Abstract
Background
Although physical activity has been shown to contribute to long-term disease control and health in breast cancer survivors, a majority of breast cancer survivors do not meet physical activity guidelines. Past research has focused on promoting physical activity components for short-term breast cancer survivor benefits, but insufficient attention has been devoted to long-term outcomes and sustained exercise adherence. We are assessing a health coach intervention (iMOVE) that uses mobile technology to increase and sustain physical activity maintenance in initially inactive breast cancer survivors.
Objective
This pilot randomized controlled trial (RCT) is an initial step in evaluating the iMOVE intervention and will inform development of a full-scale pragmatic RCT.
Methods
We will enroll 107 physically inactive breast cancer survivors and randomly assign them to intervention or control groups at the University Health Network, a tertiary cancer care center in Toronto, Canada. Participants will be women (age 18 to 74 years) stratified by age (55 years and older/younger than 55 years) and adjuvant hormone therapy (AHT) exposure (AHT vs no AHT) following breast cancer treatment with no metastases or recurrence who report less than 60 minutes of preplanned physical activity per week. Both intervention and control groups receive the 12-week physical activity program with weekly group sessions and an individualized, progressive, home-based exercise program. The intervention group will additionally receive (1) 10 telephone-based health coaching sessions, (2) smartphone with data plan, if needed, (3) supportive health tracking software (Connected Wellness, NexJ Health Inc), and (4) a wearable step-counting device linked to a smartphone program.
Results
We will be assessing recruitment rates; acceptability reflected in selective, semistructured interviews; and enrollment, retention, and adherence quantitative intervention markers as pilot outcome measures. The primary clinical outcome will be directly measured peak oxygen consumption. Secondary clinical outcomes include health-related quality of life and anthropometric measures. All outcome measures are administered at baseline, after exercise program (month 3), and 6 months after program (month 9).
Conclusions
This pilot RCT will inform full-scale RCT planning. We will assess pilot procedures and interventions and collect preliminary effect estimates.
Trial Registration
ClinicalTrials.gov NCT02620735; https://clinicaltrials.gov/ct2/show/NCT02620735 (Archived by WebCite at https://clinicaltrials.gov/ct2/show/NCT02620735)
Keywords: breast neoplasm, exercise, health coaching, RCT, telehealth
Introduction
Background
Breast cancer, the most frequently diagnosed female cancer, accounts for approximately 25% of new Canadian cancer diagnoses [1]. With improvements in early detection and treatment, breast cancer mortality has decreased significantly in the last 30 years despite increasing incidence [2,3]. Most breast cancer cases diagnosed at localized stages are associated with a mean 5-year survival rate of 96% [2,4].
Despite these improvements, breast cancer treatments can result in the long-term effects of chronic pain, fatigue, neuropathy, functional limitations, sleep disturbance, sexual dysfunction, infertility, cognitive impairment, cardiorespiratory dysfunctions, and generally reduced well-being [5-14]. Breast cancer survivors also confront elevated risks for local or distal recurrence, metastases, second primary cancers, type 2 diabetes, and cardiovascular disorders [3,15-23].
Physical activity can improve cancer outcomes and quality of life while reducing adverse effects and risks. Moderate-to-vigorous physical activity (MVPA) during or after breast cancer treatment is specifically associated with reductions in cancer-specific and all-cause mortality [23,24]. As reported in a recent systematic review of 17 breast cancer–specific observational studies, breast cancer–specific mortality reductions of 13% to 51% were observed when the highest-to-lowest physical activity categories were compared [25-32].
The American College of Sports Medicine (ACSM), the American Cancer Society, and Worldwide Cancer Research (among other national and international agencies) recommend 150 minutes per week of moderate intensity physical activity for cancer survivors [33-37]. While most breast cancer survivors believe in exercise benefits [37], physical activity levels generally reduce after a breast cancer diagnosis with the large majority of breast cancer survivors (more than 80%) not meeting recommended physical activity levels [29,37-50].
Nonetheless, the impact of a cancer diagnosis often stimulates patients to reconsider lifestyle modification [43,46,50-52], providing clinicians the opportunity to introduce physical activity promotion [53]. Substantial evidence supports the efficacy of several intervention approaches in short-term physical activity change [54-57], with findings from systematic reviews and meta-analyses of exercise studies involving cancer survivors indicating that MVPA (1) is safe and well-tolerated [35,58], (2) can significantly improve quality of life [58-61] and, (3) can improve aerobic and musculoskeletal fitness, body composition, social functioning, and mental health and reduce fatigue [36,55,56,58,61-69].
MVPA benefits following breast cancer diagnosis are only maintained for as long as exercise behaviors continue [70-72]. Therefore, the longitudinal assessment of MVPA maintenance following interventions is critical [57,72,73]. In noncancer populations, physical activity intervention effects are infrequently maintained [74-84].
Despite varying reports of barriers, the long-term maintenance of and adherence to MVPA protocols in cancer survivors has not been adequately studied [74-84]. For example, in a recent systematic review of physical and/or dietary interventions in breast cancer populations [85], only 10 of 63 trials assessed the postintervention maintenance of behavioral outcomes [85]. Of these, 4 of 10 achieved successful maintenance (defined as longer than 3 months) [85]. In one recent study of 488 long-term (more than 5 years) cancer survivors with mixed tumor types, participants were randomized to a wait-list control or to a combined diet and physical activity intervention consisting of mailed print material and 15 telephone counseling sessions over 12 months. In the intervention group, weekly physical activity levels increased significantly from baseline (37.5 minutes) to 1-year assessment (postintervention, 101.0 minutes), and these elevated levels were maintained at the 2-year follow-up assessment [86]. While these findings are encouraging, reliance on self-reported physical activity measures and low recruitment rates warrant additional studies with improved designs. Altogether, longitudinal assessments of physical activity maintenance using objective measures in breast cancer survivors are rare, and more are needed to inform physical activity interventions aimed at achieving stabilized, long-term health outcomes [85,87].
Not surprisingly, the health behavior change methods guiding counseling in long-term MVPA maintenance have been inadequately tested in breast cancer survivors. Patient-centered interventions affecting multiple factors (eg, intrinsic motivations, perceived costs and benefits, barriers, ability to change) [88,89] have been derived from evidence-based models (eg, transtheoretical model, social cognitive theory, cognitive behavior theory, and theory of planned behavior) and, in the past, their related efficacies in changing multiple lifestyle behaviors (eg, smoking, diet, chronic sedentariness) have been demonstrated [87,90-93]. While evidential support does not favor one behavior change model, successful physical activity promotion programs have included self-directed physical activity guided by a counselor, follow-up behavioral prompts [56,94-99], and more than 4 sessions of related counseling.
Implementation of theory-based behavior change models for breast cancer survivors aimed at longitudinally maintained MVPA must account for treatment-related sequelae, including adaptations that distract from or discourage health behaviors (eg, avoiding physical pain and discomfort). Accordingly, healthy physical activity promotion requires a cognitive component that emphasizes protective MVPA effects (eg, prevention of breast cancer recurrence) and a cognitive-behavioral component that assists incremental physical activity increases. Exercise prescriptions identify protective goals while carefully incremented training programs assist breast cancer survivors with immediate experiences of improved fitness, well-being, and achievement.
Counseling strategies for improving MVPA can benefit from Internet linkage, smartphone use, and wearable technologies. As of 2012, high proportions of Canadian households access broadband Internet, with mobile services adopted by nearly 80% [100]. Concomittantly, smartphone use has increased from 33% to 56% in all adult Canadians [101]. In the United States, by 2011 78% of adults used the Internet [102] and at least 64% use smartphones [103]. With increasing use, mobile technology has a rapidly increasing health care role via clinical decision making and data collection supporting chronic disease self-management [102].
In support of health behavior change, Internet linkage can provide timely reminders, assessments, behavior-tracking, and “just in time” reinforcement [101]. Supportive communications between patients and providers can occur during the critical periods of dynamic change rather than hours, days, weeks, or months later. Wearable fitness technologies have become more user-friendly with integrated feedback [104] accessed through mobile devices and Internet-linked computers [105] with reliable monitoring of physical activity at lower costs than research accelerometers [106]. Although few in number, Fitbit studies have reported 95% to 99% validity when Fitbit step counts (measured through smartphone apps) are compared with directly measured steps in healthy participants and stroke [106] and traumatic brain injury patients [107].
Despite the accumulating evidence of improved health outcomes with mobile technologies in diabetes, asthma, cardiovascular disease [102,103,108], and physical activity promotion [108], these technologies have been understudied in cancer populations [109]. To advance adoption of long-term physical activity in breast cancer survivors, our innovative health coaching intervention (iMOVE) includes applications of smartphone, computer, and wearable technologies. The pilot study will evaluate iMOVE and inform the design of a larger pragmatic randomized controlled trial (RCT).
Aims of the Pilot Study
Aim 1: To evaluate recruitment, retention, and adherence with a goal of recruiting more than 40% of eligible, contacted patients, retaining more than 75% of participants until the 6-month assessment, and seeing more than 70% of intervention components completed.
Aim 2: To evaluate acceptability feedback for intervention modification in the anticipated full-scale RCT.
Aim 3: To determine pilot estimates of intervention efficacy on fitness (primary outcome) and patient-reported, anthropometric, physical, and psychosocial outcomes (secondary outcomes).
Methods
Recruitment
This pilot RCT will enroll physically inactive breast cancer survivors stratified by age (55 years and older/younger than 55 years) and adjuvant hormone therapy (AHT) exposure (AHT or no AHT). Recruitment will be undertaken through the Princess Margaret Cancer Centre (PMCC), and interventions will occur at the Electronic Living Laboratory for Interdisciplinary Cancer Survivorship Research (ELLICSR), the Cancer Survivorship and Wellness Centre located at the Toronto General Hospital. Both institutions are members of the University Health Network in Toronto, Ontario, and research ethics board approval was obtained from the University Health Network (13-6157-DE). The trial is registered at ClinicalTrials.gov [NCT02620735].
Participants
Adult (aged 18 to 75 years) female breast cancer survivors deemed disease-free after primary cancer treatment are eligible. See Textbox 1 for selection criteria.
Inclusion and exclusion criteria for study.
Inclusion criteria:
Less than 2 years of completion of adjuvant therapy with the exception of hormone therapy for stage 0 to IIIA
Self-report of fewer than 60 minutes of weekly preplanned physical activity
Physician clearance for moderate-to-vigorous physical activity
English proficiency
Ability to attend exercise training sessions and study assessments at prescribed intervals for 9 months
Exclusion criteria:
Plans to join a weight loss or exercise program within 9 months
Current pregnancy or planned pregnancy within 9 months
Planned surgery during study duration
Unwillingness to be randomized
Recruitment and Randomization
After identification from weekly generated clinic lists and chart reviews, patients will be approached by a member of their clinical team, and interested patients will meet with a research assistant for additional study explanation and eligibility screening. Participants will also be recruited by advertisement flyers located in hospital common areas. Eligibility will be ascertained in person when possible, with written consent obtained in person prior to randomization. After participants complete baseline questionnaires and initial physiological assessments, stratification-related data (age, AHT status) will be emailed to a biostatistician in the Department of Biostatistics at PMCC who will perform randomization and then send a study identification with intervention or control group allocation.
Description of Treatment Arms
Exercise Training Program (Intervention and Control Arms)
Participants in the intervention and control groups will receive individualized exercise programming progressing toward the ACSM guidelines of 150 minutes per week of moderate intensity aerobic exercise, 2 to 3 days of resistance training, and routine flexibility training.
In the first 12 weeks, intervention and control participants have 1 supervised, facility-based group exercise class plus instruction for 2 additional unsupervised, home-based exercise sessions. Facility-based sessions will be offered at a variety of times and days weekly to accommodate schedules and increase accessibility. From weeks 13 to 26, participants will complete 3 to 5 home-based exercise sessions per week and no facility-based sessions. Each exercise program is individualized based on the initial fitness assessment, physical limitations, and exercise preferences. The exercise prescriptions are developed and monitored by a certified exercise physiologist (CEP) and a registered kinesiologist (RKin) with volume progression over the intervention course (see Figure 1). Facility-based aerobic training consists of low-impact exercises or aerobic training machines. Home-based aerobic training includes participant-selected aerobic exercises including brisk walking and cycling. Resistance exercise (facility- and home-based) is completed using resistance bands and stability balls provided to participants. The training intensity is based on exercise performance during group sessions observed by the CEP and RKin and is self-monitored via the 20-point Borg Scale for Rating of Perceived Exertion, with a target zone of 14 to 15, varying in accord with the subjective exercise experience of each participant. Adaptations to the exercise prescription based on the participant’s experiences, preferences, and changes (improvements or decrements) in physical capacity will be made by the CEP and RKin to optimize intervention efficacy and safety. All participants receive exercise manuals with exercise descriptions (eg, instructive photographs, exercise safety guidelines, stretching instructions) and a weekly exercise log to review with the CEP and RKin. This program is based on the ACSM guidelines [36] and modeled after a successful theory-based program developed by research team members [110-112].
Figure 1.
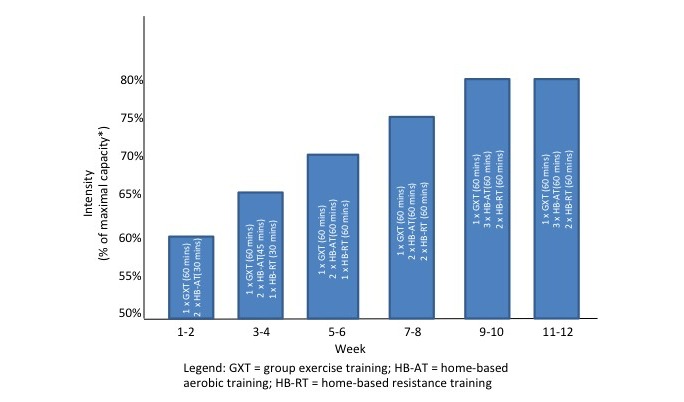
Exercise volume progression.
iMOVE Health Coach (Intervention Arm)
Intervention group participants are additionally provided with a technology-enabled health coach intervention (iMOVE) with 3 components: one-on-one telephone-based counseling, supportive health tracking smartphone software (Connected Wellness, NexJ Health Inc), and use of Fitbit and associated software (FitBit Flex, Fitbit Inc). The iMOVE intervention is intended to enhance sustained behavior change (physical activity) by integrating several active ingredients outlined in the cancer-survivor behavior change literature [113,114] and based on multiple theories, specifically motivational interviewing (MI) [115], cognitive behavioral therapy (CBT) [116], and relapse prevention therapy [117,118].
Theoretical constructs focus on promoting motivation and establishing exercise self-efficacy, exercise social support, and positive exercise-related feelings during the acute intervention (12 weeks) that are sustainable during the postexercise program period (6 months). The telephone-based health coaching component of iMOVE includes 10 30-minute telephone calls with a trained health coach, scheduled at weeks 1, 2, 3, 4, 5, 6, 8, and 12 (during the exercise program) and at weeks 20 and 28 (postexercise program booster sessions). Health coaches with a counseling background in MI and CBT are trained and supervised by a registered clinical psychologist and a Motivational Interviewing Network of Trainers–certified trainer. The training of health coaches involves instructions on breast cancer and related survivorship issues and continual exposure to the multitheoretical approach. Training proceeds weekly from the trial start to end based on case review and participant responses to the approaches implemented. Each health coach call to participants focuses on the assessment and enhancement of motivation, promotion of self-efficacy, and collaborative problem solving. Telephone-based counseling provides several advantages over face-to-face, notably the potential to reach multiregional populations, as telephone access is widely available [119] and requires no user or provider transport. The schedule provides support while building autonomy and independent motivation [120]. See Textbox 2 for a theoretical base summary.
Theoretical base of iMOVE intervention.
Multifactor focus:
Intrinsic motivations
Perceived costs-benefits
Identification of barriers
Abilities to change
Exercise self-efficacy
Exercise social support
Positive exercise-related feelings
Application of evidence-based theory:
Transtheoretical model
Social cognitive theory
Motivational interviewing
Cognitive behavioral therapy
Relapse prevention theory
Theory of planned behavior
Successful physical activity promotion features:
Self-directed physical activity with more than 4 sessions of counseling guidance
Follow-up behavioral prompts
Unique tailoring to breast cancer survivors:
Program pacing per treatment sequelae (eg, physical pain and discomfort)
Cognitive emphasis on protective physical activity effects (eg, prevention of breast cancer recurrence)
Cognitive behavioral emphasis on paced, regulated physical activity increases
Flexible exercise prescription for protective goals and incremental increases that optimize fitness and well-being
Technological assistance:
Rapidly increasing role for mobile technology in health management
Enabling patient-provider contacts during critical periods
User-friendly wearable technologies (95%-99% validity on step counts)
In the scheduled telephone-based sessions, participants will interact with the Connected Wellness software (NexJ Health Inc) by smartphone or Internet-linked computer. This software, previously found effective with participants diagnosed with type 2 diabetes [121-123], has now been tailored for breast cancer. It tracks physical activity, nutrition, pain, and psychological well-being (eg, mood, energy) and supports goal setting (with selective daily or hourly reminders). All software entries are time-stamped, allowing for graph creation that combines multiple trackers, enabling participants and health coaches to see change indicators in relation to the physical activity levels undertaken. Every initiation of contact by participants with their health coach via text messaging is recorded. Confirmations of received text messages are provided by coaches to participants. While patients are encouraged to further discuss their texts during the next phone session, there is also provision for the health coaches to text message responses immediately, responding to questions and issues raised.
Use of the Fitbit Flex provides further assistance to participants in adhering to recommended physical activity routines and tracking physical activity, notably providing real-time feedback (light-emitting diode device lights indicate percent completion of preset, daily step goals). Additional connectivity in the Health Coach program allows the participant and health coach to jointly explore daily physical activity experiences. Fitbit Flex vibrates when preset goals (eg, 10,000 steps) are reached and records the steps taken, combining them with user data to calculate distance walked, calories burned, and the duration and intensity of activity. Fitbit Flex also measures sleep quality by tracking periods of restlessness (ie, how long it takes the wearer to fall asleep per detected body movement) and the estimated sleep duration. The user can monitor their own activity on the Health Coach platform and create summaries and periodic analyses.
As is common with behavioral interventions, a handbook specifies sessional objectives and provides clinical tools for health coaches to use each session. The health coach creates a session-by-session agenda based on patient goals, monitored activity, and motivations as collected with the software during intervals between sessions and at each session. MI and CBT are the core health behavior change theories employed. MI is a collaborative counseling method that elicits and strengthens motivation for change by addressing and resolving ambivalence [124]. MI has demonstrated effectiveness in increasing physical activity in cancer survivors and those with other chronic conditions [41,95,125-132], and positive MI-related effects have been longitudinally detected (eg, at 2 years postintervention) [126,133]. In instances when self-efficacy is impeded by distorted cognitions, CBT principles will be applied, particularly to influence affect-balance through cognitive modifications that prevent or ameliorate negative mood fluctuations [117]. Telephone-based interventions have been effective and acceptable to breast cancer patients [134-137] and useful in delivering MI and CBT interventions [138-140]. Intervention fidelity will be assessed by routine reviews of implementation variables.
While there are multiple theoretical models integrated within our intervention, these models are consolidated in the focus on addressing and resolving motivational ambivalence and identification and modification of the cognitive distortions that maintain motivational ambivalence and prevent adoption of appropriate health behaviors.
Outcome Measures
Pilot Outcome Measures
These measures reflect appropriateness and effectiveness of design features:
Recruitment rate: based on Consolidated Standards of Reporting Trials criteria [141] via a screening log that enables data collection on eligible consented (pre- and post-initial screen) and eligible but nonrecruited individuals with nonrecruitment reasons documented.
Retention rate over the trial duration: the percentage of participants who complete the interventions and each data point; with reasons for drop out documented.
Capture of outcomes: recording of the proportion of participants at each time assessment point with complete or missing data.
Treatment implementation and fidelity: implementation of telephone sessions for the intervention group will be assessed by use documentation of the health coaching techniques and tools and identified barriers. Data from the health coaching software is stored on secure server and used to measure and analyze self-report and health coach activity.
Acceptability: telephone interviews will be conducted with a randomly selected subsample (n=25) of intervention participants following intervention completion. The goals are to explore participant perspectives of intervention feasibility and acceptability and to gain an understanding of experiences among those successful and unsuccessful at physical activity maintenance over differing time periods (eg, during initial 3 months of intervention, 6 months of intervention, 9 months of follow-up). An interpretive descriptive qualitative methodology will be used [142], and a record of interview participation will be kept to distinguish participants from those who don’t participate. The semistructured interviews will be about 45 minutes in duration and preceded by verbal informed consent. Interviews will be audiorecorded and transcribed verbatim.
Clinical Outcomes
Measures for fitness (primary), self-report (secondary), and anthropometric and physical outcomes (exploratory outcomes) are repeated at baseline, T1 (immediately after exercise program, month 3), and T2 (6 months after exercise program, month 9).
Primary Clinical Outcome
Cardiorespiratory fitness will be assessed by a graded exercise test using the modified Bruce protocol [143]. Directly measured peak volume of oxygen (mL/kg/minute) and anaerobic threshold will be obtained using a metabolic cart (TrueOne 2400, Parvo Medics) with continuous gas exchange analysis during incremental treadmill walking to volitional peak capacity. Blood pressure and arterial oxygen saturations are measured at rest and during exercise. Absolute and relative test termination criteria are based on standardized guidelines[144].
Secondary Clinical Outcomes
We will gather preliminary data on a number of exploratory variables which have been identified as important to understanding the potential impact of the intervention on patient-relevant and clinically-relevant outcomes. They are being collected to examine whether they are feasible to collect in a larger trial and whether they are responsive (sensitive to change) to the intervention [145,146].
Patient-Reported Clinical Outcomes
Godin-Shepherd Leisure-Time Exercise Questionnaire: a brief validated 3-item questionnaire that asks respondents to report on typical weekly exercise habits [147]
Functional Assessment of Cancer Therapy—Breast: generic quality of life measured with 44 self-report items [148]
Spielberger's State-Trait Anxiety Inventory—State [149]: a widely used 20-item measure of state anxiety
Center for Epidemiological Studies—Depression Scale short form: a 10-item self-report measure of depression
Functional Assessment of Cancer Therapy—Fatigue subscale [150]: a 13-item measure of fatigue in cancer patients
Breast Cancer Prevention Trial Symptoms Scale: a 42-item scale to assess side effects associated with the treatment of breast cancer
Fear of Recurrence Questionnaire: assesses anxiety about breast cancer recurrence [151]
Physical Activity Group Environment Questionnaire [152]: assesses group cohesion during exercise
Brief Pain Inventory [153]: a widely used measure to rapidly assess the severity of pain and its impact on functioning
Multiple Intervention Satisfaction Survey: an investigator-generated instrument that facilitates intervention participants in rank-ordering discrete intervention components with respect to how helpful they are in achieving outcomes during study participation. Additional items facilitate participant suggestions for deleting intervention components deemed (by participants) as of negligible benefit.
Anthropometric Clinical Outcomes
Body composition is assessed via body mass index, waist circumference, and body fat percentage
Waist circumference is measured according to the World Health Organization protocol (midpoint between lowest rib and iliac crest)
Body fat percentage is measured using bioelectrical impedance analysis [144]
Grip strength is measured using a Jamar dynamometer according to the Canadian Society for Exercise Physiology 2004 protocol
Results
Primary-Secondary Outcome Assessment
Recruitment and retention rates will be assessed [154] with estimates for participants with complete data per outcome and time point divided by the total number of study participants. Interpretation of the interview output (acceptability) will be based on inductive and deductive analyses and use of the constant comparative method [155].
Variability of the main and interaction effects will be examined in the primary clinical outcome (cardiovascular fitness) and each secondary outcome using separate repeated measures analysis of covariance models with Bonferroni corrections applied to the models. Hedges’ g and associated confidence intervals [156] will be calculated as an estimate of the effect size both over time (within groups) and between groups [157]. Missing data will be evaluated on a case-by-case basis such that drop-outs will be excluded. Intention-to-treat (all consented subjects) analysis will employ a last observation carried forward approach to evaluate all data collected. Per protocol analysis will evaluate data on subjects who participated in 50% of group exercise sessions (comparing intervention with control subjects), while experimental subjects will have the additional criteria of participating in 50% of health coaching calls.
Sample Size and Power
We previously conducted a simulation for a range of sample sizes and different SD values for precision of the treatment effect estimate. The precision of the estimate is represented by the inverse of the margin of error. Type I error was set at α=0.05 and power at 80%. From our simulation result, a sample size of 35 to 40 was at the elbow point of the curves, indicating the precision of estimates did not proportionally increase with a larger sample size. Therefore, our projected sample size is 80 participants (40 per arm) [158]. With an anticipated drop-out rate of up to 25% [73], we will recruit 107 participants and examine the variance in primary outcomes with precision (low standard error >0.1), while enabling further calculations of effect sizes for planning the phase 3 trial [154]. The large majority of women return to PMCC for follow-up appointments typically scheduled every 3 to 6 months. Based on data from the PMCC registry, eligibility criteria, and expected participation rates, we anticipate recruiting 8 to 10 participants per month. Study duration is estimated at 30 months.
Interpretation of Results
Interpretation of the effect size and mean difference scores and calculation of the sample size for a larger RCT (fitness outcome) will be based on a minimally important clinical difference (MCD) of 3.5 mL/kg/minute (peak volume of oxygen) between the 2 experimental groups at the 6-month T2 assessment [159,160]. We regard the MCD as a small effect size [159]. With pilot results, we will better estimate small, medium, and large effect sizes for the planned (full-scale) RCT.
Discussion
While current data suggest an important role for physical activity in disease control and the long-term health of cancer survivors, most breast cancer survivors are inactive. This discrepancy must be addressed with physical activity promotion that supports long-term exercise adherence. To date, research has focused on specific physical activity components linked to clinical benefits, but insufficient attention has been paid to factors influencing long-term physical activity maintenance. The current project employs a behavioral support intervention that assists breast cancer survivors in adopting physical activity and maintaining physical activity adherence. While multiple RCTs demonstrate effectiveness in physical activity participation during trial conduct, decreases in physical activity after trial conclusion are an important concern. It is not yet known the degree to which smartphone-enabled health coaching combined with wearable fitness technology can contribute to the lifestyle changes required for breast cancer survivors to maintain healthy physical activity over the longer term.
Our commitment to the devised intervention (combining phone-based health coaching, Fitbit step tracking, health tracking software, face-to-face exercise classes, and fitness testing) accepts the design limitation of being unable to identify which intervention components provide key contributions to significant effects; future studies may be needed to tease out what worked best in further streamlining the intervention. However, we have mitigated limitations by logging all phone counseling calls undertaken (registering time durations per call) and additionally itemizing and quantifying all use of the health tracking software, Connected Wellness (NexJ Health Inc). Furthermore, we track all Fitbit use, including use patterns per time period (day, week, and month). Additionally, use of the Multiple Intervention Satisfaction Survey facilitates each participant in subjectively ranking the intervention components on importance and suggesting deletions of components that have not been significantly helpful. These efforts will enable us to learn about the prioritization of intervention components from each subject’s perspective. Another limitation entails not knowing which allocations of staff time (to the intervention) represent a cost savings when compared to other physical activity promotion approaches. Therefore, we will carefully assess staff time, preparing for ascertaining this cost dimension in the future.
This pilot will document the implementation of the methods and intervention, preliminary outcomes, and acceptability of the interventions by qualitative interview. It will assess effect size in primary and multiple secondary outcomes with corresponding confidence intervals for more definitive sample size calculations. Although pilot results will provide a foundation for full-scale RCT planning, we anticipate challenges for which we currently have only partial or potential solutions. For example, we will only have suggestive data for assessing specific intervention components and for selecting the optimal subset for full RCT testing. Furthermore, as a pilot study, we are still refining the ultimate sample size of the planned full-scale RCT. Additionally, while control subjects receive an approximation of current standard care for exercise promotion (at ELLICSR), we cannot fully account for the attentional differences in intervention and control conditions. Nonetheless, this pilot is a distinct step forward in addressing a gap in the promotion of longer term exercise adherence for breast cancer survivors.
Abbreviations
- ACSM
American College of Sports Medicine
- AHT
adjuvant hormone therapy
- CBT
cognitive behavioral therapy
- CEP
certified exercise physiologist
- ELLICSR
Electronic Living Laboratory for Interdisciplinary Cancer Survivorship Research
- MCD
minimally important clinical difference
- MI
motivational interviewing
- MVPA
moderate-to-vigorous physical activity
- PMCC
Princess Margaret Cancer Centre
- RCT
randomized controlled trial
- RKin
registered kinesiologist
Footnotes
Conflicts of Interest: None declared.
References
- 1.Canadian Cancer Society Canadian Cancer Statistics: Prostate cancer statistics at a glance. [2017-07-17]. http://www.cancer.ca/en/cancer-information/cancer-type/prostate/statistics/?region=sk .
- 2.Altekruse S, Kosary C, Krapcho M, Neyman N, Aminou R, Waldron W, Ruhl J, Howlader N, Tatalovich Z, Cho H, Mariotto A, Eisner M, Lewis D, Cronin K, Chen H, Feuer E, Stinchcomb D, Edwards B( SEER cancer statistics review, 1975-2007. Bethesda: National Cancer Institute; [2017-07-17]. https://seer.cancer.gov/archive/csr/1975_2007/ [Google Scholar]
- 3.Lipscombe LL, Chan WW, Yun L, Austin PC, Anderson GM, Rochon PA. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia. 2013 Mar;56(3):476–483. doi: 10.1007/s00125-012-2793-9. [DOI] [PubMed] [Google Scholar]
- 4.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review, 1975-2014. Bethesda: National Cancer Institute; 2014. [Google Scholar]
- 5.Miller KD, Triano LR. Medical issues in cancer survivors—a review. Cancer J. 2008;14(6):375–387. doi: 10.1097/PPO.0b013e31818ee3dc. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA. Survivorship: adult cancer survivors. Prim Care. 2009 Dec;36(4):721–741. doi: 10.1016/j.pop.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ, ASCO Cancer Survivorship Expert Panel American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J Clin Oncol. 2007 Sep 01;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 8.Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer. 2008 May;44(8):1097–1104. doi: 10.1016/j.ejca.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Fosså SD, Vassilopoulou-Sellin R, Dahl AA. Long-term physical sequelae after adult-onset cancer. J Cancer Surviv. 2008 Mar;2(1):3–11. doi: 10.1007/s11764-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 10.Stewart A, Bielajew C, Collins B, Parkinson M, Tomiak E. A meta-analysis of the neuropsychological effects of adjuvant chemotherapy treatment in women treated for breast cancer. Clin Neuropsychol. 2006 Feb;20(1):76–89. doi: 10.1080/138540491005875. [DOI] [PubMed] [Google Scholar]
- 11.Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ. Chronic pain in the cancer survivor: a new frontier. Pain Med. 2007 Mar;8(2):189–198. doi: 10.1111/j.1526-4637.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 12.Camp-Sorrell D. Cardiorespiratory effects in cancer survivors. Cancer Nurs. 2006;29(2 Suppl):55–59. doi: 10.1097/00002820-200603002-00019. [DOI] [PubMed] [Google Scholar]
- 13.Pelusi J. Sexuality and body image. Research on breast cancer survivors documents altered body image and sexuality. Am J Nurs. 2006 Mar;106(3 Suppl):32–38. doi: 10.1097/00000446-200603003-00013. [DOI] [PubMed] [Google Scholar]
- 14.Azim HA, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011 Sep;22(9):1939–1947. doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- 15.Hewitt M, Greenfield S, Stovall EL. From Cancer Patient to Cancer Survivors: Lost in Transition. Washington: National Academies Press; 2006. [Google Scholar]
- 16.Ng AK, Kenney LB, Gilbert ES, Travis LB. Secondary malignancies across the age spectrum. Semin Radiat Oncol. 2010 Jan;20(1):67–78. doi: 10.1016/j.semradonc.2009.09.002. http://europepmc.org/abstract/MED/19959033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernstein JL, Thompson WD, Risch N, Holford TR. Risk factors predicting the incidence of second primary breast cancer among women diagnosed with a first primary breast cancer. Am J Epidemiol. 1992 Oct 15;136(8):925–936. doi: 10.1093/oxfordjournals.aje.a116565. [DOI] [PubMed] [Google Scholar]
- 18.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y, Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005 Dec 17;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan CL, Dorn PL, Fey J, Giron G, Naik A, Mendez J, Murphy C, Sclafani LM. Locoregional recurrence after mastectomy: incidence and outcomes. J Am Coll Surg. 2006 Oct;203(4):469–474. doi: 10.1016/j.jamcollsurg.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Demark-Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, Cohen HJ. Lifestyle intervention development study to improve physical function in older adults with cancer: outcomes from Project LEAD. J Clin Oncol. 2006 Jul 20;24(21):3465–3473. doi: 10.1200/JCO.2006.05.7224. http://europepmc.org/abstract/MED/16849763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011 Aug 27;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. http://linkinghub.elsevier.com/retrieve/pii/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncol. 2011 Feb;50(2):187–193. doi: 10.3109/0284186X.2010.533190. [DOI] [PubMed] [Google Scholar]
- 23.Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012 Jun 06;104(11):815–840. doi: 10.1093/jnci/djs207. http://europepmc.org/abstract/MED/22570317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemanne D, Cassileth B, Gubili J. The role of physical activity in cancer prevention, treatment, recovery, and survivorship. Oncology (Williston Park) 2013 Jun;27(6):580–585. http://www.cancernetwork.com/oncology-journal/role-physical-activity-cancer-prevention-treatment-recovery-and-survivorship. [PubMed] [Google Scholar]
- 25.Kabat GC, Kim M, Caan BJ, Chlebowski RT, Gunter MJ, Ho GYF, Rodriguez BL, Shikany JM, Strickler HD, Vitolins MZ, Rohan TE. Repeated measures of serum glucose and insulin in relation to postmenopausal breast cancer. Int J Cancer. 2009 Dec 01;125(11):2704–2710. doi: 10.1002/ijc.24609. doi: 10.1002/ijc.24609. [DOI] [PubMed] [Google Scholar]
- 26.Eliassen AH, Missmer SA, Tworoger SS, Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone concentrations and risk of breast cancer among premenopausal women. J Natl Cancer Inst. 2006 Oct 04;98(19):1406–1415. doi: 10.1093/jnci/djj376. [DOI] [PubMed] [Google Scholar]
- 27.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. http://europepmc.org/abstract/MED/12490959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003-2006) Cancer Causes Control. 2010 Feb;21(2):283–288. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 29.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, Hartwick W, Hoffman B, Hood N. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002 Jan 01;20(1):42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 30.Pollak MN. Insulin resistance, estimated by serum C-peptide level, is associated with reduced event-free survival for postmenopausal women in NCIC CTG MA.14 adjuvant breast cancer trial. J Clin Oncol. 2006;24(18):524. [Google Scholar]
- 31.Irwin ML, Aiello EJ, McTiernan A, Bernstein L, Gilliland FD, Baumgartner RN, Baumgartner KB, Ballard-Barbash R. Physical activity, body mass index, and mammographic density in postmenopausal breast cancer survivors. J Clin Oncol. 2007 Mar 20;25(9):1061–1066. doi: 10.1200/JCO.2006.07.3965. http://europepmc.org/abstract/MED/17261853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Cancer Research Fund . Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington: American Institute for Cancer Research; 2007. [Google Scholar]
- 33.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243–274. doi: 10.3322/caac.21142. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 34.Brunet J, Meterissian S, Sabiston CM. Physical activity and breast cancer survivorship: evidence-based recommendations. Am J Lifestyle Med. 2012;6(6):224–240. [Google Scholar]
- 35.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Schwartz AL. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010 Jul;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 36.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003 Apr 01;97(7):1746–1757. doi: 10.1002/cncr.11227. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irwin ML, Ainsworth BE. Physical activity interventions following cancer diagnosis: methodologic challenges to delivery and assessment. Cancer Invest. 2004;22(1):30–50. doi: 10.1081/cnv-120027579. [DOI] [PubMed] [Google Scholar]
- 38.Irwin ML, Duggan C, Wang C, Smith AW, McTiernan A, Baumgartner RN, Baumgartner KB, Bernstein L, Ballard-Barbash R. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol. 2011 Jan 01;29(1):47–53. doi: 10.1200/JCO.2010.28.4752. http://europepmc.org/abstract/MED/21115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Courneya KS, Katzmarzyk PT, Bacon E. Physical activity and obesity in Canadian cancer survivors: population-based estimates from the 2005 Canadian Community Health Survey. Cancer. 2008 Jun;112(11):2475–2482. doi: 10.1002/cncr.23455. doi: 10.1002/cncr.23455. [DOI] [PubMed] [Google Scholar]
- 40.Pinto BM, Trunzo JJ, Reiss P, Shiu S. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psychooncology. 2002;11(5):389–400. doi: 10.1002/pon.594. [DOI] [PubMed] [Google Scholar]
- 41.Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home-based physical activity intervention for breast cancer patients. J Clin Oncol. 2005 May 20;23(15):3577–3587. doi: 10.1200/JCO.2005.03.080. http://jco.ascopubs.org/cgi/pmidlookup?view=long&pmid=15908668. [DOI] [PubMed] [Google Scholar]
- 42.Blanchard CM, Courneya KS, Stein K. Cancer survivors' adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society's SCS-II. J Clin Oncol. 2008 May 1;26(13):2198–2204. doi: 10.1200/JCO.2007.14.6217. http://jco.ascopubs.org/cgi/pmidlookup?view=long&pmid=18445845. [DOI] [PubMed] [Google Scholar]
- 43.Blanchard CM, Denniston MM, Baker F, Ainsworth SR, Courneya KS, Hann DM, Gesme DH, Reding D, Flynn T, Kennedy JS. Do adults change their lifestyle behaviors after a cancer diagnosis? Am J Health Behav. 2003;27(3):246–256. doi: 10.5993/ajhb.27.3.6. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention . A National Action Plan for Cancer Survivorship: Advancing Public Health Strategies. Atlanta: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 45.Harrison S, Hayes SC, Newman B. Level of physical activity and characteristics associated with change following breast cancer diagnosis and treatment. Psychooncology. 2009 Apr;18(4):387–394. doi: 10.1002/pon.1504. [DOI] [PubMed] [Google Scholar]
- 46.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000 Feb 1;88(3):674–684. [PubMed] [Google Scholar]
- 47.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005 Aug 20;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. http://europepmc.org/abstract/MED/16043830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: examining opportunities for cancer control intervention. J Clin Oncol. 2005 Dec 1;23(34):8884–8893. doi: 10.1200/JCO.2005.02.2343. [DOI] [PubMed] [Google Scholar]
- 49.Coups EJ, Ostroff JS. A population-based estimate of the prevalence of behavioral risk factors among adult cancer survivors and noncancer controls. Prev Med. 2005 Jun;40(6):702–711. doi: 10.1016/j.ypmed.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 50.Pinto BM, Eakin E, Maruyama NC. Health behavior changes after a cancer diagnosis: what do we know and where do we go from here? Ann Behav Med. 2000;22(1):38–52. doi: 10.1007/BF02895166. [DOI] [PubMed] [Google Scholar]
- 51.Lemon SC, Zapka JG, Clemow L. Health behavior change among women with recent familial diagnosis of breast cancer. Prev Med. 2004 Aug;39(2):253–262. doi: 10.1016/j.ypmed.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 52.Blaney JM, Lowe-Strong A, Rankin-Watt J, Campbell A, Gracey JH. Cancer survivors' exercise barriers, facilitators and preferences in the context of fatigue, quality of life and physical activity participation: a questionnaire-survey. Psychooncology. 2013 Jan;22(1):186–194. doi: 10.1002/pon.2072. [DOI] [PubMed] [Google Scholar]
- 53.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005 Aug 20;23(24):5814–5830. doi: 10.1200/JCO.2005.01.230. http://europepmc.org/abstract/MED/16043830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Speck RM, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010 Jun;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 55.Schmitz KH, Holtzman J, Courneya KS, Mâsse LC, Duval S, Kane R. Controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2005 Jul;14(7):1588–1595. doi: 10.1158/1055-9965.EPI-04-0703. http://cebp.aacrjournals.org/cgi/pmidlookup?view=long&pmid=16030088. [DOI] [PubMed] [Google Scholar]
- 56.Fjeldsoe B, Neuhaus M, Winkler E, Eakin E. Systematic review of maintenance of behavior change following physical activity and dietary interventions. Health Psychol. 2011 Jan;30(1):99–109. doi: 10.1037/a0021974. [DOI] [PubMed] [Google Scholar]
- 57.Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012:CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolin KY, Schwartz AL, Matthews CE, Courneya KS, Schmitz KH. Implementing the exercise guidelines for cancer survivors. J Support Oncol. 2012;10(5):171–177. doi: 10.1016/j.suponc.2012.02.001. http://europepmc.org/abstract/MED/22579268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cramp F, James A, Lambert J. The effects of resistance training on quality of life in cancer: a systematic literature review and meta-analysis. Support Care Cancer. 2010 Nov;18(11):1367–1376. doi: 10.1007/s00520-010-0904-z. [DOI] [PubMed] [Google Scholar]
- 60.Focht BC, Clinton SK, Devor ST, Garver MJ, Lucas AR, Thomas-Ahner JM, Grainger E. Resistance exercise interventions during and following cancer treatment: a systematic review. J Support Oncol. 2013 Jun;11(2):45–60. [PubMed] [Google Scholar]
- 61.Galvão DA, Newton RU. Review of exercise intervention studies in cancer patients. J Clin Oncol. 2005 Feb 01;23(4):899–909. doi: 10.1200/JCO.2005.06.085. [DOI] [PubMed] [Google Scholar]
- 62.Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Effects of exercise on quality of life in women living with breast cancer: a systematic review. Breast J. 2009;15(1):45–51. doi: 10.1111/j.1524-4741.2008.00670.x. [DOI] [PubMed] [Google Scholar]
- 63.Pekmezi DW, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011 Feb;50(2):167–178. doi: 10.3109/0284186X.2010.529822. http://europepmc.org/abstract/MED/21091401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, Ladha AB, Proulx C, Vallance JKH, Lane K, Yasui Y, McKenzie DC. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007 Oct 1;25(28):4396–4404. doi: 10.1200/JCO.2006.08.2024. http://jco.ascopubs.org/cgi/pmidlookup?view=long&pmid=17785708. [DOI] [PubMed] [Google Scholar]
- 65.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006 Jul 04;175(1):34–41. doi: 10.1503/cmaj.051073. http://www.cmaj.ca/cgi/pmidlookup?view=long&pmid=16818906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirshbaum MN. A review of the benefits of whole body exercise during and after treatment for breast cancer. J Clin Nurs. 2007 Jan;16(1):104–121. doi: 10.1111/j.1365-2702.2006.01638.x. [DOI] [PubMed] [Google Scholar]
- 67.Sabiston CM, Brunet J, Burke S. Pain, movement, and mind: does physical activity mediate the relationship between pain and mental health among survivors of breast cancer? Clin J Pain. 2012 Jul;28(6):489–495. doi: 10.1097/AJP.0b013e31823853ac. [DOI] [PubMed] [Google Scholar]
- 68.Sabiston CM, McDonough MH, Crocker PRE. Psychosocial experiences of breast cancer survivors involved in a dragon boat program: exploring links to positive psychological growth. J Sport Exerc Psychol. 2007 Aug;29(4):419–438. doi: 10.1123/jsep.29.4.419. [DOI] [PubMed] [Google Scholar]
- 69.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005 May 25;293(20):2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 70.Stull VB, Snyder DC, Demark-Wahnefried W. Lifestyle interventions in cancer survivors: designing programs that meet the needs of this vulnerable and growing population. J Nutr. 2007 Jan;137(1 Suppl):243S–248S. doi: 10.1093/jn/137.1.243S. http://jn.nutrition.org/cgi/pmidlookup?view=long&pmid=17182834. [DOI] [PubMed] [Google Scholar]
- 71.Cramp F, Daniel J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst Rev. 2008:CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Estabrooks PA, Glasgow RE, Dzewaltowski DA. Physical activity promotion through primary care. JAMA. 2003 Jun 11;289(22):2913–2916. doi: 10.1001/jama.289.22.2913. [DOI] [PubMed] [Google Scholar]
- 73.White SM, McAuley E, Estabrooks PA, Courneya KS. Translating physical activity interventions for breast cancer survivors into practice: an evaluation of randomized controlled trials. Ann Behav Med. 2009 Feb;37(1):10–19. doi: 10.1007/s12160-009-9084-9. http://europepmc.org/abstract/MED/19255819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. A 4.5 year prospective study. Scand J Rheumatol. 1996;25(2):77–86. doi: 10.3109/03009749609069212. [DOI] [PubMed] [Google Scholar]
- 75.Rissanen A, Fogelholm M. Physical activity in the prevention and treatment of other morbid conditions and impairments associated with obesity: current evidence and research issues. Med Sci Sports Exerc. 1999 Nov;31(11 Suppl):S635–S645. doi: 10.1097/00005768-199911001-00024. [DOI] [PubMed] [Google Scholar]
- 76.Marcus BH, Dubbert PM, Forsyth LH, McKenzie TL, Stone EJ, Dunn AL, Blair SN. Physical activity behavior change: issues in adoption and maintenance. Health Psychol. 2000 Jan;19(1S):32–41. doi: 10.1037/0278-6133.19.suppl1.32. [DOI] [PubMed] [Google Scholar]
- 77.Kahn EB, Ramsey LT, Brownson RC, Heath GW, Howze EH, Powell KE, Stone EJ, Rajab MW, Corso P. The effectiveness of interventions to increase physical activity. A systematic review. Am J Prev Med. 2002 May;22(4 Suppl):73–107. doi: 10.1016/s0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 78.van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults: a review. Am J Prev Med. 2002 Feb;22(2):120–133. doi: 10.1016/s0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 79.Smith BJ. Promotion of physical activity in primary health care: update of the evidence on interventions. J Sci Med Sport. 2004 Apr;7(1 Suppl):67–73. doi: 10.1016/s1440-2440(04)80280-9. [DOI] [PubMed] [Google Scholar]
- 80.McAuley E, Morris KS, Motl RW, Hu L, Konopack JF, Elavsky S. Long-term follow-up of physical activity behavior in older adults. Health Psychol. 2007 May;26(3):375–380. doi: 10.1037/0278-6133.26.3.375. [DOI] [PubMed] [Google Scholar]
- 81.Greaney ML, Riebe D, Ewing GC, Rossi JS, Lees FD, Burbank PA, Nigg CR, Ferrone CL, Clark PG. Long-term effects of a stage-based intervention for changing exercise intentions and behavior in older adults. Gerontologist. 2008 Jun;48(3):358–367. doi: 10.1093/geront/48.3.358. [DOI] [PubMed] [Google Scholar]
- 82.Rejeski WJ, Marsh AP, Chmelo E, Prescott AJ, Dobrosielski M, Walkup MP, Espeland M, Miller ME, Kritchevsky S. The Lifestyle Interventions and Independence for Elders Pilot (LIFE-P): 2-year follow-up. J Gerontol A Biol Sci Med Sci. 2009 Apr;64(4):462–467. doi: 10.1093/gerona/gln041. http://europepmc.org/abstract/MED/19181715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hall KS, Sloane R, Pieper CF, Peterson MJ, Crowley GM, Cowper PA, McConnell ES, Bosworth HB, Ekelund CC, Morey MC. Long-term changes in physical activity following a one-year home-based physical activity counseling program in older adults with multiple morbidities. J Aging Res. 2010 Dec 26;2011:308407. doi: 10.4061/2011/308407. doi: 10.4061/2011/308407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hertogh EM, Vergouwe Y, Schuit AJ, Peeters PHM, Monninkhof EM. Behavioral changes after a 1-yr exercise program and predictors of maintenance. Med Sci Sports Exerc. 2010 May;42(5):886–892. doi: 10.1249/MSS.0b013e3181c4d964. [DOI] [PubMed] [Google Scholar]
- 85.Spark LC, Reeves MM, Fjeldsoe BS, Eakin EG. Physical activity and/or dietary interventions in breast cancer survivors: a systematic review of the maintenance of outcomes. J Cancer Surviv. 2013 Mar;7(1):74–82. doi: 10.1007/s11764-012-0246-6. [DOI] [PubMed] [Google Scholar]
- 86.Demark-Wahnefried W, Morey MC, Sloane R, Snyder DC, Miller PE, Hartman TJ, Cohen HJ. Reach out to enhance wellness home-based diet-exercise intervention promotes reproducible and sustainable long-term improvements in health behaviors, body weight, and physical functioning in older, overweight/obese cancer survivors. J Clin Oncol. 2012 Jul 01;30(19):2354–2361. doi: 10.1200/JCO.2011.40.0895. http://europepmc.org/abstract/MED/22614994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Short CE, James EL, Stacey F, Plotnikoff RC. A qualitative synthesis of trials promoting physical activity behaviour change among post-treatment breast cancer survivors. J Cancer Surviv. 2013 Dec;7(4):570–581. doi: 10.1007/s11764-013-0296-4. http://europepmc.org/abstract/MED/23888337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dacey M, Baltzell A, Zaichkowsky L. Older adults' intrinsic and extrinsic motivation toward physical activity. Am J Health Behav. 2008;32(6):570–582. doi: 10.5555/ajhb.2008.32.6.570. [DOI] [PubMed] [Google Scholar]
- 89.Mata J, Silva MN, Vieira PN, Carraça EV, Andrade AM, Coutinho SR, Sardinha LB, Teixeira PJ. Motivational spill-over during weight control: increased self-determination and exercise intrinsic motivation predict eating self-regulation. Health Psychol. 2009 Nov;28(6):709–716. doi: 10.1037/a0016764. [DOI] [PubMed] [Google Scholar]
- 90.Ory MG, Jordan PJ, Bazzarre T. The Behavior Change Consortium: setting the stage for a new century of health behavior-change research. Health Educ Res. 2002 Oct;17(5):500–511. doi: 10.1093/her/17.5.500. [DOI] [PubMed] [Google Scholar]
- 91.Pinto BM, Floyd A. Theories underlying health promotion interventions among cancer survivors. Semin Oncol Nurs. 2008 Aug;24(3):153–163. doi: 10.1016/j.soncn.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 92.Vallance J, Plotnikoff RC, Karvinen KH, Mackey JR, Courneya KS. Understanding physical activity maintenance in breast cancer survivors. Am J Health Behav. 2010;34(2):225–236. doi: 10.5993/ajhb.34.2.10. [DOI] [PubMed] [Google Scholar]
- 93.Vallance JK, Courneya KS, Plotnikoff RC, Dinu I, Mackey JR. Maintenance of physical activity in breast cancer survivors after a randomized trial. Med Sci Sports Exerc. 2008 Jan;40(1):173–180. doi: 10.1249/mss.0b013e3181586b41. [DOI] [PubMed] [Google Scholar]
- 94.Harland J, White M, Drinkwater C, Chinn D, Farr L, Howel D. The Newcastle exercise project: a randomised controlled trial of methods to promote physical activity in primary care. BMJ. 1999 Sep 25;319(7213):828–832. doi: 10.1136/bmj.319.7213.828. http://europepmc.org/abstract/MED/10496829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elley CR, Kerse N, Arroll B, Robinson E. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003 Apr 12;326(7393):793. doi: 10.1136/bmj.326.7393.793. http://europepmc.org/abstract/MED/12689976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO, Horton ES, Hoskin MA, Kriska A, Lachin J, Mayer-Davis EJ, Pi-Sunyer X, Regensteiner JG, Venditti B, Wylie-Rosett J. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res. 2004 Sep;12(9):1426–1434. doi: 10.1038/oby.2004.179. http://europepmc.org/abstract/MED/15483207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005:CD003180. doi: 10.1002/14651858.CD003180.pub2. http://europepmc.org/abstract/MED/15674903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fry JP, Neff RA. Periodic prompts and reminders in health promotion and health behavior interventions: systematic review. J Med Internet Res. 2009;11(2):e16. doi: 10.2196/jmir.1138. http://www.jmir.org/2009/2/e16/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, Saxton JM, Taylor SJC. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev. 2013 Jan;:CD010192. doi: 10.1002/14651858.CD010192.pub2. [DOI] [PubMed] [Google Scholar]
- 100.CRTC communications monitoring report: 5.0 telecommunications. [2017-07-18]. http://www.crtc.gc.ca/eng/publications/reports/PolicyMonitoring/2012/cmr5.htm#n0 .
- 101.Smyth J, Heron K. Health psychology. In: Mehl MR, Connor TS, editors. Handook of Research Methods for Studying Daily Life. New York: Guilford Press; 2011. p. 569. [Google Scholar]
- 102.Boulos MNK, Wheeler S, Tavares C, Jones R. How smartphones are changing the face of mobile and participatory healthcare: an overview, with example from eCAALYX. Biomed Eng Online. 2011;10:1–15. doi: 10.1186/1475-925X-10-24. http://www.biomedcentral.com/1475-925X/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009 Apr;15(3):231–240. doi: 10.1089/tmj.2008.0099. [DOI] [PubMed] [Google Scholar]
- 104.Lyons EJ, Lewis ZH, Mayrsohn BG, Rowland JL. Behavior change techniques implemented in electronic lifestyle activity monitors: a systematic content analysis. J Med Internet Res. 2014;16(8):e192. doi: 10.2196/jmir.3469. http://www.jmir.org/2014/8/e192/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: a cross-sectional study. Int J Behav Nutr Phys Act. 2015;12:9. doi: 10.1186/s12966-015-0201-9. http://www.ijbnpa.org/content/12//42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Takacs J, Pollock CL, Guenther JR, Bahar M, Napier C, Hunt MA. Validation of the Fitbit One activity monitor device during treadmill walking. J Sci Med Sport. 2014 Sep;17(5):496–500. doi: 10.1016/j.jsams.2013.10.241. [DOI] [PubMed] [Google Scholar]
- 107.Fulk GD, Combs SA, Danks KA, Nirider CD, Raja B, Reisman DS. Accuracy of 2 activity monitors in detecting steps in people with stroke and traumatic brain injury. Phys Ther. 2014 Feb;94(2):222–229. doi: 10.2522/ptj.20120525. http://www.ptjournal.org/cgi/pmidlookup?view=long&pmid=24052577. [DOI] [PubMed] [Google Scholar]
- 108.van den Berg MH, Schoones JW, Vliet VTPM. Internet-based physical activity interventions: a systematic review of the literature. J Med Internet Res. 2007;9(3):e26. doi: 10.2196/jmir.9.3.e26. http://www.jmir.org/2007/3/e26/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong Y, Dahlke DV, Ory M, Hochhalter A, Reynolds J, Purcell NP, Talwar D, Eugene N. Designing iCanFit: a mobile-enabled Web application to promote physical activity for older cancer survivors. JMIR Res Protoc. 2013;2(1):e12. doi: 10.2196/resprot.2440. http://www.researchprotocols.org/2013/1/e12/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Santa MD, Alibhai SMH, Matthew AG, Guglietti CL, Pirbaglou M, Trachtenberg J, Ritvo P. A randomized trial of aerobic versus resistance exercise in prostate cancer survivors. J Aging Phys Act. 2013 Oct;21(4):455–478. doi: 10.1123/japa.21.4.455. [DOI] [PubMed] [Google Scholar]
- 111.Alibhai SMH, Santa MD, Ritvo P, Sabiston C, Krahn M, Tomlinson G, Matthew A, Segal R, Warde P, Durbano S, O'Neill M, Culos-Reed N. A phase II RCT and economic analysis of three exercise delivery methods in men with prostate cancer on androgen deprivation therapy. BMC Cancer. 2015 Apr 25;15:312. doi: 10.1186/s12885-015-1316-8. https://bmccancer.biomedcentral.com/articles/10.1186/s12885-015-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Santa MD, Alibhai SMH, Matthew AG, Guglietti CL, Steele J, Trachtenberg J, Ritvo PG. Exercise in clinical cancer care: a call to action and program development description. Curr Oncol. 2012 Jun;19(3):e136–e144. doi: 10.3747/co.19.912. http://www.current-oncology.com/index.php/oncology/article/view/912/876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol. 2008 May;27(3):379–387. doi: 10.1037/0278-6133.27.3.379. [DOI] [PubMed] [Google Scholar]
- 114.Michie S, Ashford S, Sniehotta FF, Dombrowski SU, Bishop A, French DP. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol Health. 2011 Nov;26(11):1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 115.Rollnick S, Miller WR, Butler CC. Motivational Interviewing in Health Care: Helping Patients Change Behavior. New York: Guilford Press; 2007. [Google Scholar]
- 116.Brewin CR. Theoretical foundations of cognitive-behavior therapy for anxiety and depression. Annu Rev Psychol. 1996;47:33–57. doi: 10.1146/annurev.psych.47.1.33. [DOI] [PubMed] [Google Scholar]
- 117.Marlatt GA, Gordon JR. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York: Guilford Press; 1985. [Google Scholar]
- 118.Parks GA, Anderson BK, Marlatt GA. Relapse prevention therapy. In: Heather N, Peters T, editors. Handbook of Alcohol Dependence and Problems. Sussex: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- 119.Eakin EG, Lawler SP, Vandelanotte C, Owen N. Telephone interventions for physical activity and dietary behavior change: a systematic review. Am J Prev Med. 2007 May;32(5):419–434. doi: 10.1016/j.amepre.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 120.Ang DC, Kaleth AS, Bigatti S, Mazzuca S, Saha C, Hilligoss J, Lengerich M, Bandy R. Research to Encourage Exercise for Fibromyalgia (REEF): use of motivational interviewing design and method. Contemp Clin Trials. 2011 Jan;32(1):59–68. doi: 10.1016/j.cct.2010.08.014. http://europepmc.org/abstract/MED/20828634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wayne N, Perez DF, Kaplan DM, Ritvo P. Health coaching reduces HbA1c in type 2 diabetic patients from a lower-socioeconomic status community: a randomized controlled trial. J Med Internet Res. 2015;17(10):e224. doi: 10.2196/jmir.4871. http://www.jmir.org/2015/10/e224/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pludwinski S, Ahmad F, Wayne N, Ritvo P. Participant experiences in a smartphone-based health coaching intervention for type 2 diabetes: a qualitative inquiry. J Telemed Telecare. 2015 Jul 21;:172–178. doi: 10.1177/1357633X15595178. [DOI] [PubMed] [Google Scholar]
- 123.Wayne N, Ritvo P. Smartphone-enabled health coach intervention for people with diabetes from a modest socioeconomic strata community: single-arm longitudinal feasibility study. J Med Internet Res. 2014;16(6):e149. doi: 10.2196/jmir.3180. http://www.jmir.org/2014/6/e149/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Miller WR, Rollnick S. Ten things that motivational interviewing is not. Behav Cogn Psychother. 2009 Mar;37(2):129–140. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- 125.Sjöling M, Lundberg K, Englund E, Westman A, Jong MC. Effectiveness of motivational interviewing and physical activity on prescription on leisure exercise time in subjects suffering from mild to moderate hypertension. BMC Res Notes. 2011 Sep 12;4:352. doi: 10.1186/1756-0500-4-352. https://bmcresnotes.biomedcentral.com/articles/10.1186/1756-0500-4-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Burke BL, Arkowitz H, Menchola M. The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol. 2003 Oct;71(5):843–861. doi: 10.1037/0022-006X.71.5.843. [DOI] [PubMed] [Google Scholar]
- 127.Brodie DA, Inoue A. Motivational interviewing to promote physical activity for people with chronic heart failure. J Adv Nurs. 2005 Jun;50(5):518–527. doi: 10.1111/j.1365-2648.2005.03422.x. [DOI] [PubMed] [Google Scholar]
- 128.Smith DE, Heckemeyer CM, Kratt PP, Mason DA. Motivational interviewing to improve adherence to a behavioral weight-control program for older obese women with NIDDM. A pilot study. Diabetes Care. 1997 Jan;20(1):52–54. doi: 10.2337/diacare.20.1.52. [DOI] [PubMed] [Google Scholar]
- 129.Butterworth S, Linden A, McClay W. Health coaching as an intervention in health management programs. Dis Manag Health Out. 2007;15(5):299–307. [Google Scholar]
- 130.Bennett JA, Perrin NA, Hanson G, Bennett D, Gaynor W, Flaherty-Robb M, Joseph C, Butterworth S, Potempa K. Healthy aging demonstration project: nurse coaching for behavior change in older adults. Res Nurs Health. 2005 Jun;28(3):187–197. doi: 10.1002/nur.20077. [DOI] [PubMed] [Google Scholar]
- 131.Tse MMY, Vong SKS, Tang SK. Motivational interviewing and exercise programme for community-dwelling older persons with chronic pain: a randomised controlled study. J Clin Nurs. 2013 Jul;22(13-14):1843–1856. doi: 10.1111/j.1365-2702.2012.04317.x. [DOI] [PubMed] [Google Scholar]
- 132.Olsen JM, Nesbitt BJ. Health coaching to improve healthy lifestyle behaviors: an integrative review. Am J Health Promot. 2010;25(1):e1–e12. doi: 10.4278/ajhp.090313-LIT-101. [DOI] [PubMed] [Google Scholar]
- 133.Lundahl BW. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Social Work Prac. 2010;20(2):137–160. [Google Scholar]
- 134.Gotay CC, Moinpour CM, Unger JM, Jiang CS, Coleman D, Martino S, Parker BJ, Bearden JD, Dakhil S, Gross HM, Lippman S, Albain KS. Impact of a peer-delivered telephone intervention for women experiencing a breast cancer recurrence. J Clin Oncol. 2007 May 20;25(15):2093–2099. doi: 10.1200/JCO.2006.07.4674. http://jco.ascopubs.org/cgi/pmidlookup?view=long&pmid=17513815. [DOI] [PubMed] [Google Scholar]
- 135.Badger T, Segrin C, Meek P, Lopez AM, Bonham E, Sieger A. Telephone interpersonal counseling with women with breast cancer: symptom management and quality of life. Oncol Nurs Forum. 2005 Mar 05;32(2):273–279. doi: 10.1188/05.ONF.273-279. [DOI] [PubMed] [Google Scholar]
- 136.Allen SM, Shah AC, Nezu AM, Nezu CM, Ciambrone D, Hogan J, Mor V. A problem-solving approach to stress reduction among younger women with breast carcinoma: a randomized controlled trial. Cancer. 2002 Jun 15;94(12):3089–3100. doi: 10.1002/cncr.10586. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pubmed&issn=0008-543X&date=2002&volume=94&issue=12&spage=3089. [DOI] [PubMed] [Google Scholar]
- 137.Marcus AC, Garrett KM, Cella D, Wenzel L, Brady MJ, Fairclough D, Pate-Willig M, Barnes D, Emsbo SP, Kluhsman BC, Crane L, Sedlacek S, Flynn PJ. Can telephone counseling post-treatment improve psychosocial outcomes among early stage breast cancer survivors? Psychooncology. 2010 Sep;19(9):923–932. doi: 10.1002/pon.1653. http://europepmc.org/abstract/MED/19941285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wahab S, Menon U, Szalacha L. Motivational interviewing and colorectal cancer screening: a peek from the inside out. Patient Educ Couns. 2008 Aug;72(2):210–217. doi: 10.1016/j.pec.2008.03.023. http://europepmc.org/abstract/MED/18467066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Picciano J, Roffman RA, Kalichman SC, Rutledge SE, Berghuis JP. A telephone based brief intervention using motivational enhancement to facilitate HIV risk reduction among MSM: a pilot study. AIDS Behav. 2001;5(3):251–262. [Google Scholar]
- 140.Walker DD, Roffman RA, Picciano JF, Stephens RS. The check-up: in-person, computerized, and telephone adaptations of motivational enhancement treatment to elicit voluntary participation by the contemplator. Subst Abuse Treat Prev Policy. 2007 Jan 08;2:2. doi: 10.1186/1747-597X-2-2. https://substanceabusepolicy.biomedcentral.com/articles/10.1186/1747-597X-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Carroll KM. Enhancing retention in clinical trials of psychosocial treatments: practical strategies. In: Onken LS, Blaine J, Boren J, editors. Beyond the Therapeutic Alliance: Keeping the Drug-Dependent Individual in Treatment. Bethesda: National Institutes of Health; 1997. [Google Scholar]
- 142.Thorne S, Kirkham SR, MacDonald-Emes J. Interpretive description: a noncategorical qualitative alternative for developing nursing knowledge. Res Nurs Health. 1997 Apr;20(2):169–177. doi: 10.1002/(sici)1098-240x(199704)20:2<169::aid-nur9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 143.Heyward V. Advanced Fitness Assessment and Exercise Prescription. Champaign: Human Kinetics; 2002. [Google Scholar]
- 144.Heyward V. Advanced Fitness Assessment and Exercise Prescription. 6th Edition. Champaign: Human Kinetics; 2010. [Google Scholar]
- 145.Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008 Feb;61(2):102–109. doi: 10.1016/j.jclinepi.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 146.Liang MH. Evaluating measurement responsiveness. J Rheumatol. 1995 Jun;22(6):1191–1192. [PubMed] [Google Scholar]
- 147.Godin G. Godin Leisure-Time Exercise Questionnaire. Med Sci Sport Exer. 1997;29(June Supplement):S36–S38. [Google Scholar]
- 148.Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, Silberman M, Yellen SB, Winicour P, Brannon J. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993 Mar;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 149.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- 150.Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997 Jul;34(3 Suppl 2):13–19. [PubMed] [Google Scholar]
- 151.Northouse LL. Mastectomy patients and the fear of cancer recurrence. Cancer Nurs. 1981 Jun;4(3):213–220. [PubMed] [Google Scholar]
- 152.Estabrooks PA, Carron AV. The physical activity group environment questionnaire: an instrument for the assessment of cohesion in exercise classes. Group Dynamics. 2000;4(3):1089–2699. [Google Scholar]
- 153.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994 Mar;23(2):129–138. [PubMed] [Google Scholar]
- 154.Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008 Apr;31(2):180–191. doi: 10.1002/nur.20247. [DOI] [PubMed] [Google Scholar]
- 155.Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409. [Google Scholar]
- 156.Thompson B. What future quantitative social science research could look like: Confidence intervals for effect sizes. Educational Researcher. 2002;31(3):25–32. [Google Scholar]
- 157.Hedges L, Olkin I. Statistical Methods for Meta-Analysis. San Diego: Academic Press; 1985. [Google Scholar]
- 158.Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004 May;10(2):307–312. doi: 10.1111/j.2002.384.doc.x. [DOI] [PubMed] [Google Scholar]
- 159.Kavanagh T, Mertens DJ, Hamm LF, Beyene J, Kennedy J, Corey P, Shephard RJ. Prediction of long-term prognosis in 12169 men referred for cardiac rehabilitation. Circulation. 2002 Aug 06;106(6):666–671. doi: 10.1161/01.cir.0000024413.15949.ed. http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=12163425. [DOI] [PubMed] [Google Scholar]
- 160.Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res. 2011 May;45(5):626–629. doi: 10.1016/j.jpsychires.2010.10.008. http://europepmc.org/abstract/MED/21035130. [DOI] [PMC free article] [PubMed] [Google Scholar]


