incident stroke, by sex and stroke subtype
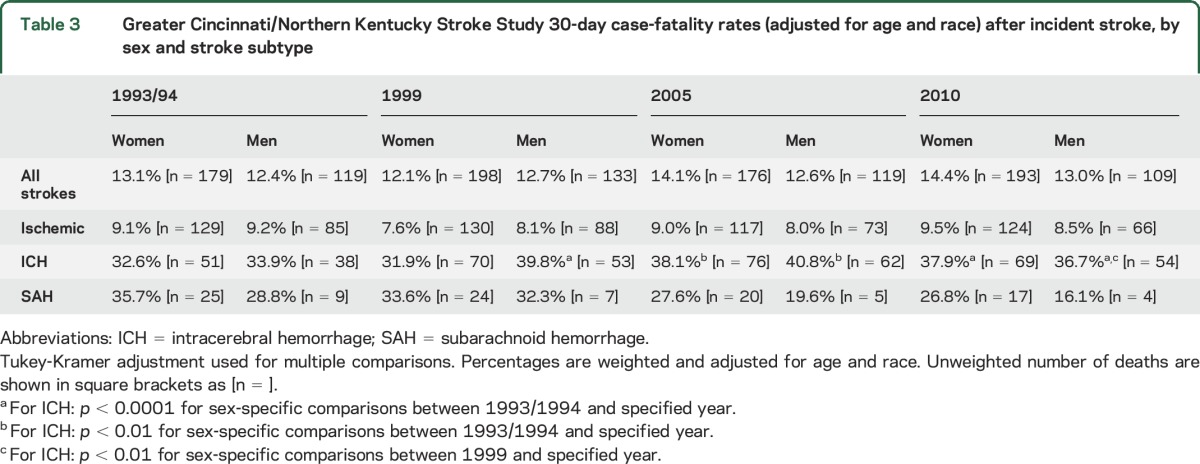
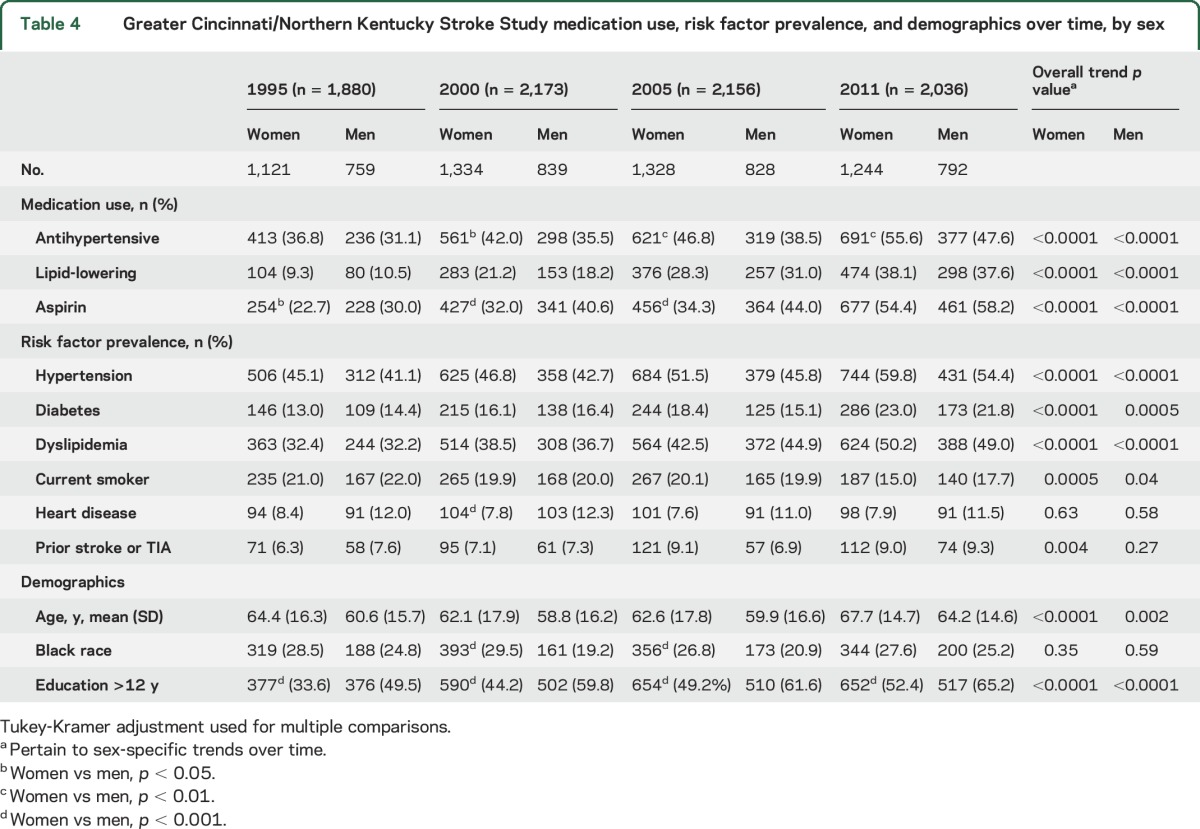
Table 4 displays risk factor prevalence and related medication use by sex over time. In all study periods, significantly fewer women reported completing at least 12 years of education compared with men. A higher proportion of women reported having a diagnosis of hypertension and taking antihypertensive medications compared with men in 2000, 2005, and 2011. A lower proportion of women reported aspirin use in 2000 and 2005. The use of antihypertensive medications, lipid-lowering agents, and aspirin increased over time in both women and men. For trends in risk factor prevalence over time, the prevalence of hypertension, diabetes mellitus, and hyperlipidemia increased, and current smoking decreased; these trends were significant in both women and men across the 4 study periods. Use of warfarin was 6.4% in women and 7.1% in men in 2011.
Table 4.
Greater Cincinnati/Northern Kentucky Stroke Study medication use, risk factor prevalence, and demographics over time, by sex
DISCUSSION
Data in recent years have shown a decrease in mortality from stroke, thought to be largely due to decreasing stroke incidence over time.5,8,11 Our study findings, however, show that the overall decrease in stroke incidence over time is primarily driven by a decrease in ischemic stroke in men. Like previous data,7 our findings show that age-adjusted stroke incidence was higher in men between 1993 and 2005; however, in 2010, stroke incidence rates were similar between the sexes, a critical and novel finding of this study. The similar incidence rates in women compared with men in 2010 may be a result of a more substantial decrease in stroke incidence in men than women over time. Our findings are in line with recent data from the Centers for Disease Control and Prevention showing that stroke has decreased to the fifth leading cause of death for men yet remains the fourth leading cause of death for women.9,10 Though case-fatality is another possible contributor to the difference in stroke mortality between women and men, our findings suggest that there is no significant difference in case-fatality by sex and that there have been few significant temporal changes in stroke case-fatality either in men or in women.
Our findings conflict to some degree with other studies, though these differences may be due largely to study design. Carandang et al.5 used the Framingham cohort and found no difference by sex but only looked through 2004, while our study includes data through 2010. The Atherosclerosis Risk in Communities (ARIC) study, a prospective cohort study, included data through 2011 and found no sex differences over time; however, it is important to note that both the Framingham and the ARIC study are cohort studies and did not investigate all strokes in a defined geographic population as ours did.5,8 Cohort studies are also prone to other biases including participation bias. A third study, the Oxford Vascular Study, was designed as a population-based study but was limited by power and only looked at incidence rates through 2004.12 Interestingly, though, their results indicate that the decrease in stroke incidence in men was more dramatic than the decrease in women.12
Contributors to the differential decrease of stroke incidence in men compared with women are unclear. Contrary to our initial hypothesis, our analysis of survey data shows that common stroke risk factors have been increasing in both sexes in our population. This contrasts with recent findings from the Framingham study, which reported a decrease in hypertension and hyperlipidemia over time.18 The discrepancies between the Framingham study and our study include a significant difference in the racial and socioeconomic distribution of the study population and differences in how vascular risk factors were defined. The Satizabal study operationalized hypertension and hyperlipidemia using numeric physical examination and laboratory values, whereas our study used a patient-reported history of risk factors. In addition, the sample for the GCNKSS survey was designed to represent the population at risk for stroke according to age, race, and sex, which may affect risk factor prevalence. It is unknown whether our findings of increasing prevalence of stroke risk factors are due to a diagnosis bias or if disease prevalence has truly increased. We also do not know whether risk factors such as hypertension are being controlled equally effectively in women and men; previous data indicate that even when treated with antihypertensive medications, women are less likely to meet blood pressure targets.19,20 Another possibility is that the same degree of risk factor control is associated with a different level of risk in men compared to women, as appears to be the case for diabetes in women.21 It will be important to conduct further research on the driving factors behind the decreasing stroke incidence in men, as such factors could potentially be applied to women as well. We also analyzed self-reported use of medications that help control stroke risk factors and found that both men and women have increased their use of antihypertensives, lipid-lowering medications, and aspirin over time. Though aspirin use doubled over time in women, fewer women than men reported aspirin use overall, a potentially concerning finding given knowledge that aspirin is recommended for women (but not men) 55–79 years old for primary prevention of ischemic stroke.22 This is an opportunity for further research.
Another potential contributor to the greater decrease in stroke incidence in men compared with women is a change in our study population over time. The proportion of patients who were functionally independent as indicated by a baseline mRS of 0 or 1 decreased significantly over time and appears to have decreased more in women than men. Reasons for this are unclear, though it may be driven by the functional status of older women. In addition, we observed a significant decrease in the mean age at time of stroke over time in both women and men and a consistent difference between sexes at each timepoint; women were always older at stroke onset. If mRS is considered a surrogate for other measures of health including comorbidities or frailty, our data may indicate that our study population in 2010, especially women, were in worse health and at higher risk for stroke than in other study periods.
Our finding that the incidence of hemorrhagic stroke did not change significantly over time is consistent with other literature.23 Reasons for this are unclear, but the protective effects of increased use of antihypertensive medications might be attenuated by the increased use of antithrombotics, leading to no net change in incidence.24,25
Our use of self-reported survey data to measure stroke risk factors may lead to an underestimate of the true prevalence of stroke risk factors; such an underestimate may limit our understanding of how stroke risk factors over time influence differential changes in stroke incidence by sex. It is also possible that stroke risk factors such as hypertension and diabetes are less well-controlled in women or that women have an underlying longer duration of such diseases, but our self-reported survey does not capture these data. Our survey instrument does not include items about risk factors unique to or more prevalent in women including atrial fibrillation, exogenous hormones, and migraine that could be changing over time and affecting stroke incidence in women. Other risk factors for which we could not account but that may affect stroke incidence in women more than men include depression and psychosocial stressors. Finally, we do not have data on the sex and demographic characteristics of participants who declined to participate in the survey.
Further discussion regarding changes of stroke incidence between adjacent study periods and of the contribution of factors such as education level of participants and changes in dietary supplements can be found in the supplemental data.
We found that the decrease in overall stroke incidence over time seems to be driven primarily by a decrease in incidence of ischemic stroke in men and that the change in stroke incidence in women over the same period was not significant. These changes may have led to the nearly equal rate of stroke in women and men in 2010. Going forward, we plan to extend our analyses to confirm whether this pattern continues beyond 2010. Our current findings, in combination with our group's past work showing that stroke incidence is not decreasing to the same extent in black compared with white participants,14 demonstrate that targeted stroke prevention efforts may be needed to effectively decrease stroke incidence (and thus mortality from stroke) across all demographic groups.
Supplementary Material
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- GCNKSS
Greater Cincinnati/Northern Kentucky Stroke Study
- ICD-9
International Classification of Diseases–9
- ICH
intracerebral hemorrhage
- mRS
modified Rankin Scale
- SAH
subarachnoid hemorrhage
Footnotes
Supplemental data at Neurology.org
Editorial, page 982
AUTHOR CONTRIBUTIONS
T.E.M. conceived the study question, designed the analysis plan, drafted the manuscript, and is responsible for the manuscript as a whole. J.K. designed the analysis plan, provided statistical guidance, analyzed the data, and contributed to manuscript revision. K.A. contributed to the overall design of the population-based study of stroke cases, supervised data collection, and contributed to manuscript revision. E.R. supervised collection of the survey data. C.J.M., M.L.F., D.W., J.M., F.D.L.R.L.R., S.M., S.F., O.A., P.K., and J.P.B. were responsible for the overall design of the population-based study of stroke cases and contributed to manuscript revision. B.M.K. obtained grant funding, was responsible for the overall design of the population-based study of stroke cases, supervised data collection, and contributed to manuscript revisions. D.K. obtained grant funding, helped to conceive the study questions, was responsible for overall design of the population-based study, supervised data collection, helped to design the analysis plan, and contributed to manuscript revision.
STUDY FUNDING
This study was funded by a grant from the National Institutes of Neurologic Disorders and Stroke (grant RO1 NS 30678).
DISCLOSURE
T. Madsen reports no disclosures relevant to the manuscript. J. Khoury is funded by a research grant (R01NS30678). K. Alwell is funded by a research grant (R01NS30678). C. Moomaw is funded by a research grant (R01NS30678). E. Rademacher is funded by a research grant (R01NS30678). M. Flaherty is funded by a research grant (R01NS30678). D. Woo is funded by a research grant (R01NS30678). J. Mackey is funded by a research grant (R01NS30678). F. De Los Rios La Rosa is a member of Boehringer Ingelheim speaker's bureau. S. Martini reports no disclosures relevant to the manuscript. S. Ferioli is funded by a research grant (R01NS30678). O. Adeoye reports no disclosures relevant to the manuscript. P. Khatri's Department of Neurology at University of Cincinnati received financial support for her research-related activities from Genentech (PRISMS Trial Lead PI), Penumbra (THERAPY Trial Neurology PI), and Biogen (DSMB member). J. Broderick reports no disclosures relevant to the manuscript. B. Kissela is funded by a research grant (R01NS30678). D. Kleindorfer is funded by a research grant (R01NS30678). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Di Carlo A, Lamassa M, Baldereschi M, et al. Sex differences in the clinical presentation, resource use, and 3-month outcome of acute stroke in Europe: data from a multicenter multinational hospital-based registry. Stroke 2003;34:1114–1119. [DOI] [PubMed] [Google Scholar]
- 2.Gall SL, Tran PL, Martin K, Blizzard L, Srikanth V. Sex differences in long-term outcomes after stroke: functional outcomes, handicap, and quality of life. Stroke 2012;43:1982–1987. [DOI] [PubMed] [Google Scholar]
- 3.Gargano JW, Reeves MJ. Sex differences in stroke recovery and stroke-specific quality of life: results from a statewide stroke registry. Stroke 2007;38:2541–2548. [DOI] [PubMed] [Google Scholar]
- 4.Lisabeth LD, Reeves MJ, Baek J, et al. Factors influencing sex differences in poststroke functional outcome. Stroke 2015;46:860–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carandang R, Seshadri S, Beiser A, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA 2006;296:2939–2946. [DOI] [PubMed] [Google Scholar]
- 6.Reeves MJ, Bushnell CD, Howard G, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 2008;7:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke 2009;40:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koton S, Schneider AL, Rosamond WD, et al. Stroke incidence and mortality trends in US communities, 1987 to 2011. JAMA 2014;312:259–268. [DOI] [PubMed] [Google Scholar]
- 9.Leading causes of death in females. In: Centers for Disease Control and Prevention Website [online]. Available at: cdc.gov/women/lcod/index.htm. Accessed April 20, 2017. [Google Scholar]
- 10.Leading causes of death in males: United States. In: Centers for Disease Control and Prevention Website [online]. Available at: cdc.gov/men/lcod/2013/index.htm. Accessed April 20, 2017. [Google Scholar]
- 11.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics: 2014 update: a report from the American Heart Association. Circulation 2014;129:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothwell PM, Coull AJ, Giles MF, et al. Change in stroke incidence, mortality, case-fatality, severity, and risk factors in Oxfordshire, UK from 1981 to 2004 (Oxford Vascular Study). Lancet 2004;363:1925–1933. [DOI] [PubMed] [Google Scholar]
- 13.Bushnell C, McCullough LD, Awad IA, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:1545–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleindorfer DO, Khoury J, Moomaw CJ, et al. Stroke incidence is decreasing in whites but not in blacks: a population-based estimate of temporal trends in stroke incidence from the Greater Cincinnati/Northern Kentucky Stroke Study. Stroke 2010;41:1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleindorfer D, Khoury J, Broderick JP, et al. Temporal trends in public awareness of stroke: warning signs, risk factors, and treatment. Stroke 2009;40:2502–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider AT, Pancioli AM, Khoury JC, et al. Trends in community knowledge of the warning signs and risk factors for stroke. JAMA 2003;289:343–346. [DOI] [PubMed] [Google Scholar]
- 17.Sekar P, Flaherty M, Woo D, Buncher R. A macro for the calculation of age, gender and race adjusted incidence and mortality rates [computer program]. In: SAS Proceedings. Available at: lexjansen.com/mwsug/2005/Pharmaceutical_Healthcare/PH600.pdf. Accessed July 15, 2015. [Google Scholar]
- 18.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aronow WS, Fleg JL, Pepine CJ, et al. ACCF/AHA 2011 expert consensus document on hypertension in the elderly: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents. Circulation 2011;123:2434–2506. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA 2005;294:466–472. [DOI] [PubMed] [Google Scholar]
- 21.Peters SA, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet 2014;383:1973–1980. [DOI] [PubMed] [Google Scholar]
- 22.Force USPST. Aspirin for the prevention of cardiovascular disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2009;150:396–404. [DOI] [PubMed] [Google Scholar]
- 23.Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case-fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 24.Bejot Y, Cordonnier C, Durier J, Aboa-Eboule C, Rouaud O, Giroud M. Intracerebral haemorrhage profiles are changing: results from the Dijon population-based study. Brain 2013;136:658–664. [DOI] [PubMed] [Google Scholar]
- 25.Lovelock CE, Molyneux AJ, Rothwell PM, Oxford Vascular S. Change in incidence and aetiology of intracerebral haemorrhage in Oxfordshire, UK, between 1981 and 2006: a population-based study. Lancet Neurol 2007;6:487–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.