Abstract
Background: Rehydration strategies in children with severe acute malnutrition (SAM) and severe dehydration are extremely cautious. The World Health Organization (WHO) SAM guidelines advise strongly against intravenous fluids unless the child is shocked or severely dehydrated and unable to tolerate oral fluids. Otherwise, guidelines recommend oral or nasogastric rehydration using low sodium oral rehydration solutions. There is limited evidence to support these recommendations.
Methods: We conducted a systematic review of randomised controlled trials (RCTs) and observational studies on 15 th June 2017 comparing different strategies of rehydration therapy in children with acute gastroenteritis and severe dehydration, specifically relating to intravenous rehydration, using standard search terms. Two authors assessed papers for inclusion. The primary endpoint was evidence of fluid overload.
Results: Four studies were identified, all published in English, including 883 children, all of which were conducted in low resource settings. Two were randomised controlled trials and two observational cohort studies, one incorporated assessment of myocardial and haemodynamic function. There was no evidence of fluid overload or other fluid-related adverse events, including children managed on more liberal rehydration protocols. Mortality was high overall, and particularly in children with shock managed on WHO recommendations (day-28 mortality 82%). There was no difference in safety outcomes when different rates of intravenous rehydration were compared.
Conclusions: The current ‘strong recommendations’ for conservative rehydration of children with SAM are not based on emerging evidence. We found no clinical trials providing a direct assessment of the current WHO guidelines, and those that were available suggested that these children have a high mortality and remain fluid depleted on current therapy. Recent studies have reported no evidence of fluid overload or heart failure with more liberal rehydration regimens. Clinical trials are urgently required to inform guidelines on routes and rates of intravenous rehydration therapy for dehydration in children with SAM.
Keywords: malnutrition, gastroenteritis, dehydration, rehydration, systematic review, Africa, Asia
Abbreviations
AGE, acute gastroenteritis; BNP, B type natriuretic peptide; CI, confidence interval; ED, Emergency Department; FEAST, Fluid As A Supportive Therapy; GEMS, Global Enteric Multi-Centre Study; HSD/D5, Half Strength Darrow’s and 5% dextrose; HR, Heart Rate; IV, Intravenous; ORS, oral rehydration solution; RCT, randomised controlled trial; ReSoMal, Rehydration Solution for Malnutrition; RL, Ringers Lactate; SAM, severe acute malnutrition; WHO, World Health Organization.
Introduction
Severe acute malnutrition (SAM) is directly responsible for over 500,000 child deaths per year and plays a significant contributing factor in the deaths of many more 1. The World Health Organization (WHO) recommends ten key steps for inpatient management of complicated SAM, and suggest that strict adherence to this protocol should reduce mortality to less than 10% 2, 3. With regard to cases complicated by diarrhoea, the 2003 WHO Guidelines for the inpatient treatment of severely malnourished children states that ‘Dehydration, usually resulting from profuse watery diarrhoea (3 or more per day), is often difficult to diagnose in malnourished children because the clinical signs usually relied on to diagnose dehydration are similar to those found in severe wasting without dehydration 2.’ Nevertheless, a prospective study of unselected Kenyan children hospitalised with SAM published in 2012 showed that diarrhoea (defined as three or more watery stools) complicated 49% of cases at admissions to hospital and developed in another 16% following admission with case fatalities of 21% and 18% respectively compared with 12% in cases without diarrhoea (χ 2 = 17.6 p<0.001) 4, 5. Furthermore, whilst the guidelines indicate that signs of severe dehydration (sunken eyes and decreased skin turgor) in SAM are unreliable markers of hydration status (and challenges arise with recognition of dehydration in children with kwashiorkor) the Kilifi study showed that signs of severe dehydration were more common in those with diarrhoea (35% versus 8%) and was a risk factor for mortality (crude odds ratio 1.7; 95% confidence interval (CI) 1.1, 2.6; p = 0.012). In the multivariate model, bacteraemia (odds ratio 6.7 (95% CI 2.5-17.8 p<0.001) and hyponatraemia (odds ratio 4.9 (95% CI 2.2-11.1 p<0.001) were key risk factors for mortality 5.
Fluids are the mainstay of treatment for dehydration; however, WHO rehydration strategies in severely malnourished children are extremely cautious. The guidelines advise oral or nasogastric rehydration using ReSoMal (rehydration solution for malnutrition, a hypo-osmolar solution with lower sodium and higher levels of potassium than standard oral rehydration solution) and strongly recommends against the use of intravenous fluids unless the child is shocked. For the shocked child (or child with severe dehydration who is unable to tolerate oral fluids), WHO guidelines recommend 15mls/Kg of intravenous fluid over 1 hour (repeated once if necessary), and a 10ml/Kg blood transfusion over 3 hours if there is no subsequent improvement ( Table 1) 3, 6. These are based on concerns regarding susceptibility to fluid overload in children with malnutrition and concerns regarding the use of additional sodium. In the section of the 2003 guidelines (under Step 4: Correct Underlying Electrolyte Imbalance/), the recommendations state ‘All severely malnourished children have excess body sodium even though plasma sodium may be low ( giving high sodium loads will kill’) 2. There is no published physiological evidence to support this contention.
Table 1. WHO recommendations for treatment of severely malnourished children with dehydration[2].
| Shock
* and severe dehydration in child unable to tolerate
oral fluid |
No shock | |
|---|---|---|
| Initial | 15ml/Kg Ringers Lactate + 5% dextrose
OR ½ Strength
Darrow’s + 5% dextrose, over 1 hour, repeated once if needed If no improvement: Transfusion 10ml/Kg over 3hours (start 4ml/Kg/hour maintenance while awaiting blood) |
ReSoMal
# Oral/Nasogastric – 5ml/kg
every 30 minutes for first 2 hours |
| Subsequent | Oral/Nasogastric ReSoMal alternating with F75 10ml/Kg/hr up
to 10hrs, and then refeeding with F75. |
Then 5–10ml/kg/hr alternating F75
$
and ReSoMal for 4–10 hours |
*Shock is defined as presence of all three of the following: prolonged capillary refill time (CRT >3s), temperature gradient and weak and fast pulse
#ReSoMal – rehydration solution for malnutrition,
$F75 – primary feeding formula for children with SAM
Researchers observing children with SAM have postulated that this group have compromised cardiovascular function and are therefore susceptible to fluid overload. Therefore, clinical practice has avoided aggressive fluid therapy in this group of children.
WHO guidelines referring to management of children with SAM were revisited in 2013 and remain unchanged with no mention of any of emerging new data 6. The recommendations continue to be based on expert opinion (strong recommendations based on weak level of evidence) rather than clinical evidence. Therefore, we have conducted an independent systematic review of the current available evidence underlying use of intravenous rehydration for children with dehydration and SAM.
Objectives
To conduct a critical appraisal of available evidence on the safety of intravenous (IV) rehydration therapy for treatment of severe dehydration in children with SAM.
Methods
We did not publish a protocol prior to conducting this review. A search of online literature was performed. There were pre-determined criteria, as detailed below for eligibility of studies, data outcomes, and an assessment of risk of bias and study method quality in each of the identified studies. English search terms were used.
Selection criteria
Population. Children aged 0 to 12 years with SAM who had received IV fluids for management of severe dehydration secondary to gastroenteritis or shock. We used the WHO definitions for malnutrition (weight for height (WHZ) <-3, mid-upper arm circumference (MUAC) <115mm or oedema consistent with kwashiorkor), gastroenteritis (three or more loose watery stools per day) and for severe dehydration (presence of two of the following signs: reduced skin turgor, sunken eyes, inability to drink, lethargy or reduced consciousness) 3. We excluded studies with chronic or persistent diarrhoea lasting ≥ 14 days.
Intervention and comparison. All studies that evaluated intravenous fluids were included with a specific focus on safety. Studies were excluded if they considered rehydration in children without malnutrition, or only considered rehydration via the oral or nasogastric route.
Outcome. Clinical studies that reported on any outcomes were included. The primary outcomes for this review were incidence of fluid overload following fluid administration (defined as clinical or echocardiographic evidence of heart failure or pulmonary oedema or the need for diuretics), as an indicator for safety, and urine output in response to rehydration therapy as a marker for efficacy. Secondary outcomes of interest were all-cause mortality, and other assessments of IV fluid safety and frequency of other fluid related adverse events: neurological compromise (defined as any reported seizure activity, altered consciousness or unequal pupils); cardiovascular compromise (defined as any worsening of blood pressure, bradycardia or tachycardia); or/and development or worsening of hyponatraemia (since WHO recommends hypo-osmolar intravenous fluids for resuscitation).
Study design. Randomised-controlled trials (RCTs) and observational studies were included.
Search methods
Online database search. A comprehensive literature search of the following databases was conducted on the 15 th June 2017 using the terms ‘fluid’ AND ‘malnutrition’ AND ‘children’ AND ‘rehydration OR dehydration’:
PubMed/ Medline
Global Health Library (Virtual Health Library)
Cochrane Database of Systematic Reviews
Cochrane Central Register of Controlled Trials
ClinicalTrials.gov
The WHO International Clinical Trials Registry Portal (ICTRP) search portal
Each of the eligible studies was assessed and a manual review of the reference lists carried out. Additionally, a Google search was performed. The search was limited to trials published in English language.
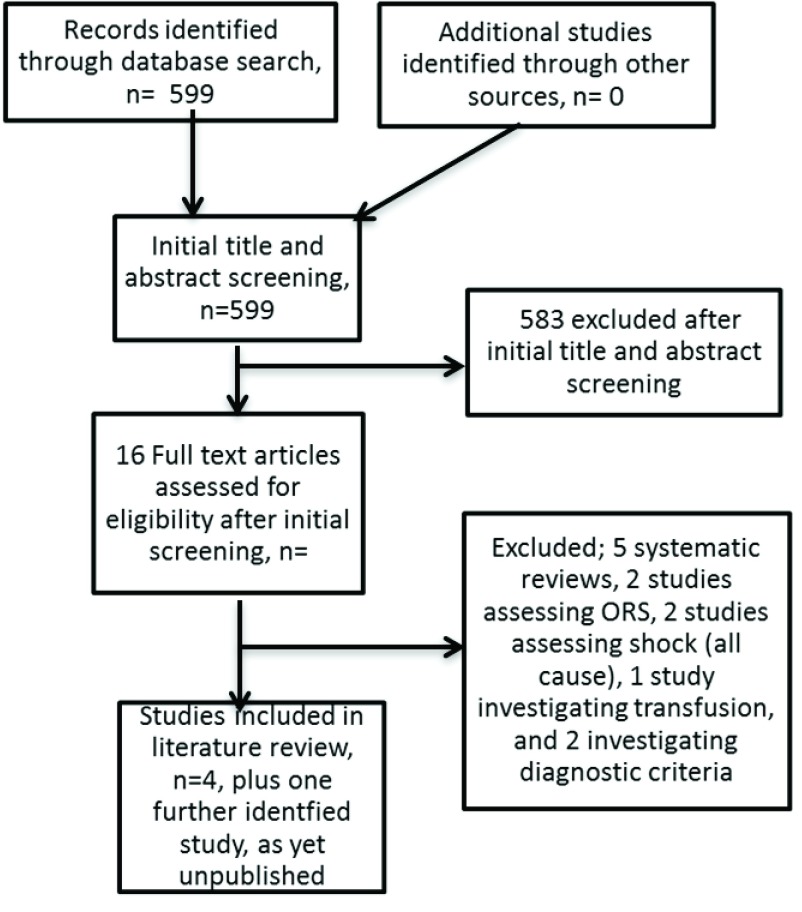
The authors screened the results of the literature search for studies that met the inclusion criteria as determined by the PICOS outline (see Figure 1).
Figure 1. Flow diagram for selection of studies and reasons for study exclusion.
Results
Study selection
The search produced 599 studies (see Figure 1.) After screening and evaluation, four studies were identified that investigate use of intravenous fluid treatment in children with severe malnutrition complicated by dehydration, incorporating a total of 883 patients. All four of these studies were conducted in low resource settings (Kenya 7, 8, Uganda 8 and Bangladesh 9, 10). Two of these studies were randomised controlled trials 7, 10, and two were observational studies 8, 9. Two considered different rates of rehydration 7, 8 and both incorporated serial assessment of haemodynamic responses, urinary output, with one including sequential detailed echocardiographic and electrocardiographic assessment before and after receipt of fluids. The third study evaluated the use of a standardised protocol for management of dehydration 9 and the fourth trial treated all children with severe dehydration with IV fluids (assessing safety) and then randomised children to one of three oral rehydration solutions (ORS) 10. One study included children with cholera only 10 (see Box 1).
Box 1. Management of cholera in children with severe acute malnutrition.
|
WHO Guidelines
(2013) 3 |
The only indication for intravenous infusion in a child with SAM is shock OR a child with severe dehydration and who cannot be rehydrated orally or by nasogastric tube. These children should receive 15ml/Kg/hour of either ½ strength Darrow’s + 5% dextrose OR Ringers lactate +5% dextrose. Children should be monitored every 5–10minutes for signs of over-hydration and congestive heart failure.
If there is no improvement, a blood transfusion (10ml/Kg over at least 3hours) should be given. |
| Evidence for intravenous rehydration | This review includes 266 children with cholera out of a possible total of 802 (33%) from two studies that identified children with cholera (Alam
et al. and Ahmed
et al.)
9,
10. Ahmed did not perform any sub-analyses on children with cholera.
Relevant findings from Alam 2009: 149 (85%) children presented with severe dehydration and required IV rehydration (mean amount of IV fluid required was 103 ml/Kg (95%CI 96-109)) No significant difference in baseline electrolyte abnormalities No children died in this study and no children developed signs of fluid overload No child developed signs of hyponatraemia (not specified) All children were clinically rehydrated within 6 hours although 31% did not pass urine within this period Rice-ORS group had significantly less stool output |
| Implications in practice | There is just one study evaluating safety of intravenous rehydration in children with SAM and cholera. This study rehydrated 149 children with a mean amount of 103ml/Kg of ‘cholera saline’ (sodium 133 mmol/L, potassium 13 mmol/L, chloride 98 mmol/L) and did not report any adverse outcome from this treatment i.e. no fluid overload, no significant difference in dysnatraemia, mortality or fluid related adverse effects.
Guidelines for cholera in children with SAM remain extremely conservative and are at risk of undertreating children. |
There was some heterogeneity in the population eligibility criteria, sample size, and methods employed by each study, and in their results. Table 2 and Table 3 show the setting, methodology and features of the included studies and their results. One further study was identified on clinicaltrials.gov that assessed two rates of IV rehydration in children with SAM. This study was conducted and completed in Bangladesh. The corresponding author was contacted and indicated that the report is currently under review for publication 11.
Table 2.
| Author | Year | Location | Study type | Population | Sample
size |
Inclusion | Exclusion | Comparison | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Obonyo et al.8 | 2017 | Kilifi, Kenya
and Mbale, Uganda |
Prospective
observational |
Children
aged 6–60mths |
20 | Severe malnutrition
(any one of; MUAC <11.5cm, WHZ <-3 or oedema indicative of kwashiorkor, with signs of severe dehydration (>3 watery stools/ 24 hours), and shock (two or more of the WHO criteria) |
Severe dermatitis of
the groin, congenital heart disease, and consent not given |
Group 1: WHO standard
protocol; 15ml/Kg Ringers lactate over 1 hour, with option to repeat once if signs of shock persist, followed by IV ½ strength Darrow’s/5% dextrose given at 4ml/Kg/hour Group 2: 10ml/kg/hour of Ringers lactate over 5 hours |
Clinical,
haemodynamic and echocardiographic data were collected |
| Akech et al.7 | 2010 | Kilifi District
Hospital, Kenya |
RCT | Children
aged >6mths |
61 | Severe acute
malnutrition (WHZ <-3 or WH% 70% or MUAC <11cm or oedema involving at least both feet (kwashiorkor) with evidence of shock (1 or more of: CRT>2 seconds, lower limb temperature gradient, weak pulse volume, deep acidotic breathing, creatinine >80μmol/L, or depressed conscious state. |
Severe anaemia
(<5g/dl), pulmonary oedema, raised intra cranial pressure or known congenital heart disease |
Group 1: Severe dehydration/
shock: randomly assigned to receive either WHO fluid resuscitation regime (HSD/5D 15mls/Kg over 1 hour, then repeat bolus and if no improvement 10mls/Kg blood) OR Ringers lactate (10mls/Kg over 30 mins repeated only twice over 1 hour if features of shock remained Group 2: Presumptive septic shock: randomly assigned to either WHO fluid resuscitation regime OR Ringers lactate OR 5% Human Albumin solution |
Primary outcome
was resolution of features of shock at 8 and 24 hours. Secondary outcomes included incidence of adverse events and mortality |
| Alam et al.10 | 2009 | ICDDR,
B Dhaka Hospital, Bangladesh |
RCT | Children
aged 6 to 60 mths |
175 | Severe malnutrition
(WHZ <70% of NCHS mean, Acute watery diarrhoea of <48hrs duration, presence of V. cholera under microscopy |
Dysentery, severe
infections |
Randomly assigned to receive
one of 3 formulations of ORS with same salt composition, but different substrates i.e. glucose, glucose plus ARS and rice powder. |
Primary outcome
was stool output Secondary outcomes was time to oedema free weight for length of 80% |
| Ahmed et al.9 | 1999 | ICDDR,B
Dhaka Hospital, Bangladesh |
Observational
Cohort study |
Children
aged 0 to 5 years |
627 | Severely malnourished
children, admitted with diarrhoea, whose WFH or WFA was less than 70% and 60% of NCHS median, respectively, or who had oedema |
None specified | Non-protocol group i.e.
children admitted between Jan 1, 1996 and June 30, 1996 (rehydration usually over 3–6hours) and standardised- protocol group (Jan 1, 1997 and June 30, 1997). Standardised protocol included slow rehydration (if severe dehydration given 20ml/Kg for 1 hour then 10ml/Kg for 1 hour, ORS was started after 1 hour) with an emphasis on oral rehydration, immediate feeding of defined diet, routine vitamins/micronutrient supplements, broad spectrum antibiotics |
Mortality rate
Others included transfers to nutritional units, and discharge rate |
MUAC Mid upper arm circumference, WHZ Weight-for-height Z score, CRT Capillary refill time, NCHS US National Centre for Health Statistics,
ICDDR, B International Centre for diarrhoeal disease research, Bangladesh
Table 3.
| Risk of bias | Methodology | Evidence of fluid overload
or cardiac failure and urine output |
other Outcomes (Fluid related adverse events and efficacy of
treatment) |
|
|---|---|---|---|---|
|
Obonyo
et al.,
2017 8 |
Low | Observation of children following WHO
standard protocol (Group 1) was halted after 10 patients were enrolled and investigators were concerned about high mortality rate so 10 patients were prospectively recruited to received 10ml/Kg/hour over 5 hours i.e. without bolus treatment. All children were switched to ReSoMal once able to tolerate oral or NGT fluids. Blood transfusion if Hb <5g/dl. And all other treatments received as per WHO guidelines for severe acute malnutrition. Serial clinical assessments, echocardiographs and ECG’s. |
None of the
children developed echocardiographic or clinical features of over-hydration or cardiac failure. Satisfactory urine output (>1ml/Kg/hr) in 8/11 (73%) in group 1 and 7/9 (78%) in group 2 |
Mortality at 48hours and Day 28 was reported
Group 1: 35% (4 deaths) and 81.8% (9 deaths) respectively Group 2: 44% (4 deaths) and 55.6% (5 deaths) respectively This difference was not statistically significant None of the patients classified as having WHO shock survived Group 1: 4 early deaths (within <24 hours) were all due to cardiovascular collapse secondary to hypovolaemia. 1 child died at 48 hours due to cardiovascular collapse. 4 late deaths occurred (48 hours to 15 days) due to aspiration of feeds and two dying in the community. Group 2: 3 early deaths due to cardiovascular collapse and 1 due to respiratory arrest (severe acidosis and hypoxaemia). Two late deaths occurred at day 4 (1 respiratory arrest associated with metabolic complications and 1 in the community) All fatal events were judged as unrelated to fluid challenges or the IV fluid regime, and largely were secondary to underlying comorbidities Bradycardia at admission (10% of patients) and low systolic blood pressure and persistent weak pulse were associated with early death, the majority within 48hr of admission. |
|
Akech
et al.,
2010 7 |
Low | Randomised 1:1 in strata i.e. severe
dehydration/shock and presumed septic shock. Unblinded trial: fluids received fluid as per randomisation. Continuous haemodynamic and clinical monitoring. Urine output measured. Blood and urine sample series taken. In all other aspects children were treated as per WHO SAM guidelines |
No children developed
clinical features of pulmonary oedema or allergic reaction during the course of study observation Oliguria at 8 hours was present in 9/22 (41%) in HSD/5D arm vs. 3/25 (12%) in RL arm, p=0.0 |
Overall 31/61 (51% died): No significant difference in mortality between
arms. 26/31 (84%) fulfilled WHO malnutrition shock definition at admission. High case fatality in this group irrespective of allocated intervention 39% (12/31) deaths occurred within 24hours of recruitment and 52% within 48hrs) No differences in mean sodium concentration at admission, 8 and 24hours between arms. Resolution of shock: at 8 and 24hours proportion of children with shock in arms was considerable, but not significantly different between groups |
|
Alam
et al.,
2009 10 |
Low | Randomised 1:1:1. Fluid as per
randomisation. Blinding of clinicians and patients. Children with severe dehydration received intravenous ‘cholera saline until recovered from shock or severe dehydration. Estimated fluid deficit was corrected with assigned ORS. Same ORS solution given to match stool losses and purging losses. Children with some dehydration were randomised to receive assigned ORS within 1 hour, and those with severe dehydration within 6hours of admission after IV rehydration. Serial blood samples, stool and urine taken. |
None of the children had or
developed clinical evidence of cardiac failure or fluid overload 31% of all children (including those receiving ORS only) remained oliguric at 6 hours, and 12% at 12 hours |
All of the children were clinical rehydrated within 6hours and 31% did not
pass urine within this period however by 12 hours 88% of the children produced urine and by 24hours all had produced urine. Hypernatraemia or severe hyponatraemia and severe hypo or hyperkalaemia was not observed in any child Groups statistically differed in stool output and in ORS and water intakes. The difference was entirely contributed by rice-ORS group children who had the greatest reduction in stool output and the ORS volume taken was less in the rice-ORS group and water intake greater. There was no statistical difference in duration of cholera. None of the children died in this study |
|
Ahmed
et al.,
1999 9 |
Moderate | Comparison of cohorts receiving two
protocols. Non-protocol i.e. prior to implementation of standardised protocol, received intravenous fluid over 3 to 6 hours if severely dehydration. Antibiotics only if clinically indicated. Feeding delayed until rehydration completed. Micronutrients not given routinely. Standardised protocol had slower IV fluids and emphasis on oral rehydration, standard antibiotics and micronutrients and standardised management of hypoglycaemia and hypothermia. |
No evidence of fluid overload or cardiac failure was reported in this study
Urine output not reported |
Twice as many children on non-protocol treatment developed
hypoglycaemia during hospital stay (6% vs. 3%) 199 (59.5%) of children in standardised protocol group were successfully rehydrated with oral rehydration rather than IV fluids, compared with 85 (29%) in the other group (p<0.0001) Total volume of IV fluids was smaller and duration shorter in standardised protocol group (p<0.0001) More children in non-protocol group became critically ill and required special care treatment or needed treatment with ceftriaxone. More children on standardised protocol were discharged uneventfully 73% vs. 63%, p=0.006. 30 (9%) mortalities in standardised protocol versus 49 (17%) in non- protocol group (odds ratio=0.49 (95% CI 0.2-0.8), p=0.003) Children who died on standardised protocol died mostly within first 48 hours. Younger age, poorer nutritional status, increased frequency of hypoglycaemia, bacteraemia and greater volume of IV fluids infused were risk factors for death |
NGT Nasogastric tube, ECG electrocardiogram, ORS Oral rehydration solution, ReSoMal Rehydration solution for malnutrition, WHO World Health Organization, SAM Severe acute malnutrition
Risk of bias
The quality of each of the included studies was assessed for risk of bias using the Cochrane collaboration’s tool in order to evaluate validity 12. Three of the studies had a low overall risk of bias 7, 8, 10, while the remaining study had a moderate risk due to limitations in the methodology 9.
Outcomes
Primary outcome
Incidence of fluid overload
This outcome was available from three studies 7, 8, 10. No evidence of fluid overload was found in any of these studies. Alam et al. (2009) defined heart failure as tachycardia, tachypnoea, enlarged liver, and prominent neck veins 11. Akech et al. (2010) reported the need for furosemide or the diagnosis of pulmonary oedema as defined by crepitations in both lungs in the presence of hypoxaemia (low oxygen saturations measured by pulse oximetry) 7. Obonyo et al. (2017) used a definition of bi-basal crepitations and worsening oxygen saturation (indicative of pulmonary oedema) gallop rhythm, raised jugular venous pressure and increasing hepatomegaly. Obonyo et al. (2017) also evaluated myocardial function using echocardiography and electrocardiographic assessment, which did not demonstrate any evidence of myocardial dysfunction indicative of biventricular failure prior to or as a result of intravenous fluid therapy 8. They demonstrated that children showed signs consistent with hypovolaemia and myocardial responses to this at baseline and following intravenous fluid therapy resulted in increases in markers of myocardial performance (fluid-responsiveness) in the majority of patients.
Urine output
Three studies reported on urine output in response to rehydration therapy. Alam et al. measured urine every 6 hours (collected in urine collectors) and reported that 31% of children remained oliguric at 6 hours, and 12% by 12 hours 10. However, it should be noted that this included all children, of which 147 had received intravenous fluids for 4 to 6 hours and 26 that received only ORS. Akech et al. measured urine output hourly for the first 8 hours (using urinary catheters) and reported frequency of oliguria (<1ml/kg/hour) at 8 hours (which also by the study’s definition included resolution of shock and absence of oliguria). Oliguria was found to be common in both arms, but more in the group receiving half strength Darrow’s and 5% dextrose (9/22, 41%) than in those receiving Ringers lactate (3/25, 12%), p=0.05 7. Obonyo et al. measured urine output using urinary catheters and reported ‘satisfactory urine output’ (>1ml/Kg/hour) in 8/11 (73%) of children group 1 and 7/9 (78%) children in group 2 8.
Secondary outcomes
Mortality
All of the studies reported on mortality rates. One study in Asia reported no deaths in 175 severely malnourished children included in the trial 10, whilst the other three reported mortality rates ranging from 9% to 81.8%. Obonyo et al. reported mortality at 48 hours and 28 days and found a universally high mortality between both groups: 35% and 81.8%, respectively, in Group 1 (receiving WHO management), and 44% and 55.6% respectively in Group 2 (receiving rehydration alone). Of note was that in children fulfilling the WHO shock criteria none survived 8. Akech et al. 7 also found a high overall mortality (31/61, 51%) with no statistically significant difference between the two arms in a Phase II trial of 61 children; 39% of these deaths occurred in the first 24 hours and 52% within 48 hours. Ahmed et al. reported 30 (9%) case fatalities in the standardised protocol 9, in which children received IV rehydration over a longer duration i.e. more than 3–6 hours, and use of IV fluids was avoided where possible (children also received a full package of care including IV antibiotics for a minimum of 48 hours, vitamin A prophylaxis, and standardised management for hypoglycaemia) versus 49 (17%) in non-protocol group, in which children received fluid as per WHO non-SAM guidelines; 75ml/Kg oral or nasogastric fluid over 4 hours for children with ‘some’ dehydration and 100ml/Kg IV fluid as per Plan ‘C’, i.e. over 3 hours in children >1 year and over 6 hours if <1 year for those with severe dehydration (odds ratio=0.49 (95% CI 0.2-0.8), p=0.003). Children in the standardised protocol mostly died within the first 48 hours following admission. Young age, poorer nutritional status, increased frequency of hypoglycaemia, bacteraemia and greater volume of IV fluids infused were all risk factors for death 9.
There was significant between-study heterogeneity in mortality rates. Mortality was greatest when signs of shock were observed 8. There was no statistical difference reported when different rates of IV rehydration were adopted.
Hyponatraemia
The definitions of hyponatraemia varied across the studies, so summary data could not be generated. Alam et al. (2009) reported an overall baseline rate of hyponatraemia (sodium <130mmol/L) in 38/175 (22%), with no cases of severe hyponatraemia (serum sodium<115mmol/L) at baseline. No child developed features of severe hyponatraemia at any time during the study (features observed were not specified and post fluid sodium level was not recorded) 10. Obonyo et al. (2017) found that 4/11 (36%) and 5/9 (63%) patients in Groups 1 and 2 respectively were found to have severe hyponatraemia (serum sodium <125mmol/L) at baseline 8. Akech et al. (2010) found 4/26 (15%) and 3/29 (10%) cases of hyponatraemia (<125mmols/L) at baseline in the half strength Darrow’s and 5% (HSD/D5) and Ringers lactate (RL) groups, respectively. They also demonstrated mean plasma sodium increases from baseline to 24 hours of 133 to 138mmol/L in the HSD/D5, and 134 to 140mmol/L in the RL group 7. Data on the post-fluid rates of hyponatraemia were not published in Alam et al. or Ahmed et al. studies 9, 10, although common at baseline.
Neurological complications
No studies reported any incidences of seizures. However, it is unclear if this outcome was predefined for inclusion in any of these studies.
Cardiovascular compromise
Two studies undertook detailed cardiovascular evaluations. Akech et al. (2010) found that, overall 19/55 (34%), 10/47 (21%) and 12/39 (30%) children were severely tachycardic (heart rate>160 beats/minute) at baseline, 8 and 24 hours after starting fluid respectively. Significantly more were tachycardic at 24 hours in the group receiving HSD/D5 group than the RL group (44% vs. 16%, p=0.04). No incidences of bradycardia (HR<60 beats/minute) were reported 7. Obonyo et al. (2017) published mean age adjusted heart rate and systolic and diastolic blood pressures of survivors and fatalities at baseline, and between 30 minutes and then 8 hour intervals up to 48 hours. They found that a low systolic blood pressure and a persistent weak pulse after IV fluid administration were associated with death in 11/20 (55%) patients. Longitudinal monitoring demonstrated decreases in heart rate in both groups over the first 48 hours with no significant differences between mortalities and survivors. Diastolic blood pressure was lower in patients in the rehydration only group who died before 4 hours, suggesting uncorrected hypovolaemia. No evidence for biventricular heart failure was found. In this study, children were monitored additionally using cardiac biomarkers (Troponin I, a highly sensitive biochemical marker of myocardial damage, and brain natriuretic peptide (BNP), a marker of volume expansion and pressure loading, which has previously been used to predict outcome in paediatric heart failure 13– 15 at admission and 48 hours. Troponin I remained within the normal range in both groups. BNP was elevated (>300pg/ml) in 6/10 (60%) in group-1 and 2/9 (22%) at baseline and remained elevated in 4/7 (57%) patients in Group 1 but not in any patients in Group 2. The interpretation of this is challenging and requires further exploration.
Discussion
All studies in the review included children with SAM, diarrhoea and some level of dehydration ranging from 41% with any dehydration 9 to 100% with severe dehydration 8. Two focused on shock (since this is the only indication for use of fluid therapy recommended by WHO), whilst two studies extended the management to children without strict WHO shock or rehydration regimes that are currently recommended by WHO in order to generate pilot data on safety and preliminary data on outcomes (efficacy) 7, 8. Critical to the evidence underpinning current treatment recommendations and contrary to the accepted opinion that the malnourished heart is susceptible to failure if intravenous fluids are administered, none of the studies demonstrated any evidence of fluid overload in their respective cohorts. These findings highlight the lack of available data to support current guidelines recommending conservative fluid therapy in rehydration of children with SAM and pave the way for further research to inform future practice.
Concerns regarding use of intravenous fluids in this vulnerable group of children reference impaired cardiovascular function and susceptibility to overload. A number of studies have been conducted over the last 30 years in Africa, Asia, the Americas and Europe that evaluate cardiovascular function in children with SAM using Echocardiography 16– 25. However, it should be noted that none of these additional studies have been conducted in the context of the child with SAM receiving rehydration therapy, with the exception of the study by Obonyo et al. 8. We summarise the findings below.
A number of early studies described reduced cardiac mass, decreased left ventricular function and cardiac and stroke indices in malnourished children when compared with healthy children. Viart et al. (1977) measured clinical and haemodynamic parameters in 43 Jamaican children with marasmic kwashiorkor and compared them with 24 convalescent children. In the malnourished children, haemodynamic parameters were abnormal when compared with convalescent patients: red cell volume and total blood volumes were 51% and 66%; cardiac and stroke indices averaged 58% and 62%, of the convalescent values, respectively. Malnourished children therefore showed prolonged circulation time with associated bradycardia and hypotension when compared with convalescent children 23. Similarly, two observational studies (Singh et al., 1989, Shoukry et al., 1986) conducted in India included 63 children with malnutrition, and found that malnourished children had smaller cardiac mass. In particular, left ventricular mass was less and indicators of left ventricular function were reduced (including cardiac output and stroke volume) 18, 19. Bergman et al (1988) observed 21 children with kwashiorkor in South Africa and also found that they had low cardiac dimensions 17. Phornphatkul et al. (1994) monitored cardiovascular status before, during and after nutritional rehabilitation and found that Thai children with SAM had features indicative of impaired ventricular function, as shown by change in fractional shortening (p= 0.015), mean velocity of circumferential fibre shortening (p= 0.038), and systolic time interval (p= 0.030) 24. More recently, Olivares (2005) compared 30 malnourished and 30 healthy Spanish children and found that left ventricular mass and left ventricular index was significantly lower in children with malnutrition (left ventricular mass: 55.3 ± 10.3 vs. 71.4 ± 6.9 g, p = 0.000; left ventricular mass index: 46.5 ± 6.6 vs. 60.5 ± 4.9 g/m2, p = 0.000) 20. However, these studies are constrained by their small sample sizes and none considered indexing the heart muscle in relation to general muscle mass.
Contradicting the findings of these earlier studies, four studies assessing cardiovascular function in children with SAM conducted between 1992 and 2016 found that, in SAM, there appears to be relative ‘cardiac- sparing’. Kothari et al. (1992) found that cardiac mass is reduced compared with that of well-nourished children; however cardiac weight: total body weight ratio is higher (4.44 +/- 1.45 vs. 2.42 +/- 0.87; p<0.001, 95% C.I. 1.28 to 2.76), and systolic function was not significantly different to well-nourished children. Cardiac output was therefore considered as appropriately reduced in proportion with body size 22. Ocal et al. (2001) found that left ventricular mass was lower in malnourished children but in proportion to body surface area, and therefore cardiac indices were not significantly different from normal ranges 21. El-Sayed et al. (2006) agreed that cardiac mass index was significantly lower in children with malnutrition, but that left ventricular function was not significantly reduced 16. The largest and most recent study (Silverman et al. 2016) evaluating baseline cardiovascular function in 272 ‘stable’ Malawian children demonstrated no significant differences in cardiac index, stroke volume index and heart rate between inpatient children with and without SAM 25.
As evidenced by the four studies included in this systematic review, there has been no reported fluid overload in children treated with IV fluid when dehydrated. To the contrary, Obonyo et al. (2017) found that ‘despite a high mortality rate, neither clinical nor echocardiographic data indicated evidence of volume overload’. There was also no evidence of gross myocardial dysfunction by echocardiographic or by specific cardiac biomarkers including Troponin I and BNP. Fluid administration in both groups led to improvements in stroke volume index and left ventricular fractional shortening, and Obonyo et al. concluded that ‘further research is required to investigate whether more liberal fluid rehydration strategies may be beneficial’ 8.
Finally, the cardiac myocardial physiological study in children with SAM (CAPMAL study) conducted in Kilifi County Hospital between May 2011 and February 2012 included 88 SAM cases (52 with marasmus and 36 with kwashiorkor phenotypes) and 22 non-malnourished disease-matched controls. Serial echocardiographic and electrocardiographic data were gathered over the course of admission and additional measurements were conducted on any SAM child requiring intravenous fluids. Overall, there was no difference in myocardial function (indexed to body surface area) in cases with either marasmus or kwashiorkor; and no differences between cases and disease matched controls (matched for comorbidities such as pneumonia, diarrhoea). Fifteen episodes of fluid resuscitation were recorded involving 12 SAM cases (75% marasmus), the majority (10/11) for hypovolaemia secondary to diarrhoea early in the course of admission. No clinical evidence of a deterioration, indicative of pulmonary oedema, was witnessed. Echocardiographic indices suggested an adequate physiological response to fluid resuscitation 26.
This body of evidence undermines the physiological rationale of fluid overload secondary to myocardial dysfunction as a mechanism of death during rehydration in paediatric SAM, and lends support to the notion that this group of children are likely to remain under-filled when following current guidelines. The most compelling data suggesting superiority of one strategy over another with regards to mortality come from Ahmed et al. (1999) 9. However, combining multiple therapies into a package of care confounds the determination of the causative intervention for improved mortality. Additionally, the pre- and post-intervention design precipitates significant performance bias. Neither Obonyo et al. (2017) nor Akech et al. (2010) were powered to detect a difference in mortality between the intervention arms. However, the high overall mortality in these studies can be explained by the high prevalence of children with shock at admission 7, 8.
There is no compelling evidence to support the conservative approach to rehydration across the spectrum of disease encompassed by the four studies identified in this systematic review. Fluid overload as a consequence of IV fluids in SAM complicated by dehydration has not been detected.
Application of guidelines
In practice, the WHO guidelines are complex, challenging to follow and open to wide interpretation (see Table 1) (3); the clinician must decide whether to rehydrate the child over 4 or 10 hours or any interval between the two limits, and at what point the child is eligible for intravenous rather than oral rehydration. Additionally, the feasibility of alternating F75 and ReSoMal every 30 minutes in a busy, pressurised and low resource setting is limited 6.
For children with shock, the fluid regimen is restricted to a maximum of two slow IV infusions over a total of two hours, with advice to transfuse if no improvement. The recommendation of a transfusion to manage hypovolaemic shock in the context of severe dehydration is extremely concerning as it will not replete the volume (water and electrolytes) loss. As detailed above, the evidence for this level of caution with IV fluid administration is limited and may risk persistent hypovolaemia and lethal shock. Additionally, there are no compelling data that support the superiority of oral over IV rehydration in non-shocked children with severe dehydration.
Difficulties also arise when the child is near to the threshold for SAM and may be misclassified due to dehydration. Prospective research has found that 20% of children hospitalised with acute gastroenteritis (AGE) and severe dehydration (10% or more loss of body weight) temporarily fulfil anthropometric criteria for SAM (MUAC <11.5cm or WHZ <-3SD), but following rehydration they are reclassified as undernourished 27. Thus, the current recommendations have much wider implications, with potentially 20% of children with severe dehydration secondary to AGE and without SAM receiving low volume low sodium rehydration, which may explain the poor outcomes that have been observed in the large case-control study Global Enteric Multicentre study (GEMS). This was conducted in Africa and Asia, and showed that patients with moderate/severe gastroenteritis are 8.5 times more likely to die than non-gastroenteritis controls 28, 29. A third of the fatalities occurred < 7 days following hospitalisation - indicating that current management strategies may not be working in practice for children with and without SAM and emphasising the need to revisit the current guidelines.
Conclusions
The WHO guidelines for rehydration in SAM are not supported by high quality evidence. There is also no evidence to support concerns regarding fluid overload in this population on the contrary, haemodynamic evaluation indicates that these children remain under-filled. In the light of recent famines across Africa and the current cholera outbreaks, we are extremely concerned that children with SAM (and potentially 20% of non-SAM children) are having intravenous rehydration fluids withheld on the grounds of strong recommendations based on low quality of evidence. New evidence on myocardial function and from clinical trials suggests they should be afforded the same standard of care as children without malnutrition. Further research should urgently evaluate safety and efficacy of a more aggressive approach to rehydration and support development of evidence based, user-friendly and directive guidelines.
Funding Statement
This work was supported by the Wellcome Trust [105603], an ISSF fellowship (Year 4 & 5 1st Oct 14 - 30 Sept 16, (2014 -2018)) awarded by Imperial College, London, to Dr K Houston; [203077], the KEMRI Wellcome Trust Programme East African Overseas Programme Award.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 3 approved]
Supplementary material
Supplementary File 1: PRISMA checklist.
References
- 1. Black RE, Cousens S, Johnson HL, et al. : Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet. 2010;375(9730):1969–1987. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- 2. Ashworth A, Khanum S, Jackson A, et al. : Guidelines for the inpatient treatment of severely malnourished children. In: Geneva: World Health Organization;2003;51 Reference Source [Google Scholar]
- 3. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Childhood Illnesses. In: Geneva: World Health Oganization;2013. [PubMed] [Google Scholar]
- 4. Maitland K, Berkley JA, Shebbe M, et al. : Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol? PLoS Med. 2006;3(12):e500. 10.1371/journal.pmed.0030500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Talbert A, Thuo N, Karisa J, et al. : Diarrhoea complicating severe acute malnutrition in Kenyan children: a prospective descriptive study of risk factors and outcome. PLoS One. 2012;7(6):e38321. 10.1371/journal.pone.0038321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guideline: Updates on the management of severe acute malnutrition in infants and children. In: Edited by Organization WH. Geneva WHO;2013. [PubMed] [Google Scholar]
- 7. Akech SO, Karisa J, Nakamya P, et al. : Phase II trial of isotonic fluid resuscitation in Kenyan children with severe malnutrition and hypovolaemia. BMC Pediatr. 2010;10:71. 10.1186/1471-2431-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Obonyo N, Brent B, Olupot-Olupot P, et al. : Myocardial and haemodynamic responses to two fluid regimens in African children with severe malnutrition and hypovolaemic shock (AFRIM study). Crit Care. 2017;21(1):103. 10.1186/s13054-017-1679-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahmed T, Ali M, Ullah MM, et al. : Mortality in severely malnourished children with diarrhoea and use of a standardised management protocol. Lancet. 1999;353(9168):1919–1922. 10.1016/S0140-6736(98)07499-6 [DOI] [PubMed] [Google Scholar]
- 10. Alam NH, Islam S, Sattar S, et al. : Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr. 2009;48(3):318–327. [DOI] [PubMed] [Google Scholar]
- 11. Slow versus Rapid Rehydration of Severely Malnourished Children. Reference Source [Google Scholar]
- 12. Higgins JP, Altman DG, Gøtzsche PC, et al. : The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Koch A, Singer H: Normal values of B type natriuretic peptide in infants, children, and adolescents. Heart. 2003;89(8):875–878. 10.1136/heart.89.8.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Auerbach SR, Richmond ME, Lamour JM, et al. : BNP levels predict outcome in pediatric heart failure patients: post hoc analysis of the Pediatric Carvedilol Trial. Circ Heart Fail. 2010;3(5):606–611. 10.1161/CIRCHEARTFAILURE.109.906875 [DOI] [PubMed] [Google Scholar]
- 15. Towbin JA, Gajarski RJ: Cardiac troponin I: a new diagnostic gold standard of cardiac injury in children? J Pediatr. 1997;130(6):853–855. [PubMed] [Google Scholar]
- 16. El-Sayed HL, Nassar MF, Habib NM, et al. : Structural and functional affection of the heart in protein energy malnutrition patients on admission and after nutritional recovery. Eur J Clin Nutr. 2006;60(4):502–510. 10.1038/sj.ejcn.1602344 [DOI] [PubMed] [Google Scholar]
- 17. Bergman JW, Human DG, De Moor MM, et al. : Effect of kwashiorkor on the cardiovascular system. Arch Dis Child. 1988;63(11):1359–1362. 10.1136/adc.63.11.1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singh GR, Malathi KE, Kasliwal RR, et al. : An evaluation of cardiac function in malnourished children by non-invasive methods. Indian Pediatr. 1989;26(9):875–881. [PubMed] [Google Scholar]
- 19. Shoukry I, Shoukry A, Ibrahim M, et al. : Cardiac atrophy and ventricular function in infants with severe protein calorie malnutrition (Kwashiorkor Disease). In: Pediatric cardiology.edn. Edited by Doyle EEM, Gersony W, Rashkind W, Talner N. New York: Springer;1986;1169–1171. 10.1007/978-1-4613-8598-1_310 [DOI] [Google Scholar]
- 20. Olivares JL, Vázquez M, Rodríguez G, et al. : Electrocardiographic and echocardiographic findings in malnourished children. J Am Coll Nutr. 2005;24(1):38–43. 10.1080/07315724.2005.10719441 [DOI] [PubMed] [Google Scholar]
- 21. Ocal B, Unal S, Zorlu P, et al. : Echocardiographic evaluation of cardiac functions and left ventricular mass in children with malnutrition. J Paediatr Child Health. 2001;37(1):14–17. 10.1046/j.1440-1754.2001.00566.x [DOI] [PubMed] [Google Scholar]
- 22. Kothari SS, Patel TM, Shetalwad AN, et al. : Left ventricular mass and function in children with severe protein energy malnutrition. Int J Cardiol. 1992;35(1):19–25. 10.1016/0167-5273(92)90050-D [DOI] [PubMed] [Google Scholar]
- 23. Viart P: Hemodynamic findings in servere protein-calorie malnutrition. Am J Clin Nutr. 1977;30(3):334–348. [DOI] [PubMed] [Google Scholar]
- 24. Phornphatkul C, Pongprot Y, Suskind R, et al. : Cardiac function in malnourished children. Clin Pediatr (Phila). 1994;33(3):147–154. 10.1177/000992289403300304 [DOI] [PubMed] [Google Scholar]
- 25. Silverman JA, Chimalizeni Y, Hawes SE, et al. : The effects of malnutrition on cardiac function in African children. Arch Dis Child. 2016;101(2):166–171. 10.1136/archdischild-2015-309188 [DOI] [PubMed] [Google Scholar]
- 26. Brent B: Cardiovascular physiology in children with acute severe malnutrition in Kenya. London: Imperial College;2015. [Google Scholar]
- 27. Mwangome MK, Fegan G, Prentice AM, et al. : Are diagnostic criteria for acute malnutrition affected by hydration status in hospitalized children? A repeated measures study. Nutr J. 2011;10:92. 10.1186/1475-2891-10-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotloff KL, Blackwelder WC, Nasrin D, et al. : The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55 Suppl 4:S232–S245. 10.1093/cid/cis753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kotloff KL, Nataro JP, Blackwelder WC, et al. : Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–222. 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]