Abstract
Background
Infection following abdominal surgery remains a major factor in morbidity among colorectal cancer (CRC) patients. Probiotic therapy has been suggested to improve the clinical and laboratory outcome of patients undergoing gastrointestinal surgery. The aim of this study was to investigate the efficacy of probiotic lactic acid bacteria in patients with CRC in the pre- and postoperative phases.
Methods
Systematic database searches identified 1,080 related articles. However, only seven articles were selected according to the eligibility criteria for qualitative and quantitative evaluation.
Results
Most of the reviewed articles presented satisfactory results related to the prevention of surgical inflammation in patients undergoing resection of CRC when using strains of Lactobacillus genus, predominantly.
Conclusions
Probiotics are suggested to prevent surgical inflammation of CRC, at the same time that the combination of particular microorganisms administered is beneficial to the treatment and surgical recovery.
Keywords: Colorectal cancer (CRC), probiotics, meta-analysis, perioperative
Introduction
Colorectal cancer (CRC) is the third most common and dangerous affection in men and women after breast, prostate and lung cancers (1). CRC is ranked as the second leading cause of death from tumors, which are primarily originate from the gastrointestinal tract (GIT), particularly the colon and rectum, and have the liver as the most common site of metastases (2).
Surgical resection remains the mainstay for treatment of CRC, with the extent of surgery and the need for chemotherapy and radiation depending on the stage and disease presentation (3). However, infection after abdominal surgery continues to be an important factor affecting patient morbidity (4). With the development of advanced surgical techniques and improved perioperative care, morbidity and mortality rates were significantly reduced (5). The occurrence of surgical site infection (SSI) extends the length of hospital stay, increasing admission costs and potentially reducing the quality of life of patients (6).
Long-term care of survivors of CRC and acute treatment are extremely relevant. Post-treatment patients suffer from various chronic symptoms, and consequently have a significant loss of quality of life (7). According to Eguchi et al. (8), an important factor inducing the perioperative SSI seems to be disorders in the intestinal flora caused by invasive stress of the surgery, administering antibiotics to prevent infection, weakness of intestinal tract motility, and intestinal mucosal atrophy due to perioperative fasting and intestinal ischemia.
Liu et al. (4) propose that probiotic therapy may improve the clinical and laboratory prognostic of patients undergoing gastrointestinal surgery. Administration of probiotics in these patients aims a competitive action against bacteria responsible for postoperative inflammation (9). Thus, it has been suggested that probiotics play an essential role in the stability of the microbiological environment (10,11).
Thus, the purpose of this study is to evaluate, through a systematic review, the efficacy of probiotics in patients with CRC in the pre- and postoperative phases, as well as combined treatments with existing chemotherapeutic agents, aiming to identify gaps in the existing knowledge base and determine the quality of the studies available in the literature.
Methods
Guidelines
For the systematic review, the guidelines established by PRISMA were followed (preferred reporting items for systematic reviews and meta-analyses) (12).
Eligibility criteria
The selected articles met the criteria of being original studies in humans; published in English, Spanish or Portuguese from 2005 to 2016; and concerning the administration of probiotics in period preoperative and postoperative in patients with CRC.
Non-original articles (reviews, editorials, letters, comments and book chapters) were excluded, as well as studies with humans, which did not meet the eligibility criteria, i.e., not presenting any information about the dosage of probiotic administered. Experimental studies using cell models and animals (mice and rats) were reported only to discuss results.
Source of information and study identification
A systematic search was conducted to evaluate the efficacy of probiotic lactic acid bacteria in the CRC prophylaxis in perioperative patients. Five electronic databases were searched, including: PubMed, Science Direct, LILACS, Scielo and Scopus. The keywords used were: “probiotic”, “perioperative”, “acid lactic bacteria” and “colon cancer”, also searched in combination with each other to improve the results.
The retrieved articles were evaluated independently. The initial phase of selection consisted of the analysis of titles, abstracts and finally reading the studies to select them based on the eligibility criteria.
To facilitate data collection and the reliability of the selection, the clinical conditions were determined according to the acronym PECO, where P = Patients: adults; E = Exposure: patients receiving probiotic bacteria supplementation (regardless of nature, method of preparation and dosage); C = Control: patients who received normal nutrition and/or milk; O = Outcome: overall mortality (13).
Statistical analysis
All Statistical analyses were performed by the MedCalc® Statistical Software, with results expressed as odds ratio (OR), according to Altman, Deeks and Sackett (14). The OR with 95% confidence interval (CI) was used to determine significant differences between treated and control groups. The calculated OR is represented in a forest-plot type graph, which shows individual and meta-analysis results (15). The Peto method was used according to the guidelines of Brazil (13).
Although the eligibility criteria for the studies and clinical conditions were similar regarding the administration of probiotics and patients with CRC, the heterogeneity was taken into account to evaluate the degree of confidence in the results. Thus, the statistical model employed was the chi-square test, according to Baglioni et al. (16). A statistical I2 value close to 0% indicates no heterogeneity among studies, close to 25% indicates low heterogeneity, close to 50% indicates moderate heterogeneity and around 75% indicates high heterogeneity between trials (15,17).
Results
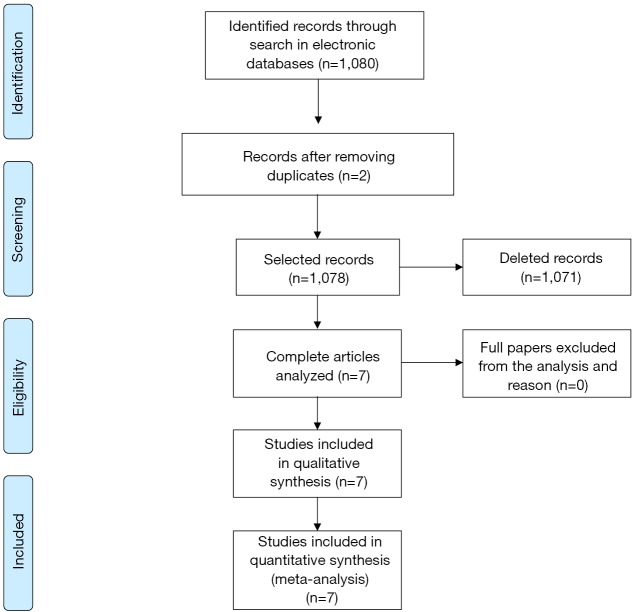
The search in ScienceDirect, PubMed and Scopus databases retrieved 581, articles 108 and 391, respectively (total of 1,080), as shown in Figure 1. The articles which did not fit the eligibility criteria were excluded (total of 1,071). Searches in LILACS and SciELO databases did not retrieve additional studies.
Figure 1.
Flow diagram for study identification and selection [adapted from Moher et al. (12)].
Therefore a number of seven peer-reviewed articles, with randomized controlled trials described in English, were selected in this review. The dates of publication in high impact journals ranged from 2010 to 2016 and the sample size ranged from 20 to 161 (mean of 90.1).
Intake of probiotics and incisional infection
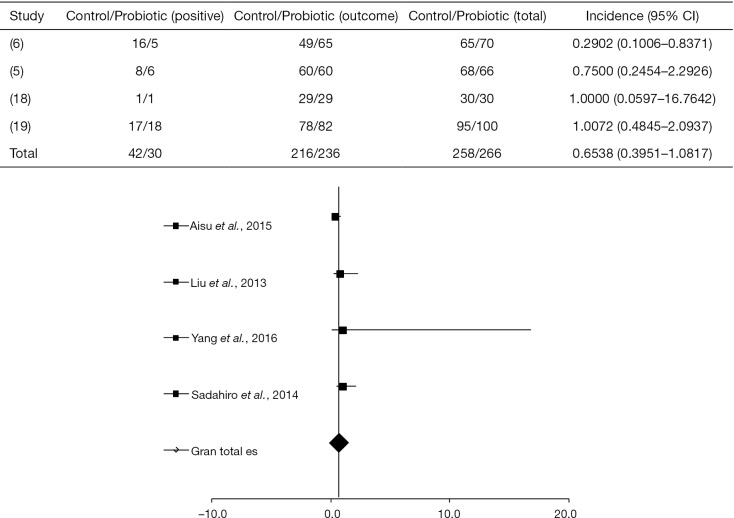
A significant difference between the probiotic-treated group and control group, regarding infection in the surgical incision, was found in only one study as shown in Figure 2 [where the four selected articles studied incisional infection (5,6,18,19) (I2=25%, P=0.2133, DF=9)].
Figure 2.
“Forest plot” effect of probiotics compared to the control group in the pre and postoperative treatment in accordance with the primary objective of the clinical trials in patients with CRC for incisional infection (5,6,18,19). CRC, colorectal cancer.
In the study by Aisu et al. (6), a total of 5 of 70 patients (7.1%) were positive for SSI when treated with probiotic, in contrast to the non-treated group where 16 of 65 patients (24.6%) presented infection (P=0.0081, 95% with confidence limit 0.8371). Other studies (5,18,19) showed a placebo response (OR =0.6928, P=0.1493, 95% CI, 0.4207 to 1.1409).
Liu et al. (5) reported that 6 of 66 patients (9%) administered with probiotics developed surgical infection, in contrast with 8 of 68 patients (11.8%) in the control group. Sadahiro et al. (19) combining probiotics with the drug therapy in 100 patients with CRC, observed a percentage of 18% of infection. Yang et al. (18) studied the use of probiotics in 30 patients and found that about 3.3% of both treated and control groups presented wound infection.
Detection of bacteria in blood
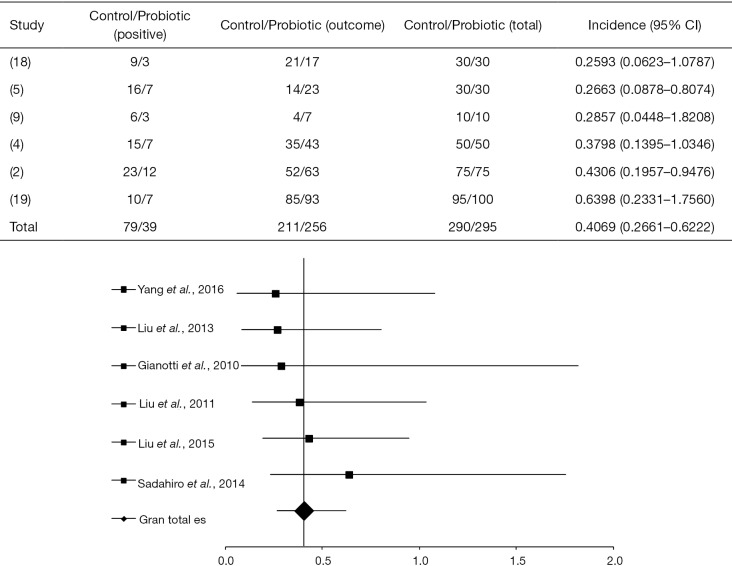
The OR between different statistical studies concerning the detection of highly pathogenic bacteria in the blood was OR =0.4069 (95% CI, 0.2662–0.6222, P<0.0001) and I2=16.7% (P=0.2851, DF=10). The forest plot of that analysis is shown in Figure 3. A total of 4 of 6 articles evaluated showed a positive response of the probiotic use (4,5,9,18), while 2 of them showed a placebo response (2,19).
Figure 3.
Detection of bacteria in the blood according to the administration of probiotics in the perioperative period, compared to the control group with the primary objective of the clinical trials in patients with CRC (2,4,5,9,18,19). CRC, colorectal cancer.
Gianotti et al. (9) used probiotics in pharmacological interventions (Table 1) during perioperative periods and observed 30% and 60% of pathogenic bacteria in the blood, in the treated and control groups, respectively. Liu et al. (4) used probiotics to treat patients with CRC and verified 14% and 30% of bacteria in the blood for the probiotic-treated and the control groups, respectively; showing a statistical significance of the intervention OR =0.3798 (95% CI, 0.1395 to 1.0346, P=0.0583).
Table 1. Summary of characteristics of the studies included in the review.
| Reference | Study design | Age (years) | Follow-up | Sample size | Microorganisms | Comments |
|---|---|---|---|---|---|---|
| (9) | Randomized, controlled, double-blind | 18–80 | 3 days pre-operative/12 postoperative days | 20: 10 control group, 10 probiotic group | Lactobacillus johnsonii; bifidobacterium longum (2×109 FCU/d) | Placebo: maltodextrin |
| (4) | Randomized, double-blind, placebo-controlled | 25–75 | 6 days pre-operative/10 days postoperative | 100: 50 control, 50 probiotic group | Lactobacillus plantarum (≥1011 FCU/g); lactobacillus acidophilus (≥7.0×1010 FCU/g); bifidobacterium longum (≥5.0×1010 FCU/g) | 2 g/days of probiotic (2.6×1014 UFC); placebo: capsules and sachet 10 g of maltodextrin |
| (6) | Randomized | 25–75 | 6 days pre-operative/10 days postoperative | 161: 80 control group, 81 probiotic group | Lactobacillus plantarum (≥1011 FCU/g); lactobacillus acidophilus (≥7.0×1010 FCU/g); bifidobacterium longum (≥5.0×1010 FCU/g) | 2 g/days of probiotic (2.6×1014 FCU); placebo: maltodextrin capsules daily |
| (19) | Randomized | 20–80 | 7 days pre-operative/5 to 10 days postoperative | 195: 95 control group, 100 probiotic group | Bifidobacterium bifidum (10 billion viable cells to nine tablets) | – |
| (2) | Randomized, double-blind | 25–75 | 6 days pre-operative/10 days postoperative | 134: 68 control group, 66 probiotic group | Lactobacillus plantarum (≥1011 FCU/g); lactobacillus acidophilus (≥7.0×1010 FCU/g); bifidobacterium longum (≥5.0×1010 FCU/g) | 2 g/days of probiotic (2.6×1014 FCU); placebo: maltodextrin capsules daily |
| (6) | – | – | Administration 3 to 15 days before surgery | 156: 81 control group, 75 probiotic group | 2 mg de Enterococcus faecalis T110; 10 mg de Clostridium butyricum TO-A; 10 mg de Bacillus mesentericus TO-A | 6 Bio-TREE® tablets daily |
| (18) | Randomized | – | 12 consecutive days: 5 days preoperative/7 days postoperatively | 60: 30 control group, 30 probiotic group | Bifidobacterium longum (≥1.0×107 UFC/g); enterococcus faecalis (≥1.0×107 FCU/g); lactobacillus acidophilus (≥1.0×107 FCU/g) | Placebo: maltodextrin and sucrose |
Liu et al. (5) observed significant statistical results for patients submitted to probiotic intervention [OR =0.2663 (95% CI, 0.08783–0.8074, P=0.0194)]. Thus, 16% of the probiotic-treated group presented pathogenic bacteria in the blood compared to 30.6% in the control group. After administration of probiotics, Yang et al. (18) verified 10% of pathogenic bacteria in the blood of patients, in contrast to 30% in the control group, with OR =0.2593 (95% CI, 0.06231–1.0787, P=0.0635).
Infectious complications
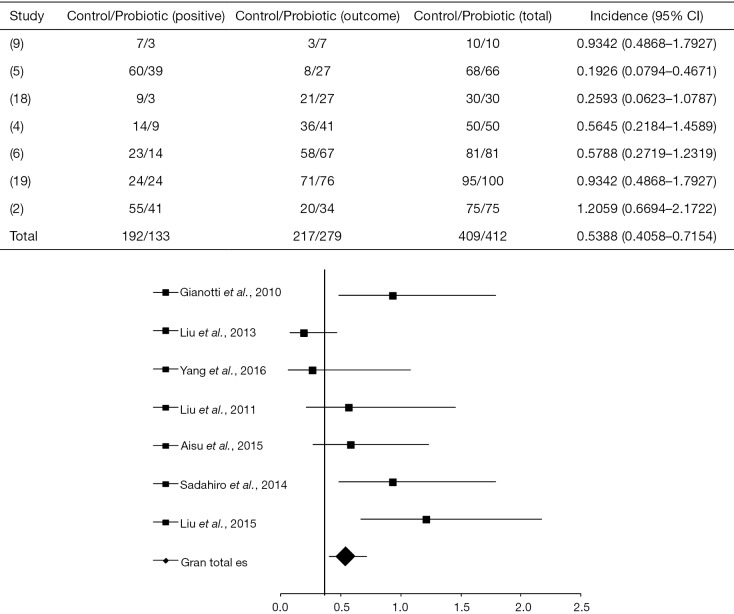
For this session, seven articles were analyzed (2,4-6,9,18,19) from which only two (5,18), after crossing data, presented a statistically positive effect of probiotics in the control of infections (as shown in the forest plot in Figure 4), with OR =0.5388 (95% CI, 0.4058–0.7154, P<0.0001) and, 6 I2=28% and 14.3% (DF=25, P=0.2338).
Figure 4.
Effect of probiotics to minimize complications of infection “Forest plot” compared to the control group in the pre and postoperative treatment in accordance with the primary objective of the clinical trials in patients with CRC (2,4-6,9,18,19). CRC, colorectal cancer.
In the study by Liu et al. (5), the administration of probiotics provided OR =0.1926 (95% CI, 0.07941 to 0.4671, P=0.0003) and had a statistically significant intervention when compared to the control group, which showed 88% of complications in surgical infection. At the same time, Yang et al. (18) showed a benefic intervention of probiotics in preventing infections, where 10% of the treated patients developed infectious complications, in contrast to 30% in the control group OR =0.2593 (95% CI, 0.06231–1.0787, P=0.0635).
Discussion
He et al. (20) found that using probiotic or symbiotic in the perioperative period in patients undergoing colorectal resection may improve clinical outcomes, although they discuss that the need for preventive strategies should still be considered in future meta-analyzes. For this purpose, the present study evaluated the prophylactic and therapeutic effects of probiotics on patients with CRC in the pre- and postoperative, and at the same time searched for preventive strategies from recent experimental models.
A large number of studies have shown that probiotics can prevent the initiation or development of cancer by changing the intestinal microbial composition, host protection against pathogenic bacteria and fungi, the production of biological substances such as short-chain fatty acid and conjugated linoleic acid, inactivation of carcinogenic compounds, improvement of gut barrier function, modulation of immune responses, apoptosis, anti-proliferative effects and antioxidant activity (21-23).
According to Zhu et al. (24) some strains of bacteria such as Lactobacillus acidophillus and Bifidobacterium longum are associated with the inhibition of carcinogenic induction and the development of CRC. At the same time, Liu et al. (5) and Yang et al. (18) observed better outcomes by using these microorganisms in the therapeutic intervention for patients with CRC. Perioperative administration of probiotics reduces the inflammatory response, which can lead to a reduction in length of hospital stay. Moreover, it is reported that the microbial imbalance induced by surgical stress and CRC can be readily improved by treatment with probiotics (6).
According to Tiptiri-Kourpeti (25), several strains of Lactobacillus have anti-proliferative effects and in vivo animal studies indicate that Lactobacillus can reduce the risk of certain cancers and have anti-carcinogenic properties. The known anti-mutagenic effects of Bifidobacterium have highlighted its use for reducing toxic levels of pro-carcinogenic enzymes CRC. Microbial metabolites such as propionates and butyrate are known to exert an antagonistic effect, especially on the proliferation of colon cancer cells, cancer cell apoptosis induction and cell lines transformed in humans (26).
To reduce surgical complications, Okazaki et al. (27) administered Lactobacillus casei and Bifidobacterium breve and obtained 12% of surgical complications compared to 36% in the control group. Tanaka et al. (28) evaluated the impact of the administration of 1×108 CFU Lactobacillus casei and Bifidobacterium breve in the perioperative period in patients with esophageal cancer and found that the incidence of infection tends to be lower in the probiotic group than in the control group, 10% and 29.4%, respectively.
Pitsouni et al. (29) reported that the use of probiotics and symbiotics in patients submitted to CRC surgery is a very promising clinical measure for the prevention of postoperative infectious complications. However, it appears that the outcome of the treatment depends on the genus of bacteria, the number of bacteria administered and the number of viable probiotic bacteria that reach and colonize the colon (30).
Postoperative infections occur despite the use of aggressive prophylaxis perioperative with powerful antibiotics, and under parenteral nutrition, in approximately 30% of patients undergoing abdominal surgery, leading to significant prolongation of hospital stay and increased costs (31). According to Pitsouni et al. (29) the duration of the hospital stay and of the antibiotic therapy in patients who received probiotics/symbiotics was significantly reduced. That is important because shorter antibiotic regimens lessen the risk of emerging antibiotic resistance. In addition, a shorter hospital stay can further reduce the risk of hospital infections and the financial expenses. However, it is already reported that the combination of preoperative and symbiotic postoperative therapies was more effective than just the post-operative therapy itself (32).
According to Okazaki et al. (27), the exclusive administration of probiotics is not enough to improve the intestinal microbiota, but the use of symbiotics leads to a synergistic effect on its growth and functions. Symbiotics have been suggested to reduce and eliminate potentially pathogenic microorganisms in the perioperative period, stimulating the growth of commensal microbiota, and subsequently increasing short chain fatty acids, which are known to stabilize the intestinal barrier and the local immune system, which can also contribute to a reduction in the incidence of surgical infections.
Following Yang et al. (18), the administration of probiotics during the perioperative period significantly influenced the recovery of bowel function and this may be of great clinical value in reducing infectious complications such as gut-origin sepsis. It has been reported that the use of probiotics can reduce perioperative and postoperative sepsis rate caused by the reduced concentrations of zonulin protein in patients undergoing surgical procedure (5).
Zonulin is able to modulate the mucosal barrier by disassembling the intercellular junctions that characterize the early phase of inflammatory states. This protein is involved in the innate immunity of the gut and its dysregulation may trigger the pathogenesis of different diseases (33). Zonulin selectively increases the intestinal permeability, and its release is induced by certain intestinal microorganisms, especially pathogens, indicating that the protein could act as a mechanical link between changes in the intestinal microbiota and intestinal barrier function (34).
The reduction of circulating zonulin is associated with a decrease of the lactulose/mannitol, an indicator of intestinal permeability, as well as less postoperative infectious complications (34). Concentrations of this protein in the postoperative period may be an early marker of sepsis. Liu et al. (5) recommended the combination of preoperative oral intake of probiotics with continued therapy in postoperative patients who need to undergo colorectal surgery.
Conclusions
The use of probiotics is promising for the prevention of infections in patients undergoing CRC surgery. It was found that the combination of more than one micro-organism such as Lactobacillus and Bifidobacterium improves the treatment and surgical recovery. Moreover, the administration of probiotics should be associated with the pre and post-operative treatments in order to intensify its beneficial effects.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Desrouillères K, Millette M, Dang Vu K, et al. Cancer preventive effects of a specific probiotic fermented milk containing Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 on male F344 rats treated with 1,2-dimethylhydrazine. J Funct Foods 2015;17:816-27. 10.1016/j.jff.2015.06.035 [DOI] [Google Scholar]
- 2.Liu Z, Li C, Huang M, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol 2015;15:34. 10.1186/s12876-015-0260-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addae JK, Gani F, Fang SY, et al. A comparison of trends in operative approach and postoperative outcomes for colorectal cancer surgery. J Surg Res 2017;208:111-20. 10.1016/j.jss.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Qin H, Yang Z, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post-operative infectious complications in colorectal cancer surgery-a double-blind study. Aliment Pharmacol Ther 2011;33:50-63. 10.1111/j.1365-2036.2010.04492.x [DOI] [PubMed] [Google Scholar]
- 5.Liu ZH, Huang MJ, Zhang XW, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr 2013;97:117-26. 10.3945/ajcn.112.040949 [DOI] [PubMed] [Google Scholar]
- 6.Aisu N, Tanimura S, Yamashita Y, et al. Impact of perioperative probiotic treatment for surgical site infections in patients with colorectal cancer. Exp Ther Med 2015;10:966-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JY, Chu SH, Jeon JY, et al. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis 2014;46:1126-32. 10.1016/j.dld.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 8.Eguchi S, Takatsuki M, Hidaka M, et al. Perioperative synbiotic treatment to prevent infectious complications in patients after elective living donor liver transplantation: a prospective randomized study. Am J Surg 2011;201:498-502. 10.1016/j.amjsurg.2010.02.013 [DOI] [PubMed] [Google Scholar]
- 9.Gianotti L, Morelli L, Galbiati F, et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J Gastroenterol 2010;16:167-75. 10.3748/wjg.v16.i2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol 2013;11:227-38. [DOI] [PubMed] [Google Scholar]
- 11.Braga M, Gianotti L, Gentilini O, et al. Early postoperative enteral nutrition improves gut oxygenation and reduces costs compared with total parenteral nutrition. Crit Care Med 2001;29:242-8. 10.1097/00003246-200102000-00003 [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Saúde M. Diretrizes metodológicas: elaboração de revisão sistemática e metanálise de ensaios clínicos randomizados. Brasília: Ministério da Saúde 2012. [Google Scholar]
- 14.Altman DG, Deeks JJ, Sackett DL. Odds ratios should be avoided when events are common. BMJ 1998;317:1318. 10.1136/bmj.317.7168.1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues CL, Ziegelmann PK. Metanálise: um guia prático. Rev HCPA 2010;30:435-46. [Google Scholar]
- 16.Baglioni C, Nissen C, Schweinoch A, et al. Polysomnographic characteristics of sleep in stroke : a systematic review and meta-analysis. PLoS One 2016;11:e0148496. 10.1371/journal.pone.0148496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Xia Y, Chen H, et al. The effect of perioperative probiotics treatment for colorectal cancer: short-term outcomes of a randomized controlled trial. Oncotarget 2016;7:8432-40. 10.18632/oncotarget.7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadahiro S, Suzuki T, Tanaka A, et al. Comparison between oral antibiotics and probiotics as bowel preparation for elective colon cancer surgery to prevent infection: Prospective randomized trial. Surgery 2014;155:493-503. 10.1016/j.surg.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 20.He D, Wang HY, Feng JY, et al. Use of pro-/synbiotics as prophylaxis in patients undergoing colorectal resection for cancer: a meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol 2013;37:406-15. 10.1016/j.clinre.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 21.Chen CC, Lin WC, Kong MS, et al. Oral inoculation of probiotics Lactobacillus acidophilus NCFM suppresses tumour growth both in segmental orthotopic colon cancer and extra-intestinal tissue. Br J Nutr 2012;107:1623-34. 10.1017/S0007114511004934 [DOI] [PubMed] [Google Scholar]
- 22.Uccello M, Malaguarnera G, Basile F, et al. Potential role of probiotics on colorectal cancer prevention. BMC Surg 2012;12 Suppl 1:S35. 10.1186/1471-2482-12-S1-S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faghfoori Z, GArgari BP, Gharamaleki AS, et al. Cellular and molecular mechanisms of probiotics effects on colorectal cancer. J Funct Foods 2015;18:463-72. 10.1016/j.jff.2015.08.013 [DOI] [Google Scholar]
- 24.Zhu Q, Gao R, Wu W, et al. The role of gut microbiota in the pathogenesis of colorectal cancer. Tumour Biol 2013;34:1285-300. 10.1007/s13277-013-0684-4 [DOI] [PubMed] [Google Scholar]
- 25.Tiptiri-Kourpeti A, Spyridopoulou K, Santarmaki V, et al. Lactobacillus casei xerts anti-Proliferative effects accompanied by apoptotic cell death and up-regulation of trail in colon carcinoma cells. Plos One 2016;11:e0147960. 10.1371/journal.pone.0147960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar A, Mandal S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol Res 2016;192:159-71. 10.1016/j.micres.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 27.Okazaki M, Matsukuma S, Suto R, et al. Perioperative synbiotic therapy in elderly patients undergoing gastroenterological surgery: a prospective, randomized control trial. Nutrition 2013;29:1224-30. 10.1016/j.nut.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K, Yano M, Motoori M, et al. Impact of perioperative administration of synbiotics in patients with esophageal cancer undergoing esophagectomy: a prospective randomized controlled trial. Surgery 2012;152:832-42. 10.1016/j.surg.2012.02.021 [DOI] [PubMed] [Google Scholar]
- 29.Pitsouni E, Alexiou V, Saridakis V, et al. Does the use of probiotics/synbiotics prevent postoperative infections in patients undergoing abdominal surgery? A meta-analysis of randomized controlled trials. Eur J Clin Pharmacol 2009;65:561-70. 10.1007/s00228-009-0642-7 [DOI] [PubMed] [Google Scholar]
- 30.Horvat M, Krebs B, Potrc S, et al. Preoperative synbiotic bowel conditioning for elective colorectal surgery. Wien Klin Wochenschr 2010;122 Suppl 2:26-30. 10.1007/s00508-010-1347-8 [DOI] [PubMed] [Google Scholar]
- 31.Rayes N, Seehofer D, Hansen S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002;74:123-7. 10.1097/00007890-200207150-00021 [DOI] [PubMed] [Google Scholar]
- 32.Sugawara G, Nagino M, Nishio H, et al. Perioperative synbiotic treatment to prevent post-operative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 2006;244:706-14. 10.1097/01.sla.0000219039.20924.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russo F, Linsalata M, Clemente C, et al. Inulin-enriched pasta improves intestinal permeability and modifies the circulating levels of zonulin and glucagon-like peptide 2 in healthy young volunteers. Nutr Res 2012;32:940-6. 10.1016/j.nutres.2012.09.010 [DOI] [PubMed] [Google Scholar]
- 34.Stenman LK, Lehtinen MJ, Meland N, et al. Probiotic with or without fiber controls body fatmass, associated with serum zonulin, in overweight and obese adults-randomized controlled trial. EBioMedicine 2016;13:190-200. 10.1016/j.ebiom.2016.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]