Abstract
The design, synthesis, and biological evaluations of eight 4-substituted 5-methyl-furo[2,3-d]pyrimidines are reported. Synthesis involved N4-alkylation of N-aryl-5-methylfuro[2,3-d]pyrimidin-4-amines, obtained from Ullmann coupling of 4-amino-5-methylfuro[2,3-d]pyrimidine and appropriate aryl iodides. Compounds 3, 4, and 9 showed potent microtubule depolymerizing activities, while compounds 6–8 had slightly lower potency. Compounds 4, 6, 7, and 9 inhibited tubulin assembly with IC50 values comparable to that of combretastatin A-4 (CA-4). Compounds 3, 4, and 6–9 circumvented Pgp and βIII-tubulin mediated drug resistance, mechanisms that can limit the efficacy of paclitaxel, docetaxel, and the vinca alkaloids. In the NCI 60-cell line panel, compound 3 exhibited GI50 values less than 10 nM in 47 of the cell lines. In an MDA-MB-435 xenograft model, compound 3 had statistically significant antitumor effects. The biological effects of 3 identify it as a novel, potent microtubule depolymerizing agent with antitumor activity.
Graphical abstract

INTRODUCTION
Microtubules are filamentous cytoskeleton protein polymers composed of αβ-tubulin heterodimers. Microtubules play an important role in many aspects of cellular function, including cellular transport, protein trafficking, and mitosis. Microtubule targeting agents (MTAs) are some of the most effective drugs used to treat cancer.1–3 MTAs are the only class of cytotoxic agents effective against p53 mutant cell lines, which constitute 39 of the 58 cell lines in the NCI 60-cell line panel.4,5 MTAs are widely used for the treatment of both solid tumors and hematological malignancies.1–3 These drugs are structurally diverse and are classified into two main groups: microtubule stabilizers and microtubule destabilizers. Microtubule stabilizers promote microtubule polymerization and include the taxanes, paclitaxel, docetaxel, and cabazitaxel, and the epothilone, ixabepilone. Microtubule destabilizers induce microtubule depolymerization and include the vinca alkaloids and colchicine-site agents. However, at low antiproliferative concentrations, both types of agents suppress microtubule dynamics leading to mitotic arrest and subsequent cell death in cells in culture.1,2 New evidence suggests that the ability of these drugs to inhibit interphase signaling events likely contributes to their anticancer actions.6
MTAs are divided into five classes based on their interactions within the taxane, vinca, colchicine, laulimalide/peloruside, or the maytansine site on tubulin (Figure 1). Paclitaxel, docetaxel, cabazitaxel, and ixabepilone bind to β-tubulin in the interior of the microtubule, a site referred to as the taxane site.7,8 The taxanes are widely used in the treatment of adult solid tumors.9,10 The vinca alkaloids, including vincristine, vinblastine, vindesine, and vinorelbine, as shown in a crystal structure with vinblastine, require two distinct tubulin αβ-heterodimers to form a binding site. The site is formed by amino acid residues from both α- and β-tubulin, and the vinblastine molecule was located between the two heterodimers. The vinca alkaloids are important in the treatment of hematological malignancies, lymphoma, and childhood cancers.9 Laulimalide and peloruside A bind to a distinct site on β-tubulin, referred to as the laulimalide/peloruside site, and both compounds cause microtubule stabilization. This site is located on the exterior of the microtubule.11 Recently, Prota et al.12 demonstrated that maytansine binds to a different site on β-tubulin, and they named this the maytansine site. Maytansine, rhizoxin F, and a polyketide PM060184, initially isolated from the marine sponge Lithoplocamia lithistoides, bind within this newly characterized site, occupancy of which prevents the addition of new subunits to the plus ends of microtubules, resulting in microtubule depolymerization.12 Maytansine is the cytotoxic component in the antibody–drug conjugate trastuzumab emtansine recently approved by the FDA for the treatment of HER2-positive advanced breast cancer.13 The fifth class of MTAs comprises a variety of small molecules that includes colchicine and combretastatins A-1 (CA-1) and A-4 (CA-4), which bind in the colchicine site located on β-tubulin at its interface with α-tubulin.14,15 Combretastatin A-4 phosphate (CA-4P, fosbretabulin) and A-1 diphosphate (CA-1P, OXi4503), 2-methoxyestradiol, and verubulin16,17 are a few of the colchicine site binding agents that have been evaluated in phase 1 and 2 clinical trials as anticancer agents alone and in combination with other drugs. Thus far, no colchicine site agent has been approved as an anticancer agent.18–24 Hence, this site provides new opportunities for drug discovery.
Figure 1.
Representative microtubule targeting agents.
Rationale and Molecular Modeling
We previously reported25 that cyclopenta[2,3-d]pyrimidine (±)-1·HCl (Figure 2) has potent microtubule depolymerization activity (EC50 in A-10 cells = 47 nM) and in vitro cytotoxic effects against MDA-MB-435 cancer cells (IC50 ± SD = 18.8 ± 0.4 nM).
Figure 2.
Parent 5-methyl-cyclopenta[2,3-d]pyrimidine (±)-1 and target 5-methyl-furo[2,3-d]pyrimidines 2 and 3.
Replacement of the cyclopentyl and the 2-methyl groups of the potent MTA (±)-1 with a furan and a 2-H moiety, respectively, afforded the 5-methyl-furo[2,3-d]pyrimidine scaffold of 2 and 3 (Figure 2). It was of interest to explore the antitumor activity of compounds with the 5-methyl-furo[2,3-d]pyrimidine scaffold without the 2-position substitution as MTAs to determine the contribution of the 2-position substitution and the furo ring to both inhibition of cancer cell proliferation and microtubule depolymerization. Additionally, these lead compounds are similar in structure to verubulin (Figure 1), which is a N4,2-dimethylquinazoline-4-amine analogue with p-methoxyphenyl substitution at N4-position.16 Similar N4-p-methoxyphenyl substitution pattern has not been explored before in 5-methyl-furo[2,3-d]pyrimidine series of compounds.
Compounds 2 and 3 were docked in the X-ray crystal structure of colchicine in tubulin at the colchicine site (PDB: 4O2B,26 2.30 Å) using Molecular Operating Environment MOE 2013.0801.27 Multiple low energy conformations were obtained on docking. Figure 3 shows the docked conformation of 3 (green) as a representative example in the colchicine site of tubulin. The furo[2,3-d]pyrimidine scaffold of 3 overlaps the C-ring of colchicine (orange) and forms hydrophobic interactions at the αβ tubulin interface with Alaα180, Asnβ258, and Lysβ352. The 5-Me group of 3 interacts with Leuβ255, Asnβ258, and Metβ259 of tubulin. The N-Me moiety of 3 mimics the bridged C5- and C6-positions of the B-ring of colchicine and forms hydrophobic interactions with Leuβ248, Alaβ250, and Lysβ254. The 4′-OMe-Ph of 3 is oriented toward the hydrophobic pocket formed by Thrβ240, Cysβ241, Alaβ316, Ileβ318, and Ileβ378 and binds in the region occupied by the A-ring of colchicine. The phenyl ring of 3 makes hydrophobic interactions with Leuβ248, Leuβ255, Alaβ316, and Alaβ354. Figure 3 also shows that the docked pose of 3 at the colchicine site is similar to that of the lead compound (S)-1. The best docked pose of 3 had a score of −5.07 kcal/mol comparable to that of colchicine (−5.54 kcal/mol) and the lead compound (S)-1 (−5.19 kcal/mol).
Figure 3.
Superimposition of the docked pose of 3 (green) in the colchicine site (colchicine in orange) of tubulin (PDB: 4O2B26).
Design of Target Compounds 4–9
The N-Me moiety of 3 forms hydrophobic interactions with Leuβ248, Alaβ250, Lysβ254, and Asnβ258 in the colchicine site (Figure 3). To optimize these hydrophobic interactions, compounds 4–7 (Figure 4) with larger alkyl groups at the N4-position were synthesized. In addition, these compounds were also designed to determine the bulk tolerance at the colchicine site for substituents at the N4-position for the furo[2,3-d]pyrimidine scaffold. The key binding interactions indicated by the docking study of the lead compound 3 in the colchicine-binding site of tubulin (Figure 3) are retained by target compounds 4–7 with docking scores (−5.60, −5.11, −4.35, and −5.12 kcal/mol, respectively) similar to that of 3 (see Supporting Information).
Figure 4.
Target compounds 4–9.
Compounds 8 and 9 were designed to improve hydrophobic interactions of the 4′-OMe of 3 within the hydrophobic pocket formed by residues Thrβ240, Cysβ241, Alaβ316, Ileβ318, and Ileβ378 at the colchicine site as suggested by the docking scores of 8 and 9 (−5.16 and −5.16 kcal/mol, respectively), both of which were similar to that of 3. Thus, compounds 8 and 9 were designed to evaluate the role of the 4′-OMe group on tubulin depolymerization and inhibition of cancer cell growth by substituting the oxygen of the 4′-OMe with a methylene and sulfur, respectively, thus decreasing the electronegative nature of the 4′-OMe substituent and increasing the hydrophobic interactions at the colchicine site.
RESULTS AND DISCUSSION
Chemistry
Target compounds 2 and 3 were synthesized as described in Scheme 1. Compound 11 was obtained by reacting hydroxyacetone 10 with malononitrile in the presence of triethylamine and was used without purification for the subsequent step.28 Treatment of 11 with formamidine hydrochloride under basic conditions provided 5-methyl-furo[2,3-d]pyrimidine 12 in 47% yield (two steps). Ullmann coupling of 12 and 4-iodo anisole 13 using CuI and l-proline afforded target compound 2. N4-Methylation of 2 with dimethyl sulfate furnished target compound 3 in 70% yield.
Scheme 1.
Synthesis of Target Compounds 2 and 3
Compounds 4–7 were synthesized from 2 (Scheme 1) by N4-alkylation with the appropriate alkyl iodides 14–17, respectively (Scheme 2).
Scheme 2.
Synthesis of Target Compounds 4–7
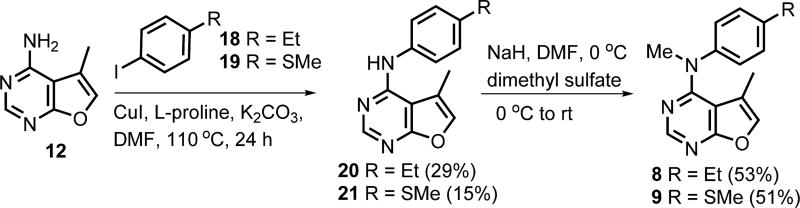
Ullmann coupling of 12 and appropriate aryl iodides 18 and 19 (Scheme 3) using CuI and l-proline afforded 20 and 21, respectively, which upon N4-methylation furnished target compounds 8 and 9, respectively.
Scheme 3.
Synthesis of Target Compounds 8 and 9
Biological Results
Effects on Microtubule Depolymerization and Cell Proliferation
Compounds 2–9 were first evaluated for their ability to depolymerize microtubules (Table 1 and Figure 5). Compounds that caused at least 50% microtubule depolymerization at a concentration of 10 µM were further evaluated to determine the EC50, the concentration that causes loss of 50% of cellular microtubules in A-10 cells and for antiproliferative effects against MDA-MB-435 cancer cells. The embryonic rat smooth muscle A-10 cells are useful to evaluate the effects of microtubule targeting agents on interphase microtubules. Unlike cancer cells, A-10 cells do not accumulate in mitosis in response to compounds that disrupt microtubules and allow effects on interphase microtubules to be easily visualized and quantified. The N-H analogue 2 was inactive in the microtubule depolymerization assay and was not evaluated further. The N-Me analogue 3 showed potent microtubule depolymerization effects and also inhibited the proliferation of MDA-MB-435 cells with potency comparable to those of CA-4 and paclitaxel. This indicates that for the furo[2,3-d]pyrimidine scaffold the N-Me moiety is important for biological activity. Compound 3 was 2.3-fold more potent for microtubule depolymerizing effects and about 4-fold more potent for antiproliferative activity (MDA-MB-435) than the lead compound (±)-1·HCl. Compounds 4 and 9 were equipotent with (±)-1·HCl as microtubule depolymerizers and were 1.4–2.1-fold more active against MDA-MB-435 cells than (±)-1·HCl. These data indicate that the 2-desmethyl furo[2,3-d]pyrimidine scaffold is conducive to both microtubule depolymerization and antiproliferative effects.
Table 1.
IC50 Values for Inhibition of Proliferation of MDA-MB-435 Cells and EC50 Values for Microtubule Depolymerization
| compd | EC50 for microtubule depolymerization in A-10 cells (nM) |
IC50 ± SD MDA- MB-435 (nM) |
|---|---|---|
| (±)-1·HCla (Lead) | 56 | 17.0 ± 0.7 |
| 2 | >10 µM | NDb |
| 3 | 24 | 4.3 ± 0.3 |
| 4 | 53 | 8.1 ± 0.5 |
| 5 | >10 µM | 504 ± 28 |
| 6 | 306 | 27.3 ± 4.5 |
| 7 | 340 | 100 ± 6.5 |
| 8 | 750 | 183 ± 13 |
| 9 | 45 | 12.3 ± 0.9 |
| 21 | >10 µM | NDb |
| paclitaxel | 4.5 ± 0.5 | |
| CA-4 | 9.8 | 4.4 ± 0.5 |
Results previously published.25
ND: Not determined.
Figure 5.
Effects of compounds 3, 4, and 6–9 on cellular microtubules. A-10 cells were treated for 18 h with 2× the EC50, and cellular microtubules were visualized by indirect immunofluorescence with a β-tubulin antibody.
Compounds 4–7 with larger alkyl groups at the N4-position had lower microtubule depolymerizing potency and higher antiproliferative IC50 values than that of the N-Me compound 3 (Table 1). The potency decreased with increased homologation and the N-Et analogue 4 was 2-fold less potent than 3 and was equipotent with 1. The N-iPr analogue 5 was ineffective at depolymerizing microtubules even at concentrations as high as 10 µM. However, compound 5 exhibited some antiproliferative activity, but it was 60-fold less potent (IC50) than the N-Et analogue 4. The N-Me analogues 8 and 9 with variations at the 4′-position had quite different microtubule depolymerization activities in that 8 had an EC50 of 750 nM while 9 was quite potent, with an EC50 of 45 nM (Table 1), and both were less potent than 3. The 4′-Et analogue 8 had a 30-fold lower potency for microtubule depolymerization than the 4′-OMe analogue 3. In the X-ray crystal structure (PDB: 4O2B26), there is a water mediated hydrogen bond between Cysβ241 of tubulin and the 3-OMe group on the A-ring of colchicine. The 4′-OMe group of 3 mimics the 3-OMe group on the A-ring of colchicine (Figure 3), suggesting its importance for the biological activity of 3. The 4′-SMe analogue 9 had a 2-fold lower microtubule depolymerizing potency than the 4′-OMe analogue 3. In addition, in the MDA-MB-435 cell antiproliferative assay, compounds 8 and 9 had 43- and 3-fold lower antiproliferative potencies, respectively, than 3. These data indicate that bioisosteric replacement of the electronegative oxygen of the 4′-OMe group with sulfur in the 2-H,5-methylfuro[2,3-d]pyrimidine scaffold is detrimental to cellular microtubule depolymerization and to antiproliferative activity. The N4-desmethyl synthetic precursor, 21, like 2, was inactive as a MTA with EC50 > 10 µM, substantiating the importance of the N4-alkyl substitution.
The effects of compounds 3, 4, and 6–9 on cellular microtubules in A-10 cells are shown in Figure 5. A concentration of two-times their respective EC50 values caused almost complete loss of the microtubule cytoskeleton, with only a few remnant microtubules remaining.
Effects on Mitotic Spindles and Cell Cycle Distribution
A common propensity of MTAs is their ability to interrupt the structure and function of mitotic spindles. The disruption of microtubule dynamics leads to the formation of aberrant mitotic spindles that are unable to align the chromosomes into the metaphase plate leading to the accumulation of cells arrested in mitosis.
The effects of the microtubule active compounds on mitotic spindles and cell cycle distribution were evaluated in HeLa cells. The results showed that cells treated with these compounds were devoid of the normal bipolar spindles that were seen in vehicle-treated cells (Figure 6). No mitotic spindles were seen in cells treated with any of the test compounds, but multiple spindle poles were apparent, and these effects are consistent with effects seen with other colchicine site agents.29 These aberrant mitotic spindles suggested that these compounds could initiate mitotic arrest, and thus, the cell cycle distribution of the HeLa cells was evaluated by flow cytometry (Figure 7).
Figure 6.
Effects of compounds 3, 4, and 6–9 on mitotic spindles. HeLa cells were treated for 18 h with a concentration five-times the respective IC50, and cellular microtubules and mitotic spindles were visualized by indirect immunofluorescence techniques with a β-tubulin antibody.
Figure 7.
Cell cycle analysis of HeLa cells treated with 3, 4, and 6–9. (A) Cell cycle distribution profiles of cells treated for 18 h with five-times the IC50 of each compound. (B) Table showing the percent of cells in G2/M in each treatment group. Values are reported as the mean ± SD from two independent experiments.
In the flow cytometry study (Figure 7), typical results were obtained for cells treated with the MTAs 3, 4, and 6–9. These compounds cause the accumulation of cells in the G2/M phase of the cell cycle, as was observed following treatment with CA-4, while a normal cell cycle distribution was observed in the vehicle-treated control cells (Figure 7A). HeLa cells treated with five-times the IC50 of 3, 4, or 6–9 exhibited a significant accumulation of cells in G2/M, as with CA-4 (Figure 7A). The percentage of cells in G2/M in each treatment group was quantified (Figure 7B).
Inhibition of Tubulin Assembly and Colchicine Binding
On the basis of their microtubule depolymerizing activities, compounds 3–7 and 9 were evaluated for their direct effects on tubulin assembly and inhibition of colchicine binding (Table 2). Except for 5, all the compounds at 5 µM inhibited [3H]colchicine binding to the protein, and the extent of inhibition was similar to that obtained with CA-4. Straight chain N4-alkyl analogues 4 (N-Et), 6 (N-Pr), and 7 (N-Bu) inhibited tubulin assembly about as well as CA-4 and were about 2-fold more potent than the lead compound (±)-1·HCl. In the colchicine inhibition assay, compounds 4 (N-Et), 6 (N-Pr) and 7 (N-Bu), at 5 µM, inhibited the binding of [3H]colchicine by 87–95%, whereas the branched alkyl N-iPr 5 showed only a 47% inhibition of [3H]colchicine binding. The lower activity of the sterically bulky isopropyl moiety could be attributed to limited steric tolerance in the colchicine site. The data indicate that N4-alkylation is tolerated to an extent as predicted by molecular modeling (Figure 3). Compound 9 (4′-SMe) inhibited tubulin assembly with an IC50 value comparable to that of CA-4, and it is 3-fold better than the 4′-OMe analogue 3. However, it is interesting to note that 3, despite its somewhat lower activity as an inhibitor of tubulin assembly, compared to CA-4, was a strong inhibitor of [3H]colchicine binding to tubulin.
Table 2.
Inhibition of Tubulin Assembly and Colchicine Binding
| inhibition of colchicine binding (% inhibition ± SD) |
|||
|---|---|---|---|
| compd | inhibition of tubulin assembly (IC50 ± SD (µM)) |
1 µM | 5 µM |
| (±)-1·HCla (lead) | 1.9 ± 0.01 | 60 ± 2 | 84 ± 3 |
| 3 | 3.3 ± 0.5 | 71 ± 6 | 96 ± 2 |
| 4 | 0.97 ± 0.09 | 84 ± 0.7 | 95 ± 0.5 |
| 5 | 3.0 ± 0.2 | ND | 47 ± 0.2 |
| 6 | 1.1 ± 0.1 | 75 ± 1 | 92 ± 0.3 |
| 7 | 1.3 ± 0.2 | 61 ± 0.4 | 87 ± 0.2 |
| 9 | 1.2 ± 0.007 | 79 ± 2 | 94 ± 0.01 |
| CA-4 | 0.96 ± 0.07 | 90 ± 1 | 99 ± 0.2 |
Results previously published.25
Ability to Overcome Multidrug Resistance Mechanisms
The clinical activity of the taxanes and the vinca alkaloids is limited by two mechanisms of drug resistance: the expression of the drug efflux pump Pgp and the βIII-isotype of tubulin.30 Pgp expression is observed clinically, particularly in patients who have received prior chemotherapy.31 The expression of Pgp was associated with poor response to paclitaxel-based chemotherapy in patients with nonsmall cell lung and breast cancer.31,32 While the use of Pgp inhibitors to overcome this resistance has been explored, it has been unsuccessful due to the side effects, which occur when using the high doses required.33 Tubulin binding agents that are not substrates for Pgp represent a viable alternate strategy for circumventing Pgp-mediated resistance. Such agents would be extremely useful for patients that develop tumor resistance due to Pgp expression.30,34,35
The expression of βIII-tubulin is involved in clinical resistance to taxanes and vinca alkaloids in nonsmall cell lung,36–38 breast,39 ovarian,40,41 and gastric42 cancers. Colchicine site agents are generally not susceptible to βIII-tubulin mediated resistance,43,44 illustrating the importance of developing anticancer agents that bind to the colchicine site. Development of tubulin binding agents that are less sensitive to Pgp and/or βIII-tubulin mediated resistance could result in broader antitumor activity and improved rates of survival with significant advantages over other MTAs.
Effect on Pgp and βIII-Tubulin Mediated Cancer Cell Resistance
The potent MTAs 3, 4, and 6–9 were evaluated for their activity in the SK-OV-3 ovarian carcinoma cell line and the Pgp expressing subline SK-OV-3 MDR1-M6/6 (Table 3).45 In these cell lines, compound 3 was the most potent compound in the series, and it had 5-fold higher potency than the lead compound (±)-1·HCl. In addition, compound 3 had potency similar to paclitaxel and CA-4 in the SK-OV-3 cell line. Comparison of the IC50 values in the parental SK-OV-3 and genetically manipulated SK-OV-3 MDR1-M6/6 cell line allows for the calculation of a relative resistance value, designated Rr. This value is calculated by dividing the IC50 value obtained in the Pgp-expressing MDR1-M6/6 cells by the IC50 obtained in the parental SK-OV-3 cells. Paclitaxel, a known Pgp substrate has an Rr value of 240, while CA-4, a poor substrate, has an Rr value of 1.3 (Table 3). Compound 3 had IC50 values in SK-OV-3 and SK-OV-3 MDR1-M6/6 cells comparable to that of CA-4 and an Rr of 1.1, indicating that it is able to overcome drug resistance mediated by Pgp. Compounds 4 and 6–9 also had Rr values less than 2, suggesting that they are all poor substrates for Pgp-mediated transport and have advantages over the taxanes and vinca alkaloids.
Table 3.
Target Compounds 3, 4, and 6–9 Circumvent Pgp and βIII-Tubulin Mediated Resistance
| IC50 ± SD (nM)
|
IC50 ± SD (nM)
|
|||||
|---|---|---|---|---|---|---|
| compd | SK-OV-3 | SK-OV-3 MDR1-M6/6 | Rr | HeLa | HeLa WTβIII | Rr |
| (±)-1·HCla (Lead) | 38.6 ± 3.1 | 44.4 ± 3.2 | 1.2 | 37.3 ± 4.1 | 23.9 ± 1.7 | 0.6 |
| 3 | 7.7 ± 0.8 | 8.4 ± 0.4 | 1.1 | 9.5 ± 0.8 | 8.1 ± 0.9 | 0.9 |
| 4 | 14.5 ± 1.0 | 18.3 ± 1.0 | 1.3 | 15.8 ± 1.4 | 14.6 ± 1.8 | 0.9 |
| 6 | 40.3 ± 2.6 | 71.0 ± 19 | 1.8 | 33.8 ± 7.4 | 35.3 ± 9.9 | 1.0 |
| 7 | 147.8 ± 2.9 | 200.0 ± 17.1 | 1.4 | 111.1 ± 19.0 | 115.9 ± 9.8 | 1.0 |
| 8 | 155.8 ± 15.0 | 161.9 ± 21.5 | 1.0 | 120.5 ± 4.2 | 159.0 ± 17.6 | 1.3 |
| 9 | 18.6 ± 0.1 | 32.1 ± 1.5 | 1.7 | 15.7 ± 1.2 | 14.9 ± 1.9 | 0.9 |
| paclitaxel | 5.0 ± 0.6 | 1,200 ± 58 | 240 | 2.8 ± 0.36 | 24.0 ± 3.0 | 8.6 |
| CA-4 | 5.5 ± 0.5 | 7.2 ± 1.1 | 1.3 | 3.3 ± 0.4 | 3.3 ± 0.3 | 1.0 |
Results previously published.25
Compounds 3, 4 and 6–9 were also evaluated for the ability to overcome βIII-tubulin mediated resistance in an isogenic HeLa cell line pair (Table 3).45 Compound 3 was the most potent compound in the series in HeLa and HeLa WT βIII cell lines with 4-fold better activity than the lead compound (±)-1· HCl. Similar to the Rr value in the SK-OV-3 isogenic cell line pair, the Rr value was calculated by dividing the IC50 of the βIII-tubulin expressing line by the IC50 obtained in the parental HeLa cells. The expression of βIII-tubulin is known to lead to paclitaxel resistance, and paclitaxel has an Rr value of 8.6 in this cell line pair (Table 3). The target compounds 3, 4, and 6–9 have Rr values ~1.0 (Table 3), suggesting that they circumvent βIII-tubulin mediated drug resistance.
Activity of Compound 3 in the NCI Cancer Cell Line Panel
The most potent compound (3) of the series was evaluated in the NCI cancer cell line panel,46 and it had GI50 values <10 nM against 47 of the 57 cancer cell lines (Table 4). Compound 3 had better potency than the lead compound (±)-1·HCl25 in 29 cancer cell lines and comparable potency to (±)-1·HCl25 in 19 cancer cell lines (Table 4).
Table 4.
Comparison of Cancer Cell Growth Inhibitory Activity (NCI) GI50 (nM) of 3 with (±)-1·HCla
| GI50 (nM)
|
GI50 (nM)
|
GI50 (nM)
|
GI50 (nM)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| panel/cell line | 3 | (±)-1· HCl |
panel/cell line |
3 | (±)-1· HCl |
panel/cell line | 3 | (±)-1· HCl |
panel/cell line | 3 | (±)-1· HCl |
| leukemia | colon cancer | melanoma | renal cancer | ||||||||
| CCRF-CEM | <10 | 16.3 | COLO 205 | <10 | 18.3 | LOX IMVI | <10 | 22.7 | 786–0 | <10 | 34.8 |
| HL-60(TB) | <10 | <10 | HCC-2998 | <10 | 24.0 | MALME-3M | 38.7b | A498 | <10 | <10 | |
| K-562 | <10 | <10 | HCT-116 | <10 | 15.1 | M14 | <10 | 10.2 | ACHN | 22.5 | 14.6 |
| MOLT-4 | <10 | 31.8 | HCT-15 | <10 | <10 | MDA-MB-435 | <10 | <10 | CAKI-1 | <10 | |
| RPMI-8226 | <10 | 15.7 | HT29 | <10 | 11.8 | SK-MEL-28 | <10 | <10 | RXF 393 | <10 | <10 |
| SR | <10 | <10 | KM12 | <10 | <10 | SK-MEL-5 | <10 | <10 | SN12C | <10 | 31.8 |
| NSCLC | <10 | SW-620 | <10 | <10 | UACC-62 | 29.6b | <10 | TK10 | 75.3b | 292 | |
| A549/ATCC | <10 | 24.4 | CNS cancer | ovarian cancer | UO-31 | <10 | 13.4 | ||||
| EKVX | <10 | 19.6 | SF-268 | 10.1 | 15.2 | IGROVI | <10 | 11.0 | prostate cancer | ||
| HOP-62 | 18.4b | 19.4 | SF-295 | <10 | <10 | OVCAR-3 | <10 | <10 | PC-3 | <10 | 14.6 |
| HOP-92 | 62.5 | 55.4 | SF-539 | <10 | 11.4 | OVCAR-4 | <10 | 26.8 | DU-145 | <10 | 21.4 |
| NCI-H226 | <10 | 31.5 | SNB-19 | <10 | 36.9 | OVCAR-5 | 12.8 | 38.5 | breast cancer | ||
| NCI-H23 | <10 | 16.4 | SNB-75 | <10 | <10 | OVCAR-8 | <10 | 32.8 | MCF7 | <10 | <10 |
| NCI-H322M | >100b | 59.0 | U251 | <10 | 12.5 | NCI/ADR-RES | <10 | <10 | MDA-MB-231/ATCC | <10 | 24.6 |
| NCI-H460 | <10 | 23.4 | SK-OV-3 | <10 | 27.5 | HS 578T | <10 | <10 | |||
| NCI-H522 | <10 | <10 | BT-549 | 59.6 | 21.9 | ||||||
| MDA-MB-468 | <10 | <10 | |||||||||
Results previously published.25
GI50 values in µM.
1H NMR Evidence for Conformational Restriction in Compound 3
The 1H NMR spectra of the N-H analogue 2 and N-Me analogue 3 in DMSO-d6 afforded additional information related to the conformational restriction in 3 (Figures 8 and 9). For 2, the “5-Me” protons appeared at δ 2.40, whereas for 3 they were significantly shielded at δ 1.05. This shielding of the “5-Me” protons in 3 was attributed to a diamagnetic anisotropic effect in 3 arising from the proximity of the phenyl ring as shown in Figure 8 (more favored anti-conformation for 3 on the basis of the N-Me and the 5-Me groups). The steric bulk of the N-Me and/or steric clash of the N-Me and the 5-Me groups in 3 restricts the conformation and positions the phenyl group on top of the 5-Me moiety (Figure 9B), resulting in the observed shielding effect, in the 1H NMR, on the 5-Me group in 3 as compared to that in 2.
Figure 8.
Conformational restriction of C4–N and N–C1′ bonds in the presence of the N-Me moiety.
Figure 9.
Conformations and 1H NMR analyses of 2 (A) and 3 (B).
This shielding effect of the phenyl group on the 5-Me group (δ ≈ 1.10) was also observed for the N4-alkyl analogues 4–7 and N4-Me analogues 8 and 9 (Figure 10).
Figure 10.
δ values (1H NMR) for compounds 2–9.
The prediction of the possible putative bound conformation of 3 gleaned from the 1H NMR was further supported by the docked pose of 3 in the colchicine site (Figure 11A, MOE 2013.080127). The phenyl ring of 3 sits on top of the 5-Me group of the furo[2,3-d]pyrimidine scaffold and exerts its anisotropic effect on the 5-Me protons. In the docked pose of 3, the distance of the 5-Me with the most proximal carbons on the phenyl ring were found to be 3.62 and 4.08 Å. Similar mode of binding was observed for compounds 4–9 when docked in the colchicine site of tubulin (Figure 11B).
Figure 11.
(A) Docked pose of 3 without the protein showing the distance (in Å) between 5-Me and the closest carbons on the phenyl ring. (B) Stereoview. Superimposition of docked poses of 3 (green), 4 (magenta), 5 (yellow), 6 (blue), 7 (brown), 8 (orange), and 9 (cyan) in the colchicine site of tubulin.
Antitumor Activity of Compound 3 in MDA-MB-435 Xenografts
Compound 3 was selected for an in vivo xenograft mouse study in light of its nanomolar potency in vitro in the NCI cancer cell line panel and its potent microtubule depolymerization activity. Compound 3 was tested for in vivo antitumor effects in the MDA-MB-435 xenograft model (Figure 12). MDA-MB-435 tumor fragments were injected sc into the flank of nude mice. Once tumors reached ~200 mm3, mice were injected three times ip with 3 (cumulative dose 170 mg/ kg) or six times with paclitaxel (120 mg/kg cumulative dose). The results show that 3 caused statistically significant inhibition of tumor growth as compared to the untreated control, and the mean tumor volume was slightly lower than that achieved with paclitaxel.
Figure 12.
Effect of 3 and paclitaxel (PTX) on MDA-MB-435 tumor growth. Nude mice bilaterally implanted with MDA-MB-435 tumor fragments were dosed with 60 mg/kg of 3 on days 0 and 2; and 50 mg/kg of 3 on day 8 or PTX at 20 mg/kg on days 0, 2, 4, 6, 8, and 11 as a positive control. (A) The average tumor volume is graphed with error bars representing the standard deviation. (B) The final tumor volumes are plotted with lines representing the mean and 95% confidence intervals. *p = 0.0425 and **p = 0.0014.
SUMMARY
A series of eight 5-methyl-furo[2,3-d]pyrimidines were designed and synthesized as microtubule depolymerizing agents. Synthesis of target compounds involved an Ullmann coupling reaction as the key step. Compounds 3, 4, and 9 showed microtubule depolymerizing activity at concentrations less than 100 nM. Compounds 3, 4, and 6–9 demonstrated the ability to cause G2/M cell cycle accumulation and induce the formation of abnormal mitotic spindles. Replacing the N-Me group with higher alkyl groups led to a 2–10-fold decrease in microtubule depolymerizing activity. N4-Alkyl-4′-OMe analogues 4, 6, and 7 and the N4-Me-4′-SMe analogue 9 inhibited tubulin assembly with activity comparable to that of CA-4. Compounds 3, 4, 6, and 9, at 5 µM, inhibited [3H]colchicine binding to tubulin about as well as did CA-4. Compounds 3, 4, and 6–9 circumvented Pgp and βIII-tubulin mediated drug resistance. Proton NMR and molecular modeling were utilized to determine the role of the N4-methyl group in tubulin inhibitory activity. The N4-alkyl group, in addition to the 5-Me group, on the furo[2,3-d]pyrimidine scaffold restricts the conformation about the C4–N and N–C1′ bonds and contributes, in part, to the biological activity of these analogues. The N4-H analogue 2, where such a conformational restriction is not attainable, is essentially devoid of activity as an MTA. Compound 3 displayed nanomolar antiproliferative activity in the NCI cancer cell line panel and thus was selected for xenograft study. In MDA-MB-435 xenografts in mice, compound 3 significantly inhibited tumor growth. Compound 3 is an important preclinical candidate as a MTA and is currently undergoing extensive preclinical evaluations.
EXPERIMENTAL SECTION
Chemistry
All evaporations were carried out in vacuum with a rotary evaporator. Analytical samples were dried in vacuo (0.2 mmHg) in a CHEM-DRY drying apparatus over P2O5 at 50 °C. Thin-layer chromatography (TLC) was performed on Whatman Sil G/UV254 silica gel plates, and the spots were visualized by irradiation with ultraviolet light (254 and 366 nm). Proportions of solvents used for TLC are by volume. All analytical samples were homogeneous on TLC in at least two different solvent systems. Column chromatography was performed on a 70–230 mesh silica gel (Fisher Scientific) column. The amount (weight) of silica gel for column chromatography was in the range of 50–100 times the amount (weight) of the crude compounds being separated. Columns were wet-packed with appropriate solvent unless specified otherwise. Melting points were determined using a digital MEL-TEMP II melting point apparatus with FLUKE 51 K/J electronic thermometer or using an MPA100 OptiMelt automated melting point system and are uncorrected. Nuclear magnetic resonance spectra for proton (1H NMR) were recorded on Bruker Avance II 400 (400 MHz) and 500 (500 MHz) systems and were analyzed using MestReC NMR data processing software. The chemical shift (δ) values are expressed in ppm (parts per million) relative to tetramethylsilane as an internal standard: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad singlet; exch, protons exchangeable by addition of D2O.
Elemental analyses or high-performance liquid chromatography (HPLC)/mass analysis were used to determine the purities of the target compounds. Elemental analyses were performed by Atlantic Microlab, Inc., Norcross, GA, USA. Elemental compositions are within ±0.4% of the calculated values and indicate >95% purity. Fractional moles of water or organic solvents frequently found in some analytical samples could not be prevented despite 24–48 h of drying in vacuo and were confirmed where possible by their presence in the 1H NMR spectra. Mass spectral data were acquired on an Agilent G6220AA TOF LC/MS system using the nano ESI (Agilent chip tube system with infusion chip). HPLC analysis was performed on a Waters HPLC system using XSelect CSH C18 column. Peak area of the major peak versus other peaks was used to determine purity. All solvents and chemicals were purchased from Sigma-Aldrich Co. or Fisher Scientific Inc. and were used as received.
N-(4-Methoxyphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (2)
A 50 mL round-bottom flask was charged with CuI (66.5 mg, 0.35 mmol), anhydrous potassium carbonate (480 mg, 3.5 mmol), l-proline (80 mg, 0.7 mmol), compound 12 (150 mg, 1 mmol), and 4-iodoanisole 13 (350 mg, 3.5 mmol). The flask was connected to a vacuum for 3 min followed by addition of anhydrous DMF (15 mL) using syringe. The flask was purged with argon for 5 min and then stirred at 110 °C. Upon heating, the color of the suspension turned blueish gray, and this color persisted for about 2 h. The reaction was stirred for another 22 h at 110 °C, at the end of which the mixture was allowed to cool to room temperature. Ethyl acetate (25 mL) was added, and the mixture was poured into water (100 mL). The product was extracted with ethyl acetate (100 mL × 2). The combined organic extracts were washed with brine (100 mL), dried (anhydrous Na2SO4), and concentrated under reduced pressure. Silica gel (500 mg) was added and the solvent evaporated to obtain a plug. Purification by column chromatography using hexanes and ethyl acetate (10:1 to 2:1) afforded 87.5 mg (33%) of 2 as a light brown solid. TLC Rf = 0.77 (CHCl3/MeOH, 10:1); mp 99.5–101.6 °C; 1H NMR (400 MHz, DMSO-d6) δ = 2.383–2.386 (d, 3H, 5-CH3, J = 1.2 Hz), 3.75 (s, 3H, OCH3), 6.92–6.94 (d, 2H, Ar, J = 8.8 Hz), 7.47–7.49 (d, 2H, Ar, J = 8.8 Hz), 7.650–7.653 (d, 1H, C6-CH, J = 1.2 Hz), 8.23 (s, 1H, C2-CH), 8.38 (s, 1H, 4-NH, exch). Anal. (C14H13N3O2) C, H, N.
N-(4-Methoxyphenyl)-N,5-dimethylfuro[2,3-d]pyrimidin-4-amine (3)
In a 25 mL round-bottom flask, compound 2 (510 mg, 2 mmol) was dissolved in DMF (10 mL). The flask was purged with argon for 5 min and cooled to 0 °C using an ice bath. NaH (144 mg, 6 mmol) was added to the solution at 0 °C, and the reaction mixture was stirred for 30 min at 0 °C under an argon atmosphere. Dimethyl sulfate (757 mg, 6 mmol) was injected into the reaction mixture, and the flask was warmed to room temperature. The mixture was stirred for 3 h, and 1 N HCl (5 mL) was added carefully to quench the reaction. Water (20 mL) was added, resulting in formation of a precipitate. The product was extracted with ethyl acetate (50 mL × 3). The combined organic extracts were washed with brine (10 mL), dried (anhydrous Na2SO4), and concentrated under reduced pressure. Silica gel (2 g) was added, and the solvent was evaporated to yield a plug. Column chromatography by elution with hexanes and ethyl acetate (5:1) afforded 3 (416 mg, 70%) as an off-white solid. TLC Rf = 0.87 (CHCl3/MeOH, 10:1); mp 87–88 °C; 1H NMR (400 MHz, DMSO-d6) δ = 1.05 (s, 3H, 5-CH3), 3.44 (s, 3H, OCH3), 3.77 (s, 3H, NCH3), 6.96–6.98 (d, 2H, Ar, J = 9.0 Hz), 7.19–7.22 (d, 2H, Ar, J = 9.0 Hz), 7.52 (s, 1H, C6-CH), 8.45 (s, 1H, C2-CH). Anal. (C15H15N3O2) C, H, N.
General Procedure for the Synthesis of Substituted Furo-[2,3-d]pyrimidines 4–7
In a 25 mL round-bottom flask, compound 2 (1 equiv) was dissolved in DMF (2 mL). The flask was purged with argon for 5 min and cooled to 0 °C using an ice bath. NaH (3 equiv) was added to the solution at 0 °C, and the reaction mixture was stirred for 20 min under argon atmosphere. The appropriate alkyl iodide (3–4 equiv) was injected into the reaction mixture, and the flask was warmed to room temperature. The mixture was stirred at room temperature until TLC showed disappearance of the reactant 2. Aqueous 1 N HCl was added dropwise to quench the reaction. Water (4 mL) was added, resulting in formation of a precipitate. The product was extracted with ethyl acetate (10 mL × 3). The combined organic extracts were washed with brine (10 mL), dried (anhydrous Na2SO4), and concentrated under reduced pressure. Silica gel (200 mg) was added, and the solvent was evaporated under reduced pressure. The resulting plug was loaded onto a silica gel column in hexanes (the silica was 20 times the weight of the plug), and the column was eluted with hexanes and ethyl acetate (5:1) to obtain 4–7 in yields of 57–85%.
N-Ethyl-N-(4-methoxyphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (4)
Using the general procedure described above, ethyl iodide 14 (61.70 mg or 0.1 mL, 1.2 mmol) was added to an ice-cold solution of 2 (100 mg, 0.4 mmol) and NaH (28.8 mg, 1.2 mmol) in 2 mL of DMF, and the reaction was run at room temperature for 2 h to provide 94 mg (85%) of 4 as an off-white solid. TLC Rf = 0.88 (CHCl3/ MeOH, 15:1); mp 77.8–78.3 °C; 1H NMR (400 MHz, CDCl3) δ = 1.064–1.067 (d, 3H, 5-CH3, J = 1.2 Hz), 1.22 (t, 3H, CH3, J = 7 Hz), 3.82 (s, 3H, OCH3), 4.06–4.11 (q, 2H, N–CH2, J = 7 Hz), 6.88–6.91 (d, 2H, Ar, J = 8.9 Hz), 7.074–7.077 (d, 1H, C6-CH, J = 1.2 Hz), 7.09–7.11 (d, 2H, Ar, J = 8.9 Hz), 8.48 (s, 1H, C2-CH). Anal. (C16H17N3O2) C, H, N.
N-Isopropyl-N-(4-methoxyphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (5)
Using the general procedure described above, isopropyl iodide 15 (266.36 mg, 1.6 mmol) was added to an ice-cold solution of 2 (100 mg, 0.4 mmol) and NaH (38.4 mg, 1.6 mmol) in 2 mL of DMF, and the reaction was run at room temperature for 6 h to provide 75 mg (63%) of 5 as an off-white solid. TLC Rf = 0.88 (CHCl3/ MeOH, 15:1); mp 95.8–96.6 °C; 1H NMR (400 MHz, CDCl3) δ = 1.02 (d, 3H, 5-CH3, J = 1.3 Hz), 1.14–1.16 (d, 6H, two CH3, J = 6.8 Hz), 3.79 (s, 3H, OCH3), 5.30–5.37 (m, 1H, N–CH), 6.85–6.87 (d, 2H, Ar, J = 8.9 Hz), 7.000–7.004 (d, 1H, C6-CH, J = 1.3 Hz), 7.02–7.04 (d, 2H, Ar, J = 8.9 Hz), 8.42 (s, 1H, C2-CH). Anal. (C17H19N3O2) C, H, N.
N-(4-Methoxyphenyl)-5-methyl-N-propylfuro[2,3-d]pyrimidin-4-amine (6)
Using the general procedure described above, propyl iodide 16 (199 mg or 0.12 mL, 1.2 mmol) was added to an ice-cold solution of 2 (100 mg, 0.4 mmol) and NaH (28.8 mg, 1.2 mmol) in 2 mL of DMF, and the reaction was run at room temperature for 2 h to provide 80 mg (69%) of 6 as an off-white solid. TLC Rf = 0.88 (CHCl3/ MeOH, 15:1); mp 113.5–114.2 °C; 1H NMR (400 MHz, CDCl3) δ = 0.94–0.97 (t, 3H, CH3), 1.095–1.098 (d, 3H, 5-CH3, J = 1.2 Hz), 1.70–1.74 (m, 2H, CH2), 3.84 (s, 3H, OCH3), 3.96–3.99 (m, 2H, N–CH2), 6.89–6.91 (d, 2H, Ar, J = 8.95 Hz), 7.092–7.095 (d, 1H, C6-CH, J = 1.2 Hz), 7.10–7.12 (d, 2H, Ar, J = 9.0 Hz), 8.49 (s, 1H, C2-CH). Anal. (C17H19N3O2) C, H, N.
N-Butyl-N-(4-methoxyphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (7)
Using the general procedure described above, butyl iodide 17 (216.26 mg or 0.13 mL, 1.2 mmol) was added to an ice-cold solution of 2 (100 mg, 0.4 mmol) and NaH (28.8 mg, 1.2 mmol) in 2 mL of DMF, and the reaction was run at room temperature for 2.5 h to provide 70 mg (57%) of 7 as a semisolid. TLC Rf = 0.88 (CHCl3/ MeOH, 15:1); 1H NMR (400 MHz, CDCl3) δ = 0.88–0.92 (t, 3H, CH3), 1.06 (s, 3H, 5-CH3), 1.30–1.39 (m, 2H, CH2), 1.59–1.67 (m, 2H, CH2), 3.81 (s, 3H, OCH3), 3.96–4.00 (m, 2H, N–CH2), 6.86–6.88 (d, 2H, Ar, J = 8.9 Hz), 7.06–7.09 (m, 3H, Ar and C6-CH), 8.45 (s, 1H, C2-CH). HRMS (ESI): m/z calculated for C18H21N3O2 + H+ [M + H+]: 312.1712. Found: 312.1709. HPLC analysis: retention time = 38.76 min; peak area, 97.33%; eluent A, H2O; eluent B, ACN; gradient elution (100% H2O to 10% H2O) over 60 min with a flow rate of 0.5 mL/min and detection at 254 nm; column temperature, rt.
General Procedure for the Synthesis of Substituted Furo-[2,3-d]pyrimidines 8 and 9
In a 25 mL round-bottom flask, the appropriate furo[2,3-d]pyrimidine (1 equiv of 20 or 21) was dissolved in DMF. The flask was purged with argon for 5 min and cooled to 0 °C using an ice bath. To the solution at 0 °C was added NaH (3 equiv), and the reaction mixture was stirred for 20 min at 0 °C under argon atmosphere. Dimethyl sulfate (3 equiv) was injected into the reaction mixture, and the flask was warmed to room temperature. The mixture was stirred at room temperature until TLC showed disappearance of the reactant 20 or 21. Aqueous 1 N HCl was added dropwise to quench the reaction, followed by water (20 mL) to yield a precipitate. The product was extracted with ethyl acetate (10 mL × 3). The combined organic extracts were washed with brine (10 mL), dried (anhydrous sodium sulfate), and concentrated under reduced pressure. Silica gel (200 mg) was added, and the solvent was evaporated to obtain a plug. The plug was loaded onto a silica gel column in hexanes (the silica was 20 times the weight of the plug), and the column was eluted with hexanes and ethyl acetate (5:1) to obtain 8 or 9.
N-Methyl-N-(4-ethylphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (8)
Using the general procedure described above, the reaction between 20 (40 mg, 0.16 mmol) and dimethyl sulfate (0.04 mL, 0.48 mmol) in DMF (2 mL) in the presence of NaH (11.5 mg, 0.48 mmol) provided 23 mg (53%) of 8 as a white solid. TLC Rf = 0.77 (CHCl3/ MeOH, 10:1); mp 116.3–116.8 °C; 1H NMR (400 MHz, CDCl3) δ = 1.09 (s, 3H, 5-CH3), 1.24–1.28 (t, 3H, CH3), 2.64–2.70 (q, 2H, CH2), 3.57 (s, 3H, NCH3), 7.10–7.12 (d, 2H, Ar), 7.19–7.21 (m, 3H, Ar and C6-CH), 8.56 (s, 1H, C2-CH). Anal. (C16H17N3O·0.07CH3CO2C2H5) C, H, N.
N,5-Dimethyl-N-(4-(methylthio)phenyl)furo[2,3-d]pyrimidin-4-amine (9)
Using the general procedure described above, the reaction between 21 (105 mg, 0.4 mmol) and dimethyl sulfate (0.08 mL, 1.2 mmol) in DMF (8 mL) in the presence of NaH (28.8 mg, 1.2 mmol) provided 56 mg (51%) of 9 as a brown solid. TLC Rf = 0.88 (CHCl3/ MeOH, 10:1); mp 95.8–96.2 °C; 1H NMR (400 MHz, DMSO-d6) δ = 1.109–1.112 (d, 3H, 5-CH3, J = 1.1 Hz), 2.48 (s, 3H, SCH3), 3.47 (s, 3H, NCH3), 7.20–7.22 (d, 2H, Ar, J = 8.65 Hz), 7.28–7.30 (d, 2H, Ar, J = 8.66 Hz), 7.567–7.570 (d, 1H, C6-CH, J = 1.2 Hz), 8.50 (s, 1H, C2-CH). Anal. (C15H15N3OS) C, H, N, S.
2-Amino-4-methylfuran-3-carbonitrile (11)
To a solution of malononitrile (1.46 g, 22.1 mmol) in anhydrous MeOH (20 mL) was added triethylamine (2.23 g, 22.1 mmol) under argon atmosphere. To this solution at 0 °C was added a solution of hydroxyacetone 10 (1.64 g, 1.08 g/mL, 22.1 mmol) in 10 mL of anhydrous methanol. The solution was warmed to room temperature and stirred for 14 h. The solvents were removed in vacuo to give 11 as a brown-colored crude solid. TLC Rf = 0.60 (hexane/EtOAc, 10:1); 1H NMR (400 MHz, CDCl3) δ = 2.01 (s, 3H, 4-CH3), 4.75 (br, 2H, 2-NH2), 6.61 (s, 1H, C5-CH). 1H NMR agreed well with the literature28 values. This material was used directly for the next step without purification.
5-Methylfuro[2,3-d]pyrimidin-4-amine (12)
In a 500 mL round-bottom flask, sodium metal (2.3 g, 0.1 mol) was added cautiously to stirred anhydrous EtOH (5.8 mL, 0.1 mol) over 10 min at room temperature. After stirring for another 5 min, formamidine hydrochloride (8.05 g, 0.1 mol) was added. The resulting slurry was stirred at room temperature for 30 min, after which a solution of 11 (13 g crude, ~0.1 mol) in anhydrous EtOH (200 mL) was added. The mixture was heated to reflux for 8 h. After cooling the reaction mixture to room temperature, silica gel (25 g) was added, and the solvent was evaporated under reduced pressure to obtain a plug. Purification was performed by flash chromatography using 1% MeOH in CHCl3. Fractions containing the product (TLC) were pooled and evaporated to provide 7.1 g (47%, two steps) of 12 as lustrous pink crystals. TLC Rf = 0.29 (CHCl3/MeOH, 10:1); mp 240.2–242.5 °C; 1H NMR (400 MHz, DMSO-d6) δ = 2.288–2.292 (d, 3H, CH3, J = 1.4 Hz), 7.02 (br, 2H, NH2, exch), 7.533–5.536 (d, 1H, C6-CH, J = 1.4 Hz), 8.13 (s, 1H, C2-CH). Anal. (C7H7N3O) C, H, N.
General Procedure for the Synthesis of N-(4-Substituted-phenyl)-5-methylfuro[2,3-d]pyrimidin-4-amines 20 and 21
A 50 mL round-bottom flask was charged with CuI (66.5 mg, 0.35 mmol), anhydrous potassium carbonate (480 mg, 3.5 mmol), l-proline (80 mg, 0.7 mmol), compound 12 (150 mg, 1 mmol), and the appropriate iodobenzene (3.5 mmol). The flask was connected to a vacuum for 3 min, followed by addition of anhydrous DMF (15 mL) using a syringe. The flask was purged with argon for 5 min and then stirred at 110 °C. On heating, the color of the suspension turned blueish gray, and this color change lasted for about 2 h. The reaction was stirred for an additional 22 h at 110 °C, at the end of which the mixture was allowed to cool to room temperature. Ethyl acetate (25 mL) was added, and the mixture was poured into water (100 mL). The product was extracted with ethyl acetate (100 mL × 2). The combined organic extracts were washed with brine (100 mL), dried (anhydrous Na2SO4), and concentrated under reduced pressure. Silica gel (500 mg) was added, and the solvent evaporated to yield a plug, which was purified by column chromatography using hexanes and ethyl acetate (10:1 to 2:1). Fractions containing the product (TLC) were pooled and evaporated to afford 20 or 21.
N-(4-Ethylphenyl)-5-methylfuro[2,3-d]pyrimidin-4-amine (20)
Using the general procedure described above, reaction between 12 (150 mg, 1 mmol) and 1-ethyl-4-iodobenzene 18 (350 mg, 3.5 mmol) provided 74 mg (29%) of 20 as a brown semisolid. TLC Rf = 0.78 (CHCl3/MeOH, 10:1); 1H NMR (400 MHz, CDCl3) δ = 1.23–1.27 (t, 3H, CH3), 2.421–2.423 (d, 3H, 5-CH3, J = 0.9 Hz), 2.65–2.69 (q, 2H, CH2), 6.85 (s, 1H, 4-NH, exch), 7.21–7.23 (d, 2H, Ar, J = 8.3 Hz), 7.296–7.298 (d, 1H, C6-CH, J = 0.9 Hz), 7.51- 7.53 (d, 2H, Ar, J = 8.3 Hz), 8.45 (s, 1H, C2-CH). Anal. (C15H15N3O·0.1CH3CO2C2H5) C, H, N.
5-Methyl-N-(4-(methylthio)phenyl)furo[2,3-d]pyrimidin-4-amine (21)
Using the general procedure described above, reaction between 12 (150 mg, 1 mmol) and 4-iodo-thioanisole 19 (350 mg, 3.5 mmol) afforded 40 mg (15%) of 21 as a brown semisolid. TLC Rf = 0.83 (CHCl3/MeOH, 10:1); 1H NMR (400 MHz, CDCl3): δ = 2.41 (s, 3H, SCH3), 2.51 (s, 3H, 5-CH3), 7.02 (s, 1H, 4-NH, exch), 7.28–7.34 (m, 3H, Ar and C6-CH), 7.57–7.59 (d, 2H, Ar), 8.47 (s, 1H, C2-CH). Anal. (C14H13N3OS·0.07CH3(CH2)4CH3) C, H, N, S.
Molecular Modeling
Docking of target compounds 2–9 was carried out in the colchicine site of tubulin (PDB: 4O2B, 2.30 Å)26 using Molecular Operating Environment (MOE 2013.0801).27 he crystal structures were obtained from the protein database and imported into MOE 2013.0801. The proteins were then prepared using the LigX function and the Amber99 forcefield for energy minimization under default settings. Ligands were prepared using the builder function in MOE and minimized using MMF94x forcefield. The ligands were then docked in the binding site using the Alpha triangle placement method. Refinement was carried out using Forcefield and scored using the Affinity dG scoring system.
After the preparation of the protein using the LigX function, chains C, D, E, and F were deleted along with Ca2+, Mg2+, GDP, GTP, and all the other bound ligands except colchicine. To validate the docking study at the colchicine site, the native ligand colchicine was redocked into the binding site using the same set of parameters as described above. The rmsd of the best docked pose was 0.5347 Å, thus validating the docking using MOE.
Biological Studies
Effects of Compounds on Cellular Microtubules
A-10 cells were used to evaluate the effects of the compounds on cellular microtubules using indirect immunofluorescence techniques. Cells were treated for 18 h with compounds, and microtubules were visualized with a β-tubulin antibody (Sigma-Aldrich, St. Louis, MO). EC50 values were calculated as previously described47 and represent an average of at least three independent experiments.
Sulforhodamine B (SRB) Assay
The SRB assay was used to evaluate the antiproliferative and cytotoxic effects of the compounds against cancer cells as previously described.45 MDA-MB-435, SK-OV-3, and HeLa cells were purchased from the American Type Culture Collection (Manassas, VA). Details about the generation of the SK-OV-3 MDR1-M6/6 and HeLa WTβIII cells were previously described.45 The IC50 values represent an average of three independent experiments using triplicate points in each experiment.
Quantitative Tubulin Studies
Bovine brain tubulin was purified as described previously.48 The tubulin assembly assay has been described in detail. 49 Briefly, 1.0 mg/mL of tubulin (10 µM) was preincubated for 15 min at 30 °C in 0.8 M monosodium glutamate (pH of 2 M stock solution adjusted to 6.6 with HCl), varying compound concentrations and 4% (v/v) DMSO as compound solvent. After preincubation, the reaction mixtures were placed on ice, and 0.4 mM GTP was added. The reaction mixtures were transferred to cuvettes at 0 °C in a recording spectrophotometer equipped with an electronic temperature controller. After baselines were established, the temperature was elevated over about 30 s to 30 °C, and changes in turbidity were monitored for 20 min. The compound concentration that caused a 50% reduction in increase in turbidity, interpolated from the values obtained with defined compound concentrations, was defined as the IC50 value. The assay to measure inhibition of [3H]colchicine binding was described in detail previously.50 Briefly, 0.1 mg/mL (1.0 µM) tubulin was incubated at 37 °C with 5.0 µM [3H]colchicine and potential inhibitors at 1.0 or 5.0 µM, as indicated. Incubation was for 10 min, at which point the reaction has reached 40–60% of the maximum colchicine that can be bound in reaction mixtures without inhibitor. The [3H]colchicine was a product of PerkinElmer. CA4 was a generous gift of Dr. G. R. Pettit, Arizona State University.
Cell Culture
HeLa, A-10, SK-OV-3, and SK-OV-3 MDR1-M6/6 cells were grown at 37 °C with 5% CO2 and maintained in Basal Medium Eagle (Sigma-Aldrich, St. Louis, MO) supplemented with 10% FBS (Hyclone, GE Life Sciences, Logan, UT), 1% GlutaMAX (Gibco, Life Technologies, Waltham, MA), and 50 µg/mL gentamycin (Life Technologies). HeLa WTβIII cells were grown at 37 °C with 5% CO2 and maintained in Dulbecco’s Modified Eagle Medium (Life Technologies) supplemented with 10% FBS (Hyclone, GE Life Sciences) and 50 µg/mL gentamycin (Life Technologies). MDA-MB-435 cells were grown at 37 °C with 5% CO2 and maintained in Improved Minimum Essential Medium (Life Technologies) supplemented with 10% FBS (Hyclone, GE Life Sciences) and 25 µg/mL gentamycin (Life Technologies).
Flow Cytometry
Cell cycle distribution was evaluated by flow cytometry. HeLa cells in log phase growth were treated with 5-times the IC50 value (Table 3) of each test compound: 16.5 nM CA-4, 47.5 nM 3, 79 nM 4, 169 nM 6, 605 nM 8, 555 nM 7, 78.5 nM 9, or vehicle (0.2% DMSO). Cells were treated for 18 h, harvested on ice and stained with Krishan’s reagent.51 DNA content was analyzed using a BD LSRII flow cytometer (BD Biosciences, San Jose, CA). ModFitLT 3.3 (Verity Software House, Topsham, ME) was used to determine the percentage of cells in each phase of the cell cycle.
MDA-MB-435 Flank Xenograft Model
MDA-MB-435 tumor fragments were implanted sc into the flanks of nude mice. Once tumors reached ~200 mm3, mice were injected ip with compound 3 (60 mg/kg on days 0 and 2 and 50 mg/kg on day 8) or paclitaxel (20 mg/kg on days 0, 2, 4, 6, 8, and 11). Compound 3 was dissolved in a 50:50 Cremophor/DMSO mixture and further dissolved in PBS for a final concentration of less than 5% (v/v) Cremophor/DMSO in PBS. Paclitaxel was dissolved in a 50:50 Cremophor/EtOH mixture and further dissolved in PBS for a final solvent concentration of less than 5% (v/v) Cremophor/EtOH in PBS. Tumor volumes and mouse weights were measured 2–3 times a week. One-way ANOVA with Dunnett’s post hoc test was used to determine significance of final tumor volumes of drug-treated groups as compared to untreated control. Other experimental agents were evaluated during this trial and the untreated and paclitaxel controls published previously.52 These animal studies were performed in compliance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio.
Supplementary Material
Acknowledgments
We acknowledge the National Cancer Institute Developmental Therapeutics Program for performing the in vitro evaluation in the NCI 60-cell line panel. This work was supported, in part, by a grant from the National Institutes of Health, National Cancer Institute (CA142868 (to A.G., S.L.M.)); by the President’s Council Research Excellence Award (to S.L.M.), Duquesne University Adrian Van Kaam Chair in Scholarly Excellence (to A.G.); the CTRC Cancer Center Support Grant P30 CA054174 and the Flow Cytometry Shared Resource; and by an NSF equipment grant for NMR instrumentation (NMR: CHE 0614785). We thank Mr. Arpit Doshi, Duquesne University, for the NMR analysis of compound 2.
ABBREVIATIONS USED
- NCI
National Cancer Institute
- MTAs
microtubule targeting agents
- CA-1
combretastatin A-1
- CA-4
combretastatin A-4
- DMSO
dimethyl sulfoxide
- DMF
dimethylformamide
- PDB
Protein Data Bank
- SRB
sulforhodamine B
- Pgp
P-glycoprotein
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmed-chem.6b00237.
Elemental analysis, high-resolution mass spectra (HRMS) (EI), and docked poses of target compounds 4–9 (PDF)
SMILES (CSV)
Figure 3 (PDB)
Figure 11b (PDB)
Figure S1 (PDB)
Figure S2 (PDB)
Figure S3 (PDB)
Figure S4 (PDB)
Figure S5 (PDB)
Figure S6 (PDB)
The content of this paper is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health.
The authors declare no competing financial interest.
References
- 1.Dumontet C, Jordan MA. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discovery. 2010;9:790–803. doi: 10.1038/nrd3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat. Rev. 2009;35:255–261. doi: 10.1016/j.ctrv.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011;31:443–481. doi: 10.1002/med.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Sun Y. Targeting p53 for novel anticancer therapy. Transl. Oncol. 2010;3:1–12. doi: 10.1593/tlo.09250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor PM, Jackman J, Bae I, Myers TG, Fan S, Mutoh M, Scudiero DA, Monks A, Sausville EA, Weinstein JN, Friend S, Fornace AJ, Kohn KW. Characterization of the p53 tumor suppressor pathway in cell lines of the National Cancer Institute anticancer drug screen and correlations with the growth-inhibitory potency of 123 anticancer agents. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 6.Komlodi-Pasztor E, Sackett D, Wilkerson J, Fojo T. Mitosis is not a key target of microtubule agents in patient tumors. Nat. Rev. Clin. Oncol. 2011;8:244–250. doi: 10.1038/nrclinonc.2010.228. [DOI] [PubMed] [Google Scholar]
- 7.Löwe J, Li H, Downing KH, Nogales E. Refined structure of αβ-tubulin at 3.5 Å resolution. J. Mol. Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 8.Rao S, He L, Chakravarty S, Ojima I, Orr GA, Horwitz SB. Characterization of the taxol binding site on the microtubule Identification of Arg(282) in beta-tubulin as the site of photo-incorporation of a 7-benzophenone analogue of taxol. J. Biol. Chem. 1999;274:37990–37994. doi: 10.1074/jbc.274.53.37990. [DOI] [PubMed] [Google Scholar]
- 9.Lee JF, Harris LN. Antimicrotubule Agents. In: DeVita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles & Practice of Oncology. 8. Lippincott Williams & Wilkins; 2008. pp. 447–456. [Google Scholar]
- 10.Altmann K-H. Microtubule-stabilizing agents: A growing class of important anticancer drugs. Curr. Opin. Chem. Biol. 2001;5:424–431. doi: 10.1016/s1367-5931(00)00225-8. [DOI] [PubMed] [Google Scholar]
- 11.Prota AE, Bargsten K, Northcote PT, Marsh M, Altmann K-H, Miller JH, Díaz JF, Steinmetz MO. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew. Chem., Int. Ed. 2014;53:1621–1625. doi: 10.1002/anie.201307749. [DOI] [PubMed] [Google Scholar]
- 12.Prota AE, Bargsten K, Diaz JF, Marsh M, Cuevas C, Liniger M, Neuhaus C, Andreu JM, Altmann K-H, Steinmetz MO. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc. Natl. Acad. Sci. U. S. A. 2014;111:13817–13821. doi: 10.1073/pnas.1408124111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh D-Y, Diéras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012;367:1783–1791. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Chen J, Xiao M, Li W, Miller D. An overview of tubulin inhibitors that interact with the colchicine binding site. Pharm. Res. 2012;29:2943–2971. doi: 10.1007/s11095-012-0828-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massarotti A, Coluccia A, Silvestri R, Sorba G, Brancale A. The tubulin colchicine domain: A molecular modeling perspective. ChemMedChem. 2012;7:33–42. doi: 10.1002/cmdc.201100361. [DOI] [PubMed] [Google Scholar]
- 16.Kasibhatla S, Baichwal V, Cai SX, Roth B, Skvortsova I, Skvortsov S, Lukas P, English NM, Sirisoma N, Drewe J, Pervin A, Tseng B, Carlson RO, Pleiman CM. MPC-6827: A small-molecule inhibitor of microtubule formation that is not a substrate for multidrug resistance pumps. Cancer Res. 2007;67:5865–5871. doi: 10.1158/0008-5472.CAN-07-0127. [DOI] [PubMed] [Google Scholar]
- 17.Tsimberidou A-M, Akerley W, Schabel MC, Hong DS, Uehara C, Chhabra A, Warren T, Mather GG, Evans BA, Woodland DP, Swabb EA, Kurzrock R. Phase I clinical trial of MPC-6827 (Azixa), a microtubule destabilizing agent, in patients with advanced cancer. Mol. Cancer Ther. 2010;9:3410–3419. doi: 10.1158/1535-7163.MCT-10-0516. [DOI] [PubMed] [Google Scholar]
- 18.Harrison M, Hahn N, Pili R, Oh W, Hammers H, Sweeney C, Kim K, Perlman S, Arnott J, Sidor C, Wilding G, Liu G. A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal® dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC) Invest. New Drugs. 2011;29:1465–1474. doi: 10.1007/s10637-010-9455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruce J, Eickhoff J, Pili R, Logan T, Carducci M, Arnott J, Treston A, Wilding G, Liu G. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest. New Drugs. 2012;30:794–802. doi: 10.1007/s10637-010-9618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu P, Qin Y, Wu L, Yang S, Li N, Wang H, Xu H, Sun K, Zhang S, Han X, Sun Y, Shi Y. A phase I clinical trial assessing the safety and tolerability of combretastatin A4 phosphate injections. Anti-Cancer Drugs. 2014;25:462–471. doi: 10.1097/CAD.0000000000000070. [DOI] [PubMed] [Google Scholar]
- 21.Ng Q-S, Mandeville H, Goh V, Alonzi R, Milner J, Carnell D, Meer K, Padhani AR, Saunders MI, Hoskin PJ. Phase Ib trial of radiotherapy in combination with combretastatin-A4-phosphate in patients with non-small-cell lung cancer, prostate adenocarcinoma, and squamous cell carcinoma of the head and neck. Ann. Oncol. 2012;23:231–237. doi: 10.1093/annonc/mdr332. [DOI] [PubMed] [Google Scholar]
- 22.Zweifel M, Jayson GC, Reed NS, Osborne R, Hassan B, Ledermann J, Shreeves G, Poupard L, Lu S-P, Balkissoon J, Chaplin DJ, Rustin GJS. Phase II trial of combretastatin A4 phosphate, carboplatin, and paclitaxel in patients with platinum-resistant ovarian cancer. Ann. Oncol. 2011;22:2036–2041. doi: 10.1093/annonc/mdq708. [DOI] [PubMed] [Google Scholar]
- 23.Nathan P, Zweifel M, Padhani AR, Koh D-M, Ng M, Collins DJ, Harris A, Carden C, Smythe J, Fisher N, Taylor NJ, Stirling JJ, Lu S-P, Leach MO, Rustin GJS, Judson I. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin. Cancer Res. 2012;18:3428–3439. doi: 10.1158/1078-0432.CCR-11-3376. [DOI] [PubMed] [Google Scholar]
- 24.Patterson DM, Zweifel M, Middleton MR, Price PM, Folkes LK, Stratford MRL, Ross P, Halford S, Peters J, Balkissoon J, Chaplin DJ, Padhani AR, Rustin GJS. Phase I clinical and pharmacokinetic evaluation of the vascular-disrupting agent OXi4503 in patients with advanced solid tumors. Clin. Cancer Res. 2012;18:1415–1425. doi: 10.1158/1078-0432.CCR-11-2414. [DOI] [PubMed] [Google Scholar]
- 25.Gangjee A, Zhao Y, Raghavan S, Rohena CC, Mooberry SL, Hamel E. Structure-activity relationship and in vitro and in vivo evaluation of the potent cytotoxic anti-microtubule agent N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H-cyclo-penta[d]-pyrimidin-4-aminium chloride and its analogues as antitumor agents. J. Med. Chem. 2013;56:6829–6844. doi: 10.1021/jm400639z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prota AE, Danel F, Bachmann F, Bargsten K, Buey RM, Pohlmann J, Reinelt S, Lane H, Steinmetz MO. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 2014;426:1848–1860. doi: 10.1016/j.jmb.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Molecular Operating Environment (MOE 2013.08) Chemical Computing Group, Inc.; Montreal, Quebec, Canada: www.chemcomp.com. [Google Scholar]
- 28.Gewald K. Heterocycles from CH-acidic nitriles. IX. Reaction of α-hydroxy ketones with malononitrile. Chem. Ber. 1966;99:1002–1007. [Google Scholar]
- 29.Tinley TL, Leal RM, Randall-Hlubek DA, Cessac JW, Wilkens LR, Rao PN, Mooberry SL. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Res. 2003;63:1538–1549. [PubMed] [Google Scholar]
- 30.Perez EA. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009;8:2086–2095. doi: 10.1158/1535-7163.MCT-09-0366. [DOI] [PubMed] [Google Scholar]
- 31.Chiou JF, Liang JA, Hsu WH, Wang JJ, Ho ST, Kao A. Comparing the relationship of taxol-based chemotherapy response with P-glycoprotein and lung resistance-related protein expression in non-small cell lung cancer. Lung. 2003;181:267–273. doi: 10.1007/s00408-003-1029-7. [DOI] [PubMed] [Google Scholar]
- 32.Trock BJ, Leonessa F, Clarke R. Multidrug resistance in breast cancer: A meta-analysis of MDR1/gp170 expression and its possible functional significance. J. Natl. Cancer Inst. 1997;89:917–931. doi: 10.1093/jnci/89.13.917. [DOI] [PubMed] [Google Scholar]
- 33.Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: Current status and future perspectives. Curr. Cancer Drug Targets. 2013;13:326–346. doi: 10.2174/15680096113139990076. [DOI] [PubMed] [Google Scholar]
- 34.Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- 35.Fojo T, Menefee M. Mechanisms of multidrug resistance: The potential role of microtubule-stabilizing agents. Ann. Oncol. 2007;18:3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 36.Rosell R, Scagliotti G, Danenberg KD, Lord RVN, Bepler G, Novello S, Cooc J, Crino L, Sanchez JJ, Taron M, Boni C, Marinis FD, Tonato M, Marangolo M, Gozzelino F, Costanzo FD, Rinaldi M, Salonga D, Stephens C. Transcripts in pretreatment biopsies from a three-arm randomized trial in metastatic non-small-cell lung cancer. Oncogene. 2003;22:3548–3553. doi: 10.1038/sj.onc.1206419. [DOI] [PubMed] [Google Scholar]
- 37.Dumontet C, Isaac S, Souquet P-J, Bejui-Thivolet F, Pacheco Y, Peloux N, Frankfurter A, Luduena R, Perol M. Expression of class III beta tubulin in non-small cell lung cancer is correlated with resistance to taxane chemotherapy. Bull. Cancer. 2005;92:25–30. [PubMed] [Google Scholar]
- 38.Sève P, Isaac S, Trédan O, Souquet P-J, Pachéco Y, Pérol M, Lafanéchère L, Penet A, Peiller E-L, Dumontet C. Expression of class III β-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin. Cancer Res. 2005;11:5481–5486. doi: 10.1158/1078-0432.CCR-05-0285. [DOI] [PubMed] [Google Scholar]
- 39.Tommasi S, Mangia A, Lacalamita R, Bellizzi A, Fedele V, Chiriatti A, Thomssen C, Kendzierski N, Latorre A, Lorusso V, Schittulli F, Zito F, Kavallaris M, Paradiso A. Cytoskeleton and paclitaxel sensitivity in breast cancer: The role of β-tubulins. Int. J. Cancer. 2007;120:2078–2085. doi: 10.1002/ijc.22557. [DOI] [PubMed] [Google Scholar]
- 40.Ferrandina G, Zannoni GF, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C. Class III β-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006;12:2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 41.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III β-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 42.Hwang J-E, Hong J-Y, Kim K, Kim S-H, Choi W-Y, Kim M-J, Jung S-H, Shim H-J, Bae W-K, Hwang E-C, Lee K-H, Lee J-H, Cho S-H, Chung I-J. Class III β-tubulin is a predictive marker for taxane-based chemotherapy in recurrent and metastatic gastric cancer. BMC Cancer. 2013;13:431–431. doi: 10.1186/1471-2407-13-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stengel C, Newman SP, Leese MP, Potter BVL, Reed MJ, Purohit A. Class III β-tubulin expression and in vitro resistance to microtubule targeting agents. Br. J. Cancer. 2010;102(102):316–324. doi: 10.1038/sj.bjc.6605489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Raghavan S, Ihnat M, Thorpe JE, Disch BC, Bastian A, Bailey-Downs LC, Dybdal-Hargreaves NF, Rohena CC, Hamel E, Mooberry SL, Gangjee A. The design and discovery of water soluble 4-substituted-2,6-dimethylfuro[2,3-d]-pyrimidines as multitargeted receptor tyrosine kinase inhibitors and microtubule targeting antitumor agents. Bioorg. Med. Chem. 2014;22:3753–3772. doi: 10.1016/j.bmc.2014.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risinger AL, Jackson EM, Polin LA, Helms GL, LeBoeuf DA, Joe PA, Hopper-Borge E, Luduena RF, Kruh GD, Mooberry SL. The taccalonolides: Microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Cancer Res. 2008;68:8881–8888. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.We thank the NCI for providing the tumor cell growth inhibitory activity in the NCI 60-cell line panel for compound 3.
- 47.Gangjee A, Zhao Y, Hamel E, Westbrook C, Mooberry SL. Synthesis and biological activities of (R)- and (S)-N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H–cyclopenta[d]-pyrimidin-4-aminium chloride as potent cytotoxic antitubulin agents. J. Med. Chem. 2011;54:6151–6155. doi: 10.1021/jm2007722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamel E, Lin CM. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry. 1984;23:4173–84. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- 49.Hamel E. Evaluation of antimitotic agents by quantitative comparisons of their effects on the polymerization of purified tubulin. Cell Biochem. Biophys. 2003;38:1–21. doi: 10.1385/CBB:38:1:1. [DOI] [PubMed] [Google Scholar]
- 50.Verdier-Pinard P, Lai J-Y, Yoo H-D, Yu J, Marquez B, Nagle DG, Nambu M, White JD, Falck JR, Gerwick WH, Day BW, Hamel E. Structure-activity analysis of the interaction of curacin A, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol. Pharmacol. 1998;53:62–76. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 51.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide. J. Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rohena CC, Telang NS, Da C, Risinger AL, Sikorski JA, Kellogg GE, Gupton JT, Mooberry SL. Biological characterization of an improved pyrrole-based colchicine site agent identified through structure-based design. Mol. Pharmacol. 2016;89:287–296. doi: 10.1124/mol.115.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.