Abstract
Background
Late-onset noninfectious pulmonary complications (LONIPCs), which occur more than 3 months after allogeneic hematopoietic stem cell transplantation (HSCT), are major causes of morbidity and mortality after transplantation. Among LONIPCs, we occasionally treat patients with late-onset severe restrictive lung defect after HSCT; however, its clinical features have not been fully elucidated.
Methods
A retrospective chart review of a single center on cases of late-onset severe restrictive lung defect after HSCT was performed. Among 453 patients who survived longer than 100 days after allogeneic HSCT with evaluable spirometry data, 12 patients (2.6%) developed late-onset severe restrictive lung defect (i.e., vital capacity percent of predicted less than 60%).
Results
Median duration from transplantation to diagnosis of late-onset severe restrictive lung defect cases was 44.5 months. Major computed tomography (CT) finding was pleuroparenchymal thickening with volume loss, an evidence of fibrosis, predominantly in upper lobes (n = 7), which was consistent with pleuroparenchymal fibroelastosis. The remaining patients showed unclassifiable interstitial pneumonia pattern (n = 2) and airway-predominant pattern (n = 3). The diffusing capacity for carbon oxide tended to decrease, while the residual volume/total lung capacity ratio tended to increase after HSCT. Of 12 patients, 8 patients died and the median month from diagnosis to death was 33.5 months. Seven patients died of pulmonary or systemic infection, and one patient died due to relapse of the primary disease.
Conclusion
Severe restrictive lung defect could develop in selected cases in the late-phase after HSCT and could be a unique clinical entity with specific radiographical findings.
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0466-7) contains supplementary material, which is available to authorized users.
Keywords: Hematopoietic stem cell transplantation, Late-onset noninfectious pulmonary complications, Pleuroparenchymal fibroelastosis, Idiopathic pneumonia syndrome
Background
Despite recent progress in allogeneic hematopoietic stem cell transplantation (HSCT) for hematological diseases, pulmonary complications have been recognized as one of the major causes of morbidity and mortality after allogeneic HSCT, occurring in about 40–70% of patients, with a high mortality rate [1–3]. Pulmonary complications after HSCT are caused by various noninfectious and infectious causes, and develop in both the early and late phases after transplantation, contingent on the day of development before or after 100 days following HSCT.
Opportunistic infection caused by bacteria, fungus, and viruses represents a major cause of infectious pulmonary complications in the early phase after HSCT [2, 4]. In contrast, noninfectious pulmonary complications are major causes of morbidity and mortality later than 100 days after allogeneic HSCT, which have been labelled late-onset noninfectious pulmonary complications (LONIPCs) [5]. LONIPCs classically include bronchiolitis obliterans (BO), cryptogenic organizing pneumonia (COP) (previously called bronchiolitis obliterans organizing pneumonia [BOOP]), diffuse alveolar hemorrhage (DAH), and idiopathic pneumonia syndrome (IPS) [6, 7].
IPS is a severe pulmonary complication occurring after HSCT, which was originally characterized by symptoms and signs of pneumonia, restrictive pulmonary function test abnormality, and alveolar injury without documented lower respiratory tract infection [8]. The American Thoracic Society (ATS) Research Statement on IPS provides a comprehensive review of IPS, the definition of which has been updated [9]. IPS is clinically defined by three criteria: widespread alveolar injury, absence of documented infection, and absence of cardiac, renal, or iatrogenic etiology. The evidence of widespread alveolar injury is based on multilobar infiltrates on chest radiographs or computed tomography (CT), symptoms and signs of pneumonia, and evidence of abnormal pulmonary physiology, including restrictive pulmonary function test abnormality. The time of onset for IPS ranges from 4 to 106 days and diffuse alveolar hemorrhage (DAH) occurs in early post-HSCT, with an indicated median onset time of 12–15 days [9]. Therefore, it seems that LONIPCs generally do not include IPS, according to the ATS statement.
Pleuroparenchymal fibroelastosis (PPFE), originally reported as an idiopathic disease [10], has previously been reported as a novel radiological and pathological feature in patients with pulmonary disease in the late phase after HSCT [11]. However, detailed clinical and radiological features of pulmonary complications after HSCT, especially in the late phase, are still largely unknown.
The primary aim of this retrospective study was to clarify the clinical and radiological features of late-onset severe restrictive lung defect after HSCT.
Methods
Patients
The retrospective study protocol was approved by the Institutional Review Board of Keio University School of Medicine (Tokyo, Japan).
We reviewed, in the institutional database, the medical records of 530 consecutive patients who underwent allogeneic HSCT at Keio University Hospital (Tokyo, Japan) between January 1990 and December 2011. The patient enrolment flowchart is shown in Fig. 1. Twelve (2.6%) of 453 patients were enrolled in the analysis as patients with late-onset severe restrictive lung defect according to % vital capacity (VC, <60%). There were 89 patients with a restrictive defect at %VC < 80%. Among the 89 patients, there were 86 patients with a restrictive defect at %VC 70–80%; their radiological findings were relatively mild and not specific. In addition, there were 3 patients with a restrictive defect at %VC 60–70%; the three patients showed very mild or no respiratory symptoms. Therefore, only patients with a severe restrictive defect at %VC < 60% were finally enrolled, because we aimed to exclude heterogeneous cases of mild to moderate restrictive ventilatory defect.
Fig. 1.
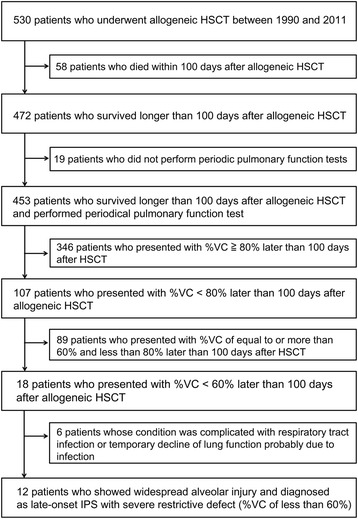
Flowchart of patient enrollment. HSCT denotes hematopoietic stem cell transplantation and VC denotes vital capacity
Radiological evaluation
Chest radiographs and chest CT images were evaluated independently by two pulmonologists and one radiologist. The conclusions were reached by consensus.
The radiological features were mainly categorized into 3 patterns: PPFE, unclassifiable interstitial pneumonia (IP), and airway-predominant (centrilobular nodules with BO), based on the consensus of the two pulmonologists and one radiologist.
Pulmonary function tests
Pulmonary function tests were performed according to established methods [12, 13].
Diagnosis of acute and chronic graft-versus-host-disease
Acute and chronic graft-versus-host-disease (GVHD) was diagnosed and graded according to established consensus criteria [14, 15].
Statistical analysis
Differences were analyzed for statistical significance using a paired t-test. P-values less than 0.05, two-tailed, were considered statistically significant.
Results
Clinical features of patients with late-onset severe restrictive lung defect
The clinical features of the patients with late-onset severe restrictive lung defect are summarized in Table 1. The median age at stem cell transplantation was 41 years. Ten patients underwent HSCT from an unrelated donor. Conditioning regimens were total body irradiation (TBI) (12 Gy)-based myeloablative regimens (n = 10), busulphan-based myeloablative regimen (n = 1), and fludarabine-based regimen in combination with melphalan (n = 1).
Table 1.
Clinical features of patients who underwent hematopoietic stem cell transplantation complicated with severe late-onset idiopathic pneumonia syndrome
| Case | Sex | Primary disease | Age at HSCT | Type of donor/Stem cells | HLA compatibility | Conditioning regimen | GVHD prophylaxis | Grades of acute GVHD | Type of chronic GVHD [1] | Organs of chronic GVHD | Months from HSCT to Dx of chronic GVHD | Smoking history | History of Infections | Out-come |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | ALL | 48 | UR/BM | DR serological 1 locus mismatch |
TBI (12 Gy) CY |
TAC + MTX | II | Extensive | M, E, L | 7.0 | – | CMV Retinitis Adenovirus cystitis Bacterial pneumonia |
Dead |
| 2 | F | MDS | 43 | UR/BM | complete match | TBI (12 Gy) CyA |
TAC + MTX | II | Extensive | S, M, E, L | 9.4 | – | Bacterial pneumonia Influenza virus bronchitis | Alive |
| 3 | M | AML | 12 | UR/BM | complete match | TBI (12 Gy) CyA, CY |
TAC + MTX | II | Extensive | S, M, E, L | 4.4 | – | MRSA pleuritis | Dead |
| 4 | F | ALL | 53 | UR/BM | complete match | TBI (12 Gy) CyA, CY |
TAC + MTX | I | Extensive | S, M | 3.7 | – | CMV enterocolitis | Alive |
| 5 | F | CML | 35 | UR/BM | DRB1 1 allele mismatch | TBI (12 Gy) CyA, CY |
TAC + MTX | III | Extensive | S, M, E, G | 4.1 | – | – | Dead |
| 6 | F | ALL | 32 | R/BM | complete match | BU, CY | CyA + MTX | 0 | Extensive | E, L | 4.8 | – | Aspergillus pneumonia Listeria bacteremia |
Dead |
| 7 | M | MM | 39 | UR/BM | DRB1 1 allele mismatch | Flu, L-PAM | TAC + MTX | I | Extensive | S, E | 2.2 | – | Bacterial pneumonia HHV6 encephalomyelitis | Dead |
| 8 | F | ALL | 49 | R/BM | complete match | TBI (12 Gy) CyA, CY |
CyA + MTX | III | Extensive | M,G | 4.9 | – | Pseudomonas pneumonia CMV infection (unknown focus) | Dead |
| 9 | M | AML | 31 | UR/BM | DRB1 1 allele mismatch | TBI (12 Gy) CyA |
TAC + MTX | II | Extensive | S, M, E | 4.0 | – | Bacterial pneumonia, CMV infection (unknown focus) | Alive |
| 10 | M | AML | 44 | UR/BM | A 1 allele mismatch | TBI (12 Gy) CyA |
TAC + MTX | I | Extensive | S, M, E, L | 4.5 | + | Cholecystitis CMV infection (unknown focus) |
Dead |
| 11 | F | CML | 33 | UR/ BM | complete match | TBI (12 Gy) CyA |
TAC + MTX | I | Extensive | S, M, E, L | 3.2 | – | Bacterial pneumonia | Alive |
| 12 | M | ALL | 43 | UR/BM | complete match | TBI (12 Gy) CyA, CY |
TAC + MTX | III | Extensive | S, M, E, L | 46.4 | – | – | Dead |
M male, F female, ALL acute lymphocytic leukemia, MDS myelodysplastic syndrome; AML acute myeloid leukemia, CML chronic myelogenous leukemia, MM multiple myeloma, HSCT hematopoietic stem cell transplantation, UR unrelated, R related, BM bone marrow cells, HLA human leukocyte antigen, TBI total body irradiation, CY cyclophosphamide, CyA cyclosporine A, BU busulphan, Flu fludarabine, L-PAM melphalan, GVHD graft-versus-host disease, TAC tacrolimus, MTX methotrexate, M mouth, E eye, L liver, S skin, G gastrointestinal tract, Dx diagnosis, HHV6 human herpesvirus 6
For GVHD prophylaxis, all patients received cyclosporine A or tacrolimus in combination with short-term methotrexate (Table 1). Eleven of 12 patients developed acute GVHD (grade I [n = 4], grade II [n = 4], and grade III [n = 3]). All 12 patients had chronic GVHD and developed the extensive-type (Table 1).
Eight of 12 patients died at a median of 33.5 months after the diagnosis of late onset severe restrictive lung defect (Tables 1 and 2). Seven of 8 patients (87.5%) died of pulmonary or systemic infection (bacterial (n = 4); fungal (n = 2), and both (n = 1)). One died of relapse of multiple myeloma.
Table 2.
Characteristics of cases with severe late-onset restrictive lung defect developed after hematopoietic stem cell transplantation
| Case | Months from HSCT to Dx of LONIPCs | Months of survival from Dx of LONIPCs | Months from HSCT to respiratory symptoms | PPFE pattern | Unclassifiable IP pattern | Airway-predominant pattern (Centrilobular nodules with BO) | Bronchiectasis | BO | Pneumothorax | Pneumomediastinum |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 90 | 42 | 103 | + | – | – | + | – | – | – |
| 2 | 26 | >84 | 67 | + | – | – | + | – | + | – |
| 3 | 41 | 132 | 62 | + | – | – | + | – | + | – |
| 4 | 40 | >33 | 54 | + | – | – | – | – | + | – |
| 5 | 109 | 60 | 109 | + | – | – | – | – | – | – |
| 6 | 60 | 16 | 64 | – | + | – | – | – | + | – |
| 7 | 16 | 3 | 16 | – | + | – | – | + | + | – |
| 8 | 22 | 44 | 19 | – | – | + | – | + | – | – |
| 9 | 50 | >80 | 69 | – | – | + | + | + | – | – |
| 10 | 126 | 10 | 124 | – | – | + | – | + | – | – |
| 11 | 36 | >117 | 30 | + | – | – | – | + | – | – |
| 12 | 48 | 25 | 54 | + | – | – | + | – | + | + |
HSCT hematopoietic stem cell transplantation, Dx diagnosis, LONIPCs late-onset noninfectious pulmonary complications, PPFE pleuroparenchymal fibroelastosis, IP interstitial pneumonia, BO bronchiolitis
Characteristics of patients with late-onset severe restrictive lung defect
The median time from transplantation to diagnosis was 44.5 months (Table 2). HRCT demonstrated pleuroparenchymal thickening with volume loss, associated with evidence of fibrosis, in the upper lobe (PPFE pattern) in 7 (58.3%) patients, bronchiectasis in 5 (41.7%) patients, BO in 5 (41.7%) patients, pneumothorax in 6 (50%) patients (Table 2). Among 5 non-PPFE pattern cases, unclassifiable IP pattern was observed in 2 patients (cases No. 6 and No. 7) and centrilobular nodules with BO, associated with the features of airway-predominant diseases (airway predominant pattern), were observed in 3 patients (cases No. 8, No. 9, and No. 10). All 5 patients complicated with BO showed air trapping on expiratory HRCT.
Representative chest radiographs and HRCT images of PPFE pattern are shown in Fig. 2a, b, and c. Bronchiectasis was observed in the left lower lobe in case No. 2 (Fig. 2a). Representative CT findings of unclassifiable IP pattern in case No. 6 (Fig. 3a, b) and airway-predominant pattern (i.e., diffuse bilateral bronchial wall thickening and centrilobular nodules) in case No. 8 (Fig. 3c, d) are also shown.
Fig. 2.
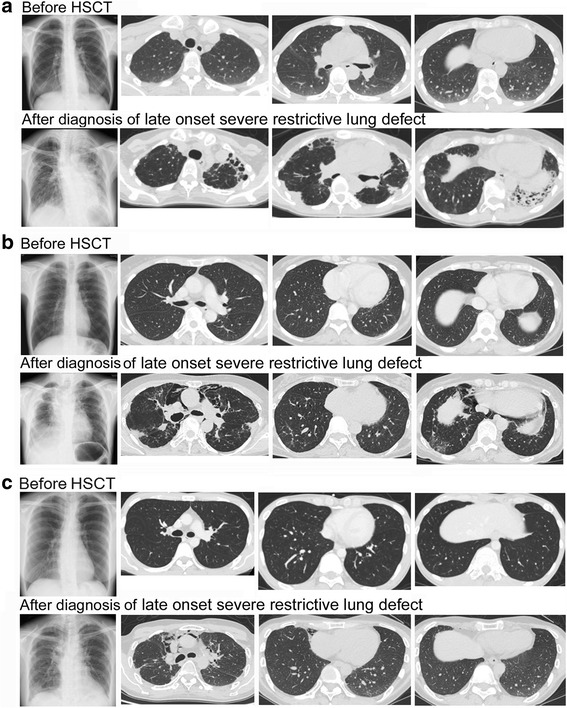
Representative CT findings of PPFE pattern in patients with late-onset severe restrictive lung defect. Chest radiograph and chest CT of representative cases [a) case No. 2, b) No. 4, and c) No. 12] with late-onset severe restrictive lung defect cases before HSCT and after diagnosis of late-onset severe restrictive lung defect are shown
Fig. 3.
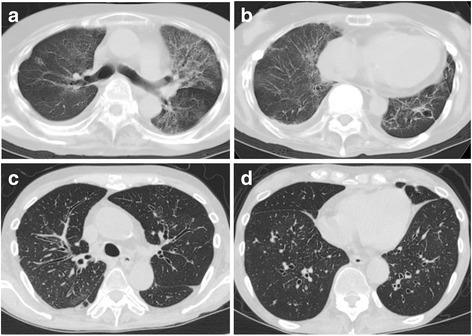
Representative CT findings of non-PPFE pattern in patients with late-onset severe restrictive lung defect. a, b CT findings of unclassifiable IP in case No. 6 are shown. c, d CT findings of airway-predominant disease with BO in case No. 8 are shown
Pulmonary function changes in patients with late-onset severe restrictive lung defect
The results of spirometry are shown in Fig. 4a. Data presented as post-diagnosis are those at diagnosis (cases No. 8 and No. 10) or those most recently examined (other cases), and data presented as pre-diagnosis were those before HSCT. The median follow up was 105.8 months. The median %VC before HSCT was 91% (range, 83–131%). However, the level of median %VC was dramatically decreased (40%, range 18–55%) after diagnosis of late onset severe restrictive lung defect. The levels of VC, forced vital capacity (FVC), and forced expiratory volume in one second (FEV1) were also decreased after HSCT.
Fig. 4.
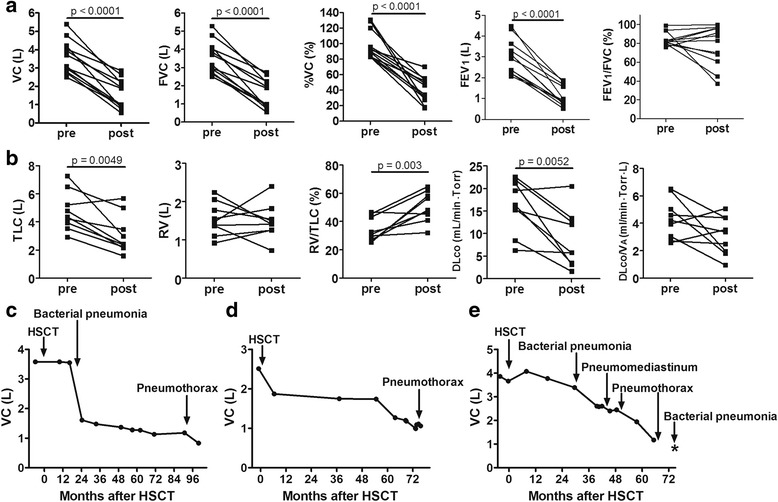
Pulmonary function in patients with late-onset severe restrictive lung defect. a Spirometry data including vital capacity (VC), forced vital capacity (FVC), vital capacity as percent of predicted (%VC), forced expiratory volume in one second (FEV1), and FEV1/ FVC ratio before HSCT (pre) and after (post) diagnosis of late-onset severe restrictive lung defect cases are shown. b Data of pulmonary function tests including total lung capacity (TLC), residual volume (RV), RV/TLC ratio, diffusing capacity of the lung for carbon monoxide (DLco), and DLco divided by the alveolar volume (DLco/VA) are shown. c, d, e Representative time-course changes of VC in patients with late-onset severe restrictive lung defect. Time course changes of VC in c) case No. 2, d) case No.4, and e) case No. 12 are shown. * indicates death
The data of lung volumes and DLco were available in 9 of 12 patients excluding cases No. 7, No. 8, and No. 12. Data presented as pre-diagnosis were those before HSCT (cases No. 4, No. 6, and No. 9) or those performed earliest after HSCT at a median of 12.6 months after HSCT (range, 5.0–40.2) but before diagnosis of late-onset severe restrictive lung defect (other cases). Data presented as post-diagnosis were those most recently examined (Fig. 4b). The median follow up was 100.3 (range, 30.5–138.0) months. The level of TLC and DLco was markedly decreased after HSCT, while RV/TLC ratio increased after HSCT. The data of lung volumes and DLco in each radiological pattern were shown in the Additional file 1: Figure S1.
Representative data of time course changes of VC in three patients (cases No. 2, No. 4, and No. 12) whose CT images are presented in Fig. 2 are shown (Fig. 4c, d, and e). In case No. 2, the episode of bacterial pneumonia resulted in marked decrease in VC, followed by further decrease in VC after the episode of pneumothorax (Fig. 4c). In case No. 4, pneumothorax as well as unknown etiology seemed to contribute to decrease in VC (Fig. 4d). In case No. 12, the episode of bacterial pneumonia reduced VC level, and pneumomediastinum and pneumothorax further decreased VC (Fig. 4e). These results indicate that the onset of pneumothorax and pulmonary infection contributed to the progressive decrease in restrictive pulmonary function and exacerbation of late onset severe restrictive lung defect, thus contributing to its poor prognosis.
Discussion
In this retrospective observational study of allogeneic HSCT recipients, we investigated the clinical features of patients with late-onset severe restrictive lung defect (%VC less than 60%) and identified 12 cases with characteristic radiological findings and pulmonary function changes.
We have shown the incidence of cases of late-onset severe restrictive lung defect (%VC less than 60%) was 2.6% in patients who survived more than 100 days after allogeneic HSCT and underwent spirometry (12 of 453 cases). The incidence of cases of late-onset severe restrictive lung defect developing later than 100 days after HSCT, which has previously been reported as IPS, was 3.5% in adult patients [6]. Another report showed the incidence of cases of late-onset severe restrictive lung defect, which has previously been reported as IPS, as 3.1% in pediatric patients with 1.6% severe restrictive ventilatory defect of %VC less than 60% [16]. The incidence of cases of late-onset severe restrictive lung defect observed in the present study is comparable to those of previous studies. A distinctive characteristic of our retrospective cohort study is that we observed, not only more than 500 consecutive HSCT cases, but also pulmonary function test data on more than 95% of these cases were available. Although this was a retrospective study in a single center, we believe that the HSCT incidence rate and other data are highly reliable and representative.
Late-onset severe restrictive lung defect cases in the present study showed the following characteristic radiological findings: pleuroparenchymal thickening with volume loss, predominantly in the upper lobe (PPFE pattern) in 7 of 12 patients. A previous report demonstrated that PPFE, originally reported as idiopathic [10], had features of late-onset lung involvement after allogeneic HSCT [11]. Although we have not confirmed PPFE by pathological examination in the present study, CT findings in 7 of 12 patients were consistent with the findings seen in patients with PPFE. The other 5 non-PPFE cases were evaluated, and we found that 3 cases were airway-predominant pattern, and 2 cases were unclassifiable IP pattern. Therefore, late-onset severe restrictive lung defect cases in the present study could be categorized into 3 groups based on radiological features: PPFE pattern IP (n = 7), airway-predominant pattern with BO (n = 3), and unclassifiable IP pattern (n = 2). In the present study, half of the patients had a history of pneumothorax, and 5 patients showed BO. Only one of the 5 patients with BO developed pneumothorax. Conversely, PPFE could have contributed to the onset of pneumothorax as subpleural fibrosis is attributed to recurrent rupturing of bullae [11]. Indeed, among 6 cases with a history of pneumothorax, 4 patients showed typical PPFE pattern on HRCT. The 2 other cases were both unclassifiable IP, suggesting that pulmonary fibrosis may be a risk factor for pneumothorax in patients with late-onset severe restrictive lung defect.
With regard to clinical features, we believe there are mainly two clinical courses leading to late-onset severe restrictive lung defect: those associated with and those not associated with BO. Late-onset severe restrictive lung defect associated with BO would display mixed ventilatory impairment after exacerbation of obstructive ventilatory impairment due to BO. In contrast, late-onset severe restrictive lung defect not associated with BO directly displays severe restrictive ventilatory impairment induced by subpleural fibrosis and rupture involving pneumothorax.
The present study demonstrated that VC tended to decrease after pneumothorax and pulmonary infection. There were two exceptional cases (cases No. 8 and No. 10) of airway-predominant diseases, of which %VC was mildly increased after the diagnosis of late-onset severe restrictive lung defect. This may be associated with cases that are not progressive PPFE pattern IP but airway-predominant diseases with BO. TLC and DLco were decreased, while RV/TLC ratio was increased in the present study. A previous report demonstrated that idiopathic PPFE showed increased RV/TLC ratio [17]. This might be due to compensated hyperinflation in the lower lobes, which is not observed in typical idiopathic pulmonary fibrosis. Therefore, increased RV/TLC ratio, in the present study, might also be due to compensated hyperinflation in the lower lobes as well as decreased TLC, especially in patients with PPFE pattern.
It has been reported that extensive chronic GVHD was a risk factor for LONIPCs, which possibly included cases of late-onset severe restrictive lung defect [5]. Consistent with these previous studies, all patients of late-onset severe restrictive lung defect, in the present study, had developed chronic GVHD. These results suggest that chronic GVHD could be a risk factor for late-onset severe restrictive lung defect cases.
The outcomes of patients with late-onset severe restrictive lung defect in the present study were unfavorable [9]; only 4 of 12 patients were alive at the time of the analysis with a median month from diagnosis to death of 33.5 months. Except for one case of death due to the relapse of multiple myeloma, all patients succumbed due to systemic or pulmonary infection, which is a novel finding in patients with late-onset severe restrictive lung defect.
There were limitations in the present study; one is that we were not able to obtain pathological specimens. Therefore, we could not pathologically confirm either PPFE or BO. However, the diagnosis of BO does not necessarily require biopsy per the 2014 NIH consensus development project [18]. Consequently, we do not believe pathological confirmation is essential to diagnose patients with late-onset severe restrictive lung defect, considering the patients’ QOL and the difficulty in performing biopsy. The second is that the range of the timing of the pulmonary function tests may be too wide as pulmonary function tests presented as pre-diagnosis were those before HSCT or those performed after HSCT at a median of 12.6 months after HSCT (range 5.0–40.2 months). The third is that VC levels might not be accurate for identifying the development of late restrictive lung defect, because the patient could have a moderate restrictive lung defect due to early BO. A large, prospective study may contribute to clarifying the late-onset severe restrictive lung defect after HSCT.
We should be more aware of the unique entities after HSCT. Although there are still few reports on these entities, more unrecognized cases are expected to happen in the real clinical setting. Considering from the perspective of HSCT, only 2.4% of HSCT patients proceed to late-onset severe restrictive lung defect, while most of the cases do not proceed to this condition. We speculate that more unknown risk factors related to late-onset severe restrictive lung defect exist. In the future, we should seek more definite risk factors of severe restrictive lung defects and interventions to prevent late-onset severe restrictive lung defect in the early stage post-HSCT by accumulating more cases.
Conclusion
In summary, we have demonstrated the clinical characteristics of late-onset severe restrictive lung defect cases. The incidence of late-onset severe restrictive lung defect cases reported in the current study is comparable to that previously reported. Major CT findings in late-onset severe restrictive lung defect cases were pleuroparenchymal thickening with volume loss predominantly in the upper lobe, which was consistent with PPFE pattern on HRCT. The major cause of death was systemic or pulmonary infection with respiratory failure. The findings of our study may help to elucidate the unique clinical and radiological features of late-onset severe restrictive lung defect after HSCT.
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
The data will not be shared with participant confidentiality.
Abbreviations
- ATS
American Thoracic Society
- BO
Bronchiolitis obliterans
- BOOP
Bronchiolitis obliterans organizing pneumonia
- BOS
BO syndrome
- COP
Cryptogenic organizing pneumonia
- CT
Computed tomography
- DAH
Diffuse alveolar hemorrhage
- GVHD
Graft-versus-host-disease
- HSCT
Hematopoietic stem cell transplantation
- IPS
Idiopathic pneumonia syndrome
- LONIPCs
Late-onset noninfectious pulmonary complications
- MDS
Myelodysplastic syndrome.
- PPFE
Pleuroparenchymal fibroelastosis
- TBI
Total body irradiation
Additional file
Pulmonary function in patients with late-onset severe restrictive lung defect in each HRCT pattern. (JPEG 528 kb)
Authors’ contributions
HN, MI, SO, TM, and TB conceived and designed the experiments; HN, MI, TM, HS, ST, MS. YK, JK, NH analyzed the data; HN, MI, TM wrote the manuscript; SO and TB edited the manuscript; all authors reviewed and approved the final manuscript.
Ethics approval and consent to participate
The protocol was approved by Institutional Review Board of Keio University School of Medicine (Tokyo, Japan). The patients’ approval or informed consent was not required for a retrospective review of their records, pursuant to the ethical guidelines of the Japanese Ministry of Health, Labor, and Welfare.; however, the present retrospective study was carried out by the opt-out method of our hospital website.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12890-017-0466-7) contains supplementary material, which is available to authorized users.
References
- 1.Chan CK, Hyland RH, Hutcheon MA. Pulmonary complications following bone marrow transplantation. Clin Chest Med. 1990;11(2):323–332. [PubMed] [Google Scholar]
- 2.Wah TM, Moss HA, Robertson RJ, Barnard DL. Pulmonary complications following bone marrow transplantation. Br J Radiol. 2003;76(906):373–379. doi: 10.1259/bjr/66835905. [DOI] [PubMed] [Google Scholar]
- 3.Soubani AO, Miller KB, Hassoun PM. Pulmonary complications of bone marrow transplantation. Chest. 1996;109(4):1066–1077. doi: 10.1378/chest.109.4.1066. [DOI] [PubMed] [Google Scholar]
- 4.Harris B, Lowy FD, Stover DE, Arcasoy SM. Diagnostic bronchoscopy in solid-organ and hematopoietic stem cell transplantation. Ann Am Thorac Soc. 2013;10(1):39–49. doi: 10.1513/AnnalsATS.201212-114FR. [DOI] [PubMed] [Google Scholar]
- 5.Sakaida E, Nakaseko C, Harima A, Yokota A, Cho R, Saito Y, Nishimura M. Late-onset noninfectious pulmonary complications after allogeneic stem cell transplantation are significantly associated with chronic graft-versus-host disease and with the graft-versus-leukemia effect. Blood. 2003;102(12):4236–4242. doi: 10.1182/blood-2002-10-3289. [DOI] [PubMed] [Google Scholar]
- 6.Solh M, Arat M, Cao Q, Majhail NS, Weisdorf D. Late-onset noninfectious pulmonary complications in adult allogeneic hematopoietic cell transplant recipients. Transplantation. 2011;91(7):798–803. doi: 10.1097/TP.0b013e31820c85fa. [DOI] [PubMed] [Google Scholar]
- 7.Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2001;28(5):425–434. doi: 10.1038/sj.bmt.1703142. [DOI] [PubMed] [Google Scholar]
- 8.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. NHLBI workshop summary. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147(6 Pt 1):1601–1606. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 9.Panoskaltsis-Mortari A, Griese M, Madtes DK, Belperio JA, Haddad IY, Folz RJ, Cooke KR. An official American Thoracic Society research statement: noninfectious lung injury after hematopoietic stem cell transplantation: idiopathic pneumonia syndrome. Am J Respir Crit Care Med. 2011;183(9):1262–1279. doi: 10.1164/rccm.2007-413ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankel SK, Cool CD, Lynch DA, Brown KK. Idiopathic pleuroparenchymal fibroelastosis: description of a novel clinicopathologic entity. Chest. 2004;126(6):2007–2013. doi: 10.1378/chest.126.6.2007. [DOI] [PubMed] [Google Scholar]
- 11.von der Thusen JH, Hansell DM, Tominaga M, Veys PA, Ashworth MT, Owens CM, Nicholson AG. Pleuroparenchymal fibroelastosis in patients with pulmonary disease secondary to bone marrow transplantation. Mod Pathol. 2011;24(12):1633–1639. doi: 10.1038/modpathol.2011.114. [DOI] [PubMed] [Google Scholar]
- 12.Standardization of Spirometry 1994 Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 13.Statement of the American Thoracic Society. Single breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique. Am Rev Respir Dis. 1987;136(5):1299–1307. [DOI] [PubMed]
- 14.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 15.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, Hackman R, Tsoi MS, Storb R, Thomas ED. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 16.Park M, Koh KN, Kim BE, Im HJ, Seo JJ. Clinical features of late onset non-infectious pulmonary complications following pediatric allogeneic hematopoietic stem cell transplantation. Clin Transpl. 2011;25(2):E168–E176. doi: 10.1111/j.1399-0012.2010.01357.x. [DOI] [PubMed] [Google Scholar]
- 17.Kusagaya H, Nakamura Y, Kono M, Kaida Y, Kuroishi S, Enomoto N, Fujisawa T, Koshimizu N, Yokomura K, Inui N, et al. Idiopathic pleuroparenchymal fibroelastosis: consideration of a clinicopathological entity in a series of Japanese patients. BMC Pulm Med. 2012;12:72. doi: 10.1186/1471-2466-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant. 2015;21(3):389–401. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be shared with participant confidentiality.


