Abstract
Tails are an intricate component of the locomotor system for many vertebrates. Leopard geckos (Eublepharis macularius) possess a large tail that is laterally undulated during steady locomotion. However, the tail is readily shed via autotomy, resulting in the loss of tail function, loss in body mass, and a cranial shift in the center of mass. To elucidate the function of tail undulations, we investigated changes in limb kinematics after manipulating the tail artificially by restricting tail undulations and naturally by removing the tail via autotomy. Restricting tail undulations resulted in kinematic adjustments similar to those that occur following tail autotomy, characterized by more flexed hind limb joints. These data suggest that effects of autotomy on locomotion may be linked to the loss of tail movements rather than the loss of mass or a shift in center of mass. We also provide empirical support for the link between lateral tail undulations and step length through the rotation of the pelvic girdle and retraction of the femur. Restriction and autotomy of the tail limits pelvic rotation, which reduces femur retraction and decreases step length. Our findings demonstrate a functional role for tail undulations in geckos, which likely applies to other terrestrial vertebrates.
Introduction
A defining feature of chordates is the post-anal tail, which has evolved many key functions across taxa1. These include courtship2, signaling3, 4, the maintenance of fat stores5, 6, and defense/combat7, 8. Tails also have functional roles in animal locomotion, most notably when used directly for propulsion, as in countless swimming animals9, 10 and when used to power pentapedal locomotion in kangaroos11. Perhaps less obvious is the tail’s role in maintaining balance and enhancing maneuverability or stability12–16. Although prehensile tails serve as an extra limb to reduce the risk of falling in arboreal environments17, 18, several taxa utilize non-prehensile tails for a similar advantage. Mice have been documented undulating the tail for balance when crossing a narrow perch19. Primates with long tails utilize sweeping movements of the tail when navigating narrow supports to alter the momentum of their body20, and cats utilize tail adjustments to realign their hips over a perch to avoid falling21. Even on broad level terrain, tails can adjust the balance of the body to counteract pitching effects of leg movements22, and tails have been shown to be useful for initiating turns and maneuvering23.
Lizards are ideal for studying tail function because all of the functions described above are represented within their tremendous diversity. The tail can be dragged behind the lizard, pushed against the substrate during climbing, raised, curled, used as a prehensile “fifth limb”, used for counter-rotation during jumping, or undulated as they walk, run, and/or climb8, 24–26. Despite the importance of the tail in various forms of locomotion15, 27–31, most lizard species voluntarily shed the tail (autotomy) as a predator-escape strategy8, 32. How tail autotomy impacts locomotion has thus become a topic of much interest in recent years15, 33–35. Performance effects are variable across species, likely due to differences in the role of the tail in locomotion25. Species for which locomotor performance is improved after autotomy generally have large fatty tails that impede faster running36, while locomotion is impaired by tail loss in species that depend on the tail for balance, stability, and/or maneuverability29, 30.
In some species, autotomy does not influence performance, but significant changes in locomotor mechanics occur. Changes in locomotor kinematics and hind limb ground-reaction forces were recently investigated in the leopard gecko, Eublepharis macularius 37, a padless desert-dwelling species and an established system for tail autotomy and regeneration38–42. Geckos lower their center of mass by taking a more sprawled posture after autotomy, a change that was attributed to a reduction in stability due to the significant loss of caudal mass and cranial shift of the center of mass (E. macularius have one of the largest tails relative to body size among lizards)37. However, it is unclear if stability is impaired by the change in mass or the loss of tail function. The tail of E. macularius serves a primary role in the storage of fats41, but unlike many other large-tailed reptiles, the tail is not dragged behind the animal as it walks. Instead, the tail is lifted off the ground and swings laterally. Undulations of the vertebral column generate a standing wave in the trunk that transforms into a traveling wave moving caudally along the tail as the lizard walks43.
The function of lateral undulations of the tail during locomotion remains unclear, although several hypotheses have been presented. Tail movements in arboreal mammals are suggested to aid in balance and stability when traversing narrow perches19–21. Recent data on green anoles demonstrate that mediolateral tail movements are most prominent on the narrowest perches and compensate for instabilities imposed by a small perch diameter15. Undulating the tail during otherwise steady locomotion may also be a useful mechanism for rapidly responding to unexpected perturbations by imparting angular momentum on the body and resisting the destabilizing motion20, 21, 31, 44. The tail may also play a role in force generation by the caudofemoralis, the muscle that retracts the femur28, 45–50. Undulating the tail could alternately lengthen the caudofemoralis muscles attached to each hind limb as the tail is swung from side to side. Lengthening the muscle to a more optimal length would lead to greater actin-myosin overlap within the muscle sarcomere, which could thus enhance the force generated for propulsion by the caudofemoralis. Tail undulations could also contribute to rotation at the pelvic girdle due to inertial effects. A large undulating tail could provide the momentum necessary for rotating the pelvic girdle in the yaw axis, which could influence both the length of a hind limb step as well as the angle at which the femur can retract to drive propulsion.
For both axial and appendicular structures that move during locomotion, function can be revealed by either removing all or some of the structure37, 51, by adding to the structure12, 52, or by restricting motion of the structure53, 54. Although the voluntary loss of the tail has been studied, little is known about the differential role of mass versus motion of the tail during locomotion. We examined how the tail is used in leopard geckos walking on level terrain and determined how these tail movements change with speed. We then disabled normal tail movements, both artificially by restricting tail undulations with a graphite rod and naturally by autotomizing the tail in the same individuals (Fig. 1). We hypothesized that restricting the motions of the tail will cause changes in locomotion that are comparable to those that occur following tail autotomy37. Thus, we predicted that autotomy-induced changes in locomotion result from the loss of tail undulations, not a loss of mass. We specifically investigated changes in limb joint angles that may augment balance or stability, as well as changes in the rotation of the pelvic girdle when an undulating tail is compromised.
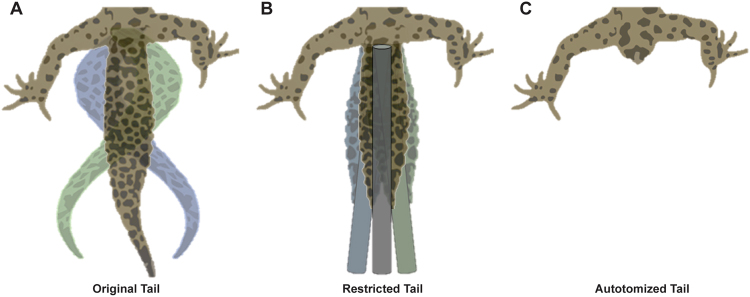
Figure 1.
Tail movements under each experimental treatment. Lateral tail undulations freely occur with original tails intact (A), while tails are reduced to a stiff rod when restricted with limited movement in the yaw axis (B). Tail movement is non-existent after autotomy (C).
Results
In running trials, geckos ran at speeds ranging from 0.59 to 3.36 SVL s−1, which was not significantly affected by restricting or autotomizing the tail (repeated measures ANOVA, F 2,8 = 4.075, P = 0.060). Lateral displacement of the tip of the tail relative to the pelvic girdle exhibited a significant negative relationship with speed (F 1,8 = 5.870, P = 0.042, R 2 = 0.423) (Fig. 2), although no relationship was observed between the height of the tail and speed (F 1,8 = 0.100, P = 0.759, R 2 = 0.012). Restricting the tail reduced the lateral displacement of the tail as intended (t = 3.112, d.f. = 9, P = 0.012) and did not affect the tail height off the ground (t = 0.734. d.f. = 9, P = 0.482).
Figure 2.
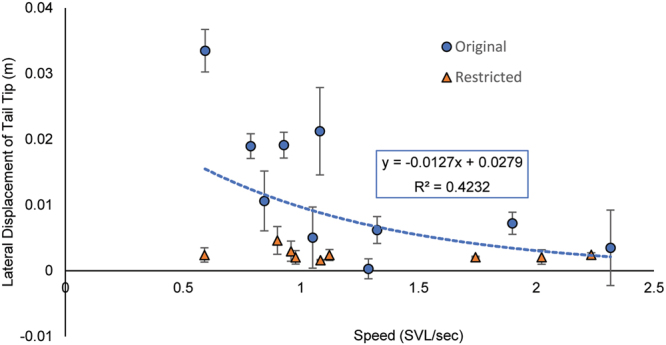
Relationships of lateral displacement of the tail tip with speed. Lateral displacement is measured as the lateral distance of the tail tip relative to the pelvic girdle, as measured on the left side of the body. Data points are means for each individual. Error bars are s.e.m. Regression analysis demonstrates a significant negative relationship of lateral displacement of the tail tip with speed when the tail is unaltered (P = 0.042). Lateral displacement of the tail is significantly reduced when the tail is restricted, with no relationship to speed.
Stride lengths, stance times, and duty factors of the fore- and hind limbs were not significantly impacted by restricting or autotomizing the tail (Table 1). Forelimb joint kinematics were also unaffected. However, step length (the distance traveled during the stance phase of the hind limb) was significantly reduced by restricting (t = 3.509, d.f. = 9, P = 0.007) and autotomizing (t = 3.447, d.f. = 9, P = 0.007) the tail. Both restricting and autotomizing the tail also significantly decreased the maximum angles of femur depression (restriction, t = 6.225, d.f. = 9, P < 0.000; autotomy, t = 7.869, d.f. = 9, P < 0.000), femur retraction (restriction, t = 2.94, d.f. = 9, P = 0.016; autotomy, t = 3.305, d.f. = 9, P = 0.009), knee flexion (restriction, t = 4.541, d.f. = 9, P = 0.001; autotomy, t = 4.627, d.f. = 9, P = 0.001), and ankle flexion (restriction, t = 3.997, d.f. = 9, P = 0.003; autotomy, t = 4.157, d.f. = 9, P = 0.002) in the hind limbs (Fig. 3). The angular excursion at each of these hind limb joints was also significantly reduced after tail restriction (femur depression, t = 3.069, d.f. = 9, P = 0.013; femur retraction, t = 3.527, d.f. = 9, P = 0.006; knee, t = 2.939, d.f. = 9, P = 0.017; ankle, t = 3.577, d.f. = 9, P = 0.006) and after tail autotomy (femur depression, t = 5.090, d.f. = 9, P = 0.001; femur retraction, t = 3.115, d.f. = 9, P = 0.012; knee, t = 5.661, d.f. = 9, P < 0.000; ankle, t = 5.825, d.f. = 9, P < 0.000). No significant differences were observed between the restricted and autotomized tail treatment groups (step length, t = 0.859, d.f. = 9, P = 0.412; maximum femur depression angle, t = 1.089, d.f. = 9, P = 0.305; maximum femur retraction angle, t = 0.051, d.f. = 9, P = 0.960; maximum knee angle, t = 1.229, d.f. = 9, P = 0.250; maximum ankle angle, t = 1.176, d.f. = 9, P = 0.270; angular excursion of femur depression, t = 2.510, d.f. = 9, P = 0.063; angular excursion of femur retraction, t = −1.157, d.f. = 9, P = 0.277; angular excursion of the knee, t = 2.123, d.f. = 9, P = 0.063; angular excursion of the ankle, t = 1.917, d.f. = 9, P = 0.087).
Table 1.
Summary of kinematic variables in the leopard gecko Eublepharis macularius across tail treatments.
| Variable | Original | Restricted | Autotomized | F-ratio | P |
|---|---|---|---|---|---|
| Forelimb | |||||
| Stride length (SVL)* | 0.63 ± 0.15 | 0.60 ± 0.05 | 0.49 ± 0.06 | 2.986 | 0.108 |
| Step length (SVL)* | 0.04 ± 0.00 | 0.05 ± 0.00 | 0.04 ± 0.00 | 1.452 | 0.290 |
| Stance time (s)* | 0.54 ± 0.03 | 0.55 ± 0.03 | 0.50 ± 0.02 | 0.640 | 0.552 |
| Duty factor* | 0.70 ± 0.01 | 0.72 ± 0.01 | 0.72 ± 0.01 | 0.765 | 0.497 |
| Humerus depression (deg) | |||||
| Maximum | 45.59 ± 8.31 | 34.29 ± 4.88 | 29.30 ± 2.91 | 0.898 | 0.445 |
| Angular excursion | 101.38 ± 15.88 | 72.67 ± 7.72 | 72.26 ± 4.30 | 1.818 | 0.223 |
| Humerus retraction (deg) | |||||
| Maximum | 54.78 ± 2.68 | 68.51 ± 2.45 | 73.02 ± 3.13 | 2.897 | 0.113 |
| Angular excursion | 44.41 ± 1.80 | 51.21 ± 2.57 | 59.23 ± 2.41 | 3.505 | 0.081 |
| Elbow angle (deg) | |||||
| Maximum | 151.04 ± 1.23 | 144.82 ± 1.91 | 142.56 ± 1.59 | 1.986 | 0.199 |
| Angular excursion | 92.49 ± 2.27 | 83.17 ± 2.64 | 93.76 ± 2.73 | 4.383 | 0.052 |
| Wrist angle (deg) | |||||
| Maximum | 165.31 ± 2.00 | 161.83 ± 1.43 | 164.83 ± 1.45 | 1.233 | 0.341 |
| Angular excursion | 71.93 ± 3.09 | 70.38 ± 2.51 | 73.33 ± 2.73 | 0.353 | 0.713 |
| Hind limb | |||||
| Stride length (SVL)* | 0.62 ± 0.15 | 0.72 ± 0.05 | 0.70 ± 0.06 | 1.275 | 0.331 |
| Step length (SVL)* | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.05 ± 0.00 | 5.836 | 0.027 |
| Stance time (s)* | 0.64 ± 0.03 | 0.60 ± 0.03 | 0.56 ± 0.01 | 0.923 | 0.436 |
| Duty factor* | 0.78 ± 0.01 | 0.78 ± 0.01 | 0.77 ± 0.01 | 0.578 | 0.583 |
| Femur depression (deg) | |||||
| Maximum | 49.44 ± 2.49 | 23.01 ± 1.44 | 20.79 ± 1.30 | 29.601 | <0.000 |
| Angular excursion | 52.42 ± 3.25 | 30.56 ± 1.63 | 23.29 ± 1.48 | 30.447 | <0.000 |
| Femur retraction (deg) | |||||
| Maximum | 55.00 ± 2.18 | 40.94 ± 1.65 | 39.82 ± 2.03 | 6.106 | 0.025 |
| Angular excursion | 82.57 ± 2.29 | 65.26 ± 2.03 | 69.50 ± 1.95 | 5.637 | 0.030 |
| Knee angle (deg) | |||||
| Maximum* | 164.49 ± 0.93 | 154.84 ± 1.47 | 151.75 ± 1.30 | 1.674 | 0.003 |
| Angular excursion* | 98.83 ± 1.39 | 88.08 ± 1.68 | 81.48 ± 2.40 | 14.282 | 0.002 |
| Ankle angle (deg) | |||||
| Maximum | 139.65 ± 2.03 | 129.69 ± 1.90 | 126.19 ± 1.97 | 9.85 | 0.007 |
| Angular excursion | 85.19 ± 2.38 | 70.41 ± 1.71 | 63.19 ± 2.61 | 16.589 | 0.001 |
| Speed (SVL/sec) | 1.23 ± 0.16 | 1.50 ± 0.22 | 1.85 ± 0.36 | 4.075 | 0.060 |
Means + residuals (±s.e.m.) for each variable are given for original, restricted, and autotomized tail treatments. Statistical significance (repeated measures ANOVA) of changes in each variable is also given. Significant results are indicated in bold type. Asterisks indicate variables that had a significant relationship (α ≤ 0.10) with speed.
Figure 3.
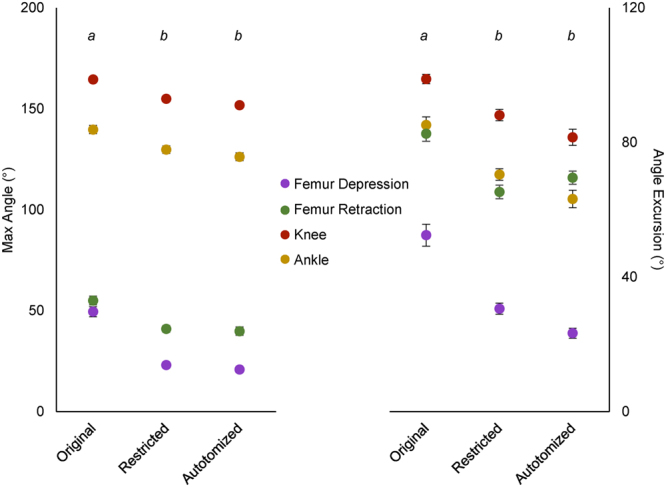
Means of maximum angles (left) and angular excursions (right) of hind limb joints during stance phase. Values for femur depression, femur retraction, knee angle, and ankle angle are means + residuals from ten individuals. Error bars are s.e.m. Letters above each treatment indicate significant differences (repeated measures ANOVA and post-hoc tests for multiple comparisons, P < 0.05).
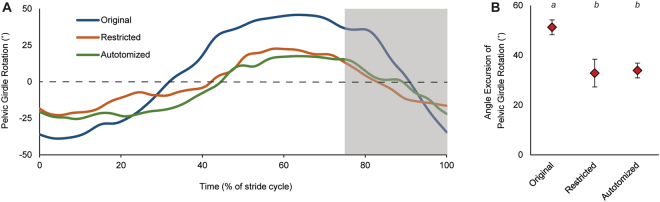
Pelvic girdle rotation decreased significantly when the tail was compromised, as indicated by a lower angular excursion in lizards with restricted (t = 2.287, d.f. = 9, P = 0.048) and autotomized (t = 3.129, d.f. = 9, P = 0.012) tails when compared to lizards with original tails intact (Fig. 4). No significant differences in pelvic girdle rotation were observed between the restricted and autotomized treatments (t = −0.247, d.f. = 9, P = 0.810).
Figure 4.
Changes in pelvic girdle rotation with restriction and autotomy. (A) Degree of rotation of the pelvic girdle over time (as a percentage of stride cycle) is provided for a representative hind limb stride of a leopard gecko with its original (blue), restricted (orange), and autotomized (green) tail. Negative values indicate that the pelvic girdle is rotated to the right (toward the hind limb being observed) and positive values indicate that the pelvic girdle is rotated to the left (toward the opposite hind limb). The non-shaded region represents the stance phase of the observed hind limb and the area shaded in gray represents the swing phase. (B) Means of angular excursion of the pelvic girdle in the yaw axis across treatments from ten individuals. Error bars are s.e.m. Letters above each treatment indicate significant differences (repeated measures ANOVA and post-hoc tests for multiple comparisons, P < 0.05).
Discussion
Tail autotomy in lizards can result in a significant loss of body mass, a cranial shift in the center of mass, and a loss of function that results from tail motion. While the loss of mass and shifted center of mass occur simultaneously and cannot be decoupled, we investigated the functional role of the tail by experimentally restricting its lateral movement. Analysis of locomotor kinematics of E. macularius under experimental conditions in which the tail was compromised revealed the function of tail motions when walking and their relationship to pelvic rotation and step length. Specifically, we observed a more sprawled posture when lateral undulations of the tail were restricted and when the tail was completely autotomized, suggesting that geckos must compensate for not only the loss of caudal mass, but also for the loss of tail motion after an autotomy event. Additionally, restricting tail undulations reduced the lateral rotation of the pelvic girdle, retraction of the femur, and step length, thereby providing evidence for a significant role of the tail in gecko locomotion. These results, elaborated below, reveal key functions of tails during locomotion that are likely applicable to any terrestrial vertebrate that relies on tail motion to move effectively.
Despite having a large fatty tail that accounts for one-fourth of the animal’s body mass, the tail of E. macularius is slightly raised and laterally undulated instead of being dragged on the ground while walking. As the base of the tail moves laterally, the femora are alternately retracted to generate propulsion. The base of the tail is flexed towards the protracted hind limb during each cycle of hind limb movement, and the remainder of the tail follows this basal movement in an undulatory manner. Interestingly, we found that lateral displacement of the tip of an intact tail exhibits a negative relationship with the speed at which the gecko walks (Fig. 2), suggesting that the tail swings less at higher speeds. This more rigid posture of the tail straightens the profile of the lizard, and is suggested to be appropriate when lizards are moving forward quickly12. It is likely that laterally undulating the tail is inefficient at higher speeds given its substantial mass. Accelerating and decelerating the large tail when moving at high speeds would require more force and power due to the reduced amount of time available for swinging the tail from side to side, which might simply not be possible for the geckos.
After losing its tail, E. macularius adopts a more sprawled posture during locomotion, as previously indicated by decreases in femur depression, femur retraction, knee angle and ankle angle37. This locomotor response to autotomy is hypothesized to augment stability and balance that may be impaired due to the altered mass distribution and/or the loss of tail as a stabilizing appendage. Restricting the tail allowed us to tease apart the locomotor effects of autotomy due to altering mass/center of mass versus losing tail function. By effectively modifying the tail into a stiff rod, the gecko was permitted to lift the tail off the ground to prevent friction, but prevented from swinging and laterally undulating the tail as it walked. This modification produced the same locomotor response as autotomizing the tail (Fig. 5). Both removing and restricting the tail can impact the location of the center of mass, with removal shifting the center of mass forward37, 55 and restriction limiting lateral displacements of the center of mass15, 28. Forelimb kinematics were unaffected by restriction and autotomy, but maximum joint angles and angular excursions in the hind limbs decreased (Fig. 3). These results suggest that tail undulations have a functional role in locomotion on level terrain, a role that is lost after autotomy and requires compensation by altering hind limb kinematics. In fact, it is likely that the impacts of autotomy on locomotion are a result of losing potentially beneficial tail movements, and not necessarily related to the loss of mass.
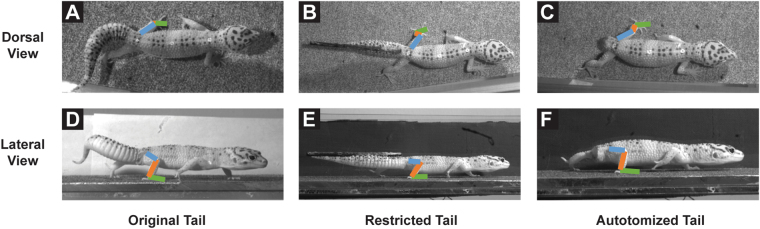
Figure 5.
Video frames of leopard geckos under each experimental treatment. Dorsal (A–C) and lateral (D–F) are shown for geckos with original (A,D), restricted (B,E), and autotomized (C,F) tails. Colored lines are superimposed over the segments of the observed hind limb to visualize changes in joint angles.
The function of tail undulations during steady locomotion is more clearly elucidated by the observed changes in pelvic rotation and its downstream effects on femur retraction and step length. Both restricting and autotomizing the tail reduced the degree of rotation of the pelvic girdle throughout the stride (Fig. 4). We hypothesize that swinging the heavy tail laterally provides momentum for rotation at the pelvic girdle via an inertial effect. As the base of the tail is rotated laterally, the length of the tail follows this movement in an undulatory manner. Given the substantial mass of the tail being shifted at the caudal end, the angular momentum of the tail contributes to rotating the pelvic girdle in the yaw axis. Lizards generally exhibit greater pelvic rotation in order to facilitate a more sprawled posture compared to most other terrestrial quadrupeds56–59. Thus, a reduction in pelvic rotation should be expected to generate a more upright posture. This is in stark contrast to what is observed after tail autotomy, in which lizards become more sprawled to maintain stability37. Decreased pelvic rotation after autotomy thus results in a reduced step length during steady locomotion to maintain the sprawled posture. Although walking speed was not affected by the observed reduction in step length, we suspect that maximal sprint speed would likely be negatively impacted. We did not assess this in our study as we were mainly interested in the impact of tail autotomy and immobilization on kinematics. Additionally, pelvic rotation influences the angle at which the femur can protract and retract28, 49, 50. The reduction in the angle of femur retraction observed in lizards with restricted and autotomized tails (Table 1) coincides with the reduction in pelvic rotation. Our data provide empirical support for the proposed link between lateral tail undulations and step length by rotation of the pelvic girdle and retraction of the femur60. Autotomy is therefore likely to impact lizards that have a functional tail that provides momentum for rotating the pelvic girdle.
Our findings demonstrate that the tail serves a functional role in locomotion by undulating and rotating the pelvic girdle, thus contributing to femur retraction and step length. To further reveal the locomotor function of tail undulations in terrestrial lizards, we propose a series of future experiments that will elucidate how the tail is used and how animals compensate for the lost appendage. First, the effects of tail loss on dynamic stability and maneuverability should be tested by examining if/how lizards utilize the tail to navigate obstacles, drops, and turns. Experiments that record the timing and intensity of muscle activation in the tail will reveal whether these movements are passively or actively controlled, providing important insight into how tail undulations are modulated. Passive control may suggest that undulating the tail occurs by simply dissipating energy from the laterally undulating body during locomotion, while active control would suggest neuromuscular input that may be necessary for regulating balance or stability. Electromyography experiments would also be insightful when testing how the tail undulations affect the activation of the caudofemoralis and its role in retracting the femur45, 46. Finally, we hope to explore the evolution of tail function by using these methods to explore the diversity of tail morphologies and their related locomotor functions across lizard taxa.
Tail autotomy in lizards provides an effective and natural system for understanding tail function. Hypothesized functions of tails commonly arise from studies on tail autotomy and locomotion. A negative impact of tail loss on performance suggests that the tail serves a role in balance, stability, maneuverability, or propulsion29, 30, 61. Other attempts at assessing tail use in locomotion involve invasive surgeries with irreparable effects on the study animals19, 21, 27. However, tail autotomy allows for a removal of the tail in a natural manner with minimal physiological effects62 in order to study its function.
Materials and Methods
Study organisms
Ten adult E. macularius (mass, 36.3 ± 1.9 g; SVL, 104.6 ± 2.1 mm) with original tails intact were obtained from commercial suppliers and housed in terraria (50.8 × 25.9 × 2.0 cm) maintained at 28–33 °C. Geckos were fed a diet of live crickets ad libitum, but fasted the day before the experiment until trials were complete. Prior to experimental trials, white nail polish was applied to the following points on the animals to visualize body and joint movements in high-speed videos: dorsal midpoint of the body, center of the pectoral/pelvic girdles, shoulder/hip, elbow/knee, wrist/ankle, and the metapodial-phalangeal joint of the middle toe. Joints were marked on the right forelimbs and hind limbs. Five points were also evenly distributed from the base of the tail to the tail tip to track the tail movements. All animal research was conducted in accordance with the University of California, Riverside Animal Care and Use Protocols (A-20110025 and A-20110038) with approval from the Institutional Animal Care and Use Committee (IACUC).
Experimental set-up
Stride kinematics were obtained from each lizard as it ran on a level trackway (1.0 × 0.13 m) with sandpaper substrate to prevent slipping. A mirror mounted at 45° above the trackway provided a dorsal view for the trials. The temperature of the experimental room was maintained at ~30 °C. Lizards were recorded moving along the trackway under three tail treatments: original, restricted, and autotomized (Fig. 1). After recording trials with a lizard’s original tail intact, a lightweight (<1.0 g) hollow graphite rod was attached along the entire length of the tail using non-toxic glue. The rod restricted undulations of the tail, while still permitting the lizard to lift its tail off the ground to prevent friction drag while walking. Locomotor trials were then repeated with the restricted tail. Following these trials, the rod was gently removed from the tail, and the base of the tail was gently pinched to initiate autotomy at the proximal-most fracture plane. Trials were then repeated for lizards with autotomized tails. Between trials for each treatment, each individual was allotted 20–30 minutes to rest in order to minimize potential effects of fatigue or stress associated with the restriction and removal of the tail62. However, we limited the amount of walking between trials to avoid any short-term adjustments.
Stride kinematics
Locomotor movements were captured at 250 frames s−1 with a shutter speed of 1/2000 s using two Photron APX-RS cameras (Photron USA, San Diego, CA, USA), one aimed at the lateral view of the lizard and the other recording a dorsal view from the mirror. Cameras were synchronized with an external trigger. A pre-measured calibration object constructed of LEGO™ blocks was used to generate 3D coordinates for digitizing. Three to five forelimb and hind limb strides were recorded for each individual under each tail treatment, providing a total of at least nine strides per individual. Each stride was representative of an individual moving at a relatively constant speed, at least two strides after the initial acceleration. We digitized the points marked on the animals using DLT DV5 custom software63 for MATLAB (version R2012a, The MathWorks, Natick, MA, USA) to obtain x, y, and z coordinates to describe antero-posterior, medio-lateral, and dorso-ventral movements, respectively. These coordinates were then used to calculate speed, stride length, stance time, duty factor, and joint angles for the fore- and hind limb throughout each stride. Details of these calculations are available elsewhere37, 64. Tail coordinates were used to calculate the height of the tail off the ground and lateral displacement of the tail (measured as the lateral displacement of the tail tip relative to the pelvic girdle) throughout each stride. Only the movements of the tail in the yaw axis were considered here.
Statistical analyses
Averages of each kinematic variable for each individual per tail treatment were used for all statistical analyses. For the tail variables (tail height and lateral displacement), a regression analysis was used to examine the relationship between tail movements and walking speed. The effects of speed on fore- and hind limb joint kinematics were removed by regressing the variables against body speed. Residuals of the variables that had a significant relationship (α ≤ 0.10) with speed were used for subsequent statistical analyses, while all other data were analyzed in their original form. A repeated-measures ANOVA was used to compare each variable between original, restricted, and autotomized tail treatments, and post hoc tests with Bonferroni corrections were used for pair-wise comparisons among the treatments. Assumptions for normality and equal variances were not violated for any of the variables measured. All statistical analyses were performed using SYSTAT 13.00.05.
Acknowledgements
We thank all past and present members of the Higham Lab for assistance with animal care and for providing helpful feedback on this project. Anthony Russell provided valuable suggestions as the project idea was being conceived. A.V. Birn-Jeffery assisted with coding for limb and tail kinematics. Financial support was provided by a National Science Foundation grant (IOS-1147043) to T.E.H., and the UCR Newell Award presented to K.J.
Author Contributions
K.J. and T.E.H. developed the idea and approach for the study. K.J. performed the experiments, analyzed the data, and drafted the manuscript. T.E.H. contributed to the interpretation of the findings and revisions of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hickman GC. The mammalian tail: a review of functions. Mammal Rev. 1979;9:143–157. doi: 10.1111/j.1365-2907.1979.tb00252.x. [DOI] [Google Scholar]
- 2.Dakin R, McCrossan O, Hare JF, Montgomerie R, Amador Kane S. Biomechanics of the peacock’s display: how feather structure and resonance influence multimodal signaling. PloS ONE. 2016;11:e0152759. doi: 10.1371/journal.pone.0152759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper WE. Reactive and anticipatory display to deflect predatory attack to an autotomous lizard tail. Can. J. Zool. 1998;76:1507–1510. doi: 10.1139/z98-093. [DOI] [Google Scholar]
- 4.Barbour MA, Clark RW. Ground squirrel tail-flag displays alter both predatory strike and ambush site selection behaviours of rattlesnakes. Proc. R. Soc. B. 2012;279:3827–3833. doi: 10.1098/rspb.2012.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young JW, Patel BA, Stevens NJ. Body mass distribution and gait mechanics in fat-tailed dwarf lemurs (Cheirogaleus medius) and patas monkeys (Erythrocebus patas) J. Hum. Evol. 2007;53:26–40. doi: 10.1016/j.jhevol.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Lemelin P, Schmitt D. Seasonal variation in body mass and locomotor kinetics of the fat-tailed dwarf lemur (Cheirogaleus medius) J. Morphol. 2004;260:65–71. doi: 10.1002/jmor.10214. [DOI] [PubMed] [Google Scholar]
- 7.Arbour VM. Estimating impact forces of tail club strikes by ankylosaurid dinosaurs. PloS ONE. 2009;4:e6738. doi: 10.1371/journal.pone.0006738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold EN. Evolutionary aspects of tail shedding in lizards and their relatives. J. Nat. Hist. 1984;18:127–169. doi: 10.1080/00222938400770131. [DOI] [Google Scholar]
- 9.Fish FE. Kinematics of undulatory swimming in the American alligator. Copeia. 1984;1984:839–843. doi: 10.2307/1445326. [DOI] [Google Scholar]
- 10.Lauder GV. Function of the caudal fin during locomotion in fishes: kinematics, flow visualization, and evolutionary patterns. Amer. Zool. 2000;40:101–122. [Google Scholar]
- 11.O’Connor SM, Dawson TJ, Kram R, Donelan JM. The kangaroo’s tail propels and powers pentapedal locomotion. Biol. Lett. 2014;10:20140381. doi: 10.1098/rsbl.2014.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carrier DR, Walter RM, Lee DV. Influence of rotational inertia on turning performance of theropod dinosaurs: clues from humans with increased rotational inertia. J. Exp. Biol. 2001;204:3917–3926. doi: 10.1242/jeb.204.22.3917. [DOI] [PubMed] [Google Scholar]
- 13.Walter RM, Carrier DR. Scaling of rotational inertia in murine rodents and two species of lizard. J. Exp. Biol. 2002;205:2135–2141. doi: 10.1242/jeb.205.14.2135. [DOI] [PubMed] [Google Scholar]
- 14.Ballinger RE. Experimental evidence of the tail as a balancing organ in the lizard. Anolis carolinensis. Herpetologica. 1973;29:65–66. [Google Scholar]
- 15.Hsieh ST. Tail loss and narrow surfaces decrease locomotor stability in the arboreal green anole lizard (Anolis carolinensis) J. Exp. Biol. 2016;219:364–373. doi: 10.1242/jeb.124958. [DOI] [PubMed] [Google Scholar]
- 16.Boistel R, et al. Assisted walking in Malagasy dwarf chamaeleons. Biol. Lett. 2010;6:740–743. doi: 10.1098/rsbl.2010.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.German RZ. The functional morphology of caudal vertebrae in New World monkeys. Am. J. Phys. Anthropol. 1982;58:453–459. doi: 10.1002/ajpa.1330580414. [DOI] [PubMed] [Google Scholar]
- 18.Lemelin P. Comparative and functional myology of the prehensile tail in New World monkeys. J. Morphol. 1995;224:351–368. doi: 10.1002/jmor.1052240308. [DOI] [PubMed] [Google Scholar]
- 19.Buck CW, Tolman N, Tolman W. The tail as a balancing organ in mice. J. Mammal. 1925;6:267–271. doi: 10.2307/1373415. [DOI] [Google Scholar]
- 20.Larson SG, Stern JT., Jr. Maintenance of above-branch balance during primate arboreal quadrupedalism: coordinated use of forearm rotators and tail motion. Am. J. Phys. Anthropol. 2006;129:71–81. doi: 10.1002/ajpa.20236. [DOI] [PubMed] [Google Scholar]
- 21.Walker C, Vierck CJ, Jr., Ritz LA. Balance in the cat: role of the tail and effects of sacrocaudal transection. Behav. Brain Res. 1998;91:41–47. doi: 10.1016/S0166-4328(97)00101-0. [DOI] [PubMed] [Google Scholar]
- 22.Alexander RM, Vernon A. The mechanics of hopping by kangaroos (Macropodidae) J. Zool. Lond. 1975;177:265–303. doi: 10.1111/j.1469-7998.1975.tb05983.x. [DOI] [Google Scholar]
- 23.Wilson AM, et al. Locomotion dynamics of hunting in wild cheetahs. Nature. 2013;498:185–189. doi: 10.1038/nature12295. [DOI] [PubMed] [Google Scholar]
- 24.Higham, T. E. & Anderson, C. V. In The biology of chameleons (eds Tolley, K. A. & Herrel, A.) 63–83 (University of California Press, 2013).
- 25.Vitt LJ, Congdon JD, Dickson NA. Adaptive strategies and energetics of tail autotomy in lizards. Ecology. 1977;58:326–337. doi: 10.2307/1935607. [DOI] [Google Scholar]
- 26.Higham TE, Davenport MS, Jayne BC. Maneuvering in an arboreal habitat: the effects of turning angle on the locomotion of three sympatric ecomorphs of Anolis lizards. J. Exp. Biol. 2001;204:4141–4155. doi: 10.1242/jeb.204.23.4141. [DOI] [PubMed] [Google Scholar]
- 27.Snyder RC. Bipedal locomotion of the lizard Basiliscus basiliscus. Copeia. 1949;1949:129–137. doi: 10.2307/1438487. [DOI] [Google Scholar]
- 28.Snyder RC. Adaptations for bipedal locomotion of lizards. Am. Zool. 1962;2:191–203. doi: 10.1093/icb/2.2.191. [DOI] [Google Scholar]
- 29.Ballinger RE, Nietfeldt JW, Krupa JJ. An experimental analysis of the role of the tail in attaining high running speed in Cnemidophorus sexlineatus (Reptilia: Squamata: Lacertilia) Herpetologica. 1979;35:114–116. [Google Scholar]
- 30.Punzo F. Tail autotomy and running speed in the lizards Cophosaurus texanus and Uma notata. J. Herpetol. 1982;16:329–331. doi: 10.2307/1563731. [DOI] [Google Scholar]
- 31.Jusufi A, Goldman DI, Revzen S, Full RJ. Active tails enhance arboreal acrobatics in geckos. Proc. Natl. Acad. Sci. USA. 2008;105:4215–4219. doi: 10.1073/pnas.0711944105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bateman PW, Fleming PA. To cut a long tail short: a review of lizard caudal autotomy studies carried out over the last 20 years. J. Zool. 2009;277:1–14. doi: 10.1111/j.1469-7998.2008.00484.x. [DOI] [Google Scholar]
- 33.McElroy EJ, Bergmann PJ. Tail autotomy, tail size, and locomotor performance in lizards. Physiol. Biochem. Zool. 2013;86:669–679. doi: 10.1086/673890. [DOI] [PubMed] [Google Scholar]
- 34.Higham TE, Russell AP, Zani PA. Integrative biology of tail autotomy in lizards. Physiol. Biochem. Zool. 2013;86:603–610. doi: 10.1086/673875. [DOI] [PubMed] [Google Scholar]
- 35.Gillis G, Higham TE. Consequences of lost endings: caudal autotomy as a lens for focusing attention on tail function during locomotion. J. Exp. Biol. 2016;219:2416–2422. doi: 10.1242/jeb.124024. [DOI] [PubMed] [Google Scholar]
- 36.Daniels CB. Running: an escape strategy enhanced by autotomy. Herpetologica. 1983;39:162–165. [Google Scholar]
- 37.Jagnandan K, Russell AP, Higham TE. Tail autotomy and subsequent regeneration alter the mechanics of locomotion in lizards. J. Exp. Biol. 2014;217:3891–3897. doi: 10.1242/jeb.110916. [DOI] [PubMed] [Google Scholar]
- 38.Higham TE, Russell AP. Time-varying motor control of autotomized leopard gecko tails: multiple inputs and behavioral modulation. J. Exp. Biol. 2012;215:435–441. doi: 10.1242/jeb.054460. [DOI] [PubMed] [Google Scholar]
- 39.Russell AP, Lynn SE, Powell GL, Cottle A. The regenerated tail of juvenile leopard geckos (Gekkota: Eublepharidae: Eublepharis macularius) preferentially stores more fat than the original. Zoology. 2015;118:183–191. doi: 10.1016/j.zool.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Delorme SL, Lungu IM, Vickaryous MK. Scar-free wound healing and regeneration following tail loss in the leopard gecko. Eublepharis macularius. Anat. Rec. 2012;295:1575–1595. doi: 10.1002/ar.22490. [DOI] [PubMed] [Google Scholar]
- 41.Lynn SE, Borkovic BP, Russell AP. Relative apportioning of resources to the body and regenerating tail in juvenile leopard geckos (Eublepharis macularius) maintained on different dietary rations. Physiol. Biochem. Zool. 2013;86:659–668. doi: 10.1086/673312. [DOI] [PubMed] [Google Scholar]
- 42.McLean KE, Vickaryous MK. A novel amniote model of epimorphic regeneration: the leopard gecko. Eublepharis macularius. BMC Dev. Biol. 2011;11:50. doi: 10.1186/1471-213X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamley T. Functions of the tail in bipedal locomotion of lizards, dinosaurs and pterosaurs. Mem. Qd. Mus. 1990;28:153–158. [Google Scholar]
- 44.Libby T, et al. Tail-assisted pitch control in lizards, robots and dinosaurs. Nature. 2012;481:181–184. doi: 10.1038/nature10710. [DOI] [PubMed] [Google Scholar]
- 45.Reilly SM. Quantitative electromyography and muscle function of the hind limb during quadrupedal running in the lizard Sceloporus clarki. Zoology. 1994;98:263–277. [Google Scholar]
- 46.Irschick DJ, Jayne BC. Comparative three-dimensional kinematics of the hindlimb for high-speed bipedal and quadrupedal locomotion of lizards. J. Exp. Biol. 1999;202:1047–1065. doi: 10.1242/jeb.202.9.1047. [DOI] [PubMed] [Google Scholar]
- 47.Russell AP, Bauer AM. The m. caudifemoralis longusand its relationship to caudal autotomy and locomotion in lizards (Reptilia: Sauria) J. Zool. Lond. 1992;227:127–143. doi: 10.1111/j.1469-7998.1992.tb04349.x. [DOI] [Google Scholar]
- 48.Nelson FE, Jayne BC. The effects of speed on the in vivo activity and length of a limb muscle during the locomotion of the iguanian lizard Dipsosaurus dorsalis. J. Exp. Biol. 2001;204:3507–3522. doi: 10.1242/jeb.204.20.3507. [DOI] [PubMed] [Google Scholar]
- 49.Snyder RC. The anatomy and function of the pelvic girdle and hindlimb in lizard locomotion. Am. J. Anat. 1954;95:1–45. doi: 10.1002/aja.1000950102. [DOI] [PubMed] [Google Scholar]
- 50.Snyder RC. Quadrupedal and bipedal locomotion of lizards. Copeia. 1952;1952:64–70. doi: 10.2307/1438533. [DOI] [Google Scholar]
- 51.Higham TE, Malas B, Jayne BC, Lauder GV. Constraints on starting and stopping: behavior compensates for reduced pectoral fin area during braking of the bluegill sunfish Lepomis macrochirus. J. Exp. Biol. 2005;208:4735–4746. doi: 10.1242/jeb.01966. [DOI] [PubMed] [Google Scholar]
- 52.Wickler SJ, et al. Energetic and kinematic consequences of weighting the distal limb. Equine Vet. J. 2004;36:772–777. doi: 10.2746/0425164044848046. [DOI] [PubMed] [Google Scholar]
- 53.Carr JH, Gentile AM. The effect of arm movement on the biomechanics of standing up. Hum. Mov. Sci. 1994;13:175–193. doi: 10.1016/0167-9457(94)90035-3. [DOI] [Google Scholar]
- 54.Ashby BM, Delp SL. Optimal control simulations reveal mechanisms by which arm movement improves standing long jump performance. J. Biomech. 2006;39:1726–1734. doi: 10.1016/j.jbiomech.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 55.Gillis GB, Kuo CY, Irschick D. The impact of tail loss on stability during jumping in green anoles (Anolis carolinensis) Physiol. Biochem. Zool. 2013;86:680–689. doi: 10.1086/673756. [DOI] [PubMed] [Google Scholar]
- 56.Reilly SM, Delancey MJ. Sprawling locomotion in the lizard Sceloporus clarkii: quantitative kinematics of a walking trot. J. Exp. Biol. 1997;200:753–765. doi: 10.1242/jeb.200.4.753. [DOI] [PubMed] [Google Scholar]
- 57.Reilly SM, Delancey MJ. Sprawling locomotion in the lizard Sceloporus clarkii: the effects of speed on gait, hindlimb kinematics, and axial bending during walking. J. Zool. Lond. 1997;243:417–433. doi: 10.1111/j.1469-7998.1997.tb02791.x. [DOI] [Google Scholar]
- 58.Reilly SM, Willey JS, Biknevicius AR, Blob RW. Hindlimb function in the alligator: integrating movements, motor patterns, ground reaction forces and bone strain of terrestrial locomotion. J. Exp. Biol. 2005;208:993–1009. doi: 10.1242/jeb.01473. [DOI] [PubMed] [Google Scholar]
- 59.Nyakatura JA, Andrada E, Curth S, Fischer MS. Bridging “Romer’s gap”: limb mechanics of an extant belly-dragging lizard inform debate on tetrapod locomotion during the early Carboniferous. Evol. Biol. 2013;41:175–190. doi: 10.1007/s11692-013-9266-z. [DOI] [Google Scholar]
- 60.Peterson JA. The locomotion of Chamaeleo (Reptilia: Sauria) with particular reference to the forelimb. J. Zool. Lond. 1984;202:1–42. doi: 10.1111/j.1469-7998.1984.tb04286.x. [DOI] [Google Scholar]
- 61.Gillis GB, Bonvini LA, Irschick DJ. Losing stability: tail loss and jumping in the arboreal lizard Anolis carolinensis. J. Exp. Biol. 2009;212:604–609. doi: 10.1242/jeb.024349. [DOI] [PubMed] [Google Scholar]
- 62.Langkilde T, Shine R. How much stress do researchers inflict on their study animals? A case study using a scincid lizard. Eulamprus heatwolei. J. Exp. Biol. 2006;209:1035–1043. doi: 10.1242/jeb.02112. [DOI] [PubMed] [Google Scholar]
- 63.Hedrick TL. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir. Biomim. 2008;3:034001. doi: 10.1088/1748-3182/3/3/034001. [DOI] [PubMed] [Google Scholar]
- 64.Foster KL, Higham TE. How forelimb and hindlimb function changes with incline and perch diameter in the green anole. Anolis carolinensis. J. Exp. Biol. 2012;215:2288–2300. doi: 10.1242/jeb.069856. [DOI] [PubMed] [Google Scholar]