Abstract
Allogeneic hematopoietic cell transplantation (HCT) is potentially curative for patients with chronic myelomonocytic leukemia (CMML), however, few data exist regarding prognostic factors and transplant outcomes. We performed this retrospective study to identify prognostic factors for post-transplant outcomes. The CMML-specific prognostic scoring system (CPSS) has been validated in subjects receiving non-transplant therapy and was included in our study. From 2001–2012, there were 209 adult subjects who received HCT for CMML reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). The median age at transplant was 57 years (range 23–74). Median follow up was 51 months (range, 3–122). On multivariate analyses, CPSS scores, Karnofsky performance status (KPS), and graft source were significant predictors of survival (p=0.004, p=0.01, p=0.01, respectively). Higher CPSS scores were not associated with disease-free survival, relapse, or transplant-related mortality. In a restricted analysis of subjects with relapse following HCT, those with intermediate-2/high risk had a nearly two-fold increased risk of death after relapse compared to those with low/intermediate-1 CPSS scores. Respective 1, 3 and 5-year survival rates for low/intermediate-1 risk subjects were 61% (95% confidence interval [CI], 52%–72%), 48% (95% CI, 37%–59%), and 44% (95% CI, 33%–55%), and for intermediate-2/high risk subjects were 38% (95% CI, 28%–49%), 32% (95% CI, 21% – 42%), and 19% (95% CI,8%–29%). We conclude that higher CPSS score at time of transplant, lower KPS, and a bone marrow (BM) graft are associated with inferior survival after HCT. Further investigation of CMML disease-related biology may provide insights into other risk factors predictive of post-transplant outcomes.
Keywords: Chronic Myelomonocytic Leukemia, Allogeneic Hematopoietic Cell Transplant, Transplant Outcomes
Introduction
Chronic myelomonocytic leukemia (CMML) is a clonal hematopoietic stem cell disorder with both myelodysplastic and myeloproliferative properties. In the original French-American-British (FAB) classification, it was included under myelodysplastic syndromes (MDS) with 2 subtypes based upon white blood cell count, an MDS variant (CMML-MD) and a myeloproliferative variant (CMML-MP). However, these concomitant properties made it difficult to classify, prompting a new category of myeloproliferative/myelodysplastic disorders (MPD/MDS) that was formed in the World Health Organization (WHO) classification of myeloid disorders in 2001.1,2 The diagnosis of CMML is characterized by a peripheral blood monocytosis, absence of Philadelphia chromosome, absence of rearrangements of PDGFRA or PDGFRB, presence of <20% blasts in the blood and bone marrow, and evidence of dysplasia in at least one precursor cell lineage (although if myelodyspasia is absent, the diagnosis of CCML can still be made if there is a clonal abnormality or persistent monocytosis and all other causes have been excluded). CMML is further divided into two subcategories with prognostic significance: CMML-1 (presence of <5% blasts in the peripheral blood and <10% blasts in the bone marrow) and CMML-2 (presence of 5–19% blasts in the peripheral blood and 10–19% in the bone marrow). The diagnosis of CMML-2 can also be made if Auer rods are present, irrespective of blast count.3,4
CMML has a heterogeneous clinical course, with much variability in survival and rates of transformation to acute myeloid leukemia. Expected survival ranges from months to several years.5–7 Rates of transformation to acute myeloid leukemia (AML) range from 4% to 44%.5–7 In a study reported from MD Anderson Cancer Center (MDACC) of 213 patients, the median survival was 12 months with 19% progressing to AML after a median of 7 months (range, 1 to 96 months).5 Given this wide variability, studies have focused on identifying important risk factors for prognosis and outcomes. A CMML-specific prognostic scoring system (CPSS) assessed at the time of diagnosis has been validated in the non-transplant setting.8 The CPSS incorporates CMML FAB type, CMML WHO type, CMML-specific cytogenetics, and RBC transfusion dependence.
Unfortunately, effective treatment options for CMML are limited. There are no specific therapies for CMML and the optimal treatment is not yet defined. Several studies in patients with MDS receiving azacitidine and decitabine have included CMML patients, however, the number of CMML patients included is small and results are difficult to interpret for this population.9,10 Allogeneic hematopoietic cell transplant (HCT) remains the only potentially curative treatment and outcomes following transplant are sparse.11–18 Some of these reports suggest that the percentage of blasts present in the peripheral blood, cytogenetic abnormalities, and transplant type may have prognostic importance following transplant. However, the studies are limited by small numbers of patients from single institutions and no definitive conclusions have been made.
Our retrospective study assessed the outcomes of 209 consecutive adult subjects who underwent HCT for CMML reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) registry from 2001 through 2012. The purpose of our study was to identify prognostic risk factors for post-transplant outcomes.
Patients and Methods
Data Sources
The CIBMTR is a combined research program of the Medical College of Wisconsin and the National Marrow Donor Program. CIBMTR comprises a voluntary network of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic allogeneic and autologous HCT to a centralized Statistical Center. Observational studies conducted by CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected health information issued in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the Health Insurance Portability and Accountability Act Privacy Rule. Additional details regarding the data source are described elsewhere.19
Subject Eligibility
Between 2001 and 2012, 209 adult patients (18 years of age or older) who underwent first HCT from HLA-identical sibling or adult unrelated-donor for CMML were identified for this analysis. Patients receiving cord blood transplants (N=20), ex-vivo T cell depletion (N=6), CD34-selection (N=6), or post-transplant cyclophosphamide (N=1) as part of their graft-versus-host disease (GvHD) prophylaxis were excluded. Subjects missing 100-day follow-up data were also excluded.
Study Endpoints
Primary endpoints were treatment-related mortality (TRM), relapse/progression, disease-free survival (DFS) and survival. TRM was defined as death from any cause in the first 28 days post transplantation, irrespective of relapse status, or death beyond day +28 without any evidence of disease recurrence; relapse was considered a competing event. Relapse/progression was defined as reported by the transplantation centers. DFS is defined as time to relapse or death from any cause. Survival is defined as time to death from any cause. Subjects were censored at time of last follow-up. Secondary endpoints included hematopoietic recovery, acute and chronic graftversus- host disease (GvHD). Hematopoietic recovery was defined as time to absolute neutrophil count ≥0.5 × 109 /L for ≥3 consecutive days and time to platelets ≥20 × 109 /L without transfusions for 7 days, using the first of 3 consecutive results obtained on different days. Acute and chronic GvHD were diagnosed and graded using consensus criteria.20,21 For hematopoietic recovery and GvHD, death without the event was considered a competing event. The transplantation conditioning regimen intensity was determined according to the CIBMTR Reduced-Intensity Conditioning (RIC) Regimen Workshop.22 CPSS scores were calculated at the time of transplant and were based on information from CIBMTR registry. The CPSS scoring system incorporates CMML FAB type, CMML WHO type, CMML-specific cytogenetics, and RBC transfusion dependence.8 Within the CPSS scoring system, there are 4 risk groups: low (score = 0), intermediate-1 (score =1), intermediate-2 (score = 2–3, and high (score = 4–5). each variable is assigned the same weight. A score is calculated by adding together the points according to risk factors. WHO subtype CMML-1 and CMML-2 are assigned 0 and 1 points, respectively. FAB subtype CMML-MD and CMML-MP are assigned 0 and 1 points, respectively. CMML-specific cytogenetic risk classification is as follows: low, normal and isolated -Y (0 points); intermediate, other abnormalities (1 point); high (2 points), trisomy 8, complex karyotype (≥3 abnormalities), and abnormalities of chromosome 7. Of note, the CPSS scoring system also include red blood cell transfusion defined as having at least 1 RBC transfusion every 8 weeks over a period of 4 months. The CIBMTR registry includes information about transfusion dependency, but does not specify the frequency of transfusion.
Statistical Analysis
Descriptive tables of donor- and collection-related variables were prepared. Probabilities of DFS and survival at 1, 3, and 5 years were calculated using the Kaplan-Meier estimator, with lost follow-up treated as a censoring event. Incidence rates for other outcomes were generated using the cumulative incidence estimates to adjust for competing risks (death without the event of interest). Point-wise p-values were calculated to evaluate the differences at specified time points.
Multi-variate analyses for survival, TRM, relapse, and GVHD were performed using the Cox proportional hazard model adjusting for the effects of covariates. Logistic regression was utilized to analyze neutrophil engraftment at 28 days and platelet recovery at 100 days. Covariates considered for prognostic value included: patient-related variables (patient age, gender, and Karnofsky score), disease-related variables (time from diagnosis to transplant, CPSS prior to transplant, treatment prior to transplant), and transplant-related variables (graft source, donor type, donor age, antithymocyte globulin (ATG)/alemtuzumab use, GvHD prophylaxis, donor/recipient sex match, donor/recipient CMV status, year of transplantation). Adjusted analyses of the outcomes were performed where additional covariates and interactions were determined by stepwise selection. We attempted to identify a profile for high vs. low risk prognosis for survival and relapse. Due to the small sample size available, the entire cohort was used for training to select the model and five-fold cross-validation was used to assess out-ofsample performance. We also performed multi-variate analysis for OS restricted to patients who relapsed following HCT. Adjusted cumulative incidence curves were produced for TRM and relapse of the high vs. low risk groups. SAS 9.3 (SAS Inc.) was used for all analyses.
Results
Transplantation Subjects
Subject- and disease-related characteristics are presented in Table 1. Between 2001 and 2012, 209 consecutive adult patients from 94 institutions underwent HCT for CMML. The median ages at transplant for patients with low/intermediate-1 and intermediate-2/high were 59 years and 55 years, respectively. The majority of patients were male (71% in patients with low/intermediate-1 and 66% in intermediate-2/high). Most patients had Karnofsky Performance Scores (KPS) of 90–100%. CPSS scores at the time of transplant (HCT specific CPSS scores) were available for 80% of subjects. Cytogenetic data were available for 86% of subjects. Median time from diagnosis to transplant was 8 months. Approximately one-third of subjects were transplanted from an HLA-identical sibling. The remaining two-thirds were transplanted from unrelated donors; a majority of these subjects (70%) were from well-matched unrelated donors. Peripheral blood (PB) was used as the graft source in 84% of subjects. Myeloablative conditioning regimens were given to 51% of subjects. Almost all patients received non-total body irradiation (TBI) based therapies (only 5 patients received TBI). GVHD prophylaxis mostly consisted of tacrolimus-based regimens (61%). The median follow up of surviving patients was 51 months.
Table 1.
Characteristics of patients received allogeneic HCT for CMML between 2001 and 2012
| Variable | N (%) |
|---|---|
| Number of patients | 209 |
| Number of centers | 94 |
| Patient-related | |
| Age, median | 57 (23–74) |
| Gender | |
| Male | 146 (70) |
| Female | 63 (30) |
| Karnofsky score | |
| 90–100% | 127 (61) |
| < 90% | 74 (35) |
| Missing | 8 (4) |
| Disease-related | |
| Time from diagnosis to transplant, months | 8 (2–170) |
| HMA and chemotherapy prior to transplant | |
| HMA | 74 (35) |
| Chemo | 19 (9) |
| HMA & chemo | 6 (3) |
| No HMA or chemo | 106 (51) |
| Missing | 4 (2) |
| CMML-1 vs. CMML-2 | |
| CMML-1 | 140 (67) |
| CMML-2 | 52 (25) |
| Missing | 17 (8) |
| Blast in marrow prior to transplant | |
| ≤ 5% | 136 (65) |
| > 5% | 56 (27) |
| Missing | 17 (8) |
| HCT Specific CPSS | |
| Low | 38 (18) |
| Intermediate-1 | 52 (25) |
| Intermediate-2 | 63 (30) |
| High | 16 (8) |
| Missing | 40 (19) |
| Platelet count prior to transplant | |
| ≥ 100 × 109/L | 88 (42) |
| < 100 × 109/L | 121 (58) |
| ANC prior to transplant | |
| ≥ 1500 /uL | 143 (68) |
| < 1500 /uL | 54 (26) |
| Missing | 12 (6) |
| Transplant-related | |
| Graft type | |
| Bone marrow | 33 (16) |
| Peripheral blood | 176 (84) |
| Type of donor | |
| HLA-identical sibling | 73 (35) |
| Well-matched unrelated | 95 (45) |
| Partially-matched unrelated | 32 (15) |
| Mis-matched unrelated | 4 (2) |
| Unrelated (matching indeterminable) | 5 (2) |
| Donor age, median | |
| HLA-identical sibling | 54 (27–74) |
| URD | 34 (19–61) |
| D-R sex match | |
| M-M | 96 (46) |
| M-F | 40 (19) |
| F-M | 50 (24) |
| F-F | 22 (11) |
| Missing | 1 (<1) |
| D-R CMV status | |
| +/+ | 47 (22) |
| +/− | 24 (11) |
| −/+ | 63 (30) |
| −/− | 65 (31) |
| Missing | 10 (5) |
| Year of transplant | |
| 2001–2003 | 39 (19) |
| 2004–2006 | 51 (24) |
| 2007–2009 | 53 (25) |
| 2010–2012 | 66 (32) |
| Conditioning regimen combination | |
| Myeloablative | 105 (50) |
| RIC/NMA | 99 (48) |
| Missing | 5 (2) |
| Serotherapy used | |
| ATG alone | 58 (28) |
| CAMPATH alone | 8 (4) |
| No ATG or CAMPATH | 132 (63) |
| Missing | 11 (5) |
| GVHD prophylaxis | |
| CSA based | 78 (37) |
| TAC based | 127 (61) |
| MTX alone | 2 (<1) |
| Missing | 2 (<1) |
| Median follow-up of survivors (range), months | 51 (3–122) |
Hematopoietic Recovery
On univariate analysis, rates of neutrophil recovery at days 28 and 100 were comparable between subjects with low/intermediate-1 and those with intermediate-2/high HCT specific CPSS scores (94% [95% CI, 86%–98%] and 89% [95% CI, 79% to 95%] at day 28, respectively, p=0.40; 99% [95% CI, 87% to 100%] and 96% [95% CI, 80% to 99%] at day 100, respectively, p=0.51). Platelet recovery at day 28 was comparable between groups. However, more subjects in the low/intermediate-1 group achieved platelet recovery at day 100 compared to the intermediate-2/high risk group (94% [95% CI, 86% to 98%] compared to 80% [95% CI, 69% to 87%] (p=0.007). There were no primary graft failures. (Table 2)
Table 2.
Univariate analysis for patients who received allogeneic HCT for CMML between 2001 and 2012
| Study population (N = 209) | ||
|---|---|---|
| Outcomes | N Eval | Prob (95% CI) |
| Neutrophil engraftment | 206 | |
| 28-day | 92 (88–95)% | |
| 100-day | 97 (95–99)% | |
| Platelet recovery | 207 | |
| 28-day | 66 (59–72)% | |
| 100-day | 86 (81–90)% | |
| Acute GVHD | 209 | |
| 100-day | 36 (30–43)% | |
| Chronic GVHD | 209 | |
| 1-year | 45 (38–52)% | |
| 3-year | 47 (40–54)% | |
| 5-year | 47 (40–54)% | |
| Relapse | 200 | |
| 1-year | 46 (39–53)% | |
| 3-year | 50 (43–57)% | |
| 5-year | 52 (45–59)% | |
| Treatment related mortality | 200 | |
| 1-year | 19 (14–25)% | |
| 3-year | 23 (18–30)% | |
| 5-year | 28 (21–35)% | |
| Disease free survival | 200 | |
| 1-year | 35 (28–42)% | |
| 3-year | 27 (21–33)% | |
| 5-year | 20 (14–27)% | |
| Overall survival | 209 | |
| 1-year | 50 (43–57)% | |
| 3-year | 38 (31–45)% | |
| 5-year | 30 (23–37)% | |
Neutrophil engraftment and platelet recovery between subjects receiving PB and BM graft were also compared. Neutrophil engraftment at day 28 was lower for subjects in the BM group; however by day 100, groups were similar: BM group 78% (95% CI, 59%–89%) and PB group 94% (95% CI, 89%–97%) at day 28, BM group 94%(95% CI, 69%–99%) and PB group 98%(93%–99%) at day 100. Platelet recovery at day 28 was again lower for subjects in the BM group; however by 100, groups were again similar: BM group 44% (95% CI, 26%–60%) and PB group 70% (95% CI, 62%–76%) at day 28, BM group 73%(95% CI, 51%–86%) and PB group 88%(95% CI, 82%–92%) at day 100.
Acute and Chronic GvHD
On univariate analysis, the cumulative incidence of grades ≥2 to 4 acute GvHD at day 100 were comparable between those with low/intermediate-1 and intermediate-2/high risk disease groups (34% [95% CI, 24% to 44%] and 38% [95% CI, 27% to 49%], respectively). On multivariate analysis, only donor type was associated with acute GvHD (p=0.002). The cumulative incidence of chronic GVHD at 1, 3, and 5 years were also comparable between groups (50% [95% CI, 38% to 60%] and 41% [95% CI 30% to 52%] at 1 year; 51% [95% CI, 40% to 61%] and 41% [95% CI, 30% to 52%] at 3 years; 51% [95% CI 40% to 61%] and 41% [95% CI, 30% to 52%], respectively). (Table 2) On multivariate analysis, only donor type was associated with acute GvHD (p=0.002). (Table 3)
Table 3.
Multi-variate analysis for adult CMML subjects who received allogeneic HCT between 2001 and 20121
| 1. Survival | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 88 | 1 | 0.0045 | ||
| Intermediate 2 & High | 79 | 1.927 | 1.299–2.858 | 0.0011 | |
| Missing | 42 | 1.571 | 0.976–2.529 | 0.0627 | |
| KPS | |||||
| 90–100% | 127 | 1 | 0.0119 | ||
| <90% | 74 | 1.717 | 1.200–2.457 | 0.0031 | |
| Missing | 8 | 1.444 | 0.625–3.336 | 0.39 | |
| Graft type | |||||
| BM | 33 | 1 | 0.0196 | ||
| PB | 176 | 0.584 | 0.371–0.917 | 0.0196 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 1.2263 | 0.774–1.942 | 0.3845 | ||
| <90% vs. Missing | 1.1893 | 0.508–2.784 | 0.6896 | ||
|
| |||||
| 2. DFS | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 85 | 1 | 0.2065 | ||
| Intermediate 2 & High | 76 | 1.38 | 0.965–1.972 | 0.0772 | |
| Missing | 39 | 1.137 | 0.729–1.773 | 0.5722 | |
| KPS | |||||
| 90–100% | 119 | 1 | 0.0183 | ||
| <90% | 74 | 1.607 | 1.156–2.234 | 0.0047 | |
| Missing | 7 | 1.297 | 0.562–2.997 | 0.5423 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 1.2138 | 0.781–1.886 | 0.3885 | ||
| <90% vs. Missing | 1.2387 | 0.530–2.894 | 0.621 | ||
|
| |||||
| 3. TRM | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 85 | 1 | 0.0884 | ||
| Intermediate 2 & High | 76 | 1.485 | 0.762–2.895 | 0.2455 | |
| Missing | 39 | 2.183 | 1.089–4.375 | 0.0277 | |
| KPS | |||||
| 90–100% | 119 | 1 | 0.0301 | ||
| <90% | 74 | 2.15 | 1.219–3.790 | 0.0081 | |
| Missing | 7 | 1.348 | 0.315–5.768 | 0.6869 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 0.6803 | 0.344–1.343 | 0.2668 | ||
| <90% vs. Missing | 1.5943 | 0.369–6.887 | 0.5321 | ||
|
| |||||
| 4. Relapse | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 85 | 1 | 0.118 | ||
| Intermediate 2 & High | 76 | 1.321 | 0.869–2.009 | 0.1929 | |
| Missing | 39 | 0.719 | 0.393–1.316 | 0.2851 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 1.8369 | 1.001–3.372 | 0.0498 | ||
|
| |||||
| 5. Acute GVHD | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 88 | 1 | 0.592 | ||
| Intermediate 2 & High | 79 | 1.266 | 0.779–2.057 | 0.3418 | |
| Missing | 42 | 1.014 | 0.544–1.890 | 0.9656 | |
| Donor | |||||
| HLA identical sibling | 73 | 1 | 0.0017 | ||
| Well-matched URD | 95 | 1.063 | 0.633–1.785 | 0.8165 | |
| Partially-matched URD or MM URD | 36 | 2.836 | 1.560–5.156 | 0.0006 | |
| Missing (URD) | 5 | 2.304 | 0.694–7.651 | 0.1727 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 1.2483 | 0.677–2.304 | 0.478 | ||
| Well-matched URD vs. Partially-matched or MM URD | 0.3749 | 0.211–0.665 | 0.0008 | ||
| Well-matched URD vs. Missing (URD) | 0.4614 | 0.140–1.516 | 0.2024 | ||
| Partially-matched or MM URD vs. Missing (URD) | 1.2308 | 0.361–4.198 | 0.7401 | ||
|
| |||||
| 6. Chronic GVHD | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 88 | 1 | 0.9131 | ||
| Intermediate 2 & High | 79 | 1.087 | 0.681–1.734 | 0.7263 | |
| Missing | 42 | 1.107 | 0.623–1.967 | 0.7289 | |
| Contrast | |||||
| Intermediate 2 & High vs. Missing | 0.9819 | 0.535–1.802 | 0.953 | ||
|
| |||||
| 7. OS after relapse2 | Overall | ||||
| HCT Specific CPSS | N | RR | 95% CI | p-value | p-value |
| Low & Intermediate 1 | 46 | 1 | 0.0262 | ||
| Intermediate 2 & High | 44 | 1.993 | 1.199–3.311 | 0.0078 | |
| Missing | 14 | 1.737 | 0.836–3.608 | 0.1389 | |
The majority of the patients achieved neutrophil engraftment by day 28 and platelet recovery by day 100 (Table 2), therefore multi-variate analysis was not performed for them.
List of abbreviations: disease free survival (DFS), overall survival (OS), transplant related mortality (TRM), non-relapse mortality (NRM), bone marrow (BM), myeloablative (MAC), second primary malignancy (SPM), lactate dehydrogenase (LDH), red blood cell (RBC), peripheral blood (PB)
Overall survival was compared with relapse instead of using the left-truncated model, so analysis was performed starting at the time of relapse and non-relapse patients were excluded in this model.
Treatment Related Mortality
On univariate analysis, there was no significant difference in TRM at 1, 3, or 5 years between low/intermediate-1 (15% [95% CI, 9% to 24%], 20% [95% CI, 12% to 29%] and 22% [95% CI, 13% to 32%]) and intermediate-2/high risk groups (19% [95% CI, 11% to 29%], 21% [95% CI, 12% to 31%], and 26% [16% to 37%], respectively). (Table 2) On multivariate analysis, higher HCT specific CPSS scores and KPS scores were not associated with TRM (p=0.08 and p=0.03, respectively). (Table 3)
Relapse
On univariate analysis, relapse rates at 1, 3, and 5 years between low/intermediate-1 and intermediate-2/high groups were comparable (46% [95% CI, 35% to 56%], 50% [95% CI, 39% to 61%], 52% [95% CI, 40% to 63%], respectively, and 54% [95%CI, 41% to 64%], 56% [95% CI, 44% to 67%], and 60% [95% CI, 47 % to 70%], respectively). On multivariate analysis, HCT specific CPSS scores were not associated with relapse (p=0.112). (Table 3)
Survival Outcomes
On univariate analysis, DFS rates were comparable: for low/intermediate-1 risk groups, at 1, 3, and 5 years were 38% (95% CI, 28% to 49%), 30% (95% CI, 20% to 40%), and 26% (95% CI, 17% to 37%), respectively, and for intermediate-2/high risk groups were 28% (95% CI, 18% to 38%), 23% (95% CI, 14% to 33%), and 14% (95% CI, 6% to 24%), respectively. On multivariate analysis, CPSS scores did not impact DFS (p=0.21), however higher KPS scores were associated with improved DFS (p=0.02). (Table 3)
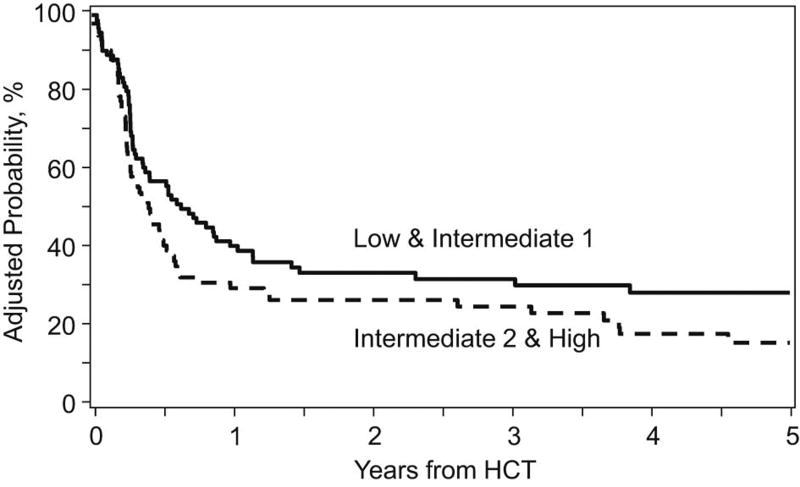
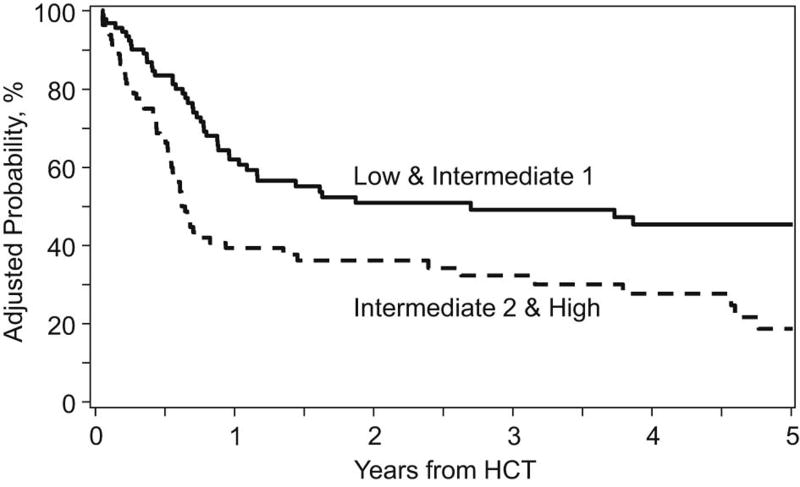
On univariate analysis, low/intermediate-1 risk groups had higher rates of OS at 1, 3, and 5 years: corresponding rates for low/intermediate-1 risk groups were 61% (95% CI, 51% to 71%), 48% (95% CI, 37% to 59%), and 44% (95% CI, 33% to 56%) respectively and for intermediate-2/high risk groups were 38% (95% CI 27% to 49%), 31% (95% CI, 21% to 42%), and 18% (95% CI, 8% to 30%) respectively. (Table 2) On multivariate analysis, HCT specific CPSS scores, KPS and graft source were significant predictors of survival (p=0.005, p=0.01, and p=0.02, respectively). Patients receiving PB had more favorable outcome. (Table 3) Adjusted OS and DFS starting at time of transplant, based on HCT specific scores, are shown in Figures 1 and 2.
Figure 1.
Adjusted disease free survival and overall survival, starting at the time of transplant, by HCT Specific CPSS
Figure 2.
Adjusted disease free survival and overall survival, starting at the time of transplant, by HCT Specific CPSS
To investigate why higher HCT specific CPSS scores were associated with higher mortality but not DFS, we performed multivariate analysis restricted to patients with relapse following HCT. Those with intermediate-2/high risk had nearly two-fold increased risk of death after relapse compared to those with low/intermediate-1 HCT specific CPSS scores.
On multivariate analysis, survival of patients who received pre-HCT treatment with hypomethylating agents (HMA), chemotherapy, or both was not different compared to those who received no prior therapy (p=0.96).
Discussion
Allogeneic HCT remains the only potentially curative treatment for patients with CMML. Few data exist regarding transplant outcomes and there are no randomized clinical trials comparing transplant to non-transplant approaches. Most studies are limited by their retrospective nature and small sample size. (Table 4) While our study is also retrospective, it represents a large series with a long median follow up. The median age of our patients was 57 years, older than in other reported studies.11–18, 22 The median follow up in our study is 51 months, longer than in most other reported studies.11–13, 15–18, 23–27 Subjects underwent either myeloablative or RIC preparative regimen. The majority of patients received PB as their graft source. Our study is unique in that we not only describe transplant outcomes, but we also validated a predictive model for survival and relapse. Patient stratification according to HCT specific CPSS scores was prognostic for transplant outcomes.
Table 4.
Allogeneic hematopoietic transplant studies in patients with CMML
| Patien ts |
Medi an age |
Conditioni ng |
Cell source |
DF S/ PFS % |
OS % |
TRM/NR M % |
Relapse % |
Median follow up, months |
Factors predictive of OS |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Zang et al (2000)10 | 21 | 47 | MAC: 21 RIC: 0 | BM: 21 PB: 0 | 25 (3 yrs) | 39 (3 yrs) | 34 | 23 | Unknown | Patients transplanted early (< 12 months from diagnosis) had better survival. |
| Kroger et al (2002)11 | 50 | 44 | MAC: 50 RIC:0 | BM: 40 PB: 9 | 18 (5 yrs) | 21 (5 yrs) | 52 | 49 | 40 (range, 11 to 110) | No correlation |
| Mittal et al (2004)12 | 8 | 51 | MAC: 4 RIC: 4 | BM: 4 PB: 4 | 37 (2 yrs) | 47 (2 yrs) | 13 | 63 | 17.5 | Number too small |
| Kerbauy et al (2005)13 | 43 | 48 | MAC: 41 RIC: 2 | BM: 23 PB: 20 | 41 (4 years) | 41 (4 years) | 34 | 23 (4 years) | 69 (range, 7 to 171) | MDAPS not correlative. Higher comorbidity scores associated with worse OS |
| Elliot et al (2006)14 | 17 | 50 | MAC:16 RIC: 1 | BM: 8 PB: 7 | 18 (3 yrs) | 18 (3 yrs) | 41 | 41 | 34.5 | No correlation |
| Laport et al (2008)15 | 7 | 59 | MAC: 0 RIC: 7 | Unknown, likely PB | 43 (3 yrs) | 43 (3 yrs) | 32 (3 years) | 57 | 47 (range, 6 to 89) | Number too small |
| Krishnamur thy et al (2010)14 | 18 | 54 | MAC:1 RIC 17 | BM: 18 PB: 36 | 31% (3 yrs) | 22 | 44 | 40 (range, 1 to 59) | None | |
| Eissa et al (2011)23 | 85 | 51 | MAC: 58 RIC: 27 | BM: 32 PB: 53 | 40 (10 yrs) | 42 (5 years) | 35 (10 years) | 27 (10 yrs) | 62 (range, 6 to 229) | MDAPS not correlative. Mortality negatively correlated with pre-HCT hematocrit and increased high-risk cytogenetics, higher HCT comorbidity index, and increased age |
| Lim et al (2013)24 | 7 | 43 | MAC: 3 RIC: 7 | BM: 2 PB: 7 | 51 (5 years) | 42% (5 years) | 14 | 29 (5 years) | 47.5 (range, 4.6 to 98.8) | Number too small |
| Park et al (2013)25 | 73 | 53 | MAC: 30 RIC: 43 | BM: 27 PB: 46 | 29 (3 years) | 42 (2 years) 32 (3 years) | 36 (3 years) | 29 (3 years) | 23 (range, 1–145) | Palpable SPM, transplant performed prior to 2004 correlated with poorer OS |
| Bajel et al (2014)26 | 57 | 56 | MAC:28 RIC: 29 | BM: 3 PB: 54 | 40 (6 years) | 27 (6 years) | 39 (6 years) | 35 (6 years) | 15.3 (range, 0.6 to 154) | In multivariate analysis, age < 50yo, non-sibling donor, and lymphocyte count > 2.9 × 109/L were associated with worse OS and PFS. Bone marrow blasts pre-transplant were associated with higher risk of relapse. |
| Sanchez et al (2014)27 | 28 | 60 | MAC: 16 (T-cell depleted) RIC: 12 | BM: 2 PB: 23 Cord: 3 | 74 (3 years) | 71 (3 years) | 7 (1 year) | 13 (1 year) | 39.6 (range, 3 to 35) | |
| Symeonidis et al (2015)17 | 513 | 53 | MAC: 249 (52%) RIC: 226 (48%) | BM: 119 (23%) PB: 394 (77%) | 27% (4 years) | 33% (4 years) | 31% (1 year) 41% (4 years) | 32 (4 years) | Patients transplanted in CR had lower probability for non-relapse death and longer survival. | |
| Kongtim et al (2016)18 | 83 | 57 | MAC: 64 (77%) | BM: 35 (42%) PB: 48 (58%) | 34% (3 years) | CMML-1/2: 36% (3 years) CMML/A ML: 32% (3 years) | 25% (day 100) 31% (1 year) | 33% (3 years) | 48 | Use of HMA therapy was associated with lower relapse at 3 years (22% compared to 35%, p=0.03) and higher PFS at 3 years (43% compared to 27%, p=0.04) |
List of abbreviations: myeloablative (MAC), reduced intensity conditioning (RIC), bone marrow (BM), peripheral blood (PB), disease free survival (DFS), progression free survival (PFS), overall survival (OS), transplant related mortality (TRM), nonrelapsed mortality (NRM), MDAPS (MD Anderson Prognostic Score)
Given the heterogeneity of clinical outcomes for patients with CMML, it is important to better define and stratify risk for patients with CMML. The International Prognostic Scoring System (IPSS) is widely used for myelodysplastic syndrome. However, for CMML patients, analysis and validation were restricted to patients with WBC < 12 × 109/L, excluding patients with myeloproliferative characteristics and not applicable for all patients with CMML.28 There are several prognostic scoring systems developed for CMML, however, they each have limitations and have not been validated in the setting of transplant.5,6,29–31 (Table 5) The CPSS incorporates CMML FAB type, CMML WHO type, CMML-specific cytogenetics, and RBC transfusion dependence. The CPSS calculated at diagnosis is a simple scoring system that was developed in the large patient sample size and was externally validated, in the non-transplant setting.8 The CPSS score at diagnosis has been shown to be predictive of survival and risk of progression to AML. Our study sought to validate the CPSS, calculated at the time of transplant, in the setting of HCT. In multivariate analysis, higher HCT specific CPSS scores were associated with inferior survival. It was not, however, associated with DFS, relapse, or TRM. In order to further investigate why higher HCT specific CPSS scores were associated with higher mortality, but not with DFS, we performed an analysis restricted to subjects who relapsed after transplant. This revealed that subjects with intermediate-2/high risk HCT specific CPSS scores had a nearly two-fold increased risk of death after relapse compared to those with low/intermediate-1 HCT specific CPSS scores. Intermediate-2/high risk patients do have higher disease burden and poorer risk cytogenetic abnormalities. Higher HCT specific CPSS scores are predictive of poorer treatment response and more aggressive biology. Interestingly, regardless of HCT specific CPSS scores, the main cause of death was primary disease. (Table 6) Post-transplant donor lymphocyte infusion and/or 2nd HSCT were similar between groups. Other post-transplant strategies, such as azacitidine maintenance in patients with myelodysplastic syndromes or acute myeloid leukemia, may be beneficial for these patients and warrants further investigation.32
Table 5.
Prognostic scoring systems in CMML patients
| Patients | External Validation |
Variables included in final scoring system |
|
|---|---|---|---|
| MD Anderson prognostic score5 | 213 | No |
|
| Dusseldorf score6,29 | 288 | No |
|
| Spanish cytogenetic risk stratification system30 | 414 | No |
|
| CMML-specific prognostic scoring system8 | 578 | Yes |
|
| Mayo prognostic model31 | 226 | Yes |
|
CMML-specific cytogenetic risk classification: low, normal and isolated –Y; intermediate, other abnormalities; and high, trisomy 8, complex karyotype (≥3 abnormalities), and abnormalities of chromosome 7
Table 6.
Causes of death, according to HCT Specific CPSS
| Cause of death | Low / Intermediate-1 | Intermediate-2 / High |
|---|---|---|
| Primary disease | 21 (46) | 23 (41) |
| Graft failure | 0 | 2 (4) |
| GVHD | 5 (11) | 13 (23) |
| Infection | 3 (7) | 7 (13) |
| IPn/ARDS | 3 (7) | 0 |
| Organ failure | 4 (9) | 8 (14) |
| Secondary malignancy | 3 (7) | 0 |
| Other cause | 3 (7) | 2 (4) |
| Unknown | 3 (7) | 1 (2) |
| Missing | 1 (2) | 0 |
We observed favorable survival with PB graft compared to BM. The majority of subjects received PB grafts. While the incidence of acute or chronic GVHD was comparable between those who received PB or bone marrow grafts, subjects who received PB grafts had improved survival compared with those who received bone marrow. This is contrary to what has been reported in other studies.33–36 It is also interesting to note that no deaths in the bone marrow graft group were due to graft failure. It is unclear why those patients with BM had poorer survival; however our study is limited in that only a small number of subjects received bone marrow grafts (16%).
We also evaluated the effect of prior therapy on transplant. Few published studies have included information on use of HMA and transplant outcomes. Over the last decade, hypomethylating agents have become a cornerstone of therapy for MDS and CMML.37–42 We cannot determine whether pre-transplant HMA therapy or chemotherapy affected transplant eligibility. However, our data shows that pre-transplant treatment with HMA therapy or chemotherapy had no impact on transplant outcomes. This is contrary to a recent publication from Kongtim et al that reports lower relapse and improved progression-free survival for patients treated with hypomethylating agents prior to alloHCT.18
Our registry-based study is limited to the data contained in the CIBMTR database. Transplantations were performed at many different institutions, with varying conditioning regiments and GvHD prophylaxis. We recognize that the original CPSS score was calculated at time of diagnosis. We use the same variables that are part of the original CPSS, now calculated at the time of transplant, to attempt to validate this scoring system in the HCT setting. Another limitation of our study is that data regarding CPSS was missing for many of our subjects (20%). Another limitation is regarding missing details of transfusion dependence; as part of criteria for the CPSS, transfusion dependence is defined as requiring at least 1 red blood cell transfusion every 8 weeks over a period of 4 months.8 While patients may meet this minimal criteria, we do not have data on how many transfusions and how frequently these transfusions were required for our subjects. We also do not have data on whether subjects had splenomegaly prior to HCT, which has been suggested to also have prognostic significance.25
We conclude that allogeneic HCT remains an important treatment that is curative for some patients with CMML. Higher HCT specific CPSS scores, lower KPS, and bone marrow graft source are associated with inferior outcomes following allogeneic HCT. Future investigation to further elucidate the biology of CMML may help identify other risk factors that better predict which patients benefit most from transplant.
Highlights.
Hematopoietic cell transplant is an important and potentially curative treatment option for patients with chronic myelomonocytic leukemia.
Higher CPSS scores, lower performance status, and bone marrow graft are associated with inferior survival post-BMT.
Treatment with hypomethylating agents or chemotherapy prior to transplant did not impact transplant outcomes.
Acknowledgments
I. CIBMTR Support List
The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Atara Biotherapeutics, Inc.; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; Cerus Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
*Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vardiman JW, Pierre R, Bain B, et al. Chronic myelomonocytic leukaemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. pp. 49–51. [Google Scholar]
- 2.Orazi A, Bennett JM, Germing U, et al. Chronic myelomonocytic leukaemia. In: Swerdlow S, Campos E, Lee Harris N, et al., editors. World Health Organization Classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC press, World Health Organization; 2008. pp. 76–81. [Google Scholar]
- 3.Emanuel PD. Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia. 2008;22:1335–1342. doi: 10.1038/leu.2008.162. [DOI] [PubMed] [Google Scholar]
- 4.Vardiman JW, Harris NL, Brunning RD, et al. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 5.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: a retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 6.Germing U, Kundgen A, Gattermann N. Risk assessment in chronic myelomonocytic leukemia (CMML) Leukemia Lymphoma. 2004;45:1311–1318. doi: 10.1080/1042819042000207271. [DOI] [PubMed] [Google Scholar]
- 7.Elliott MA. Chronic neutrophilic leukemia and chronic myelomonocytic leukemia: WHO defined. Best Pract Res Clin Haematol. 2006;19:571–93. doi: 10.1016/j.beha.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood. 2013;121:3005–3015. doi: 10.1182/blood-2012-08-452938. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomized, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang DY, Deeg HJ, Gooley T, et al. Treatment of chronic myelomonocytic leukaemia by allogeneic marrow transplantation. Br J Haematol. 2000;110:217–222. doi: 10.1046/j.1365-2141.2000.02133.x. [DOI] [PubMed] [Google Scholar]
- 11.Kroger N, Zabelina T, Guardiola P, et al. Allogeneic stem cell transplantation of adult chronic myelomonocytic leukaemia. A report on behalf of the Chronic Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 2002;118:67–73. doi: 10.1046/j.1365-2141.2002.03552.x. [DOI] [PubMed] [Google Scholar]
- 12.Mittal P, Saliba RM, Giralt SA, et al. Allogeneic transplantation: a therapeutic option for myelofibrosis, chronic myelomonocytic leukemia and Philadephia-negative/BCR-ABLnegative chronic myelogenous leukemia. Bone Marrow Transplant. 2004;33:1005–1009. doi: 10.1038/sj.bmt.1704472. [DOI] [PubMed] [Google Scholar]
- 13.Kerbauy DM, Chyou F, Gooley T, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2005;11:713–720. doi: 10.1016/j.bbmt.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Elliott MA, Tefferi A, Hogan WJ, et al. Allogeneic stem cell transplantation and donor lymphocyte infusions for chronic myelomonocytic leukemia. Bone Marrow Transplant. 2006;37:1003–1008. doi: 10.1038/sj.bmt.1705369. [DOI] [PubMed] [Google Scholar]
- 15.Laport GG, Sandmaier BM, Storer BE, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–255. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krishnamurthy P, Lim ZY, Nagi W, et al. Allogeneic haematopoietic SCT for chronic myelomonocytic leukaemia: a single-centre experience. Bone Marrow Transplant. 2010 Jan; doi: 10.1038/bmt.2009.375. ePub. [DOI] [PubMed] [Google Scholar]
- 17.Symeonidis A, van Biezen A, de Wreede L, et al. Achievement of complete remission predicts outcome of allogeneic haematopioetic stem cell transplantation in patients with chronic myelomonocytic leukaemia. A study of the Chronic Malignancies Working Party of the European Group for Blood and Marrow Transplantation. British J Haematology. 2015;171:239–246. doi: 10.1111/bjh.13576. [DOI] [PubMed] [Google Scholar]
- 18.Kongtim P, Popat U, Jimenez An, et al. Treatment with hypomethylating agents before allogeneic stem cell transplant improves progression-free survival for patients with chronic myelomonocytic leukemia. Biol Blood Marrow Transplant. 2016;(22):47–53. doi: 10.1016/j.bbmt.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz M. The role of registries in facilitating clinical research in BMT: examples from the Center for International Blood and Marrow Transplant Research. Bone Marrow Transplant. 2008;42(Suppl 1):S1–2. doi: 10.1038/bmt.2008.101. [DOI] [PubMed] [Google Scholar]
- 20.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997;97:855–864. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 21.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eissa H, Gooley TA, Sorror M, et al. Allogeneic hematopoietic cell transplantation for chronic myelomonocytic leukemia: relapse-free survival is determined by karyotype and comorbidities. Biol Blood Marrow Transplant. 2011;17:908–915. doi: 10.1016/j.bbmt.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim SN, Lee JH, Lee JH, et al. Allogeneic hematopoietic cell transplantation in adult patients with myelodysplastic/myeloproliferative neoplasms. Blood Res. 2013;48:178–184. doi: 10.5045/br.2013.48.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S, Labopin M, Yakoub-Agha I, et al. Allogeneic stem cell transplantation for chronic myelomonocytic leukemia: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Eur J Haematology. 2013;90:355–364. doi: 10.1111/ejh.12073. [DOI] [PubMed] [Google Scholar]
- 26.Bajel A, Curley C, Ming Lim AB, et al. Allogeneic stem cell transplantation (allo-SCT) for chronic myelomonocytic leukemia – a Multiceentre Australian Experience: Prognostic Factors for Survival and Relapse. American Society for Hematology Annual Meeting Abstracts. :1927. [Google Scholar]
- 27.Sanchez ME, Singh Abbi KK, Tamari R, et al. Allogeneic transplantation for chronic myelomonocytic leukemia (CMML) is associated with high disease-free survival even in the setting of high risk disease. American Society for Hematology Annual Meeting Abstracts. :2575. [Google Scholar]
- 28.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079–88. [PubMed] [Google Scholar]
- 29.Germing U, Strupp CA, Gattermann N. New prognostic parameters for chronic myelomonocytic leukemia. Blood. 2002;100(2):731–732. doi: 10.1182/blood-2002-01-0330. [DOI] [PubMed] [Google Scholar]
- 30.Such E, Cervera J, Costa D, et al. Cytogenetic risk stratification in chronic myelomonocytic leukemia. Haematologica. 2011;96:375–383. doi: 10.3324/haematol.2010.030957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patnaik MM, Padron E, LaBorde RR, et al. Mayo prognostic model for WHO-defined chronic myelomonocytic leukemia: ASXL1 and spliceosome component mutations and outcomes. Leukemia. 2013;27:1504–1510. doi: 10.1038/leu.2013.88. [DOI] [PubMed] [Google Scholar]
- 32.De Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;(116):5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craddock C, Jilani N, Siddique S, et al. Tolerability and clinical activity of posttransplantation azacitidine in patients allografted for acute myeloid leukemia treated on the RICAZA trial. Biol Blood Marrow Transplant. 2016:385–390. doi: 10.1016/j.bbmt.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Remberger M, Ringden O, Blau IW, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98:1739–1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 35.Eapen M, Logan BR, Confer DL, et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic GVHD without improved survival. Biol Blood Marrow Transplant. 2007;13:1461–1468. doi: 10.1016/j.bbmt.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khera N, Majhail NS, Brazauskas R, et al. Comparisons of characteristics and outcomes of trial participants and non-participants: example of Blood and Marrow Transplant Clinical Trials Network 0201 Trial. Biol Blood Marrow Transplant. 2015;21:1815–1822. doi: 10.1016/j.bbmt.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 38.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 39.Wijermans P, Lubbert M, Verhoef G, et al. Low-dose 5-aza-2’-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol. 2000;18:956–962. doi: 10.1200/JCO.2000.18.5.956. [DOI] [PubMed] [Google Scholar]
- 40.Costa R, Abdulhaq H, Haq B, et al. Activity of azacitidine in chronic myelomonocytic leukemia. Cancer. 2011;117(12):2690–6. doi: 10.1002/cncr.25759. [DOI] [PubMed] [Google Scholar]
- 41.Braun T, Itzykson R, Renneville A, et al. Molecular predictors of response to decitabine in advanced chronic myelomonocytic leukemia: a phase 2 trial. Blood. 2011;118(14):3824–31. doi: 10.1182/blood-2011-05-352039. [DOI] [PubMed] [Google Scholar]
- 42.Ades L, Sekeres MA, Wolfromm A, et al. Predictive factors of response and survival among chronic myelomonocytic leukemia patients treated with azacitidine. Leuk Res. 2013;37(6):609–13. doi: 10.1016/j.leukres.2013.01.004. [DOI] [PubMed] [Google Scholar]