Apoptosis and necroptosis are two types of programmed cell death with distinct morphological features. Necroptosis has been assumed to be more inflammatory than apoptosis, but a recent paper1 concluded that necroptotic cells cannot elicit cross-priming of CD8+ T cells and that NF-κB activation in necroptotic cells is required for maximal CD8+ T-cell cross-priming. In contrast, our data have shown that necroptotic cells are sufficient to elicit robust CD8+ T-cell cross-priming and that NF-κB activation is not required for necroptotic cell-mediated CD8+ T-cell cross-priming. We have concluded that compared with apoptosis, necroptosis is a much more potent promoter of CD8+ T-cell cross-priming.
Necroptosis is a form of programmed cell death with a different morphology and regulatory mechanism from those of the best-characterized form of programmed cell death, apoptosis. Apoptosis is caspase dependent, and its typical morphological features include nuclear condensation, shrinkage and fragmentation of cells into membrane-bound apoptotic bodies. Unlike apoptosis, necroptosis, also called necrosis, is a lytic cell death program. Both apoptosis and necroptosis can be induced by tumor necrosis factor-α (TNF). Upon TNF treatment, TNF receptor 1 trimerizes and recruits several effector proteins, including receptor-interacting protein (RIP) 1, which interacts with caspase-8 via FADD and initiates caspase cascade-mediated apoptosis. However, when cells have a high concentration of RIP3, RIP1 can interact with RIP3 and trigger a necrosis pathway, now termed necroptosis. The RIP1–RIP3 complex, also known as the necrosome (ripoptosome), triggers phosphorylation and activation of an effector pseudokinase, mixed lineage kinase domain-like (MLKL). Upon activation, MLKL multimerizes and translocates to the cell membrane, where it induces cation influx, leading to cell swelling and rupture.2 Oligomerization is a common mechanism to activate cell death mediator proteins. Accordingly, inducible dimerization systems based on FK506 binding domain F36V mutant (Fv) or estrogen-induced homodimerization of hormone-binding domain G521R mutant (HBD*) have been successfully used to initiate apoptosis and necroptosis by inducing caspase-8 or RIP3 oligomerization, respectively.3, 4 MCA205 cells stably transfected with Fv-caspase-8, Fv-RIP3, or Fv-RIP3 RHIM-AAA (QIG449-451AAA mutant) expression vectors (Supplementary Figure S1a) underwent cell death upon AP20187 treatment. Regarding the forms of cell death, caspase-8 oligomerization-induced apoptosis or RIP3 oligomerization-induced necroptosis can be distinguished by Annexin V staining and membrane permeabilization of propidium iodide (PI) (Supplementary Figure S1b), as well as the cleavage of caspase-3/PARP and phosphorylation of MLKL (Supplementary Figure S1c). Apoptosis does not induce inflammation because the cell contents are restricted within the intact plasma membrane during this process. Conversely, necroptosis is lytic cell death and is assumed to stimulate immune responses. Indeed, compared with apoptotic cells, necroptotic MCA205 cells released more damage-associated molecular patterns (DAMPs), such as ATP and chromatin-binding protein high-mobility group B1 (HMGB-1) (Supplementary Figure S1d and e), and promoted more DC maturation (Supplementary Figure S1f) and cross-presentation to CD8+ T cells (Supplementary Figure S1g and h), which results in T-cell-mediated cytotoxicity (Supplementary Figure S1i and j). However, necroptosis may not be more immunogenic than apoptosis in all aspects because unlike apoptosis, there is no surface exposure of calreticulin in necroptotic cells (Supplementary Figure S1k). While our study was under way, a report stated that necroptotic cells were not sufficient for CD8+ T-cell cross-priming.1 Using an approach similar to that used in our study to induce oligomerization of RIP3 and caspase-8, the authors showed that reverse signaling from RIP3 to RIP1 via RHIM domain interaction initiated RIP1-dependent NF-κB activation, which is required for dying cells to elicit maximal CD8+ T-cell cross-priming. The major evidence for this conclusion is that necroptotic cells induced by full-length RIP3 oligomerization but not by a RIP3 C-terminal RHIM domain deleted mutant can elicit cross-priming. We used a RIP3 RHIM-AAA mutant but, intriguingly, did not observe any difference between the mutant and wild-type RIP3 in the induction of ATP and HMGB-1 release, DC maturation, CD8+ T-cell cross-priming and T-cell-mediated cytotoxicity (Supplementary Figure S1d–j). We further analyzed NF-κB activation and IL-6 secretion but did not find any difference between RIP3 and RIP3 RHIM-AAA mutant-mediated necroptotic cells (Supplementary Figure S2a and b).
The discrepancy between the published report and our study could be because the published study used a C-terminal Fv fusion RIP3ΔC mutant and NIH3T3 cells, whereas we used an N-terminal Fv fusion RHIM-AAA mutant and MCA205 cells. To address this possibility, we used the same RIP3-ΔC mutant that was used in the published study, fused Fv to the C-terminal of RIP3 and its mutant (Figure 1a), and stably expressed each individual construct in NIH3T3 cells. In addition, we also included the necroptosis of NIH3T3 cells induced by MLKL N-terminal domain oligomerization (Figure 1a).
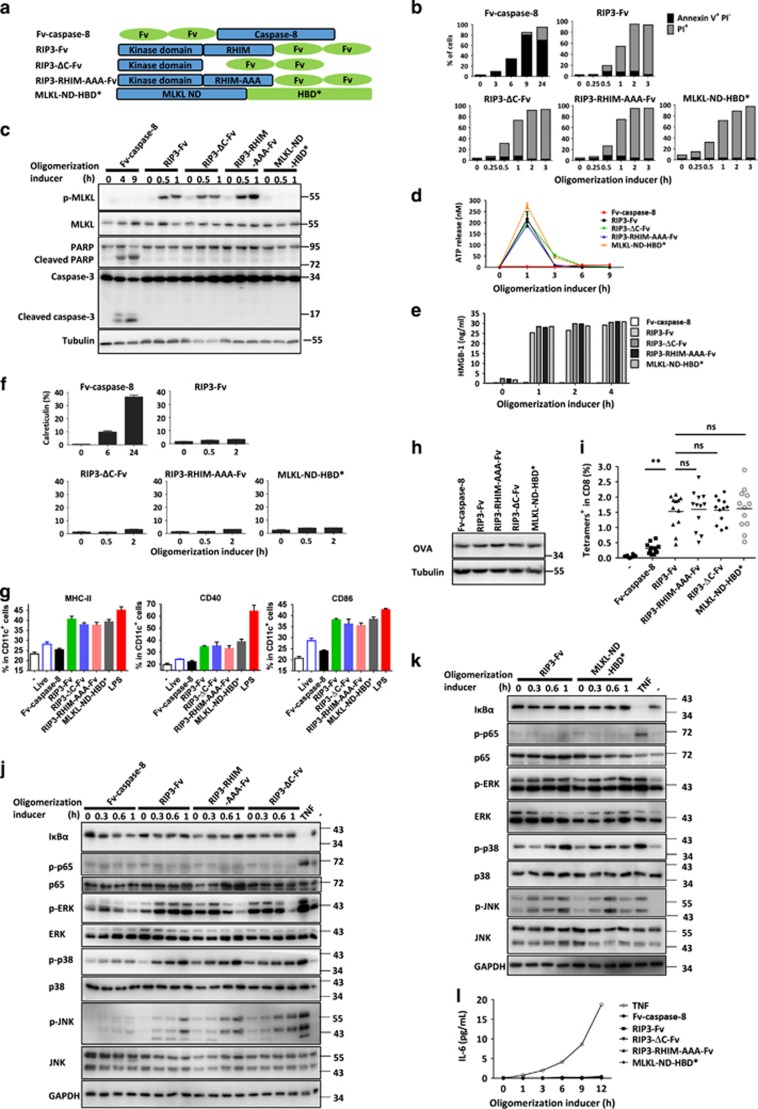
Figure 1.
RIP3–RIP1–NF-κB signaling is not required for the efficient cross-priming of CD8+ T cells by necroptotic cells. (a) Schematic representation of oligomerizable caspase-8 protease domain, wild-type RIP3, RHIM deleted RIP3, RHIM-AAA mutant RIP3 and MLKL N domain. Fv (FK506 binding domain F36V mutant) and HBD* (hormone-binding domain G521R mutant) are dimerization domains. (b) NIH3T3 cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv or MLKL-ND-HBD* were collected after an oligomerization inducer (AP20187, 50 nM; or 4-OHT, 1 μM) induced oligomerization of the corresponding fusion protein in each cell line for different time periods and were stained with Annexin V and PI and analyzed by flow cytometry. (c) The cells in b were treated the same as those in b. Cell lysates were collected and separated by SDS-PAGE and immunoblotted for the detection of phospho-MLKL, MLKL, poly (ADP-ribose) polymerase (PARP) and caspase-3. Tubulin served as the loading control. Phospho-MLKL, Ser345 phosphorylated MLKL. (d, e) The levels of ATP and HMGB-1 in the culture media of NIH3T3 cells were measured after oligomerization of the indicated fusion proteins. (f) Exposure levels of calreticulin in NIH3T3 cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv or MLKL-ND-HBD* after oligomerization of the indicated fusion proteins for different time periods. (g) BMDCs were co-cultured with oligomerization inducer-treated NIH3T3 cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv or MLKL-ND-HBD* for 24 h. Cell surface expression of MHCII, CD40 and CD86 on DCs were analyzed by FACS. BMDCs treated with LPS served as positive controls. (h) Western blot analyses of OVA expression in NIH3T3-OVA cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv or MLKL-ND-HBD*. Tubulin served as the loading control. (i) C57BL/6 mice were immunized with oligomerization inducer-treated NIH3T3-OVA cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv or MLKL-ND-HBD* nine days later, the frequency of OVA-specific (Tetramer+) CD8+ T cells was determined. Each dot corresponds to an individual mouse. (j) Western blot analyses of MAPKs and NF-κB activation in NIH3T3-OVA cells expressing Fv-caspase-8, RIP3-Fv, RIP3-ΔC-Fv, RIP3-RHIM-AAA-Fv after oligomerization. NIH3T3-OVA cells treated with TNF served as a positive control. (k) Western blot analyses of MAPKs and NF-κB activation in NIH3T3-OVA cells expressing RIP3-Fv, MLKL-ND-HBD* after oligomerization. NIH3T3-OVA cells treated with TNF served as a positive control. (l) Secretion of IL-6 was measured in the cells described in b using an enzyme linked immunosorbent assay (ELISA) detection kit. NIH3T3-OVA cells treated with TNF served as a positive control. All the abovementioned data are represented as the mean±s.e.m. of three independent experiments. Statistical analysis was conducted using Kruskal–Wallis test and Dunn’s post-test. **P<0.01; ns, no significant difference.
As expected, AP20187-induced oligomerization of Fv-caspase-8 and RIP3-Fv or their mutants triggered apoptosis and necroptosis, respectively (Figures 1b and c), and 4-hydroxytamoxifen (4-OHT)-induced oligomerization of MLKL-ND-HBD* led to necroptosis (Figures 1b and c). Necroptosis released much more ATP and HMGB-1 than apoptosis, regardless of whether RIP3 was in the wild-type, RHIM-AAA mutated, or C-terminal truncated form (Figures 1d and e). MLKL oligomerization-mediated necroptosis showed similar ATP and HMGB-1 release to that mediated by RIP3 oligomerization (Figures 1d and e). Moreover, surface exposure of calreticulin occurred only in caspase-8-mediated apoptotic cells but not in necroptotic cells induced by the other molecules (Figure 1f). We next examined the effects of these forms of cell death on DC maturation by co-culturing the dying NIH3T3 cells with primary bone marrow-derived dendritic cells (BMDCs). Dying RIP3-Fv-, RIP3-ΔC-Fv-, RIP3-RHIM-AAA-Fv- and MLKL-ND-HBD*-expressing cells, but not the same amount of dying Fv-caspase-8-expressing cells or live cells, induced a high degree of MHC-II and co-stimulatory molecules CD40 and CD86 expression on DCs (Figure 1g). However, no difference in the induction of DC maturation was observed among the necroptotic cells induced by oligomerized RIP3, RIP3-ΔC-Fv, RIP3-RHIM-AAA, or MLKL N domain (Figure 1g). To further examine the role of necroptosis in CD8+ T-cell cross-priming, we used a cross-priming model that relies on the intrinsic immunogenicity of dying cells to induce CTL responses to a cell-associated model antigen. For this experiment, the cell lines were transduced to express a truncated and non-secreted OVA protein (Figure 1h). We then immunized C57BL/6 mice with OVA-expressing dying cells and measured OVA peptide-specific CD8+ T cells by tetramer staining 9 days later. Dying cells expressing RIP3-Fv, RIP3-ΔC-Fv and RIP3-mut-Fv but not Fv-caspase-8 induced robust antigen-specific CD8+ T-cell responses (Figure 1i). Again, no difference was found among the necroptotic NIH3T3 cells induced by RIP3, RIP3-RHIM-AAA and RIP3-ΔC oligomerization. We also expressed MLKL-ND-HBD* in NIH3T3 cells, a cell line that lacks endogenous RIP3 expression and is therefore unable to form the ripoptosome-like complex. Compared with RIP3-Fv-expressing dying cells, MLKL-ND-HBD*-expressing dying cells have similar effects on the induction of CD8+ T-cell responses (Figure 1i). Taken together, these results indicate that necroptotic cells can efficiently induce cross-presentation of dying-cell-associated antigens, and ripoptosome-like complex formation was not required for necroptotic cells to induce cross-priming. We also examined activation of mitogen-activated protein kinases (ERK, JNK and p38) and NF-κB in dying cells induced by oligomerization of caspase-8, RIP3, RIP3-RHIM-AAA, RIP3-ΔC or MLKL-ND, and found that all were indistinguishable in their degradation of IκBα and phosphorylation of p65, p38, JNK and ERK (Figures 1j and k). We also did not detect IL-6 in the supernatants of the dying cells (Figure 1l). Collectively, our data do not support the conclusion that RIP1 and NF-κB signaling in dying cells determines cross-priming of CD8+ T cells.
Reverse signaling from RIP3 to RIP1 was proposed when RIP1-dependent apoptosis was observed in cells expressing the D161 to N mutant of RIP35 or when the RIP3 inhibitor GSK’872 was applied.6 The reversal of the signaling from necroptosis to apoptosis was thought to be due to loss/inhibition of RIP3 kinase activity, but another kinase-dead mutant of RIP3 (51 K to A) did not reverse the signaling to apoptosis and only lost its function in necroptosis.6 Whether the reversed signaling from RIP3 can activate the NF-κB pathway via RIP1 was not reported until Yatim et al.1 reported that this signaling pathway in necroptotic cells determines the cross-priming of CD8+ T cells. Activation of the NF-κB pathway by overexpression of RIP3 has been reported,5 which supports the idea that RIP3–RIP1–NF-κB signaling exists. However, this signaling pathway is clearly not present in our experimental systems. The conflicting results cannot be due to differences between the methods or cell lines used in previously published studies and our experiments. Nonetheless, the possibility that these conflicting results were caused by different cell conditions, animal hosts or other issues related to the experiments cannot be excluded. For example, RIP1-mediated NF-κB activation requires RIP1 ubiquitination, and the expression level of the proteins related to RIP1 ubiquitination may determine the activation of RIP3-RIP1-NF-κB signaling. Regardless of whether RIP3–RIP1–NF-κB signaling exists, our data demonstrated that this signaling is not required for the efficient cross-priming of CD8+ T cells by necroptotic dying cells. Although it does not address the exact same issue, a study by Aaes et al.7 of vaccination with necroptotic cells also showed that the immunogenicity of necroptotic cells does not correlate with the extent of NF-κB activation.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91029304, 31420103910, 31330047 and 81630042), the National Basic Research Program of China (973 Program; 2015CB553800, 2013CB944903 and 2014CB541804), the 111 Project (B12001), and the National Science Foundation of China for Fostering Talents in Basic Research (J1310027).
Footnotes
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website (http://www.nature.com/cmi)
The authors declare no conflict of interest.
Supplementary Material
References
- Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reis e Sousa C et al. RIPK1 and NF-kappaB signaling in dying cells determines cross-priming of CD8(+) T cells. Science 2015; 350: 328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Li W, Ren J, Huang D, He WT, Song Y et al. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res 2014; 24: 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Chang HY, Baltimore D. Autoproteolytic activation of pro-caspases by oligomerization. Mol Cell 1998; 1: 319–325. [DOI] [PubMed] [Google Scholar]
- Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y et al. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ 2014; 21: 1709–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazdernik NJ, Donner DB, Goebl MG, Harrington MA. Mouse receptor interacting protein 3 does not contain a caspase-recruiting or a death domain but induces apoptosis and activates NF-kappaB. Mol Cell Biol 1999; 19: 6500–6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal P, Berger SB, Pillay S, Moriwaki K, Huang C, Guo H et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol Cell 2014; 56: 481–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep 2016; 15: 274–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.