Abstract
Objective:
To investigate the effect of postoperative delirium on longitudinal brain microstructural changes, as measured by diffusion tensor imaging.
Methods:
We studied a subset of the larger Successful Aging after Elective Surgery (SAGES) study cohort of older adults (≥70 years) without dementia undergoing elective surgery: 113 participants who had diffusion tensor imaging before and 1 year after surgery. Postoperative delirium severity and occurrence were assessed during the hospital stay using the Confusion Assessment Method and a validated chart review method. We investigated the association of delirium severity and occurrence with longitudinal diffusion changes across 1 year, adjusting for age, sex, vascular comorbidity, and baseline cognitive performance. We also assessed the association between changes in diffusion and cognitive performance across the 1-year follow-up period, adjusting for age, sex, education, and baseline cognitive performance.
Results:
Postoperative delirium occurred in 25 participants (22%). Delirium severity and occurrence were associated with longitudinal diffusion changes in the periventricular, frontal, and temporal white matter. Diffusion changes were also associated with changes in cognitive performance across 1 year, although the cognitive changes did not show significant association with delirium severity or occurrence.
Conclusions:
Our study raises the possibility that delirium has an effect on the development of brain microstructural abnormalities, which may reflect brain changes underlying cognitive trajectories. Future studies are warranted to clarify whether delirium is the driving factor of the observed changes or rather a correlate of a vulnerable brain that is at high risk for neurodegenerative processes.
Postoperative delirium is among the most frequent complications of surgery in older patients,1 and may lead to progressive cognitive decline and dementia.2–5 The neural correlates of the consequences of delirium are largely unknown. Previous neuroimaging studies suggest that delirium may contribute to the development of brain abnormalities underlying cognitive decline. Duration of delirium was associated with global and regional brain atrophy of the frontal lobe and hippocampus, as well as diffusion tensor imaging (DTI) abnormalities of the corpus callosum in intensive care unit patients.6,7 Structural MRI abnormalities of the corpus callosum,8–10 and changes in network connectivity between the dorsolateral prefrontal cortex and posterior cingulate on resting-state functional MRI,11 have been reported during delirium episodes. Due to confounders and lack of baseline neuroimaging assessment associated with the challenge of conducting neuroimaging studies in patients with delirium,12 the previous studies were not able to address the key question of whether delirium contributes to brain injury or serves only as an indicator of preexisting or coexisting brain abnormalities.
We demonstrated presurgical DTI abnormalities that predispose to postoperative delirium.13 DTI is a sensitive MRI tool for measuring microstructural abnormalities by quantifying the mobility and directionality of water within the brain parenchyma.14,15 In the present study, we investigated the effect of postoperative delirium on longitudinal microstructural changes, as measured by DTI before and 1 year after surgery, in the same cohort of older individuals without dementia undergoing elective surgery. We also investigated the relationship between changes in diffusion and cognitive performance across 1 year.
METHODS
Study design and cohort assembly.
Our study population is a subsample of the ongoing Successful Aging after Elective Surgery (SAGES) prospective cohort study. The study design and methods have been described previously.16,17 Eligible participants were age 70 years and older, English-speaking, scheduled to undergo elective major noncardiac surgery at 1 of 2 Harvard-affiliated academic medical centers, with an anticipated length of stay of at least 3 days. Eligible surgical procedures were total hip or knee replacement; lumbar, cervical, or sacral laminectomy; lower extremity arterial bypass surgery; open abdominal aortic aneurysm repair; and colectomy. Exclusion criteria included evidence of dementia, delirium, hospitalization within 3 months, terminal condition, legal blindness, severe deafness, history of schizophrenia or psychosis, and history of alcohol abuse.17 A total of 560 study participants were enrolled between June 18, 2010, and August 8, 2013. A subset of 147 participants underwent MRI within 1 month prior to surgery. One participant was excluded since the baseline MRI protocol was not completed due to noncompliance. Of the remaining 146 participants, 126 underwent a follow-up scan 1 year after surgery: 12 refused to perform the follow-up scan, 2 dropped out of the overall study, 5 were unable to perform the follow-up scan due to pacemaker implantation or death following the baseline scan, and 1 could not be scheduled for the follow-up scan during the allowable time period. Of the 126 participants who had MRI before and after surgery, 114 had DTI at both time points. DTI failures primarily reflected participant inability to tolerate the full duration of scan (∼45 minutes), since DTI was the final sequence of the MRI protocol. The algorithm used for DTI analysis failed in one participant due to baseline anatomical abnormalities, and this participant was also excluded, yielding a total of 113 participants for the present study.
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all participants according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Women's Hospital, the 2 study hospitals, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
Assessment of postoperative delirium severity and occurrence.
Postoperative delirium severity and occurrence were assessed daily during the hospital stay. Delirium severity was assessed by the Confusion Assessment Method–Severity (CAM-S) Long Form, with scores ranging from 0 to 19 (19 = most severe).18 The sum of all daily CAM-S scores on the daily hospital assessments, regardless of whether or not the patient was delirious, was utilized in the analyses.19
We used both the Confusion Assessment Method (CAM)20 and a validated chart review method21,22 to detect the occurrence of delirium. A patient was deemed to have incident delirium if either or both methods indicated its presence. The CAM is a standardized method for identification of delirium with high sensitivity, specificity, and interrater reliability.16,23 It is based on structured interviews, including formal cognitive testing. The validated chart review method21,22 was performed to enhance sensitivity in detecting delirium episodes across each 24-hour period. Each chart rating was adjudicated independently by 2 experts according to prespecified criteria to determine possible, probable, or definite delirium, or no delirium.22 A patient was deemed to have delirium if a rating of definite, probable, or possible delirium was assigned by both raters (any discrepancies were resolved during a consensus conference).
Assessment of general cognitive performance.
General cognitive performance (GCP) is a composite score based on a battery of neuropsychological tests administered to assess attention, memory, learning, and executive functioning.24 The battery was administered before (median 5, interquartile range 1–8 days prior to surgery) and 1 year after surgery (median 376, interquartile range 365–392 days after surgery) in 110 out of 113 participants. The assessment included the Hopkins Verbal Learning Test,25 Visual Search and Attention Task,26 Trail Making Tests A and B,27 Digit Symbol Substitution and Copy tests,28 Digit Span forward and backward,28 and 15-item Boston Naming Test.29 The GCP score was scaled to reflect population-based norms30 with a mean value of 50 (SD 10). A higher GCP score indicates better cognitive performance. The GCP score at 1 year was adjusted for retest effects using an accepted method,5 which involved subtracting a correction derived from repeated administrations in a comparison sample of 119 age-matched primary care patients. The difference between 1-year retest adjusted and baseline GCP scores was used as measure of cognitive changes.
MRI acquisition.
Study participants were imaged before (median 7, interquartile range 4–13 days before surgery) and 1 year after surgery (median 378, interquartile range 364–407 days after surgery) at the Beth Israel Deaconess Medical Center Radiology Department on a 3T HDxt MRI scanner (General Electric Medical Systems, Milwaukee, WI) using a standard 8-channel head coil. Diffusion-weighted echoplanar imaging was performed using a double echo diffusion preparation sequence: repetition time 16,000 ms, echo time 84.4 ms, acquisition matrix 96 × 96, flip angle 90°, field of view 240 × 240 mm, slice thickness 2.6 mm, in-plane resolution 2.5 × 2.5 mm. Whole brain images were acquired with a b value of 1,000 s/mm2 at each of 25 optimized diffusion directions.
DTI analysis.
TRACULA (FreeSurfer 5.2)31 was used to process the diffusion-weighted images and extract the fractional anisotropy (FA) and mean diffusivity (MD) maps. We ran 6 iterations of a nonlinear normalization routine in SPM-8 (fil.ion.ucl.ac.uk/spm) to align the individual resampled 1 × 1 × 1 mm diffusion maps to the average map obtained from all individual diffusion maps. In successive iterations, FA and MD images were alternately used for the normalization to improve spatial alignment across participants, using a higher than default 1.5 cm cutoff for spatial warping. Differential diffusion maps for each participant were obtained by subtracting the baseline from the follow-up signal of the FA and MD maps. The differential diffusion maps were then smoothed using a Gaussian kernel of 6 mm full width at half maximum. Voxel-wise analysis of the whole brain (including both the gray and white matter) was performed using statistical parametric mapping (SPM-8). Statistical nonparametric mapping (SnPM-13) (warwick.ac.uk/snpm) and a region of interest (ROI) approach were used to confirm the main findings obtained by SPM. For ROI analysis, we used an atlas-based method32 to measure FA and MD changes of the medulla oblongata, pons, midbrain, anterior cerebellum, posterior cerebellum, subcortical/basal ganglia region, occipital lobe, limbic lobe, parietal lobe, temporal lobe, frontal lobe, and frontal-temporal boundary.
MRI analyses were performed by operators (M.C., W.D., D.C.A.) who were blinded to all demographic and clinical data, including delirium outcomes.
Statistical analysis.
For our primary analysis, we investigated the voxel-wise association between longitudinal DTI changes and delirium severity in our 113 study participants. We performed multiple linear regression adjusting for age, sex, and vascular comorbidity. Age refers to the participant's age (years) at the time of surgery. For vascular comorbidity, the participants were subdivided in 2 categories according to the presence or absence of at least one of the following conditions: confirmed or history of myocardial infarction, congestive heart failure, peripheral vascular disease, diabetes (with or without end organ damage), cerebrovascular disease (carotid stenosis, history of stroke or transient ischemic attack), or hemiplegia.33
In secondary analysis, we included additional covariables to the regression models to factor into our analysis preoperative levels of cognitive functioning (baseline GCP), as well as preoperative MRI-derived measures of brain abnormalities (baseline white matter hyperintensities volume, or regional diffusion values associated with delirium at baseline), in addition to age, sex, and vascular comorbidity. Baseline white matter hyperintensities volume was taken from our previous study on the relationship between preoperative MRI abnormalities and delirium in the same cohort.34 To account for baseline diffusion abnormalities, we used scalar values of a region that showed highly significant association with delirium (i.e., MD of the right parietal lobe) in our previous work in the same cohort.13
In addition, we investigated the voxel-wise association between longitudinal DTI changes and delirium occurrence in separate multiple linear regression models, using the same analysis framework and covariables employed for delirium severity analyses.
We assessed the spatial distribution of DTI changes across 1 year regardless of delirium status using multiple linear regression adjusted for delirium severity, age, sex, vascular comorbidity, and baseline GCP.
The voxel-wise association between GPC changes and DTI changes across 1 year, as well as the association of GPC changes with delirium severity and occurrence, was assessed in the 110 participants who completed the neuropsychological assessment at 1 year. We performed multiple linear regression analyses, adjusting for age, sex, years of education, and baseline GCP. To investigate the effect of delirium on the relationship between GCP and DTI changes, we also performed multiple linear regression adjusting for delirium severity (in addition to age, sex, education, and baseline GCP).
In voxel-wise analyses, we determined empirically the cluster size that provided significant results (p value <0.05 after correction for multiple comparison within each cluster) using a one-tailed p value threshold of 0.05 at the voxel level, in line with our overarching hypothesis of a deleterious effect of delirium towards accrual of brain microstructural abnormalities associated with increase in MD and decrease in FA over time.14,15 To improve accuracy in localizing the diffusion changes over 1 year regardless of delirium status, we used a one-tailed p value threshold of 0.01 at the voxel level.
Since one-tailed p values are prone to type I error, to verify the robustness of our findings we supported the results of SPM with nonparametric randomization/permutation testing (number of permutations 1,000) in SnPM-13, as well as by an ROI-based approach, using the identical multivariable approaches used in SPM analyses.
JMP Pro 12 (jmp.com) was used for all non-voxel-based statistical analyses. The threshold for statistical significance was set to p < 0.05 for all analyses.
RESULTS
Baseline characteristics of the 113 study participants are summarized in the table. The subsample investigated in the present study presented no overall differences in the baseline characteristics with respect to the baseline MRI cohort.34 While the 33 participants who did not contribute to the present study were on average slightly older than the 113 participants who did contribute (mean ± SD age 77 ± 4 vs 76 ± 5 years; Wilcoxon rank-sum test p = 0.03), and showed higher prevalence of vascular comorbidity (58% vs 35%; χ2 test p = 0.02), we found no differences in sex distribution (58% vs 60% female; χ2 test p = 0.79), GCP (mean ± SD 58 ± 8 vs 59 ± 7; Student t test p = 0.71), or delirium incidence (21% vs 22%; χ2 test p = 0.91) between the 2 groups. No differences in the baseline characteristics were found between participants with and without delirium, with the exception of GCP, which was lower in the delirium group (table). Postoperative delirium occurred in 25 out of 113 participants (22%) during hospitalization.
Table.
Characteristics of the study participants

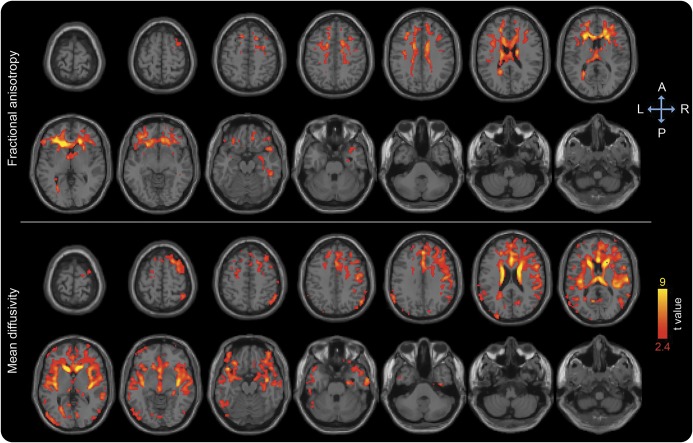
Our primary analysis showed association between delirium severity and longitudinal diffusion changes controlling for age, sex, and vascular comorbidity (figure 1). Delirium was associated with decrease in FA and increase in MD, predominantly in the cerebral white matter of the frontal, parietal, and temporal lobes, slightly more prominent in the right hemisphere (figure 1). MD increase was also detected in periventricular areas, inside the lateral ventricles, and in the lower brainstem (figure 1). The observed associations and spatial patterns did not change with addition of baseline GCP as a covariable (figure e-1 at Neurology.org). Analyses adjusted for preoperative MRI abnormalities, as measured by either white matter hyperintensities volume or diffusion, also showed similar results.
Figure 1. Longitudinal diffusion changes associated with delirium severity in multiple linear regression analysis adjusted for age, sex, and vascular comorbidity.
Areas showing decrease in fractional anisotropy and increase in mean diffusivity are overlaid to canonical T1-weighted images. Colors refer to t values of significant diffusion tensor imaging changes (one-tailed p < 0.05 after correction for multiple comparison within each cluster, cluster size ≥10,000) on a scale of 1.7 (red) to 5 (yellow).
Delirium occurrence showed association with DTI changes with a similar spatial distribution (figures e-2 and e-3), although delirium severity showed a broader spatial pattern and more prominent association with the diffusion changes. FA and MD changes over 1 year regardless of delirium status were distributed throughout the cerebral white matter, with more prominent involvement of the periventricular and frontal regions (figure 2). The confirmatory, nonparametric voxel-wise, and ROI analyses showed qualitatively similar results.
Figure 2. Diffusion tensor imaging (DTI) changes over 1 year regardless of delirium status in multiple linear regression analysis adjusted for delirium severity, age, sex, vascular comorbidity, and baseline general cognitive performance.
Areas showing decrease in fractional anisotropy and increase in mean diffusivity are overlaid to canonical T1-weighted images. Colors refer to t values of significant DTI changes (one-tailed p < 0.05 after correction for multiple comparison within each cluster, cluster size ≥5,000) on a scale of 2.4 (red) to 9 (yellow).
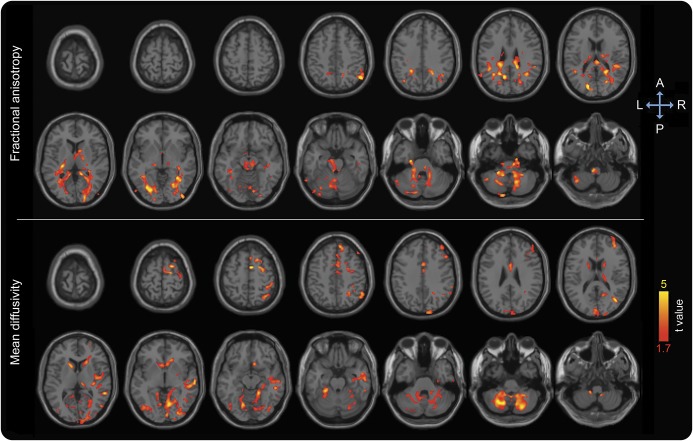
We found a positive association between GCP changes over 1 year and FA changes, and a negative association with MD changes, predominantly in the posterior temporal, parietal, and occipital white matter (figure 3). Longitudinal GCP changes were not associated with delirium severity or occurrence.
Figure 3. Diffusion changes associated with cognitive changes across 1 year in multiple linear regression analysis adjusted for age, sex, education, and baseline general cognitive performance.
Areas showing decrease in fractional anisotropy and increase in mean diffusivity are overlaid to canonical T1-weighted images. Colors refer to t values of significant diffusion tensor imaging changes (one-tailed p < 0.05 after correction for multiple comparison within each cluster, cluster size ≥10,000) on a scale of 1.7 (red) to 5 (yellow).
DISCUSSION
Our main finding was the association between postoperative delirium and longitudinal brain microstructural changes as measured by DTI. As the study participants were imaged shortly before and then 1 year after surgery, with incident delirium occurring in 22%, the finding raises the possibility that delirium may contribute to the development of the observed brain microstructural abnormalities.
Development of these abnormalities might result from neurodegenerative phenomena, which may coexist with or result from delirium.35 Moreover, delirium may represent either the result of or serve as a mediator of many factors, such as anesthesia, surgery, or psychoactive drugs, which may initiate inflammatory or neurotoxic cascades that lead to the observed diffusion changes.36,37 Our ability to establish a causal relationship between delirium and the observed diffusion changes is limited by our own previous finding of presurgical DTI abnormalities predisposing to delirium in the same cohort.13 While not completely overlapping, their spatial pattern included brain areas that also showed longitudinal changes associated with delirium in the present study (e.g., the frontal, parietal, and temporal white matter). The secondary analysis adjusting for preoperative diffusion abnormalities suggests that delirium may have an effect on the accrual of brain microstructural abnormalities. Since our study design includes only one MRI data point before surgery, we are unable to estimate premorbid trajectories of diffusion changes and therefore to establish whether the occurrence of delirium had a deleterious effect per se.
In the present study, only the association between MD and delirium severity was robust throughout the different analysis approaches, including nonparametric and ROI-based analyses. This finding suggests that the effect of delirium on subsequent diffusion changes is relatively mild. This is further supported by comparison of t values between the observed diffusion changes regardless of delirium status (figure 2; t values ranging from 2.4 to 9) and the delirium-associated diffusion changes (figure 1; t values ranging from 1.7 to 5). Our relatively healthy population of highly educated elective surgery patients without dementia at baseline may explain the proportionately modest effect observed in our study.
Notably, the diffusion changes do not seem to be related to major focal cerebrovascular events, as we observed no major MRI signs of occurrence of de novo ischemic lesions at 1 year. Rather, they likely represent accrual of more diffuse damage to the cerebral white matter. This is further supported by the association between diffusion changes and delirium in our secondary analysis adjusting for white matter hyperintensities volume.
The diffusion changes observed around and within the lateral ventricles likely reflect expansion of the CSF compartment associated with brain atrophy, similar to previous observations in normal aging.38 We plan additional structural MRI analysis (e.g., voxel-based morphometry, cortical thickness) to investigate more in depth the association between brain atrophy and delirium in our cohort.
We found an association between diffusion and cognitive changes over 1 year. The finding may reflect both improved structural connectivity associated with improvements in cognitive performance39 and impaired structural connectivity associated with preclinical trajectories of cognitive decline.3–5,40 The observed association seems to reflect predominantly the effect of aging, hospitalization, and surgery, since we found no association between longitudinal cognitive changes and delirium. This is further supported by the observed association between diffusion and cognitive changes when adjusting for delirium severity. Since analysis of the full SAGES cohort and other studies did show a deleterious effect of delirium on longer term cognitive decline,3–5 the lack of association between cognitive changes at 1 year and delirium may be explained by the relatively short follow-up, exclusion of individuals with dementia, and smaller sample size of the MRI subcohort. As this is an ongoing study, we will investigate the long-term effect of delirium and the associated diffusion changes on cognitive trajectories in future studies.
Limitations of our study include generalizability of our findings obtained from an elective surgical population of older people without dementia in a single geographic area. In prioritizing adjustment for relevant baseline covariables and avoiding overcontrolling given the limited number of outcome events, we did not account for other potential confounders, such as perioperative factors related to the surgical procedure. Future studies with more MRI data points before surgery are warranted to assess whether delirium may accelerate the trajectory of diffusion changes. Studies using more advanced MRI acquisition and analysis approaches may also improve the anatomical detail of the observed association between brain changes and delirium.
We observed a modest yet significant association between delirium and brain microstructural changes in our cohort of older individuals without dementia undergoing elective noncardiac surgery. The observed diffusion changes may reflect diffuse damage to the cerebral white matter, consistent with the global pathophysiology of delirium. Our findings raise the possibility that delirium, or the underlying neuropathology, has an effect on the development of brain microstructural abnormalities.
Supplementary Material
GLOSSARY
- CAM
Confusion Assessment Method
- CAM-S
Confusion Assessment Method–Severity
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- GCP
General Cognitive Performance
- MD
mean diffusivity
- ROI
region of interest
- SAGES
Successful Aging after Elective Surgery
- SnPM
statistical nonparametric mapping
- SPM
statistical parametric mapping
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: SAGES Study Group, Sharon K. Inouye, David Alsop, Richard Jones, Thomas Travison, Edward R. Marcantonio, Steven Arnold, Zara Cooper, Bradford Dickerson, Tamara Fong, Eran Metzger, Alvaro Pascual-Leone, Eva M. Schmitt, Mouhsin Shafi, Michele Cavallari, Weiying Dai, Simon T. Dillon, Janet McElhaney, Charles Guttmann, Tammy Hshieh, George Kuchel, Towia Libermann, Long Ngo, Daniel Press, Jane Saczynski, Sarinnapha Vasunilashorn, Margaret O’Connor, Eyal Kimchi, Jason Strauss, Bonnie Wong, Michael Belkin, Douglas Ayres, Mark Callery, Frank Pomposelli, John Wright, Marc Schermerhorn, Asha Albuquerque, Amanda Brown, Amy Callahan, Sarah Dowal, Meaghan Fox, Jacqueline Gallagher, Rebecca Anna Gersten, Ariel Hodara, Ben Helfand, Jennifer Inloes, Jennifer Kettell, Aleksandra Kuczmarska, Jacqueline Nee, Emese Nemeth, Lisa Ochsner, Kerry Palihnich, Katelyn Parisi, Margaret Puelle, Sarah Rastegar, Margaret Vella, Guoquan Xu, Margaret Bryan, Jamey Guess, Dee Enghorn, Alden Gross, Yun Gou, Daniel Habtemariam, Ilean Isaza, Cyrus Kosar, Christopher Rockett, Douglas Tommet, Ted Gruen, Meg Ross, Katherine Tasker, James Gee, Ann Kolanowski, Margaret Pisani, Sophia de Rooij, Selwyn Rogers, Stephanie Studenski, Yaakov Stern, Anthony Whittemore, Gary Gottlieb, John Orav, and Reisa Sperling
AUTHOR CONTRIBUTIONS
Michele Cavallari: data analysis and interpretation, manuscript writing. Weiying Dai: data analysis and interpretation. Charles R.G. Guttmann: critical revision of the manuscript for important intellectual content. Dominik S. Meier: critical revision of the manuscript for important intellectual content. Long H. Ngo: study design, data management. Tammy T. Hshieh: critical revision of the manuscript for important intellectual content. Tamara G. Fong: critical revision of the manuscript for important intellectual content. Eva Schmitt: acquisition of data. Daniel Z. Press: critical revision of the manuscript for important intellectual content. Thomas G. Travison: study concept and design, data management. Edward R. Marcantonio: study concept and design. Richard N. Jones: study concept and design. Sharon K. Inouye: study concept and design, acquisition of data, study supervision. David C. Alsop: study concept and design, acquisition, analysis and interpretation of data.
STUDY FUNDING
Study supported by grants no. P01AG031720 (S.K.I.), K07AG041835 (S.K.I.), and R01AG044518 (S.K.I./R.N.J.) from the National Institute on Aging. Dr. Hshieh was supported by an NIH-funded T32 Training Grant (AG000158). Dr. Marcantonio is supported by K24AG035075 from the National Institute on Aging. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the design, conduct, or reporting of this study.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet 2014;383:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinlan N, Rudolph JL. Postoperative delirium and functional decline after noncardiac surgery. J Am Geriatr Soc 2011;59(suppl 2):S301–S304. [DOI] [PubMed] [Google Scholar]
- 3.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–451. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement 2016;12:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunther ML, Morandi A, Krauskopf E, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med 2012;40:2022–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morandi A, Rogers BP, Gunther ML, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study. Crit Care Med 2012;40:2182–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okumura A, Hayakawa F, Kato T, et al. Callosal lesions and delirious behavior during febrile illness. Brain Dev 2009;31:158–162. [DOI] [PubMed] [Google Scholar]
- 9.Takanashi J, Tada H, Kuroki H, Barkovich AJ. Delirious behavior in influenza is associated with a reversible splenial lesion. Brain Dev 2009;31:423–426. [DOI] [PubMed] [Google Scholar]
- 10.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004;63:1854–1858. [DOI] [PubMed] [Google Scholar]
- 11.Choi SH, Lee H, Chung TS, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry 2012;169:498–507. [DOI] [PubMed] [Google Scholar]
- 12.Alsop DC, Fearing MA, Johnson K, Sperling R, Fong TG, Inouye SK. The role of neuroimaging in elucidating delirium pathophysiology. J Gerontol A Biol Sci Med Sci 2006;61:1287–1293. [DOI] [PubMed] [Google Scholar]
- 13.Cavallari M, Dai W, Guttmann CRG, et al. Neural substrates of vulnerability to postsurgical delirium as revealed by presurgical diffusion MRI. Brain Epub 2016 Feb 26. [DOI] [PMC free article] [PubMed]
- 14.Charlton RA, Schiavone F, Barrick TR, Morris RG, Markus HS. Diffusion tensor imaging detects age related white matter change over a 2 year follow-up which is associated with working memory decline. J Neurol Neurosurg Psychiatry 2010;81:13–19. [DOI] [PubMed] [Google Scholar]
- 15.Barrick TR, Charlton RA, Clark CA, Markus HS. White matter structural decline in normal ageing: a prospective longitudinal study using tract-based spatial statistics. Neuroimage 2010;51:565–577. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: the Successful Aging After Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc 2012;13:818.e1–818.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitt EM, Saczynski JS, Kosar CM, et al. The Successful Aging After Elective Surgery Study: cohort description and data quality procedures. J Am Geriatr Soc 2015;63:2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014;160:526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasunilashorn SM, Marcantonio ER, Gou Y, et al. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. J Gen Intern Med Epub 2016 Jun 3. [DOI] [PMC free article] [PubMed]
- 20.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- 21.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc 2005;53:312–318. [DOI] [PubMed] [Google Scholar]
- 22.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc 2014;62:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The confusion assessment method: a systematic review of current usage. J Am Geriatr Soc 2008;56:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones RN, Rudolph JL, Inouye SK, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsychol 2010;32:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991;5:125–142. [Google Scholar]
- 26.Trenerry M, Crosson B, DeBoe J, Leber W. Visual Search and Attention Test (VSAT). Odessa, FL: Psychological Assessment Resources; 1990. [Google Scholar]
- 27.Trail Making Tests A and B. Washington, DC: War Department Adjutant General's Office; 1944. [Google Scholar]
- 28.Wechsler D. Manual: Wechsler Adult Intelligence Scale–Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 29.Mack WJ, Freed DM, Williams BW, Henderson VW. Boston Naming Test: shortened versions for use in Alzheimer's disease. J Gerontol 1992;47:P154–P158. [DOI] [PubMed] [Google Scholar]
- 30.Gross AL, Jones RN, Fong TG, Tommet D, Inouye SK. Calibration and validation of an innovative approach for estimating general cognitive performance. Neuroepidemiology 2014;42:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 2011;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 33.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 34.Cavallari M, Hshieh TT, Guttmann CRG, et al. Brain atrophy and white-matter hyperintensities are not significantly associated with incidence and severity of postoperative delirium in older persons without dementia. Neurobiol Aging 2015;36:2122–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inouye SK. Delirium in older persons. N Engl J Med 2006;354:1157–1165. [DOI] [PubMed] [Google Scholar]
- 36.Vasunilashorn SM, Ngo L, Kosar CM, et al. Does apolipoprotein E genotype increase risk of postoperative delirium? Am J Geriatr 2015;23:1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: a longitudinal nested case-control study. Biol Psychiatry Epub 2016 Mar 25. [DOI] [PMC free article] [PubMed]
- 38.Helenius J, Soinne L, Perkiö J, et al. Diffusion-weighted MR imaging in normal human brains in various age groups. Am J Neuroradiol 2002;23:194–199. [PMC free article] [PubMed] [Google Scholar]
- 39.Cao X, Yao Y, Li T, et al. The impact of cognitive training on cerebral white matter in community-dwelling elderly: one-year prospective longitudinal diffusion tensor imaging study. Sci Rep 2016;6:33212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frings L, Dressel K, Abel S, et al. Longitudinal cerebral diffusion changes reflect progressive decline of language and cognition. Psychiatry Res 2013;214:395–401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.