Abstract
Alternative oxidase (AOX) is a mitochondrial inner-membrane oxidase that accepts electrons directly from ubiquinol and reduces oxygen to water without involving cytochrome-linked electron transport chain. It is highly conserved in many non-vertebrate taxa and may protect cells against hypoxia and oxidative stress. We identified two AOX mRNAs in eastern oyster Crassostrea virginica, CvAOXA and CvAOXB, which differ by 170 bp but encode AOXs of the same size. Sequence analyses indicate that CvAOX has 10 exons with a tandem duplication of exon 10, and 3′ alternative splicing using either the first or second exon 10 produces the two variants CvAOXB or CvAOXA, respectively. The second exon 10 in CvAOXA is more conserved across taxa, while the first exon 10 in CvAOXB contains novel mutations surrounding key functional sites. Both variants are expressed in all organs with the expression of CvAOXA higher than that of CvAOXB under normal condition. Under stress by air exposure, CvAOXB showed significantly higher expression than CvAOXA and became the dominant variant. This is the first case of alternative splicing of duplicated exon in a mollusc that produces a novel variant adaptive to stress, highlighting genome’s versatility in generating diversity and phenotypic plasticity.
Introduction
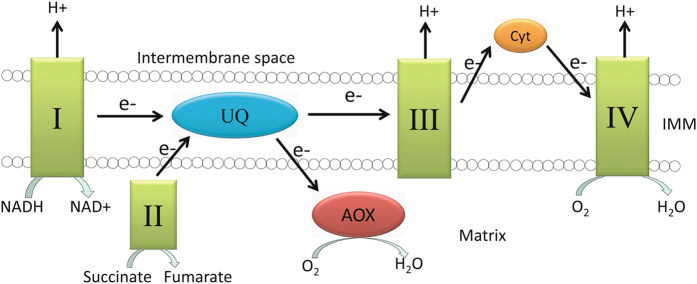
The electron transport chain (ETC) is an essential part of cellular respiration where biochemical nutrients are converted to adenosine triphosphates (ATPs), the main energy source for cellular activities. In eukaryotes, ETC consists of a series of protein complexes that are located on the inner membrane of mitochondria. Electrons from donors such as nicotinamide adenine dinucleotide (NADH) and FADH2 are transferred through Complex I (NADH dehydrogenase) and II (succinate dehydrogenase) to ubiquinone, and then through Complex III to cytochrome c, which is subsequently oxidized by Complex IV (cytochrome c oxidase), reducing oxygen to water1, 2 (Fig. 1). Coupled with redox reactions in Complex I, III and IV, protons are transferred from the mitochondrial matrix to the inner membrane space creating a proton gradient that drives ATP synthesis. In most non-vertebrate taxa, an alternative pathway exists that takes electrons directly from the ubiquinone pool and reduces oxygen to water, bypassing complexes III, IV and cytochrome c and generating heat but few ATPs3, 4. The reaction is catalyzed by alternative oxidase (AOX)3, a diiron carboxylate protein5 located at the surface of the mitochondrial inner membrane.
Figure 1.
The respiratory electron transport chain of mitochondria showing the position of alternative oxidase (AOX), which introduces an alternative pathway at the point of ubiquinol (UQ). Complexes I, III and IV move protons across the inner mitochondrial membrane into the intermembrane space, producing a proton gradient which drives ATP synthesis.
AOX was first discovered in aroids6 and it was thought that heat generated by AOX activity was used by thermogenic plants to volatilize primary amines to attract pollinating insects7, 8. Subsequently AOX has been widely found in plants, fungi, protists and many non-vertebrate animal taxa suggesting that the alternative pathway may have more general and important functions4, 9. Besides thermogenesis, AOX in plants plays a critical role in responding to stress, balancing carbon metabolism and electron transport9, 10, regulating reactive oxygen species (ROS) generated by respiration2, 11–13, and resisting to potent inhibitors of the cytochrome pathway such as cyanide (CN)14, sulfide15, nitric oxide (NO)16, azide17, and antimycin A18. The function of AOX in animals are not well understood, although cyanide-resistant respiration has been observed in annelid worms Nereis pelagica and Marenzelleria viridis 19, 20 and molluscs Arctica islandica and Geukensia demissa 20, 21.
The eastern oyster Crassostrea virginica Gmelin 1791, is a bivalve mollusc native to Atlantic and Gulf Coasts of North America. It is an important fishery and aquaculture species as well as a keystone species for estuary ecology. As a filter-feeder that thrives in the intertidal zone, the eastern oyster can tolerate wide fluctuations in salinity, temperature and air exposure. Nevertheless, eastern oyster populations in the mid-Atlantic region have greatly declined due to overharvesting, diseases and environmental changes22, 23. Genomic research has been conducted to understand disease and stress resistance in oysters24–27. In analyzing transcriptome data, we identified two AOX mRNAs that differed by 170 bp. In this study, we studied the origin of the two AOX variants and their expression under air exposure. Here we report that these variants are produced by alternative splicing of a duplicated exon and may play a role in oyster’s adaptation to environmental stress.
Results
Characterization of CvAOX gene and protein
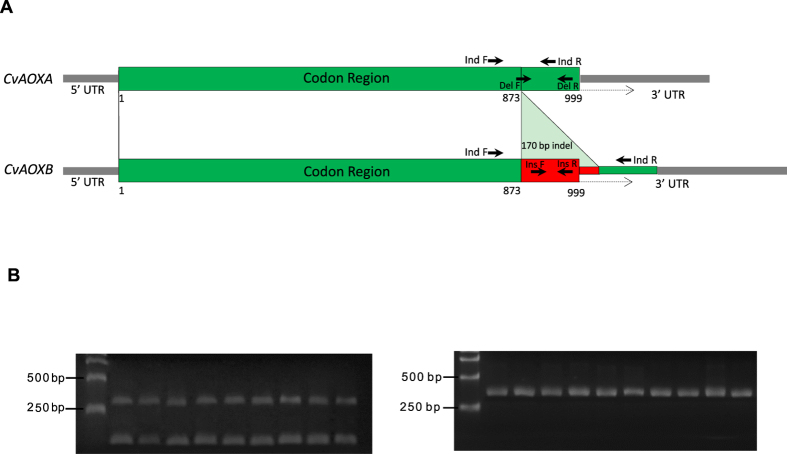
We identified seven CvAOX mRNAs by searching published and unpublished EST and transcriptome databases with C. gigas AOX sequence (CGI_10020743). Alignment of the seven sequences revealed a 170 bp indel with three sequences having the deletion (CvAOXA, short) and four sequences having the insertion (CvAOXB, long) (Fig. 2A). Open reading frame analysis indicated that the longer CvAOXB had a stop codon within the 170 bp insertion, producing a polypeptide of 332 amino acids, which is identical in length as AOX encoded by the shorter CvAOXA. Amplification of the indel region consistently produced two fragments from cDNA that differed by about 170 bp, but only one long fragment from genomic DNA (Fig. 2B), indicating that the two variants are not genomic mutations but produced through alternative splicing. Sequencing of the two mRNA fragments confirmed that they were identical except for the 170 bp indel.
Figure 2.
(A) Diagram of cDNAs corresponding to the two mRNA variants of C. virginica with the position of the indel, and the positions of primers used in this study. (B) PCR products from amplification of the indel using cDNA (left) and genomic DNA (right) as templates (the uncropped images are included in Supplementary Fig. S2). The expected size of the amplicon is 267 bp for the long variant and 97 bp for the short variant.
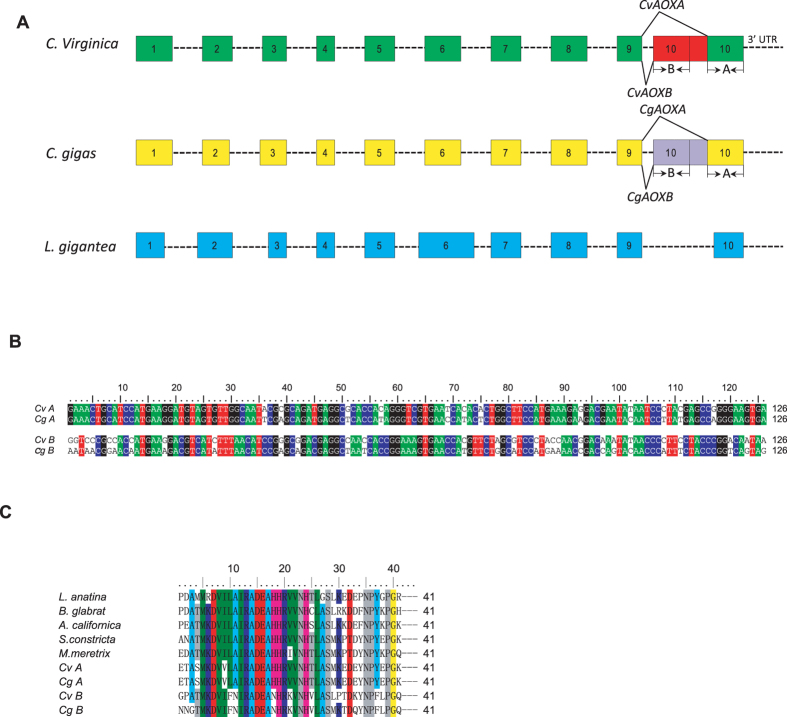
To identify intron-exon structure and confirm alternative splicing, we sequenced the gene body of CvAOX. Like known AOXs of other molluscs, CvAOX consisted of 10 exons and 9 introns (Fig. 3A). However, CvAOX contained a tandem duplication of exon 10 and part of the 3′ UTR, and an alternative 3′ splicing site that produces CvAOXA and CvAOXB by incorporating either the second exon 10 or both duplicated exons, respectively. The duplicated exon 10s showed significant homology in DNA (e-value = 9e−14) and protein (e-value = 4e−20) sequences. Because the duplicated exon 10s were terminal and contain stop codons, CvAOXB and CvAOXA encoded AOXs of the same size (332 aa), despite their difference in length.
Figure 3.
(A) Structure of C. virginica, C. gigas and L. gigantea AOX gene and mode of alternative splicing (alternative 3′ splicing site) in C. virginica and C. gigas. Blocks represent exons, dash represent introns; A and B are duplicated exon 10s. (B) Alignment of nucleotide sequences of the duplicated exon 10s in C. virginica (Cv) and C. gigas (Cg). (C) Alignment of peptide sequences of duplicated exon 10s in C. virginica and C. gigas, and exon 10s of other molluscs.
We examined available genome and transcriptome data from other species and found the same duplication and alternative splicing in C. gigas but not in Lottia gigantea, Aplasia californica, Meretrix meretrix and Sinonovacula constricta, suggesting these events may be unique to oysters. DNA sequences of the duplicated exon 10 s showed significant divergence. Similarity between the two duplicated copies of exon 10 was 63.5% in C. virginica and 65.9% in C. gigas. Between the two species, the second exon 10 used by AOXA was more conversed (similarity = 90.5%) than the first exon 10 used by AOXB (similarity = 77.0%; Fig. 3B).
The 41-amino acid peptides coded by the duplicated exon 10s differed at 15 positions in C. virginica and at 14 positions in C. gigas. Peptide sequence coded by the second exon 10 used by CvAOXA was completely conserved between C. gigas and C. virginica, while peptides coded by the first exon 10 used by CvAOXB differed at 6 positions between the two species (Fig. 3C). Further, alignment of these 41 amino acids with that from other molluscs showed that the peptide coded by the second exon 10 was more similar with that of other species than that of the first exon 10 (Fig. 3C). Compared with that of two other bivalves, clams Sinonovacula constricta and Meretrix meretrix, the peptide coded by the second exon 10 differed by 7 amino acids while that coded by the first exon 10 differed by 14 amino acids. If only the 33 highly conserved amino acid positions were considered, the peptide coded by the second exon 10 diverged at 2 amino acid positions while that coded by the first exon 10 diverged at 8 positions, and the difference is significant (p = 0.039). These findings suggest that, after duplication, the second exon is completely conserved in protein sequences between the two oyster species, while the first exon 10 went through significant divergence probably because of relaxed selection pressure from infrequent usage. Further analysis revealed a small Ka/Ks value (0.04) between AOX sequences of the two oyster species, suggesting that the gene is under strong purifying selection.
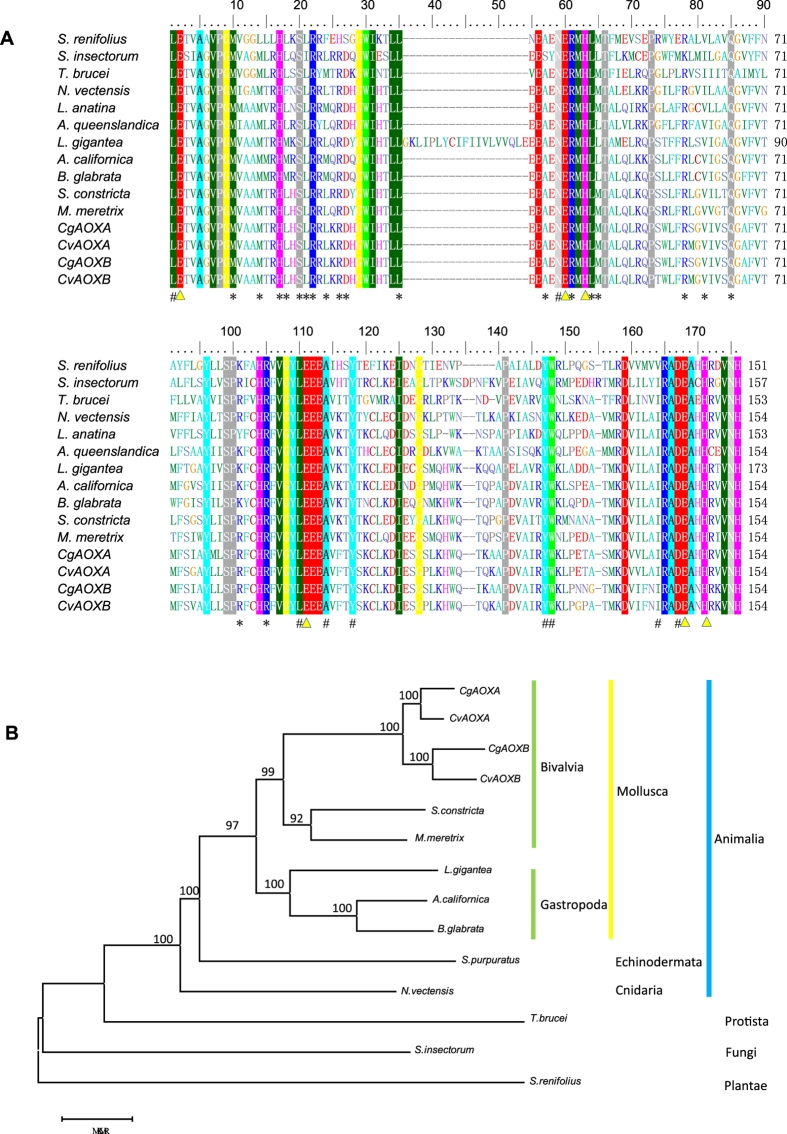
Sequence alignment of the AOX domain from ten species representing Protista, Fungi, Plantae, and Animalia, showed that key functional sites were complete conserved among species and between the two variants, although AOXB had several novel amino acids next to the functional sites that may affect site activity (Fig. 4A). Phylogenetic analysis of the entire AOX protein revealed a clade of molluscs and a closer relationship between the two oyster species, rather than between the two duplicated exons within species (Fig. 4B).
Figure 4.
(A) Alignment of AOXs of C. virginica, C. gigas and 10 other species showing conservation of functional sites. Triangles represent the diiron binding motifs, # indicates the probable sites associated with activities of AOX, and asterisks are sites involved in interaction between monomers55. (B) Phylogenetic relationships among AOXs from C. virginica, C. gigas and 10 other species.
Expression of CvAOX under air exposure
To determine whether AOX is involved in stress response in C. virginica and if the two variants respond differently, we measured mRNA expression of the two variants in oysters exposed to air exposure. Under normal conditions, both CvAOXA and CvAOXB were expressed in all six organs examined in this study: gill, mantle, digestive, adductor (striated), labial and gonad. The level of expression varied among organs, and the highest expression for both variants was found in gill, although the differences among most organs were not significant (Fig. 5A). In all organs, the expression level of CvAOXA was consistently higher than that of CvAOXB under normal conditions, and the difference was significant in gill (p = 0.014) and mantle (p = 0.049, Fig. 5A). When all organs were considered together, paired t-test showed that the expression of CvAOXA was significantly (p = 6.52E-06) higher than that of CvAOXB.
Figure 5.
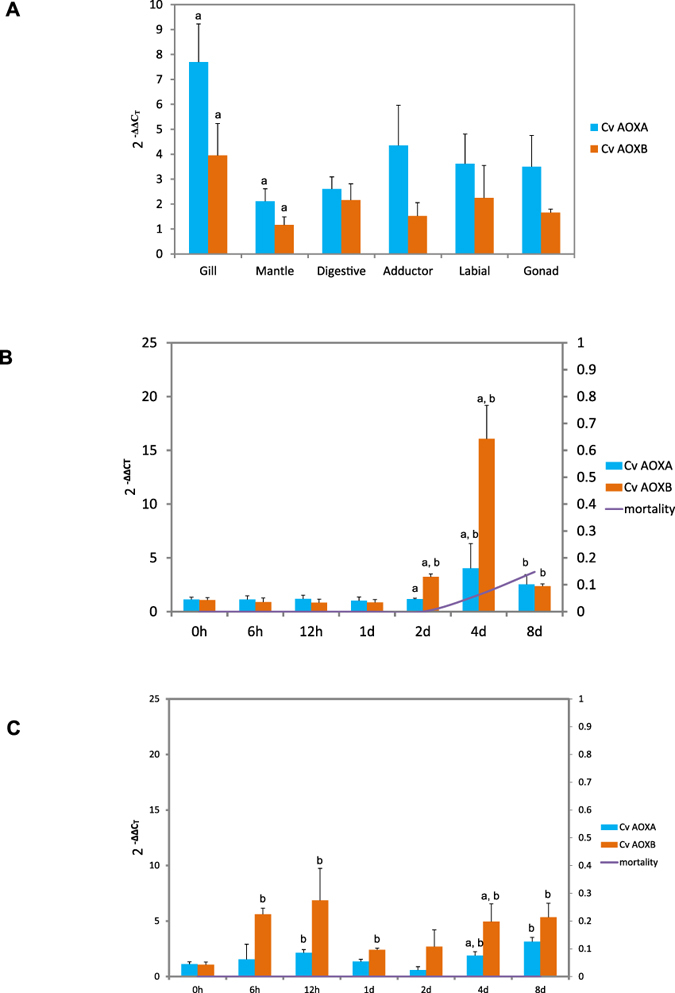
(A) Expression profile of CvAOXA and CvAOXB in different organs of C. virginica. Relative expression (y-axis) is calculated using 2−ΔΔCт standardized by the lowest expression of CvAOXB in mantle. Error bars are standard errors. (B) Relative expression of CvAOXA and CvAOXB in gill under air exposure at 25 °C. (C) Relative expression of CvAOXA and CvAOXB in gill under air exposure at 5 °C. Expression levels of control samples (in water) in each time set are used as references. Lines and the secondary axis (right) show the mortality. Letter a designates significant difference between CvAOXA and CvAOXB. Letter b designates significant difference between challenged and control groups. Significance level is set at p < 0.05.
The expression of both CvAOXA and CvAOXB was up-regulated by prolonged air exposure. At 25 °C, CvAOXB showed significantly higher expression than CvAOXA at Day 2 (p = 0.005) and Day 4 (p = 0.043) after air exposure (Fig. 5B). At Day 4, the expression of CvAOXB was up-regulated by 16 folds, while the expression of CvAOXA was up-regulated by 4 folds (Fig. 5B). The up-regulation of both variants was reduced at Day 8 when oysters began to die. At 5 °C, CvAOXB was significantly up-regulated at 6 h, 12 h, 1 day (d), 4 d and 8 d after air exposure, and CvAOXA was up-regulated at 12 h, 4 d and 8 d after air exposure (Fig. 5C). The expression of CvAOXB was consistently higher than that of CvAOXA, although the difference was only significant (p = 0.014) at Day 4. When all oysters under air exposure were considered together regardless of exposure time, expression of CvAOXB was significantly (p = 0.001) higher than that of CvAOXA. These findings suggest that CvAOXA is preferentially expressed under normal conditions (Fig. 5A), while CvAOXB is preferentially expressed under stress by prolonged air exposure.
Discussion
In plants, AOX is highly up-regulated when plants are exposed to stresses including temperature changes, drought, saline, hypoxia and infection by pathogens28–32. The up-regulation of AOX expression alleviates oxidative stress and maintains metablolic homeostasis in plants under stress33, 34. Two subfamilies of AOX exist in plants: AOX1 that has been found in all angiosperms examined to date and mainly functions in stress response, and AOX2 appears limited to dicot species where its physiological significance remains to be determined. For AOX1 subfamily, there are more than one gene loci found in many monocots such as rice Oryza sativa and wheat Triticum aestivum 35. Likewise, multiple AOX genes are also observed in many fungi and protists36–38. The existence of multiple AOX genes in these organisms may indicate gene and function diversification. In contrast, no more than one AOX gene has been identified in animals so far, and AOXs from animals are highly conserved38. The finding of two variants of AOX mRNA showing a 170 bp indel is therefore unexpected and interesting.
Our results show that there is only one AOX gene in C. virginica genome, and the two variants of AOX are not from insertion-deletion of genomic DNA, but due to alternative splicing involving the duplicated terminal exon 10. The duplication of exon 10 is supported by high homology in DNA (e-value = 9e−14) and protein (e-value = 4e−20) sequences and the same coding length between the duplicated copies. Gene duplication is an important mechanism for the emergence of new genes and functions during evolution. Exon duplication is also common in eukaryotes, and about 10% of all genes in human, fly and worm contain tandemly duplicated exons39, which may be an underestimate because some duplicates of short exons may have diverged beyond recognition. Exon duplication is often associated with alternative splicing of the duplicated exons40. Alternative splicing is the primary mechanism through which the genome generates mRNA and protein diversity from a given coding repertoire. The vast majority of eukaryotic genes go through alternative splicing41. Alternative splicing plays important roles in regulating development, physiology and homeostatis. One of the most important functions of alternative splicing is to provide alternative programs in response to environmental stress. Alternative splicing in plants is often associated with stress-related genes42 and may also play a role in stress response in bivalve molluscs43, 44.
AOX is a diiron oxidase located at mitochondrial inner membrane that reduces O2 to water without involving the cytochrome c pathway of the ETC and producing additional ATPs45–47. Mitochondrial ETC is a significant source of ATPs as well as reactive oxygen species (ROS). Stress causes electron leakage from ETC and generates excess ROS that damage cellular components48–50. AOX may participate in stress response by scavenging ROS and maintaining homeostasis51. Transgenic plant cells lacking AOX display higher rates of mitochondrial ROS generation11. AOX activation in durum wheat mitochondria leads to a decrease in the rate of superoxide anion generation52. In a marine worm and bivalve, it is also suggested excess oxygen can be eliminated by the alternative parthway to minimize oxidative stress20. In this study, expression of both variants of AOX is up-regulated by air exposure, suggesting AOX is important for stress response in C. viginica also. However, the up-regulation of AOX declined at day 8 at the on-site of mortalities indicates that there is a point when AOX response is no longer sufficient. Low temperature stress generates ROS53 but also reduces respiratory and metabolic rates, which may explain why AOX has an early but mild response to air exposure at 5 °C.
While CvAOXA shows higher expression than CvAOXB under normal condition (Fig. 5A) and both variants are up-regulated by stress, the expression of CvAOXB is significantly higher that of CvAOXA under prolonged air exposure (Fig. 5B and C), suggesting CvAOXB is prefered in response to stress. It is not clear why CvAOXA is preferentially expressed under normal conditions while CvAOXB is preferentially expressed under stress. The function of AOX may depend on the presence of the diiron binding motif (E2, E60, H63, E111, E168, H171), tyrosine residues especially Y118 for catalytic reaction9, 54, sites associated with ubiquinol binding and oxidization (L1, N59, L110, A114, Y147, W148, I164, D167), and twenty residues (M10, M14, H17, L18, S20, L21, R22, L24, R26, D27, L35, A57, R61, L64, M65, R78, Q85, V86, R101, R105) for interactions between the two monomers55. Although protein sequences of the two variants differed due to the use of the alternative exon, all sites that are known to be critical to AOX’s function are completely conserved. CvAOXB has several novel residues surrounding the second EXXH motif of the diiron center, although it is unknown if and how these novel mutations affects the function of CvAOXB. Further studies on the structure, cellular location and catalytic activity of this novel CvAOXB variant may provide insights into molecular mechansims of AOX’s function. Nevertheless, our results suggest that the alternative splicing variant CvAOXB is probably adaptive and preferred by oysters under stress. It should be pointed out that air exposure represents multiple stressors that may include hypoxia, changes in salinity, temperature and physical state, and response to air exposure may differ from that to other stressors.
The significant divergence between CvAOXA and CvAOXB and the relatively low divergence of both variants between C. virginica and C. gigas suggest that the duplication event occurred long before the divergence of the two species some 83 mya56. The finding that the duplication is not found in gastropods and other bivalves suggests that the duplication may be specific to oysters or certain subclasses of Bivalvia. Further studies in other oysters, bivalves and molluscs may provide better estimates of when the duplication occurred. The fact that the peptide coded by the second exon 10 in CvAOXA is completely conserved between the two oysters, while the peptide coded by the first exon 10 in CvAOXB diverged at 6 amino acid positions indicates that the second exon 10 is more important and subjected to stronger purifying selection pressure. Exon duplication may have relaxed selection pressure and allowed rapid accumulation of mutations in the first exon 10 in CvAOXB that is only preferred under stress. This is also supported by the observation that the second exon 10 in CvAOXA is more conserved than the first exon 10 in CvAOXB compared with other bivalve species. The preferential expression of CvAOXB under air exposure indicates that alternative splicing and the use of CvAOXB variant is beneficial to oysters under stress. Alternative splicing is known to participate in stress response in bivalve molluscs43, 44, and this study further demonstrates for the first time in a mollusc that exon duplication and alternative splicing in combination create a novel variant that is adaptive to environmental stress. Alternative splicing of duplicated exons40 may be an important mechanism of generating protein diversity and responding to stress in molluscs, which deserves further investigation.
Materials and Methods
Characterization of C. virginica AOX (CvAOX)
CvAOX mRNA sequences were obtained by searching Crassostrea gigas AOX (CgAOX) cDNA sequence (GenBank: ACL31211) in published and unpublished transcriptome databases of C. virginica. CvAOX sequences were aligned using Clustal W 57 which revealed two variants differed by ~170 bp (Fig. 2A).
To verify if the size difference is due to an indel in genomic DNA or from alternative splicing, primers (Table 1, Fig. 2A) were designed to amplify the region in question using both cDNA and genomic DNA as templates. Genomic DNA and cDNA from gills of nine C. virginica as well as genomic DNA of 31 oysters from four wild populations: Rhode Island (8), Delaware Bay (8), Florida (7) and Texas (8), were extracted and used for amplification. Polymerase Chain Reaction (PCR) was carried out in 10 µl volume containing 20 ng of DNA, 1 × PCR buffer, 1.5 mM of MgCl2, 0.2 mM of dNTP, 200 nM of each primer and 0.2 U of GoTaq polymerase (all from Promega) using the following profile: 95 °C for 5 min; 35 cycles of 95 °C for 30 sec, 57 °C for 30 sec, and 72 °C for 45 sec; and a final extension at 72 °C for 10 min. PCR products were analyzed in 1% agarose gels.
Table 1.
Primers used for amplification of AOX in C. virginica.
| Primer name | Sequence 5′-3′ | usage |
|---|---|---|
| Ind F | AAAACACTGGCAGACTCAGAAAGC | Amplify the indel |
| Ind R | GCGTATTGCCAACACTACATCC | Amplify the indel |
| Del F | GCTACTGGAAACTTCCGGAAA | RT-PCR for CvAOXA |
| Del R | CATGGAAGCCAGTGTGTGATTC | RT-PCR for CvAOXA |
| Ins F | AAGGACGTCATCTTTAACATCC | RT-PCR for CvAOXB |
| Ins R | GTAGGAAGGGGTTATATTTGTC | RT-PCR for CvAOXB |
| G1 F | GGGAAGTTTACGACAAATTACG | Amplify the genomic DNA coding sequence |
| G1 R | ACATTATGGAGTTCTTCCTCTGAC | Amplify the genomic DNA coding sequence |
| G2 F | CACAGAGACACTTGTGGAGTCGCT | Amplify the genomic DNA coding sequence |
| G2 R | TTTCTCTGATCTTTCACCCCAGTTG | Amplify the genomic DNA coding sequence |
| G3 F | CTCCAGAGGGCTTTGTTGACAAG | Amplify the genomic DNA coding sequence |
| G3 R | CAGTCTCCAGGAAACAGATTCGT | Amplify the genomic DNA coding sequence |
| G4 F | CTTGCCTTCCGATCTGTGAAG | Amplify the genomic DNA coding sequence |
| G4 R | GACGCCCATTCTGAATAACCAAG | Amplify the genomic DNA coding sequence |
| G5 F | CTCAAACGAGACCACGGATGGAT | Amplify the genomic DNA coding sequence |
| G5 R | AGTTTCCAGTAGCGGATAGCCACG | Amplify the genomic DNA coding sequence |
| G6 F | CTTTACCTACTCCAAGTGTTTGAAG | Amplify the genomic DNA coding sequence |
| G6 R | GTGTCCTTAGTTTCCGGCTGTT | Amplify the genomic DNA coding sequence |
| EF-1 F | ATCAACTTCCACTGGCCATC | Reference gene for RT-PCR |
| EF-1 R | TTTTCCCATCTCAGCTGCTT | Reference gene for RT-PCR |
To determine gene intron-exon structure, primers were designed to amplify genomic sequence of CvAOX based on cDNA sequences (Table 1). PCR was carried out as described above using genomic DNA and the following profile: 95 °C for 5 min; 35 cycles of 95 °C for 45 sec, 56 °C for 45 sec, and 72 °C for 90 sec; and a final extension at 72 °C for 10 min. PCR products were purified and sequenced at GenScript Inc., USA. Sequences were assembled using SeqMan (DNASTAR Inc. http://www.dnastar.com). Introns were identified by sequence alignment and the GT-AG rule. Ten protein sequences of AOX representing Protista, Fungi, Plantae, and Animalia were obtained from NCBI (Table 2) and aligned with the protein sequences of CvAOX and CgAOX. Active and functional sites in the protein sequence were determined according to Shiba et al. 201355. Phylogenetic tree was constructed with Bayesian model using the Markov Chain Monte Carlo (MCMC) approach58. The parameters for MCMC sampling were: generations = 2,000,000 (standard deviation of split frequencies was < 0.01) with sample frequency = 1000, chains = 4, and burn-in value = 250 (corresponding to 25% of the samples). The method was implemented in MrBayes 3.158.
Table 2.
Species and AOX sequences used for phylogenetic analysis.
| Kingdom | Plylum | Species | Genbank no | Reference |
|---|---|---|---|---|
| Protista | Euglenozoa | Trypanosoma brucei | XP_822944 | Berriman et al.61 |
| Fungi | Ascomycota | Sporothrix insectorum | OAA58516 | Shang et al.62 |
| Plantae | Angiosperms | Symplocarpus renifolius | BAD83866 | Onda et al.63 |
| Animalia | Cnidaria | Nematostella vectensis | XM_001635879 | Putnam et al.64 |
| Echinodermata | Strongylocentrotus purpuratus | XP_011669099 | Sodergren et al.65 | |
| Mollusca | Aplysia californica | XP_005099181 | Lowe and Eddy66 | |
| Biomphalaria glabrata | XP_013086260 | Yoshino et al.67 | ||
| Lottia gigantea | XP_009054933 | Simakov et al.68 | ||
| Sinonovacula constricta | Dong et al., unpublished | |||
| Meretrix meretrix | Dong et al., unpublished | |||
| Crassostrea gigas AOXA | NP_001292289 | Medeiros et al.69 | ||
| Crassostrea gigas AOXB | XP_011419856 | Zhang et al.60 | ||
| Crassostrea virginica AOXA | This study | |||
| Crassostrea virginica AOXB | This study |
Expression of two CvAOX mRNA variants under air exposure
Adult eastern oysters (n = 436) from the Rutgers NEHTM strain were used to study the expression of CvAOX under air exposure stress. To determine the basic transcription profile of CvAOX, six different organs (gill, mantle, digestive, striated adductor muscle, labial and gonad) of four oysters were collected and fixed in RNAlater (Life Technologies) before the challenge experiment. The remaining oysters were equally divided into three groups. Oysters in two groups were subjected to air exposure at 25 °C and 5 °C. Oysters in the third group were kept in seawater at 25 °C as control. Each group was further divided into four replicates. Three organs (gill, mantle and digestive) from four oysters were sampled from each group and stored in RNAlater at 6 h, 12 h, 1 day (d), 2 d, 4 d, and 8 d after exposure. RNA was extracted using RNeasy Mini Kit (Qiagen) and treated with DNase I (Qiagen) to prevent DNA contamination. First-strand cDNA was synthesized from 1 µg of total RNA using oligo dT, random 6 mers and reverse transcriptase in a volume of 25 µl using the PrimeScript™ RT reagent Kit (Perfect Real Time, Takara) following supplied protocol. Single-strand cDNA was used directly for real-time RT-PCR.
For expression analysis, two pairs of primers were designed to specifically amplify the two variants (Table 1, Fig. 2). Products for the two variants were verified by sequencing. Real-time quantitative RT-PCR was performed on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). We tested three housekeeping genes, beta-actin, elongation factor 1 (EF-1) and 18S 59 and checked the expression profile of EF-1, beta-actin and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) under air exposure in C. gigas transcriptome data60. EF-1 exhibited the most stable expression and was used as the reference gene for this study. Quantitative RT-PCR was carried out in biological triplicate, each in a volume of 20 ml containing 10 μl of 2X Power SYBR Green PCR mater mix (Applied Biosystems), 1 μl cDNA, 0.8 μl each of 5 µM primer and 7.4 μl of deionized water. The amplification was programmed as: 2 min at 50 °C, 2 min at 95 °C, and 40 cycles at 95 °C for 15 s and 60 °C for 1 min with the fluorescent signal collection. Expression of CvAOXA and CvAOXB was measured using the 2−ΔΔCt method, whereas Ct is threshold cycle number determined by the real-time PCR system and reflecting the concentration of target gene in the reaction, ΔCt is the difference in Ct between target and reference gene which provides a relative quantification for the target gene in each sample, and ΔΔCt is the difference that ΔCt of each challenged sample minus ΔCt of the control sample. The expression level was then calculated by 2−ΔΔCt which represent an n-fold difference relative to the control. Differences between groups were tested with two-sample t-test and differences between CvAOXA and CvAOXB within the same oyster and organ were tested with paired t-test on the ΔCt values using GraphPad Prism version 5.00 (GraphPad Software).
Electronic supplementary material
Acknowledgements
This study is partly supported by US NSF EEID Program (Award #1216220), New Jersey Sea Grant Consortium (#6410-0010) and USDA/NJAES Project 1004475/NJ32920. This is NJSGC publication 2017-000.
Author Contributions
X.G. discovered the mRNA variants and conceived the study. X.G. and M.L. designed experiments. M.L. performed the experiments. M.L. and X.G. analyzed data and wrote the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10976-w
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray, R. K., Granner, D. K., Mayes, P. A. & Rodwell, V. W. Harper’s illustrated biochemistry. (McGraw-Hill, 2014).
- 2.McDonald AE, Vanlerberghe GC. Branched mitochondrial electron transport in the Animalia: presence of alternative oxidase in several animal phyla. IUBMB life. 2004;56:333–341. doi: 10.1080/1521-6540400000876. [DOI] [PubMed] [Google Scholar]
- 3.Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- 4.McDonald AE, Vanlerberghe GC, Staples JF. Alternative oxidase in animals: unique characteristics and taxonomic distribution. J Exp Biol. 2009;212:2627–2634. doi: 10.1242/jeb.032151. [DOI] [PubMed] [Google Scholar]
- 5.Berthold DA, Stenmark P. Membrane-bound diiron carboxylate proteins. Annu Rev Plant Physiol Plant Mol Biol. 2003;54:497–517. doi: 10.1146/annurev.arplant.54.031902.134915. [DOI] [PubMed] [Google Scholar]
- 6.Meeuse BJ. Thermogenic respiration in aroids. Annu Rev Plant Physiol Plant Mol Biol. 1975;26:117–126. doi: 10.1146/annurev.pp.26.060175.001001. [DOI] [Google Scholar]
- 7.Skubatz H, Nelson TA, Meeuse BJ, Bendich AJ. Heat Production in the Voodoo Lily (Sauromatum guttatum) as Monitored by Infrared Thermography. Plant Physiol. 1991;95:1084–1088. doi: 10.1104/pp.95.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watling JR, Robinson SA, Seymour RS. Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiol. 2006;140:1367–1373. doi: 10.1104/pp.105.075523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Affourtit C, Albury MS, Crichton PG, Moore AL. Exploring the molecular nature of alternative oxidase regulation and catalysis. FEBS Lett. 2002;510:121–126. doi: 10.1016/S0014-5793(01)03261-6. [DOI] [PubMed] [Google Scholar]
- 10.Millenaar F. & Lambers, H. The alternative oxidase: in vivo regulation and function. Plant Biol. 2003;5:2–15. doi: 10.1055/s-2003-37974. [DOI] [Google Scholar]
- 11.Maxwell DP, Wang Y, McIntosh L. The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA. 1999;96:8271–8276. doi: 10.1073/pnas.96.14.8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moller IM. Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Plant Mol Biol. 2001;52:561–591. doi: 10.1146/annurev.arplant.52.1.561. [DOI] [PubMed] [Google Scholar]
- 13.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 14.Bendall DS, Bonner WD. Cyanide-insensitive Respiration in Plant Mitochondria. Plant Physiol. 1971;47:236–245. doi: 10.1104/pp.47.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Azcon-Bieto J, Ribas-Carbo M, Gonzalez-Meler MA, Penuelas J. Sulfide-Resistant Respiration in Leaves of Elodea canadensis Michx: Comparison with Cyanide-Resistant Respiration. Plant Physiol. 1989;90:1249–1251. doi: 10.1104/pp.90.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang X, von Rad U, Durner J. Nitric oxide induces transcriptional activation of the nitric oxide-tolerant alternative oxidase in Arabidopsis suspension cells. Planta. 2002;215:914–923. doi: 10.1007/s00425-002-0828-z. [DOI] [PubMed] [Google Scholar]
- 17.Baurain D, Dinant M, Coosemans N, Matagne RF. Regulation of the alternative oxidase Aox1 gene in Chlamydomonas reinhardtii. Role of the nitrogen source on the expression of a reporter gene under the control of the Aox1 promoter. Plant Physiol. 2003;131:1418–1430. doi: 10.1104/pp.013409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chae MS, et al. Identification of an alternative oxidase induction motif in the promoter region of the aod-1 gene in Neurospora crassa. Genetics. 2007;175:1597–1606. doi: 10.1534/genetics.106.068635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hahlbeck E, Arndt C, Schiedek D. Sulphide detoxification in Hediste diversicolor and Marenzelleria viridis, two dominant polychaete worms within the shallow coastal waters of the southern Baltic Sea. Comp Biochem Physiol B Biochem Mol Biol. 2000;125:457–471. doi: 10.1016/S0305-0491(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 20.Tschischka K, Abele D, Portner HO. Mitochondrial oxyconformity and cold adaptation in the polychaete Nereis pelagica and the bivalve Arctica islandica from the Baltic and White Seas. J Exp Biol. 2000;203:3355–3368. doi: 10.1242/jeb.203.21.3355. [DOI] [PubMed] [Google Scholar]
- 21.Parrino V, Kraus DW, Doeller JE. ATP production from the oxidation of sulfide in gill mitochondria of the ribbed mussel Geukensia demissa. J Exp Biol. 2000;203:2209–2218. doi: 10.1242/jeb.203.14.2209. [DOI] [PubMed] [Google Scholar]
- 22.Newell RI. Ecological changes in Chesapeake Bay: are they the result of overharvesting the American oyster, Crassostrea virginica. Understanding the estuary: advances in Chesapeake Bay research. 1988;129:536–546. [Google Scholar]
- 23.Ford, S. E. & Tripp, M. R. Diseases and defense mechanisms in The eastern oyster: Crassostrea virginica (ed. Kennedy, V. S., Newell, R. I., Eble, A. F.) 581 (Maryland Sea Grant College, 1996).
- 24.Guo, X., Wang, Y., Wang, L. & Lee, J.H. Genome mapping and genomics in fishes and aquatic animals in Oysters (ed. Guo, X., Wang, Y., Wang, L. & Lee, J.H.) 163–175 (Springer, 2008).
- 25.Gomez-Chiarri M, Guo X, Tanguy A, He Y, Proestou D. The use of -omic tools in the study of disease processes in marine bivalve mollusks. J Invertebr Pathol. 2015;131:137–154. doi: 10.1016/j.jip.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Guo X, He Y, Zhang L, Lelong C, Jouaux A. Immune and stress responses in oysters with insights on adaptation. Fish Shellfish Immunol. 2015;46:107–119. doi: 10.1016/j.fsi.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Guo, X. & Ford, S. E. Infectious diseases of marine molluscs and host responses as revealed by genomic tools. Philos Trans R Soc Lond B Biol Sci 371 (2016). [DOI] [PMC free article] [PubMed]
- 28.Purvis AC, Shewfelt RL. Does the alternative pathway ameliorate chilling injury in sensitive plant tissues? Physiol Plant. 1993;88:712–718. doi: 10.1111/j.1399-3054.1993.tb01393.x. [DOI] [PubMed] [Google Scholar]
- 29.Hilal M, Zenoff AM, Ponessa G, Moreno H, Massa EM. Saline stress alters the temporal patterns of xylem differentiation and alternative oxidase expression in developing soybean roots. Plant Physiol. 1998;117:695–701. doi: 10.1104/pp.117.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djajanegara I, et al. Regulation of alternative oxidase gene expression in soybean. Plant Mol Biol. 2002;50:735–742. doi: 10.1023/A:1019942720636. [DOI] [PubMed] [Google Scholar]
- 31.Yan YC, Lin HH, Liang HG, Zhang NH. Comparison of the effects of different low temperature stresses on the induction of the cyanide-resistant alternative pathway and the expression of alternative oxidase in tobacco callus. Chin Bull Bot. 2004;21:296–305. [Google Scholar]
- 32.Costa JH, et al. Stress-induced co-expression of two alternative oxidase (VuAox1 and 2b) genes in Vigna unguiculata. J Plant Physiol. 2010;167:561–570. doi: 10.1016/j.jplph.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Sugie A, Naydenov N, Mizuno N, Nakamura C, Takumi S. Overexpression of wheat alternative oxidase gene Waox1a alters respiration capacity and response to reactive oxygen species under low temperature in transgenic Arabidopsis. Genes Genet Syst. 2006;81:349–354. doi: 10.1266/ggs.81.349. [DOI] [PubMed] [Google Scholar]
- 34.Vanlerberghe GC. Alternative Oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci. 2013;14:6805–6847. doi: 10.3390/ijms14046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Considine MJ, Holtzapffel RC, Day DA, Whelan J, Millar AH. Molecular distinction between alternative oxidase from monocots and dicots. Plant Physiology. 2002;129:949–953. doi: 10.1104/pp.004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh WK, Kang SO. Characterization of the gene family encoding alternative oxidase from Candida albicans. Biochem J. 2001;356:595–604. doi: 10.1042/bj3560595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dinant M, Baurain D, Coosemans N, Joris B, Matagne RF. Characterization of two genes encoding the mitochondrial alternative oxidase in Chlamydomonas reinhardtii. Curr Genet. 2001;39:101–108. doi: 10.1007/s002940000183. [DOI] [PubMed] [Google Scholar]
- 38.McDonald AE, Vanlerberghe GC. Origins, evolutionary history, and taxonomic distribution of alternative oxidase and plastoquinol terminal oxidase. Comp Biochem Physiol Part D Genomics Proteomics. 2006;1:357–364. doi: 10.1016/j.cbd.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Letunic I, Copley RR, Bork P. Common exon duplication in animals and its role in alternative splicing. Hum Mol Genet. 2002;11:1561–1567. doi: 10.1093/hmg/11.13.1561. [DOI] [PubMed] [Google Scholar]
- 40.Kondrashov FA, Koonin EV. Origin of alternative splicing by tandem exon duplication. Hum Mol Genet. 2001;10:2661–2669. doi: 10.1093/hmg/10.23.2661. [DOI] [PubMed] [Google Scholar]
- 41.Kelemen O, et al. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastrangelo AM, Marone D, Laidò G, De Leonardis AM, De Vita P. Alternative splicing: enhancing ability to cope with stress via transcriptome plasticity. Plant Science. 2012;185:40–49. doi: 10.1016/j.plantsci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Guevelou E, et al. Regulation of a truncated isoform of AMP-activated protein kinase alpha (AMPK alpha) in response to hypoxia in the muscle of Pacific oyster Crassostrea gigas. J Comp Physiol B. 2013;183:597–611. doi: 10.1007/s00360-013-0743-6. [DOI] [PubMed] [Google Scholar]
- 44.Huang B, Zhang L, Tang X, Zhang G, Li L. Genome-wide analysis of alternative splicing provides insights into stress adaptation of the Pacific oyster. Mar Biotechnol. 2016;18:598–609. doi: 10.1007/s10126-016-9720-x. [DOI] [PubMed] [Google Scholar]
- 45.Broadwater JA, Ai J, Loehr TM, Sanders-Loehr J, Fox BG. Peroxodiferric intermediate of stearoyl-acyl carrier protein delta 9 desaturase: oxidase reactivity during single turnover and implications for the mechanism of desaturation. Biochemistry. 1998;37:14664–14671. doi: 10.1021/bi981839i. [DOI] [PubMed] [Google Scholar]
- 46.Gassner GT, Lippard SJ. Component interactions in the soluble methane monooxygenase system from Methylococcus capsulatus (Bath) Biochemistry. 1999;38:12768–12785. doi: 10.1021/bi990841m. [DOI] [PubMed] [Google Scholar]
- 47.Gomes CM, Le Gall J, Xavier AV, Teixeira M. Could a Diiron‐Containing Four‐Helix‐Bundle Protein Have Been a Primitive Oxygen Reductase? Chembiochem. 2001;2:583–587. doi: 10.1002/1439-7633(20010803)2:7/8<583::AID-CBIC583>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 48.Hur E, Chang KY, Lee E, Lee SK, Park H. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not the stabilization or DNA binding ability of hypoxia-inducible factor-1alpha. Mol Pharmacol. 2001;59:1216–1224. doi: 10.1124/mol.59.5.1216. [DOI] [PubMed] [Google Scholar]
- 49.Chen J, Sharma S, Quiocho FA, Davidson AL. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc Natl Acad Sci USA. 2001;98:1525–1530. doi: 10.1073/pnas.98.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharkey TD. Effects of moderate heat stress on photosynthesis: importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant, Cell & Environment. 2005;28:269–277. doi: 10.1111/j.1365-3040.2005.01324.x. [DOI] [Google Scholar]
- 51.McDonald AE. Alternative oxidase: an inter-kingdom perspective on the function and regulation of this broadly distributed ‘cyanide-resistant’terminal oxidase. Funct Plant Biol. 2008;35:535–552. doi: 10.1071/FP08025. [DOI] [PubMed] [Google Scholar]
- 52.Pastore D, Trono D, Laus MN, Di Fonzo N, Passarella S. Alternative oxidase in durum wheat mitochondria. Activation by pyruvate, hydroxypyruvate and glyoxylate and physiological role. Plant Cell Physiol. 2001;42:1373–1382. doi: 10.1093/pcp/pce174. [DOI] [PubMed] [Google Scholar]
- 53.Ito Y, Saisho D, Nakazono M, Tsutsumi N, Hirai A. Transcript levels of tandem-arranged alternative oxidase genes in rice are increased by low temperature. Gene. 1997;203:121–129. doi: 10.1016/S0378-1119(97)00502-7. [DOI] [PubMed] [Google Scholar]
- 54.Moore AL, Albury MS. Further insights into the structure of the alternative oxidase: From plants to parasites. Biochem Soc Trans. 2008;36:1022–1026. doi: 10.1042/BST0361022. [DOI] [PubMed] [Google Scholar]
- 55.Shiba T, et al. Structure of the trypanosome cyanide-insensitive alternative oxidase. Proc Natl Acad Sci USA. 2013;110:4580–4585. doi: 10.1073/pnas.1218386110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ren J, Liu X, Jiang F, Guo X, Liu B. Unusual conservation of mitochondrial gene order in Crassostrea oysters: evidence for recent speciation in Asia. BMC Evolutionary Biology. 2010;10:394. doi: 10.1186/1471-2148-10-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Larkin MA, et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 58.Ronquist F, Huelsenbeck JP. Mayas 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 59.Araya MT, et al. Selection and evaluation of housekeeping genes for haemocytes of softshell clams (Mya arenaria) challenged with Vibrio splendidus. J Invert Pathol. 2008;99:326–331. doi: 10.1016/j.jip.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Zhang G, et al. The oyster genome reveals stress adaptation and complexity of shell formation. Nature. 2012;490:49–54. doi: 10.1038/nature11413. [DOI] [PubMed] [Google Scholar]
- 61.Berriman M, et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- 62.Shang Y, et al. Divergent and Convergent Evolution of Fungal Pathogenicity. Genome Biol Evol. 2016;8:1374–1387. doi: 10.1093/gbe/evw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Onda Y, et al. Pyruvate-sensitive AOX exists as a non-covalently associated dimer in the homeothermic spadix of the skunk cabbage. Symplocarpus renifolius. FEBS Lett. 2007;581:5852–5858. doi: 10.1016/j.febslet.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 64.Putnam NH, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 65.Sodergren E, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoshino TP, Dinguirard N, Kunert J, Hokke CH. Molecular and functional characterization of a tandem-repeat galectin from the freshwater snail Biomphalaria glabrata, intermediate host of the human blood fluke Schistosoma mansoni. Gene. 2008;411:46–58. doi: 10.1016/j.gene.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simakov O, et al. Insights into bilaterian evolution from three spiralian genomes. Nature. 2013;493:526–531. doi: 10.1038/nature11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Medeiros ID, et al. Differential gene expression in oyster exposed to sewage. Mar Environ Res. 2008;66:156–157. doi: 10.1016/j.marenvres.2008.02.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.