Abstract
The traditional identification of plant-parasitic nematode species by morphology and morphometric studies is very difficult because of high morphological variability that can lead to considerable overlap of many characteristics and their ambiguous interpretation. For this reason, it is essential to implement approaches to ensure accurate species identification. DNA barcoding aids in identification and advances species discovery. This study sought to unravel the use of the mitochondrial marker cytochrome c oxidase subunit 1 (coxI) as barcode for Longidoridae species identification, and as a phylogenetic marker. The results showed that mitochondrial and ribosomal markers could be used as barcoding markers, except for some species from the Xiphinema americanum group. The ITS1 region showed a promising role in barcoding for species identification because of the clear molecular variability among species. Some species presented important molecular variability in coxI. The analysis of the newly provided sequences and the sequences deposited in GenBank showed plausible misidentifications, and the use of voucher species and topotype specimens is a priority for this group of nematodes. The use of coxI and D2 and D3 expansion segments of the 28S rRNA gene did not clarify the phylogeny at the genus level.
Introduction
The phylum Nematoda comprises one of the largest and most diverse groups of animals. Most species are found in oceanic, freshwater and soil ecosystems, and only a few are pathogens of animals and plants1. Plant-parasitic nematodes (PPNs) have a diverse morphology and parasitic habits2. PPNs are distributed between the classes Chromadorea and Enoplea within very restricted orders (Rhabditida, Dorylaimida and Triplonchida)3. The order Dorylaimida, which belongs to Enoplea, includes several genera of PPNs in the family Longidoridae (Australodorus, Longidoroides, Longidorus, Paralongidorus, Paraxiphidorus, Xiphidorus and Xiphinema)3. These nematodes are of particular scientific and economic interest because they directly damage the roots of the host plant and some are vectors of several plant viruses (genus Nepovirus) that cause severe damage to a wide variety of crops4. Because of its great morphological diversity, the genus Xiphinema has been divided into two species groups5–8: (i) the Xiphinema americanum group, which comprises a complex of approximately 60 species, and (ii) the Xiphinema non-americanum group, which comprises a complex of more than 200 species. The traditional identification of these species by morphology and morphometric studies is very difficult because of their high intra-specific morphological variability, which can lead to considerable overlap of many characteristics and ambiguous interpretation6, 9. For this reason, new approaches are needed to ensure accurate species identification. Recently, numerous species from Longidoridae (44.4%) were molecularly characterized by ribosomal RNA genes (rDNA), i.e. partial 18S, ITS regions, or the D2 and D3 expansion segments of the 28S rRNA gene, as well as by the protein-coding mitochondrial gene cytochrome c oxidase subunit 1 (coxI), constituting a useful tool for species identification and the establishment of phylogenetic relationships within PPNs6, 10–14. Several studies conducted with 18S rRNA gene sequences11, 15, 16 did not provide taxonomic clarity among Longidoridae, since this gene seems to evolve too slowly to be useful as an appropriate marker for phylogenetic studies at the species level. The ITS region, D2–D3 of 28S rDNA sequences, and the coxI gene could be considered good markers for species identification. However, due to molecular variability in the ITS region, it appears better suited for species identification than for phylogenetic analysis17. Additionally, recent studies showed that mtDNA genes evolve much more quickly than rRNA genes, revealing low intra-specific and high inter-specific molecular variability for Longidoridae12, 16, 18–21. Therefore, it seems to be the most promising marker to relieve taxonomic confusion within this group. The coxI gene is frequently used as an efficient marker for species identification in the animal kingdom and may also be used to estimate species richness, particularly in understudied faunas22.
Therefore, the objectives of this research were to evaluate the variability of the mitochondrial marker gene coxI and partial sequence of the 28S rRNA gene within Longidoridae, as well as their usefulness as markers for barcoding and for reconstructing the phylogeny of the group.
Results and Discussion
coxI amplification in Longidoridae
A total of 136 new accessions belonging to 82 species for coxI were obtained for the first time in this study (Tables 1 and S1). Taxon coverage (species/genus species) of 11.9%, 8.3%, and 1.5% was achieved for Xiphinema, Longidorus and Paralongidorus, respectively. PCR amplification and sequencing for the partial coxI were carried out by combining several primers (Table 1). The best set of primers were COIF/XIPHR221, followed by JB3/JB423, COIF/COIR and COIF/XIPHR121. These sets of primers amplified a single fragment of approximately 500 bp. We did not find amplification of pseudogenes using these sets of primers. However, we did not perform a systematic analysis of primer amplification, as we started with the combination COIF/XIPHR2 in the majority of the studied samples; this combination was reported to be efficient in previous studies21. All new partial coxI sequences were obtained using voucher specimens identified by integrative taxonomy, with the combination of morphological characteristics and unequivocal molecular markers from the same individual nematode, viz. the D2–D3 region (Tables 1 and S1) and ITS1 in some cases.
Table 1.
Taxa sampled for dagger and needle nematodes species of the family Longidoridae and sequences of cytochrome c oxidase subunit 1 (coxI) used in this study. Species identifications were based on morphology and barcoding using D2–D3 expansion segments of 28S rDNA1.
| Nematode species | Sample code | Locality | Host plant | GenBank accession numbers | |
|---|---|---|---|---|---|
| 28S | coxI | ||||
| Genus Xiphinema | |||||
| 1.Xiphinema adenohystherum | SORIA | Arévalo de la Sierra, Soria province, Spain | european holly | KC567164 | KY816588 |
| Xiphinema adenohystherum | ALMAG | Almagro, Ciudad Real province, Spain | wild olive | *2 | KY816589 |
| Xiphinema adenohystherum | AR086 | Prado del Rey, Cádiz province, Spain | wild olive | * | KY816590 |
| Xiphinema adenohystherum | AR078 | Almodóvar, Córdoba province, Spain | wild olive | * | KY816591 |
| Xiphinema adenohystherum | IASNB | Jerez de la Frontera, Cádiz province, Spain | wild olive | * | KY816592 |
| 2.Xiphinema andalusiense | ARO93 | Belmez,Córdoba, Spain | wild olive | KX244884 | KY816593 |
| Xiphinema andalusiense | 00419 | Andújar, Jaén, Spain | wild olive | KX244885 | KY816594 |
| Xiphinema andalusiense | AR108 | Villaviciosa de Córdoba, Córdoba, Spain | wild olive | KX244888 | KY816595 |
| 3.Xiphinema baetica | LOMAS | Hinojos, Huelva province, Spain | stone pine | KC567165 | KY816596 |
| Xiphinema baetica | HATRA | Villamanrique de la Condesa, Huelva, Spain | cork oak | KC567166 | KY816597 |
| 4.Xiphinema belmontense | MOUCH | Merza, Pontevedra province, Spain | chestnut | KC567171 | KY816598 |
| 5.Xiphinema cadavalense | ST077 | Espiel, Córdoba province, Spain | cultivated olive | KX244932 | KY816599 |
| 6.Xiphinema celtiense | AR083 | Adamuz, Córdoba province, Spain | wild olive | KX244889 | KY816600 |
| Xiphinema celtiense | AR082 | Adamuz, Córdoba province, Spain | wild olive | KX244890 | KY816601 |
| 7.Xiphinema cohni | J0126 | Puerto de Sta. María, Cádiz province, Spain | grapevine | KC567173 | KY816602 |
| 8.Xiphinema conurum | ST45V | Sorbas, Almería province, Spain | cultivated olive | KX244892 | KY816603 |
| 9.Xiphinema costaricense | ACC86 | Guayabo, Turrialba, Cartago, Costa Rica | forest | KX931056 | KY816604 |
| Xiphinema costaricense | ACC46 | Santa Rosa, Limón, Limón | cocoa | KX931057 | KY816605 |
| 10 Xiphinema coxi europaeum | AR020 | Hinojos, Huelva province, Spain | wild olive | KC567174 | KY816606 |
| Xiphinema coxi europaeum | H0027 | Almonte, Huelva province, Spain | cork oak | KC567177 | KY816607 |
| 11.Xiphinema cretense | AR039 | Hersonisos province, Crete, Greece | wild olive | KJ802878 | KY816608 |
| 12.Xiphinema duriense 3 | ST02C | Gibraleón, Huelva province, Spain | cultivated olive | KP268963 | KY816609 |
| 13.Xiphinema gersoni | H0059 | Almonte, Huelva province, Spain | eucalyptus | KC567180 | KY816610 |
| 14.Xiphinema herakliense | OLEA8 | Vathy Rema, Crete, Greece | wild olive | KM586345 | KY816611 |
| Xiphinema herakliense | OLEA17 | Agiofarago, Crete, Greece | wild olive | KM586346 | KY816612 |
| Xiphinema herakliense | OLE18 | Agiofarago, Crete, Greece | wild olive | KM58634 9 | KY816613 |
| 15.Xiphinema hispanum | 00419 | Andújar, Jaén province, Spain | wild olive | GU725074 | KY816614 |
| 16.Xiphinema hispidum | AR098 | Bollullos par del Condado, Huelva province, Spain | grapevine | KC567181 | KY816615 |
| Xiphinema hispidum | H0026 | Rociana del Condado, Huelva province, Spain | grapevine | HM921366 | KY816616 |
| 17.Xiphinema insigne | MIYA1 | Miyazaki, Japan | Prunus sp. | * | KY816617 |
| 18.Xiphinema israeliae | AR013 | Roufas province, Greece | wild olive | KJ802883 | KY816618 |
| 19.Xiphinema italiae | AR041 | Las Tres Villas, Almería province, Spain | wild olive | KX244911 | KY816619 |
| Xiphinema italiae | AR091 | Puerto Real, Cádiz province, Spain | wild olive | KX244912 | KY816620 |
| Xiphinema italiae | TUNIS | Sbitla, Kasserine, Tunisia | cultivated olive | KX062674 | KY816621 |
| Xiphinema italiae | TUN11 | Sbiba, Kasserine, Tunisia | cultivated olive | KX062677 | KY816622 |
| Xiphinema italiae | APUL | Bari, Bari province, Italy | grapevine | * | KY816623 |
| 20.Xiphinema iznajarense | JAO25 | Iznájar, Córdoba province, Spain | cultivated olive | KX244892 | KY816624 |
| 21.Xiphinema krugi | ACC47 | Sucre, Ciudad Quesada, Alajuela, Costa Rica | Robust star-grass | KX931061 | KY816625 |
| Xiphinema krugi | ACC13 | Santa Gertrudis, Grecia, Alajuela, Costa Rica | Sugar-cane | KX931060 | KY816626 |
| 22.Xiphinema luci | IAGRQ | Benacazón, Sevilla province, Spain | rose | KP268965 | KY816627 |
| 23.Xiphinema lupini | H0050 | Hinojos, Huelva province, Spain | grapevine | KC567183 | KY816628 |
| Xiphinema lupini | 388GD | Bollullos par del Condado, Huelva province, Spain | grapevine | HM921352 | KY816629 |
| Xiphinema lupini | 388GD | Bollullos par del Condado, Huelva province, Spain | grapevine | * | KY816630 |
| 24.Xiphinema macroacanthum | ITAL | Brindisi province, Italy | cultivated olive | * | KY816631 |
| 25.Xiphinema macrodora | AR097 | Santa Mª de Trassierra, Córdoba province, Spain | wild olive | KU171044 | KY816632 |
| 26.Xiphinema mengibarense | O3C04 | Mengíbar, Jaen province, Spain | cultivated olive | KX244893 | KY816633 |
| Xiphinema mengibarense | O30V5 | Mengíbar, Jaen province, Spain | cultivated olive | KX244894 | KY816634 |
| 27.Xiphinema meridianum | 11R16 | Sbitla, Kasserine, Tunisia | cultivated olive | KX062678 | KY816635 |
| 28.Xiphinema nuragicum | ST012 | Espejo, Córdoba province, Spain | grapevine | * | KY816636 |
| Xiphinema nuragicum | AR054 | Medina Sidonia, Cádiz province, Spain | wild olive | * | KY816637 |
| Xiphinema nuragicum | ST106 | La Puebla de los Infantes, Sevilla province, Spain | cultivated olive | * | KY816638 |
| Xiphinema nuragicum | JAO28 | Antequera, Málaga province, Spain | cultivated olive | * | KY816639 |
| Xiphinema nuragicum | AR113 | Alcolea, Córdoba province, Spain | wild olive | * | KY816640 |
| 29.Xiphinema opisthohysterum | AR031 | Tarifa, Cádiz province, Spain | wild olive | KP268967 | KY816641 |
| Xiphinema opisthohysterum | 00418 | Andújar, Jaén province, Spain | grasses | JQ990040 | KY816642 |
| 30.Xiphinema pseudocoxi | AR095 | Alcaracejos, Córdoba province, Spain | wild olive | KX244915 | KY816643 |
| 31.Xiphinema pyrenaicum | ESMEN | Cahors, Quercy province, France | grapevine | GU725073 | KY816644 |
| 32.Xiphinema rivesi | CASLO | Castillo de Locubín, Jaén province, Spain | cherry tree | JQ990037 | KY816645 |
| Xiphinema rivesi | 00518 | Moriles, Córdoba province, Spain | grapevine | HM921357 | KY816646 |
| 33.Xiphinema robbinsi | 12R28 | Sbitla, Kasserine, Tunisia | cultivated olive | KX062683 | KY816647 |
| 34.Xiphinema setariae | ACC09 | Pueblo Nuevo de Duacarí, Limón, Costa Rica | banana | KX931066 | KY816648 |
| 35.Xiphinema sphaerocephalum | AR063 | Coto Ríos, Jaén province, Spain | wild olive | * | KY816649 |
| 36.Xiphinema turcicum | ST149 | San José del Valle, Cádiz province, Spain | wild olive | * | KY816650 |
| 37.Xiphinema turdetanense | AR0015 | Sanlúcar de Barrameda, Cádiz province, Spain | wild olive | KC567186 | KY816651 |
| 38.Xiphinema vallense | AR0027 | Bolonia, Cádiz province, Spain | wild olive | KP268960 | KY816652 |
| Xiphinema vallense | H00003 | Hinojos, Huelva province, Spain | cultivated olive | KP268961 | KY816653 |
| 39.Xiphinema sp. | P0011 | Sbitla, Kasserine, Tunisia | cultivated olive | KX062686 | KY816654 |
| Genus Longidorus | |||||
| 40.Longidorus aetnaeus | CD1138 | Varenikovskaya, Krymsk, Krasnodar Terr., Russia | silver poplar | KF242324 | KY816655 |
| Longidorus aetnaeus | CD1108 | Varenikovskaya, Krymsk, Krasnodar Terr., Russia | Populus sp. | KF242323 | KY816656 |
| Longidorus aetnaeus | CD1111 | Varenikovskaya, Krymsk, Krasnodar Terr., Russia | Salix fragilis | KF242318 | KY816657 |
| Longidorus aetnaeus | CD1129 | Varenikovskaya, Krymsk, Krasnodar Terr., Russia | Acer tataricum | KF242321 | KY816658 |
| Longidorus aetnaeus | CD1143 | Varenikovskaya, Krymsk, Krasnodar Terr., Russia | Salix alba | KF242322 | KY816659 |
| 41.Longidorus africanus | P00011 | Chott-mariem province, Tunisia | cultivated olive | KX062665 | KY816660 |
| 42.Longidorus alvegus | ALNOR | Andújar, Jaén province, Spain | black alder | KT308867 | KY816661 |
| 43.Longidorus andalusicus | J0172 | Sanlúcar de Barrameda, Cádiz province, Spain | pickle weed | JX445118 | KY816662 |
| 44.Longidorus apulus | BARLE | Barletta, Bari province, Italy | artichoke | AY601571 | KY816663 |
| 45.Longidorus artemisiae | CD1127 | Shestikhino, Myshkin district, Yaroslavl, Russia | Poa sp. | KF242314 | KY816664 |
| 46.Longidorus asiaticus | LARGE | Bari province, Italy | crape myrtle | KR351254 | KY816665 |
| 47.Longidorus baeticus | M0121 | Montemayor, Córdoba province, Spain | grapevine | JX445106 | KY816666 |
| 48.Longidorus closelongatus | 23CRE | Mires, Heraklion province, Crete, Greece | grapevine | KJ802865 | KY816667 |
| 49.Longidorus crataegi | M0156 | Montemayor, Córdoba province, Spain | grapevine | JX445114 | KY816668 |
| Longidorus crataegi | M0156 | Montemayor, Córdoba province, Spain | grapevine | * | KY816669 |
| 50.Longidorus cretensis | TOCRE | Pentamodi, Heraklion province, Crete, Greece | cultivated olive | KJ802868 | KY816670 |
| 51.Longidorus distinctus | CD1128 | Pyatigorsk, Stavropol Territory, Russia | Salix sp. | KF242317 | KY816671 |
| 52.Longidorus euonymus | CD1118 | Bolshoy Vyas, Lunino district, Russia | Asparagus cicer | KF242333 | KY816672 |
| Longidorus euonymus | CD1130 | Anapa, Anapa district, Krasnodar Territory, Russia | Juglans regia | KF242332 | KY816673 |
| 53.Longidorus fasciatus | M0063 | Monturque, Córdoba province, Spain | grapevine | JX445108 | KY816674 |
| 54.Longidorus indalus | ST042 | Las Tres Villas, Almería province, Spain | cultivated olive | KT308854 | KY816675 |
| 55.Longidorus intermedius | CD1122 | Kamennomostsky, Adygeya, Russia | Fagus orientalis | KF242312 | KY816676 |
| 56.Longidorus iranicus | GRECD | Harakas province, Crete, Greece | grapevine | KJ802875 | KY816677 |
| 57.Longidorus iuglandis | H0183 | Bonares, Huelva province, Spain | grapevine | JX445104 | KY816678 |
| 58.Longidorus jonesi | MIY03 | Miyazaki, Japan | Prunus sp. | KF552069 | KY816679 |
| 59.Longidorus kuiperi | BOLOI | Bolonia, Cádiz province, Spain | marram grass | * | KY816680 |
| 60.Longidorus laevicapitatus | ACC01 | La Virgen de Sarapiquí, Heredia, Costa Rica | Sugar cane | KX136865 | KY816681 |
| 61.Longidorus leptocephalus | CD1119 | Potrosovo, Kozelsk district, Kaluga region, Russia | common nettle | KF242326 | KY816682 |
| 62.Longidorus lignosus | CD1120 | Sukko, Anapa district, Krasnodar Territory, Russia | Acer campestre | KF242345 | KY816683 |
| 63.Longidorus lusitanicus | J0212 | Sanlúcar de Barrameda, Cádiz province, Spain | wild olive | KT308869 | KY816684 |
| 64.Longidorus macrodorus | JAO06 | La Grajuela, Córdoba province, Spain | cultivated olive | KT308855 | KY816685 |
| Longidorus macrodorus | JAO06 | La Grajuela, Córdoba province, Spain | cultivated olive | KT308856 | KY816686 |
| 65.Longidorus magnus | M0130 | Aguilar de la Frontera, Córdoba province, Spain | cultivated olive | * | KY816687 |
| Longidorus magnus | M0017 | Lucena, Córdoba province, Spain | grapevine | JX445113 | KY816688 |
| Longidorus magnus | M0079 | Monturque, Córdoba province, Spain | grapevine | * | KY816689 |
| Longidorus magnus | J0164 | Jerez de la Frontera, Cádiz province, Spain | grapevine | * | KY816690 |
| Longidorus magnus | ST077 | Espiel, Córdoba province, Spain | cultivated olive | * | KY816691 |
| Longidorus magnus | JAO01 | Villaviciosa de Córdoba, Córdoba province, Spain | cultivated olive | * | KY816692 |
| Longidorus magnus | JAO31 | Antequera, Málaga province, Spain | cultivated olive | * | KY816693 |
| Longidorus magnus | CASLO | Castillo de Locubin, Jaén province, Spain. | cherry tree | * | KY816694 |
| 66.Longidorus onubensis | ST005 | Niebla, Huelva province, Spain | cultivated olive | KT308857 | KY816695 |
| 67.Longidorus persicus | ESMAE | Gilan-e-Gharb, Kermanshah province, Iran | rose | KT149799 | KY816696 |
| 68.Longidorus pisi | 0IRAN | Markazi province, Iran | apple tree | JQ240274 | KY816697 |
| 69.Longidorus pseudoelongatus | AR034 | Voutes province,Crete, Greece | cultivated olive | KJ802870 | KY816698 |
| Longidorus pseudoelongatus | AR040 | Hersonisos province, Crete, Greece | cultivated olive | KJ802871 | KY816699 |
| 70.Longidorus rubi | H0026 | Almonte, Huelva province, Spain | Pinus pinea | JX445116 | KY816700 |
| 71.Longidorus silvestris | AR027 | Bolonia, Cádiz province, Spain | cultivated olive | KT308859 | KY816701 |
| 72.Longidorus vallensis | AR055 | San José del Valle, Cádiz province, Spain | wild olive | KT308861 | KY816702 |
| Longidorus vallensis | M0012 | Cabra, Córdoba province, Spain | grapevine | KT308862 | KY816703 |
| 73.Longidorus vineacola | AR031 | Tarifa, Cádiz province, Spain | wild olive | KT308873 | KY816704 |
| Longidorus vineacola | AR113 | Alcolea, Córdoba province, Spain | wild olive | * | KY816705 |
| Longidorus vineacola | TRASI | Santa Mª de Trassierra, Córdoba province, Spain | cultivated olive | * | KY816706 |
| Longidorus vineacola | M0124 | Montemayor, Córdoba province, Spain | Portuguese oak | * | KY816707 |
| Longidorus vineacola | M0124 | Montemayor, Córdoba province, Spain | Portuguese oak | * | KY816708 |
| Longidorus vineacola | 0419B | Andújar, Jaen province, Spain | wild olive | * | KY816709 |
| Longidorus vineacola | H0089 | Almonte, Huelva province, Spain | Stone pine | * | KY816710 |
| Longidorus vineacola | ST117 | Setenil de las Bodegas, Cádiz province, Spain | cultivated olive | * | KY816711 |
| Longidorus vineacola | ST016 | El Saucejo, Sevilla province, Spain | cultivated olive | KT308872 | KY816712 |
| 74.Longidorus vinearum | AR097 | Santa Mª de Trassierra, Córdoba province, Spain | wild olive | KT308876 | KY816713 |
| 75.Longidorus wicuolea | AR0101 | Bonares, Huelva province, Spain | wild olive | KT308865 | KY816714 |
| 76.Longidorus sp.3 | CD1112 | Natukhaevskaya, Krasnodar Territory, Russia | Prunus divaricata | KF242335 | KY816715 |
| 77.Longidorus sp.4 | CD1117 | Proletarka, Krasnosulinsk, Rostov region, Russia | Salix babylonica | KF242334 | KY816716 |
| 78.Longidorus sp.6 | CD876 | Point Reyes, Marin county, California, USA | unknown | KF242328 | KY816717 |
| Genus Paralongidorus | |||||
| 79.Paralongidorus bikanerensis | BAMIR | Bam, Kerman province, Iran | Palm | JN032584 | KY816718 |
| 80.Paralongidorus iranicus | NOURI | Nour, Mazandaran province, Iran | Pine | JN032587 | KY816719 |
| 81.Paralongidorus litoralis | ZAHAR | Zahara de los Atunes, Cádiz province, Spain | mask tree | EU026155 | KY816720 |
| 82.Paralongidorus paramaximus | ALGUC | Alcalá de Guadaira, Sevilla province, Spain | citrus | EU026156 | KY816721 |
| Paralongidorus paramaximus | ALGUC | Alcalá de Guadaira, Sevilla province, Spain | citrus | * | KY816722 |
| Paralongidorus paramaximus | ALGUC | Alcalá de Guadaira, Sevilla province, Spain | citrus | * | KY816723 |
1For species identification see refs 9, 19, 20, 25, 27, 39, 40, 43–47, 63–69. 2(*) Sequenced population but not deposited in GenBank database, since was identical to other sequences of the same species already deposited in GenBank. 3The previous Accession JQ990053 reported as belonging to X. duriense was a mistake, and has been already corrected in NCBI, and replaced here by the correct one (accurately sequenced from the same specimen than D2–D3) and replaced by the new correct sequence KY816609 in this study.
mtDNA and rDNA molecular variability
To our knowledge, the present study is the largest survey ever conducted for Longidoridae mtDNA and rDNA molecular variability. It covers 44 species (268 sequences), 112 species (577 sequences) and 64 species (252 sequences) for partial coxI, D2–D3 and ITS respectively, with more than one sequence per species as available in GenBank or obtained in this study (Tables S2–S4). However, some genera of Longidoridae were underrepresented (e.g., Paralongidorus and Xiphidorus) (Table S1).
For the partial coxI gene, 14 species (101 sequences) from the X. americanum group were studied, of which 7 showed a percent similarity lower than 90%: X. americanum (78.82%), X. brevicolle ‘complex’ (76.67%), X. californicum (89.83%), X. incognitum (86.61%), X. rivesi (70.94%), X. peruvianum (79.71%) and Xiphinema sp. 1 (82.66%). In the X. non-americanum group, intra-specific molecular variability of coxI was analysed in 18 species (89 sequences), but only two species within this group showed similarity values lower than 90%: X. adenohystherum (88.40%) and X. italiae (69.73%). The intra-specific molecular variability detected in 11 studied Longidorus species (52 sequences) was high; 4 of them showed a percentage of similarity below 85%: L. magnus (78.70%), L. orientalis (78.78%), L. poessneckensis (84.62%), and L. vineacola (68.91%). Finally, only one species from the genus Paralongidorus with available partial coxI sequences was found—Paralongidorus paramaximus—with 99% similarity between the three sequences analysed.
The majority of sequence variability in all the studied genera appears at the third codon position, as for L. helveticus, which showed a sequence similarity of 92.66% with all variations at silent sites24, or L. poessneckensis, which showed an 81% sequence similarity with all molecular variability at silent sites, except for two nucleotides that caused changes in the amino acid sequence25. In the majority of the studied cases, mean Kimura 2-parameter distance (K2P) values did not exceed the interspecific distance mean, except for 5 species from the X. americanum group: X. americanum, X. brevicolle ‘complex’, X. peruvianum, X. rivesi, and Xiphinema sp. 1. However, these species comprise species complexes that must be further studied, as recently proposed by Orlando et al., because some of them may have been misidentified26. In contrast, intra-specific molecular variability detected in X. italiae and X. adenohystherum was accurate and correct. In both cases, these species were identified by integrative taxonomic approaches, and molecular analyses were performed using the same DNA extraction of single individuals for different markers (D2–D3 and coxI). Integrative identification of the X. non-americanum group is apparently less difficult due to more taxonomically informative traits (e.g., uterine differentiation) and the higher number of species molecularly studied. Similarly, Longidorus spp. with higher intra-specific variability were clearly delineated in this study (viz. L. vineacola and L. magnus) and previous studies (viz. L. orientalis 27, L. poessneckensis 25 and L. helveticus 24), using integrative taxonomy and the combination of unequivocal molecular markers (D2–D3 and partial coxI) from single individuals. Our results suggest that intra-specific variation in the partial coxI gene may be higher than expected. However, more species and more populations should be studied in the future to clarify the real molecular variability among species within Longidoridae.
In contrast, the D2–D3 region showed low intra-specific molecular variability, since no similarity value below 95% was detected for any of the studied species (except X. americanum, with 94.65% similarity), even though there are more sequences from this region than for the partial coxI (112 species for D2–D3 vs 43 species for coxI) (Table S3). However, this lower intra-specific molecular variability may confound species identification, especially within the X. americanum group, where seven species showed molecular similarity values of 99% (X. rivesi, X. santos, X. citricolum, X. americanum, X. thornei, X. pacificum and X. georgianum) (data not shown). High inter-specific similarity values were detected in the other species—L. wicuolea and L. silvestris or X. pseudocoxi and X. globosum—which showed a similarity value of 97%. Hence, in these species, this marker could not provide clear species identification, and other sequences and integrative taxonomic approaches must be applied28.
The ITS1 maker showed low intra-specific molecular variability in the majority of the species studied; only some species showed a significantly low similarity (below 90%), such as X. brasiliense (89%), X. inaequale (80%), X. chambersi (87%), and L. biformis (85%). Unfortunately, because no data were available to confirm that these cases were misidentifications, further research is needed to confirm this high molecular variability. ITS sequences have been a prominent choice for species identification because this region is one of the most variable nuclear loci, and the availability of universal primers that work with most nematodes29 has contributed to its extensive use (Table S4). However, the high length and sequence variability between Longidoridae species complicates the construction of a plausible alignment of this region. Thus, this region appears to be better for species delimitation than for phylogenetic studies17, 29.
Maximum intra- and minimum inter-specific distances for each coxI and D2–D3 sequences are shown in Fig. 1, which shows that higher molecular variability for K2P distance was associated with partial coxI than with D2–D3 region for intra- and inter-specific comparisons. As discussed above, the range of intra- and inter-specific distances in the X. americanum group was minimal for the D2–D3 region. Importantly, the difference between intra- and inter-specific distances in the X. non-americanum is large and non-overlapping. The intra-specific variability in coxI is largely attributable to X. italiae in this group.
Figure 1.
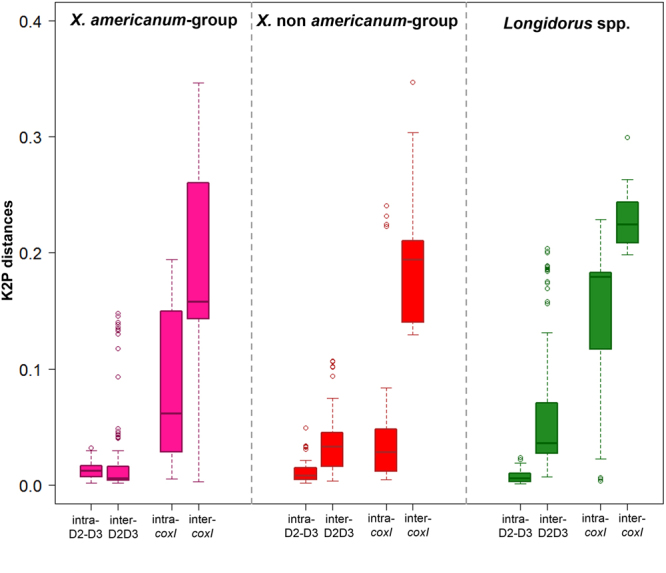
Intra- and inter-specific distance (K2P) for D2–D3 region and coxI markers for different groups of species within Longidoridae. Distances calculated using the biggest distance for intra-specific variability for each individual (sequence) among the sequences for the same species and the smallest distance among species for each individual. The box shows the third (Q3) and first (Q1) quartile range of the data and the median. Whiskers indicate minimum and maximum values of the data. Data falling outside the box and whiskers (circle) range are plotted but considered outliers.
Barcoding
To evaluate how well various barcoding tools perform for Longidoridae, we analyzed datasets for species that had been previously identified using integrative taxonomy and in addition data for Longidoridae deposited in GenBank. Three software packages were tested: Weka, Spider and phylogenetic trees topology based on MrBayes. We included and excluded the X. americanum group to understand the effect of these close-related species in our analysis. Our results suggest that DNA barcoding could be a powerful tool for the majority of species in Longidoridae using several approaches: (a) supervised machine learning methods; (b) distance threshold methods and (c) monophyly for species with more than two sequences in phylogenetic trees. However, barcoding results were highly dependent on the selected molecular marker and the technique used (Tables 2 and 3). Both mitochondrial and ribosomal sequences have been used as barcoding regions for nematodes in studies with smaller sample sizes and a larger phylogenetic range30, 31. Since our sequences were all derived from single vouchered specimens and are of high quality because we sequenced PCR products from both ends, the present reference database could also be a valuable tool for validating field collections32. The marker could also be used for soil nematode metabarcoding33, 34. The majority of our sequences for partial coxI are 400 bp long, which is in the range of appropriate size suggested by iBOL data quality: length of finished sequence must be >75% of approved marker length (e.g., 500 bp for coxI), with an expectation of 2X coverage (http://ibol.org/about-us/how-ibol-works/). With this sequence, we could clearly re-identify the majority of species, except for closely related species in the X. americanum group or species that were probably misidentified. The D2–D3 marker showed considerable sequence similarity in the X. americanum group, and for this reason two datasets were studied—one with all sequences and other excluding these sequences—to check the validity for the X. non-americanum-group species (Tables 2 and 3).
Table 2.
Accuracies (% correctly identified sequences from the test dataset) for barcoding in Longidoridae using the program Weka v.3.8.0. The datasets included all sequences of accessions that were identified to the species level and was divided into 80% as train set and 20% as test.
| Dataset1 | Jrip | J48 | Naïve Bayes | Iterative Classifier Optimizer |
|---|---|---|---|---|
| Cytochrome oxidase 1 | 78.43 | 82.35 | 80.39 | 88.24 |
| D2 and D3 expansion segments of the 28S | 63.06 | 84.69 | 36.03 | 94.59 |
| D2 and D3 expansion segments of the 28S (excluding X. americanum-group) | 69.74 | 88.16 | 36.84 | 96.05 |
1 X. brevicolle species complex was excluded from the analysis.
Table 3.
Accuracies for barcoding in Longidoridae using SPIDER package and tree-based comparison for monophyly using Bayesian inference.
| Dataset | Number of species | Number of sequences | Near Neighbour | Best Close Match1 | Sequences with inter-intra < = 0 | Optimal differences for barcoding2 | MrBayes phylogeny3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| False | True | Ambiguous | Correct | Incorrect | No id | ||||||
| Cytochrome oxidase 1 | 42 | 253 | 3 | 250 (99.9%) | 0 | 189 (74.7%) | 2 | 62 | 58 (22.9%) | 6.36% | 92.9% (39/42) |
| D2 and D3 expansion segments of the 28S4 | 111 | 560 | 24 | 536 (95.7%) | 18 | 503 (89.8%) | 19 | 20 | 138 (24.7%) | 2.87% | 90.1% (100/111) |
| D2 and D3 expansion segments of the 28S (excluding X. americanum-group) | 88 | 384 | 11 | 373 (99.9%) | 7 | 354 (92.2%) | 6 | 17 | 37 (9.6%) | 2.04% | 100% (88/88) |
Accuracy is defined as the percentage of sequences correctly assigned to their species in the case of Near Neighbour and Best Close Match. For the tree-based method, the accuracy was expressed as the percentage of species with more than one sequence that grouped as monophyletic in their respective molecular marker tree. 1Threshold based criterion of 1%. 2Experimental script in SPIDER. 3Percentage of species monophyletic to the respective tree. 4 X. brevicolle species complex excluded from the analysis.
The coxI and D2–D3 markers performed differently depending on the barcoding techniques used. The learning methods implemented in the Weka package achieved similar results for the coxI marker, ranging from 78.43% to 88.24% (Table 2). The performance of classification by machine learning was not strongly influenced by the presence of X. americanum-group sequences (384 vs. 560 sequences in D2–D3) (Table 2). The Bayesian-based method naïve Bayes classifier35 did not perform well with the D2–D3 data including or excluding the X. americanum group (36.03 and 36.84% of sequences assigned to correct species). The best classifier was the iterative classifier optimizer36 with 94.59 to 96.05% of sequences assigned to the correct species, followed by the decision tree C4.5 (J48)37 and the rule-based RIPPER (Jrip)38.
Using the Spider package, the Near Neighbour method showed very good accuracy for coxI, with almost 100% of correct identifications. Best Close Match performed less well. For both methods, the exclusion of the X. americanum group increased accuracy (Table 3). These results showed the potential for barcoding with these software packages for the majority of our species using both markers. In the case of MrBayes, phylogenetic analysis for species with more than one sequence showed that 92.9% of our species presented a monophyletic position in the tree for coxI. This performance was similar for the D2–D3 marker when both including (90.1%) and excluding the X. americanum group in Longidoridae (100%) (Table 3).
The knowledge of intra- and inter-specific molecular variability is important to detect misidentifications or cryptic speciation in different nematodes groups. Approximately a quarter of the sequences for coxI and D2–D3 region including X. americanum group showed a larger intra-specific than inter-specific molecular diversity; while an approximately 10% of the sequences was for D2–D3 region excluding X. americanum group (Table 3). Even with these differences, the performance was good and probably these molecular differences included the important molecular variability of some species, low intra-specific variability in others (species from the X. americanum group), poorly corrected sequences from chromatograms or sequences from PCR cloning products and, in some cases, incorrect identifications deposited in GenBank. Using an experimental script provided by the R package Spider, we were able to calculate the approximate optimal molecular differences for barcoding, which were 6.36% for coxI and 2.87% and 2.04% for D2–D3 when including the X. americanum group or excluding it, respectively (Table 3). Although this script is experimental and should be used with caution, our integrative taxonomic identifications in Longidoridae support these values9, 20, 28, 39, 40.
Phylogeny of Longidoridae using nuclear and mitochondrial sequence data
The phylogeny obtained using the coxI fragment (583 sequences) showed a monophyletic clade for the X. non-americanum-group species and a clade for Paralongidorus and Longidorus species, while the X. americanum group was paraphyletic (Fig. 2). However, all clades were weakly supported (<0.95 Bayesian probability values (BPP)). The phylogenies at the species level relationship generally supported the phylogenetic relationships among groups of species in Xiphinema more than in Longidorus reported in former papers (Fig. S1)6, 9, 11, 28, 39, 40. Nevertheless, in this wider analysis, we could not clearly determine groupings such as X. brevicolle ‘complex’ (nested among X. diffusum, X. taylori, and X. incognitum), and some entries for X. rivesi (from different geographical locations) following the corrections performed by Orlando et al. for the X. americanum group (Fig. S1), as one X. rivesi sequence (AM086697) was considered as X. floridae (AM086696)26. In addition, Xiphinema sp. 5 studied by Orlando et al.26 nested inside Longidorus. However, when BLASTn was performed on GenBank, this sequence matched as a Xiphinema sp. The separation among species was remarkable, with the exception of a few species in the X. americanum group, using a phylogenetic approach. The base saturation (third nucleotide position in each codon) and the short fragment used in this study could be responsible for this lack of phylogenetic resolution at the genus level and between X. americanum and X. non-americanum group inside the genus Xiphinema. Additionally, different mutation rates in the mitochondrial genome and the wide evolutionary differences within these studied groups could complicate the phylogeny. A dataset excluding the third codon position did not improve the phylogeny, and in fact made it worse because of the low phylogenetic signal (Fig. S2). Probably, a possible improvement in the phylogenetic relationships among genera in Nematoda could be obtained using full mitochondrial genomes41, 42.
Figure 2.
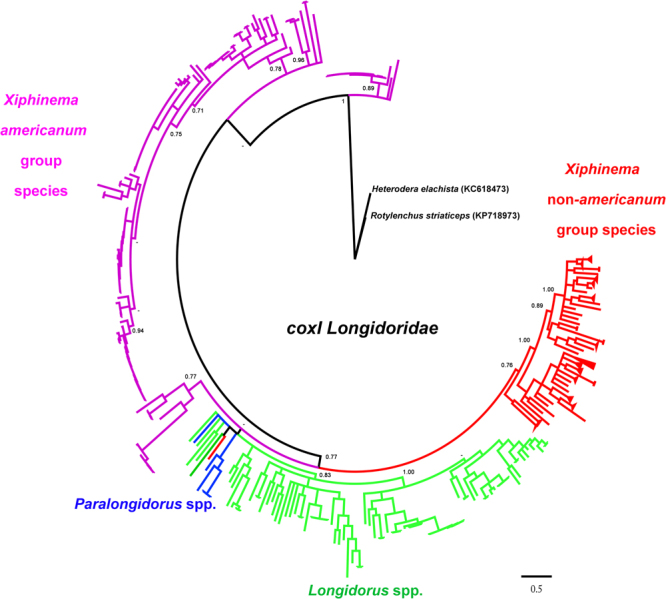
Phylogenetic relationships within Longidoridae. Bayesian 50% majority rule consensus tree as inferred from analysis of the partial coxI sequence alignment under a TrN + I + G model. Posterior probabilities more than 0.70 are given for appropriate clades.
The phylogeny of nuclear ribosomal marker (D2–D3) based on 1085 sequences of Longidoridae showed a similar pattern of separation among genera (Figs 3 and S3) after corrections for some misidentified species (X. cretense and X. diversicaudatum)43, 44. However, here, the separation for some species was better than in the coxI tree, since the X. non-americanum-group species and Longidorus-Paralongidorus (with the exception of L. laevicapitatus) were clearly separated into two well-supported clades (Figs 3 and S3). However, the X. americanum group formed a clade that is, however, weakly supported (≤0.90 BPP). As in the analysis with coxI, the genus Paralongidorus was nested among the Longidorus spp. clade. Xiphinema americanum s. s. species formed a low supported clade (0.77) (Fig. S3). As mentioned before, this group of species showed low nucleotide variability, probably because of a short speciation time among these species. Paralongidorus species formed a well-supported clade (1.00 BPP) inside Longidorus, with the exception of P. bikanerensis. This phylogeny is similar to others for Longidoridae9, 39, 45–47. Longer sequences probably need to be added in order to address this problem of deep resolution, but major clades have been clearly resolved using a more slowly evolving gene such as 18S. Recently, the sequencing of four additional mitogenomes of Longidoridae supported a similar phylogenetic pattern of Paralongidorus being most closely related to Longidorus, both associated with the Xiphinema species48.
Figure 3.
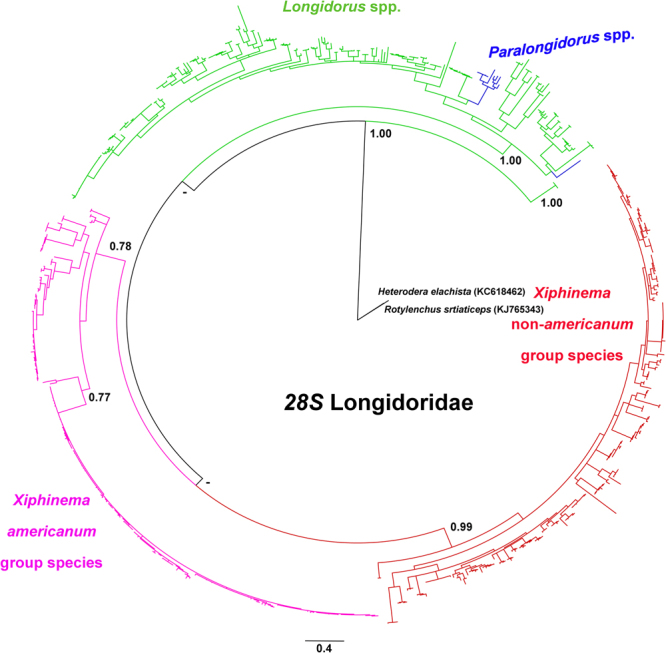
Phylogenetic relationships within Longidoridae. Bayesian 50% majority rule consensus tree as inferred from analysis of the D2–D3 region alignment under a GTR + I + G model. Posterior probabilities more than 0.70 are given for appropriate clades.
Conclusions
This is the first broad study of the variability of molecular markers used for phylogenetic relationships and the identification of Longidoridae. This research significantly increases the number of coxI sequences available for Longidoridae using integrative taxonomic approaches with voucher specimens and the combination of several unequivocal molecular markers (coxI, D2–D3 region and ITS1, in some cases) from one individual nematode. The ITS1 region showed promise for barcoding and species identification because of the clear molecular variability among species. However, difficulties with obtaining an unequivocal alignment limit its usefulness beyond BLASTn-like searches. In addition, we revealed problems for species delimitation in Longidoridae, as well as phylogenetic relationships using coxI and D2–D3 regions. However, in shallow phylogenetic relationships (close to the external branches of the tree) or for a restricted number of species, these markers gave good results. Several barcoding methods showed the utility of coxI and D2–D3 for species identification, except for some species in the X. americanum group (for which more studies are necessary for longer sequences or different markers). Our results suggest that the use of more than one molecular marker is essential for the correct identification of Longidoridae unless integrative taxonomical approaches are employed.
Material and Methods
Samples and nematode extraction
Nematode soil samples were collected from 2007 to 2016, mainly in Spain but also in Greece, Japan, the USA, Russia and Italy, from the rhizosphere of a wide variety of plants, including both agriculture and natural ecosystems (Tables 1 and S1). At each site, several subsampling points were randomly selected for soil sampling in an area of 5 m2. Soil samples were collected with a shovel discarding the upper 5-cm top soil profile from a 5- to 40-cm depth, in the close vicinity of active roots. To obtain a representative soil sample per site, all subsample soils were thoroughly mixed before nematode extraction. Nematodes from the soil were extracted from a 500-cm3 sub-sample using the magnesium sulphate centrifugal-flotation method49. The extracted nematodes were identified by selecting adult nematode specimens belonging to Longidoridae. Nematodes were fixed in 4% formaldehyde, processed with glycerin50, and identified by morphological traits to the genus or species level. Some additional nematodes from the same morphotype were not fixed and were used for molecular studies from each site.
DNA extraction and PCR conditions
For molecular analyses, to avoid complications from mixed species populations in the same sample, at least two live nematodes from each sample were temporarily mounted on a drop of 1 M NaCl containing glass beads (to avoid crushing the nematode). Here, diagnostic morphological characteristics were observed and measurements were taken to confirm species identity. The slides were dismantled and DNA was extracted. Nematode DNA was extracted from single individuals and PCR assays were conducted as described by Castillo et al.51. The portion of the partial coxI gene was amplified, as described by Lazarova et al.21 using the primers COIF (5′-GATTTTTTGGKCATCCWGARG-3′), COIR (5′-CWACATAATAAGTATCATG-3′), XIPHR1 (5′-ACAATTCCAGTTAATCCTCCTACC-3′) or XIPHR2 (5′-GTACATAATGAAAATGTGCCAC-3′) and as Bowles et al.23 using primers JB3 (5′-TTTTTTGGGCATCCTGAGGTTTAT-3′) and JB4 (5′-TAAAGAAAGAACATAATGAAAATG-3′). PCR cycle conditions for mtDNA were as described by Lazarova et al.: 1 cycle of 94 °C for 1 min, 50 °C for a further 1 min and 72 °C for 2 min. This was followed by 40 cycles of 94 °C for 1 min, 45 °C for 1 min and 72 °C for 2 min. The PCR was completed with a final extension phase of 94 °C for 1 min, 45 °C for 1 min and 72 °C for 5 min21. The D2–D3 region was obtained using a protocol and primers described in Archidona-Yuste et al.9, 39. PCR products were purified after amplification using ExoSAP-IT (Affmetrix, USB products) and used for direct sequencing in both directions. The resulting products were run on a DNA multicapillary sequencer (Model 3130XL genetic analyser; Applied Biosystems, Foster City, CA, USA), using the BigDye Terminator Sequencing Kit v.3.1 (Applied Biosystems, Foster City, CA, USA), at the Stab Vida sequencing facilities (Caparica, Portugal). The newly obtained sequences were submitted to the GenBank database under accession numbers indicated on the phylogenetic trees and Tables 1 and S1.
Nucleotide variability analyses
A total of 577, 257, and 261 sequences from 112, 65 and 44 species of Longidoridae were used to calculate the intra- and inter-specific molecular variability of 28S, ITS1 and coxI, respectively. For intra-specific molecular variability, one dataset from each species with more than one available sequence (Tables S2–S4) was created and aligned using MAFFT v. 7.252 with default parameters. Then, pairwise divergence among taxa were computed as a percentage of sequence similarity, singletons sites and parsimony informative sites using the program MEGA v. 7.053 (Tables S2–S4). Additionally, for coxI, p-distance was calculated for each codon position. For inter-specific molecular variability, four datasets were created, including sequences from the X. non-americanum group, X. americanum group, Longidorus spp. and Paralongidorus spp. Nucleotide variability indices were calculated in the same way as the intra-specific molecular variability after grouping the different species in each dataset (MEGA v.7.0). “Spider” package54 with R version 3.1.1 freeware (R Core Development Team; CRAN, http://cran.r-project.org)55 generates two statistics for each sequence (individual) in the dataset: the furthest intra-specific distance among its own species and the closest, non-conspecific (i.e., inter-specific distance). These data were used to create Fig. 1 among makers and species groups.
Barcoding analyses
Species without clear taxonomic status (X. brevicolle) and sequences considered misidentifications using several phylogenetic analyses9, 26, 39, 43, 44, as well as sequences with less than 300 bp in the D2–D3 fragment, were excluded from the analysis. Two datasets were used, corresponding to the coxI and D2–D3 regions. Several barcoding methods were used to test the utility of these molecular markers for species identification: (i) supervised machine learning methods to classify species following the method explained by Weitschek et al.56 using the Weka machine learning software55, which includes a collection of supervised classification methods. Jrip, J48, and naïve Bayes were used as supervised classification methods. The dataset included all species identified with all molecular variability using a test option for the dataset with a percentage split of 80% train set of sequences and 20% as test sequences, this option is allowed in Weka v.3.8.057 using the following Weka classifiers: (1) the rule-based RIPPER (Jrip)38; (2) the decision tree C4.5 (J48)37; (3) the Iterative Classifier Optimizer57; and (4) the Bayesian-based method naïve Bayes35. (ii) Tests of barcoding “best close match”58, nearest-neighbour identification59, and a standard threshold cut-off for species separation was determined using the function “localMinima” (this function determines possible thresholds from the distance matrix for an alignment) using a dataset for both the coxI and D2–D3 regions (including and excluding the X. americanum group) using the indications and principal functions implemented in the “spider” package54 with R version 3.1.1 freeware (R Core Development Team; CRAN, http://cran.r-project.org)55. Additionally, iii) phylogenetic trees conducted using MrBayes were analysed for species monophyly and species congruence for species with more than one available sequence. For this analysis, species not forming a monophyletic clade were considered not well identified, and the number of divergent sequences was annotated.
ITS1 sequences were excluded from all analyses because of the high divergence degree and difficulties with regard to phylogenies and correct alignments. However, a molecular variability table was considered in order to elucidate the molecular diversity of this marker in Longidoridae.
Phylogenetics analyses
Nucleotide data sets consisted of the partial coxI fragments for barcoding species in Longidoridae and of protein coding fragments. Outgroup taxa were Heterodera elachista and Rotylenchus striaticeps. The newly obtained and published sequences for each gene were aligned using MAFFT v. 7.252 with default parameters. Sequence alignments were manually edited using BioEdit57. Phylogenetic analyses of the sequence data sets were performed based on Bayesian inference (BI) using MrBayes 3.1.260. The best fitting model of DNA evolution was obtained using jModelTest v. 2.1.761 with the Akaike Information Criterion (AIC). The Akaike-supported model, the base frequency, the proportion of invariable sites, and the gamma distribution shape parameters and substitution rates in the AIC were then used in phylogenetic analyses. BI analysis under a Tamura-Nei with a proportion of invariable sites and a gamma-shaped distribution (TrN + I + G) model for coxI mtDNA was run for 4 × 106 generations, while for the first and second nucleotide for each codon a transversion model with a proportion of invariable sites and a gamma-shaped distribution (TVM + I + G) was used, with 10 × 106 generations. The general time reversible model with a proportion of invariable sites and a gamma-shaped distribution (GTR + I + G) using 10 × 106 generations was used for the D2–D3 maker. The Markov chains were sampled at intervals of 100 generations. Two runs were performed for each analysis. After discarding burn-in samples and evaluating convergence, the remaining samples were retained for further analyses. The topologies were used to generate a 50% majority rule consensus tree. Posterior probabilities (PP) are given in appropriate clades. Trees were visualized using TreeView62 and FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Electronic supplementary material
Acknowledgements
The authors thank J. Martín-Barbarroja (IAS-CSIC) and G. León Ropero (IAS-CSIC) for their excellent technical assistance. We thank Dr. E.A. Tzortzakakis (N.AG.RE.F., Hellenic Agricultural Organization-DEMETER, Greece); Dr. N. Vovlas (Istituto per la Protezione delle Piante, Italy); Dr. Peraza Padilla (Escuela de Ciencias Agrarias, Universidad Nacional, Costa Rica); I. Guesmi-Mzoughi (Higher Institute of Agronomy, University of Sousse, Tunisia); M. Esmaeili (College of Agriculture and Natural Resources, University of Tehran, Iran) and Dr. M. Pedram (College of Agriculture, Tarbiat Modares University, Iran) for providing nematodes for some of the species studied. This research was supported by grants P12-AGR 1486 and AGR-136 from the ‘Consejeria de Economia, Innovacion y Ciencia’ of the Junta de Andalucia, and Union Europea, Fondo Europeo de Desarrollo regional, ‘Una manera de hacer Europa’, grant 201740E042, “Análisis de diversidad molecular, barcoding, y relaciones filogenéticas de nematodos fitoparásitos en cultivos mediterráneos” from Spanish National Research Council (CSIC), grant 219262 ArimNET_ERANET FP7 2012–2015 Project PESTOLIVE ‘Contribution of olive history for the management of soil-borne parasites in the Mediterranean basin’ from the Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (INIA), and Project AGL-2012-37521 from the ‘Ministerio de Economía y Competitividad’ of Spain.
Author Contributions
Conceived and designed the experiments: J.E.P.R., P.C., C.C.N., A.A.Y. and S.A.S. Performed the experiments: J.E.P.R. and C.C.N. Analysed the data: J.E.P.R., C.C.N. and P.C. Contributed reagents/materials/analysis tools: J.E.P.R., C.C.N., A.A.Y. and S.A.S. Wrote the paper: J.E.P.R., P.C., A.A.Y., C.C.N. and S.A.S.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-11085-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baldwin JG, Nadler SA, Adams BJ. Evolution of plant parasitism among nematodes. Annu Rev Phytopathol. 2004;42:83–105. doi: 10.1146/annurev.phyto.42.012204.130804. [DOI] [PubMed] [Google Scholar]
- 2.Hunt, D. & Palomares-Rius, J. E. In Practical Plant Nematology (ed. R.H. Manzanilla-López, Marbán-Mendoza, N.) 25–62 (Mundi-Prensa-España, 2012).
- 3.Manzanilla-López, R. H. & Hunt, D. In Practical Plant Nematology (ed R.H. Manzanilla-López, Marbán-Mendoza, N.) 65–87 (Mundi-Prensa-España, 2012).
- 4.Decraemer, W. & Chaves, E. In Practical Plant Nematology (ed R.H. Manzanilla-López, Marbán-Mendoza, N.) 25–62 (Mundi-Prensa-España, 2012).
- 5.Coomans, A., Huys, R., Heyns, J., Luc, M. Character analysis, phylogeny, and biogeography of the genus Xiphinema Cobb, 1973 (Nematoda, Longidoridae) (Tervuren, Belgique., 2001).
- 6.He Y, et al. A molecular phylogenetic approach to Longidoridae (Nematoda: Dorylaimida) Nematology. 2005;7:111–124. doi: 10.1163/1568541054192108. [DOI] [Google Scholar]
- 7.Lamberti F, Molinari S, Moens M, Brown DJF. The Xiphinema americanum group. I. Putative species, their geographical occurrence and distribution, and regional polytomous identification keys for the group. Russian J Nematol. 2000;8:65–84. [Google Scholar]
- 8.Loof PAA, Luc M. A revised polytomous key for the identification of species of the genus Xiphinema Cobb, 1913 (Nematoda: Longidoridae) with exclusion of the X. americanum-group. Syst Parasitol. 1990;16:35–66. doi: 10.1007/BF00009600. [DOI] [Google Scholar]
- 9.Archidona-Yuste A, Navas-Cortés JA, Cantalapiedra-Navarrete C, Palomares-Rius JE, Castillo P. Remarkable diversity and prevalence of dagger nematodes of the genus Xiphinema Cobb, 1913 (Nematoda: Longidoridae) in olives revealed by integrative approaches. PLoS ONE. 2016;11:e0165412. doi: 10.1371/journal.pone.0165412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsi L, De Luca F. Morphological and molecular characterisation of two putative Xiphinema americanum group species, X. parasimile and X. simile (Nematoda: Dorylaimida) from Serbia. Nematology. 2008;10:15–25. doi: 10.1163/156854108783360212. [DOI] [Google Scholar]
- 11.Gutiérrez-Gutiérrez C, et al. Molecular analysis and comparative morphology to resolve a complex of cryptic Xiphinema species. Zool Scr. 2010;39:483–498. doi: 10.1111/j.1463-6409.2010.00437.x. [DOI] [Google Scholar]
- 12.Kumari S, Decraemer W, De Luca F, Tiefenbrunner W. Cytochrome c oxidase subunit 1 analysis of Xiphinema diversicaudatum, X. pachtaicum, X. simile and X. vuittenezi (Nematoda, Dorylaimida) Eur J Plant Pathol. 2010;127:493–499. doi: 10.1007/s10658-010-9614-0. [DOI] [Google Scholar]
- 13.Wang X, et al. Multiplex polymerase chain reaction identification of single individuals of the Longidorid nematodes Xiphinema index, X. diversicaudatum, X. vuittenezi, and X. italiae using specific primers from ribosomal genes. Phytopathology. 2003;93:160–166. doi: 10.1094/PHYTO.2003.93.2.160. [DOI] [PubMed] [Google Scholar]
- 14.Ye W, Szalanski AL, Robbins RT. Phylogenetic relationships and genetic variation in Longidorus and Xiphinema species (Nematoda: Longidoridae) using ITS1 sequences of nuclear ribosomal DNA. J Nematol. 2004;36:14–19. [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira CMG, et al. Phylogenetic relationships among Xiphinema and Xiphidorus nematode species from Brazil inferred from 18S rDNA sequences. J Nematol. 2004;36:153–159. [PMC free article] [PubMed] [Google Scholar]
- 16.Zasada IA, et al. Using mitogenomic and nuclear ribosomal sequence data to investigate the phylogeny of the Xiphinema americanum species complex. PLoS ONE. 2014;9:e90035. doi: 10.1371/journal.pone.0090035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luca F, et al. Characterization and sequence variation in the rDNA region of six nematode species of the genus Longidorus (Nematoda) J Nematol. 2004;36:147–152. [PMC free article] [PubMed] [Google Scholar]
- 18.Chizhov VN, Pridannikov MV, Peneva V, Subbtoin AS. Morphological and molecular characterisation of the Saratov population of the European dagger nematode, Xiphinema diversicaudatum (Nematoda: Dorylaimida), with notes on phylogeography of the species. Nematology. 2014;16:847–862. doi: 10.1163/15685411-00002813. [DOI] [Google Scholar]
- 19.Gutiérrez-Gutiérrez C, et al. Genetic structure of Xiphinema pachtaicum and X. index populations based on mitochondrial DNA variation. Phytopathology. 2011;101:1168–1175. doi: 10.1094/PHYTO-07-10-0194. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez-Gutiérrez C, et al. Phylogeny, diversity, and species delimitation in some species of the Xiphinema americanum-group complex (Nematoda: Longidoridae), as inferred from nuclear and mitochondrial DNA sequences and morphology. Eur J Plant Pathol. 2012;134:561–597. doi: 10.1007/s10658-012-0039-9. [DOI] [Google Scholar]
- 21.Lazarova SS, Malloch G, Oliveira CMG, Hübschen J, Neilson R. Ribosomal and mitochondrial DNA analyses of Xiphinema americanum-group populations. J Nematol. 2006;38:404–410. [PMC free article] [PubMed] [Google Scholar]
- 22.Clare EL, Lim B, Fenton MB, Hebert PDN. Neotropical bats: estimating species diversity with DNA barcodes. PLoS ONE. 2011;6:e22648. doi: 10.1371/journal.pone.0022648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasit. 1992;54:165–174. doi: 10.1016/0166-6851(92)90109-W. [DOI] [PubMed] [Google Scholar]
- 24.Kumari S, Subbotin SA. Characterization of Longidorus helveticus (Nematoda: Longidoridae) from the Czech Republic. Eur J Plant Pathol. 2012;133:923–933. doi: 10.1007/s10658-012-9959-7. [DOI] [Google Scholar]
- 25.Kumari S, Decraemer W, Traversa D, Lišková M. Molecular and morphological delineation of Longidorus poessneckensis Altherr, 1974 (Nematoda: Dorylaimida) Eur J Plant Pathol. 2009;123:125–137. doi: 10.1007/s10658-008-9348-4. [DOI] [Google Scholar]
- 26.Orlando V, et al. Molecular and morphological characterisation of Xiphinema americanum-group species (Nematoda: Dorylaimida) from California, USA, and other regions, and co-evolution of bacteria from the genus Candidatus Xiphinematobacter with nematodes. Nematology. 2016;18:1015–1043. doi: 10.1163/15685411-00003012. [DOI] [Google Scholar]
- 27.Subbotin SA, et al. Characterisation of populations of Longidorus orientalis Loof, 1982 (Nematoda: Dorylaimida) from date palm (Phoenix dactylifera L.) in the USA and other countries and incongruence of phylogenies inferred from ITS1 rRNA and coxI genes. Nematology. 2015;17:459–477. doi: 10.1163/15685411-00002881. [DOI] [Google Scholar]
- 28.Archidona-Yuste A, Navas-Cortes JA, Cantalapiedra-Navarrete C, Palomares-Rius JE, Castillo P. Cryptic diversity and species delimitation in the Xiphinema americanum-group complex (Nematoda: Longidoridae) as inferred from morphometrics and molecular markers. Zool J Linn Soc-Lond. 2016;176:231–265. doi: 10.1111/zoj.12316. [DOI] [Google Scholar]
- 29.Blouin MS. Molecular prospecting for cryptic species of nematodes: mitochondrial DNA versus internal transcribed spacer. Int J Parasitol. 2002;32:527–531. doi: 10.1016/S0020-7519(01)00357-5. [DOI] [PubMed] [Google Scholar]
- 30.De Ley P, et al. An integrated approach to fast and informative morphological vouchering of nematodes for applications in molecular barcoding. Philos Trans R Soc Lond B Biol Sci. 2005;360:1945–1958. doi: 10.1098/rstb.2005.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derycke S, Vanaverbeke J, Rigaux A, Backeljau T, Moens T. Exploring the use of cytochrome oxidase c subunit 1 (COI) for DNA barcoding of free-living marine nematodes. PLoS ONE. 2010;5:e13716. doi: 10.1371/journal.pone.0013716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borisenko AV, Lim BK, Ivanova NV, Hanner RH, Hebert PDN. DNA barcoding in surveys of small mammal communities: a field study in Suriname. Mol Ecol Resources. 2008;8:471–479. doi: 10.1111/j.1471-8286.2007.01998.x. [DOI] [PubMed] [Google Scholar]
- 33.Carew ME, Pettigrove VJ, Metzeling L, Hoffmann AA. Environmental monitoring using next generation sequencing: rapid identification of macroinvertebrate bioindicator species. Front Zool. 2013;10:45. doi: 10.1186/1742-9994-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shokralla S, et al. Pyrosequencing for mini-barcoding of fresh and old museum specimens. PLoS ONE. 2011;6:e21252. doi: 10.1371/journal.pone.0021252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.John, G.H. & Langley, P. Estimating continuous distributions in bayessian classfiers. In Eleventh Conference on Uncertainty in Artificial Intelligence, San Mateo, CA. 338–345 (1995).
- 36.Hall M, et al. The WEKA data mining software: an update. SIGKDD Explorations. 2009;11:10–18. doi: 10.1145/1656274.1656278. [DOI] [Google Scholar]
- 37.Quinlan, R.: C4.5: Programs for Machine Learning. Morgan Kaufmann Publishers. San Mateo, CA, USA (1993).
- 38.Cohen, W. W.: Fast effective rule induction. Twelfth International Conference on Machine Learning (ICML) 95, 115–123 (1995).
- 39.Archidona-Yuste A, Navas-Cortés JA, Cantalapiedra-Navarrete C, Palomares-Rius JE, Castillo P. Unravelling the biodiversity and molecular phylogeny of needle nematodes of the genus Longidorus (Nematoda: Longidoridae) in olive and a description of six new species. PLoS ONE. 2016;11:e0147689. doi: 10.1371/journal.pone.0147689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gutiérrez-Gutiérrez C, Palomares Rius JE, Cantalapiedra-Navarrete C, Landa BB, Castillo P. Prevalence, polyphasic identification, and molecular phylogeny of dagger and needle nematodes infesting vineyards in southern Spain. Eur J Plant Pathol. 2011;129:427–453. doi: 10.1007/s10658-010-9705-y. [DOI] [Google Scholar]
- 41.Humphreys-Pereira DA, Elling AA. Mitochondrial genomes of Meloidogyne chitwoodi and M. incognita (Nematoda: Tylenchina): Comparative analysis, gene order and phylogenetic relationships with other nematodes. Mol Biochem Parasit. 2014;194:20–32. doi: 10.1016/j.molbiopara.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Palomares-Rius JE, Cantalapiedra-Navarrete C, Archidona-Yuste A, Blok V, Castillo P. Mitochondrial genome diversity in dagger and needle nematodes (Nematoda: Longidoridae) Sci Rep-UK. 2017;7:41813. doi: 10.1038/srep41813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gutiérrez-Gutiérrez C, et al. New insight into the identification and molecular phylogeny of dagger nematodes of the genus Xiphinema (Nematoda: Longidoridae) with description of two new species. Zool J Linn Soc-Lond. 2013;169:548–579. doi: 10.1111/zoj.12071. [DOI] [Google Scholar]
- 44.Tzortzakakis EA, et al. Integrative diagnosis and molecular phylogeny of dagger and needle nematodes of olives and grapevines in the island of Crete, Greece, with description of Xiphinema cretense n. sp. (Nematoda, Longidoridae) Eur J Plant Pathol. 2014;140:563–590. doi: 10.1007/s10658-014-0488-4. [DOI] [Google Scholar]
- 45.Gutiérrez-Gutiérrez C, Cantalapiedra-Navarrete C, Montes-Borrego M, Palomares-Rius JE, Castillo P. Molecular phylogeny of the nematode genus Longidorus (Nematoda: Longidoridae) with description of three new species. Zool J Linn Soc-Lond. 2013;167:473–500. doi: 10.1111/zoj.12019. [DOI] [Google Scholar]
- 46.Palomares-Rius JE, Subbotin SA, Landa BB, Vovlas N, Castillo P. Description and molecular characterisation of Paralongidorus litoralis sp. n. and P. paramaximus Heyns, 1965 (Nematoda: Longidoridae) from Spain. Nematology. 2008;10:87–101. doi: 10.1163/156854108783360186. [DOI] [Google Scholar]
- 47.Palomares-Rius JE, Cantalapiedra-Navarrete C, Gutiérrez- Gutiérrez C, Liebanas G, Castillo P. Morphological and molecular characterisation of Paralongidorus plesioepimikis n. sp. (Nematoda: Longidoridae) from southern Spain. Nematology. 2013;15:363–378. doi: 10.1163/15685411-00002680. [DOI] [Google Scholar]
- 48.Palomares-Rius JE, Cantalapiedra-Navarrete C, Archidona-Yuste A, Blok VC, Castillo P. Mitochondrial genome diversity in dagger and needle nematodes (Nematoda: Longidoridae) Scientific Reports. 2017;7:41813. doi: 10.1038/srep41813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coolen, W. A. In Root-knot nematodes (Meloidogyne species). Systematics, biology and control (eds F. Lamberti & C.E. Taylor) 317–329 (Academic Press, 1979).
- 50.Seinhorst JW. On the killing, fixation and transferring to glycerine of the nematodes. Nematologica. 1962;8:29–32. doi: 10.1163/187529262X00981. [DOI] [Google Scholar]
- 51.Castillo P, Vovlas N, Subbotin S, Troccoli A. A new root-knot nematode, Meloidogyne baetica n. sp. (Nematoda: Heteroderidae), parasitizing wild olive in Southern Spain. Phytopathology. 2003;93:1093–1102. doi: 10.1094/PHYTO.2003.93.9.1093. [DOI] [PubMed] [Google Scholar]
- 52.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tamura K, et al. Estimating divergence times in large molecular phylogenies. Proc Natl Acad Sci USA. 2012;109:19333–19338. doi: 10.1073/pnas.1213199109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown SDJ, et al. SPIDER: An R package for the analysis of species identity and evolution, with particular reference to DNA barcoding. Mol Ecol Resour. 2012;12:562–565. doi: 10.1111/j.1755-0998.2011.03108.x. [DOI] [PubMed] [Google Scholar]
- 55.R Development Core Team (2014). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, http://www.R-project.org.
- 56.Weitschek E, Fiscon G, Felici G. Supervised DNA barcodes species classification: analysis, comparisons and results. BioData Mining. 2014;7:4. doi: 10.1186/1756-0381-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall M, et al. The WEKA data mining software: an update. SIGKDD Explorations. 2009;11:10–18. doi: 10.1145/1656274.1656278. [DOI] [Google Scholar]
- 58.Meier R, Shiyang K, Vaidya G, Ng PKL. DNA barcoding and taxonomy in Diptera: a tale of high intraspecific variability and low identification success. Syst Biol. 2006;55:715–728. doi: 10.1080/10635150600969864. [DOI] [PubMed] [Google Scholar]
- 59.Austerlitz F, et al. DNA barcode analysis: a comparison of phylogenetic and statistical classification methods. BMC Bioinformatics. 2009;10:S10. doi: 10.1186/1471-2105-10-S14-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ronquist F, Huelsenbeck JP. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 61.Darriba D, Taboada GL, Doallo R, Posada D. jModelTest 2: more models, new heuristics and parallel computing. Nat Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Page RD. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 63.Archidona-Yuste A, Navas-Cortés JA, Cantalapiedra-Navarrete C, Palomares-Rius JE, Castillo P. Molecular phylogenetic analysis and comparative morphology resolve two new species of olive-tree soil related dagger nematodes of the genus Xiphinema (Dorylaimida: Longidoridae) from Spain. Invertebrate Systematics. 2016;30:547–565. [Google Scholar]
- 64.De Luca F, et al. Molecular characterisation of Longidorus kuiperi Brinkman, Loof Barbez, 1987 (Nematoda: Longidoridae) from the Mediterranean Basin. Nematology. 2009;11:155–160. doi: 10.1163/156854108X398534. [DOI] [Google Scholar]
- 65.Pedram M, et al. Molecular and morphological characterisation of Paralongidorus iranicus n. sp. and P. bikanerensis (Lal Mathur, 1987) Siddiqi, Baujard Mounport, 1993 (Nematoda: Longidoridae) from Iran. Nematology. 2012;14:427–443. doi: 10.1163/156854111X610272. [DOI] [Google Scholar]
- 66.Palomares-Rius JE, Landa BB, Tanha Maafi Z, Hunt DJ, Castillo P. Comparative morphometrics and ribosomal DNA sequence analysis of Longidorus orientalis Loof, 1983 (Nematoda: Longidoridae) from Spain and Iran. Nematology. 2010;12:631–640. doi: 10.1163/138855410X12628646276041. [DOI] [Google Scholar]
- 67.Palomares-Rius JE, Kikuchi T, Cantalapiedra-Navarrete C, Castillo P. Integrative diagnosis of the needle nematode Longidorus jonesi affecting forest in southern Japan. Forest Pathol. 2014;44:246–249. doi: 10.1111/efp.12108. [DOI] [Google Scholar]
- 68.Subbotin S, Rogozhin E, Chizhov V. Molecular characterisation and diagnostics of some Longidorus species (Nematoda: Longidoridae) from Russia and other countries using rRNA genes. Eur J Plant Pathol. 2014;138:377–390. doi: 10.1007/s10658-013-0338-9. [DOI] [Google Scholar]
- 69.Pedram M, et al. Molecular and morphological characterisation of Paralongidorus iranicus n. sp. and P. bikanerensis (Lal Mathur, 1987) Siddiqi, Baujard Mounport, 1993 (Nematoda: Longidoridae) from Iran. Nematology. 2012;14:427–443. doi: 10.1163/156854111X610272. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


