Abstract
Background:
A correlation exists between the microbial flora of the upper and lower airways in patients with cystic fibrosis (CF) or with primary ciliary dyskinesia (PCD). The sinuses can function as a bacterial reservoir where gram-negative bacteria adapt to the airways and repeatedly are aspirated to and colonize the lungs according to the theory of the united (unified) airways. Whereas the pattern of bacterial flora in the lower airways has been extensively studied, the upper airways have drawn limited attention.
Objective:
Our aim was to review the literature that reported bacterial flora in the sinuses and nasal cavities of patients with CF or PCD.
Methods:
A number of medical literature data bases were systematically searched between January 1960 and July 2016. We applied the following inclusion criteria: a minimum of one case of PCD (or Kartagener syndrome) or CF, and microbiology analyses from the nose or paranasal sinuses.
Results:
We included 46 studies (1823 patients) from 16 countries. Staphylococcus aureus was found in 30% of the noses and sinuses of patients with CF. Other common bacteria found included Pseudomonas aeruginosa, coagulase negative staphylococci, and Haemophilus influenzae. In PCD, H. influenzae was the most common bacteria (28%), followed by Streptococcus pneumoniae and P. aeruginosa. If studies that included nonsurgical swab and blowing samples were excluded, then P. aeruginosa was the most common bacterium in patients with CF (34%) and in patients with PCD (50%), followed by S. aureus and H. influenza.
Conclusion:
S. aureus, P. aeruginosa, coagulase negative staphylococci, and H. influenzae dominated in the upper airways of patients with CF. In patients with PCD, H. influenzae, S. pneumoniae, and P. aeruginosa dominated. When studies that included swab and blowing samples were excluded, P. aeruginosa was the most common bacterium in both groups. Direct comparisons among the studies were restricted due to very heterogeneous methods, and a better standardization of procedures and outcomes is needed.
Keywords: Cystic fibrosis, primary ciliary dyskinesia, bacteria, microbiology, nose, sinus, upper airways, unified airways, united airways
Cystic fibrosis (CF) is an autosomal recessive genetic disease caused by mutations in the CF transmembrane conductance regulator gene.1 The CF transmembrane conductance regulator gene is responsible for chloride transport over the cell membrane.2 The defect causes tenacious and viscous mucus, which further impairs the movement of cilia.3,4 In Europe, the incidence of CF is ∼1 in 2300,5 and it is the most common genetic lethal disease among whites.6 Primary ciliary dyskinesia (PCD) is similarly an autosomal recessive genetic disorder caused by mutations in genes responsible for the function and structure of cilia.7 PCD affects ∼1 in 15,000 individuals,8 and ∼50% of patients with PCD have Kartagener syndrome, characterized by the triad of chronic rhinosinusitis (CRS), bronchiectasis, and situs inversus.9 The incidence may be higher because patients with mild phenotypes may remain undiagnosed and the diagnostic procedures require advanced and expensive equipment. Both diseases impair the sinonasal and pulmonary mucociliary clearance, which promotes recurrent colonization and infection with microorganisms in the upper and lower airways.10,11
The airways, according to the united (unified) airways concept, can be seen as a single unit. A strong correlation exists between the upper and lower airway bacteriology in patients with CF and PCD. Bacteria isolated from the lungs and sinuses in the same patient often have the same genotype,12–14 which supports the theory that the sinuses function as a bacterial reservoir where bacteria can adapt to the airways and become aspirated to the lungs and vice versa.12 In the early stages of lung colonization, the migration of pathogens may mainly occur in a downward direction, with the sinuses as a primary focus.12 Several studies found concordance between upper and lower airway bacteriology in both patients with CF13–17 and those with PCD.11 Although the pattern of bacterial flora in the lower airways has been extensively studied, the upper airways have drawn limited attention. The primary aim of this study was to review existing studies that cover the bacterial flora in sinuses and the nasal cavity in patients with CF and patients with PCD, and to discuss factors that may influence the result.
METHODS
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement.18
Systematic Literature Search and Eligibility Criteria
In February 2016, one author (M.E.M.) systematically searched the PubMed, Embase (Elsevier B.V. Registered Office, Amsterdam, the Netherlands), and Cochrane Library (London, UK) data bases for articles written in the English language by using the following search strategy, including Medical Subject Heading terms and keywords: “nose” or “paranasal sinus” or “paranasal sinuses” or “sinus” and “microbiology” or “bacteria” or “mucociliary transport” and “cystic fibrosis” or “cf” or “primary ciliary dyskinesia” or “pcd” or “ciliary motility disorder” or “immotile cilia syndrome” or “kartagener syndrome” or “kartagener's syndrome.” One author (M.E.M.) independently reviewed abstracts, accessed the full-text copies of relevant articles, and reviewed the reference lists.
The inclusion criteria were as follows: one or more cases of PCD (or Kartagener syndrome) or CF and bacterial flora analysis from the nose or the paranasal sinuses. The criteria were restricted to January 1960 to July 2016, and animal experiments were excluded. Articles or parts of the results were excluded if the number of bacterial species were not counted (e.g., listed as follows: e.g., Pseudomonas aeruginosa, Staphylococcus aureus) or nonspecific (e.g., other bacteria, other). Furthermore, to avoid patient group overlap, only one article was chosen among the articles with the same authors, patients from the same institution, or within a possible overlap period. The article chosen was consequently the one with the greatest number of patients included based on the sinonasal procedures. Four studies presented their results in relation to the number of procedures instead of per patient. The proportions were given in percentages, which were recalculated to provide a more general estimate according to the number of patients (e.g., S. aureus accounted for 47% of the samples ×46 patients/100 = 22 patients). The general estimates were accepted. The data were calculated and presented as before and after exclusion of studies that used blowing samples and swabs as methods that were not described as a part of a surgical procedure and, therefore, most likely represented the contaminating flora of the anterior nares. Some studies did not describe the methods applied for collecting samples.
Data Analysis
One author (M.E.M.) extracted the relevant data from the articles and entered them into a spreadsheet that included the institution and country, age, year of publication, number of patients, type of bacteria, anatomic location(s) (e.g., maxillary sinus), sampling method (e.g., endoscopy), use of antibiotics (yes or no and, if yes, antibiotic specified), and culture or molecular methods (e.g., agar or polymerase chain reaction [PCR]). Included studies were divided into two groups based on patients with PCD and patients with CF.
RESULTS
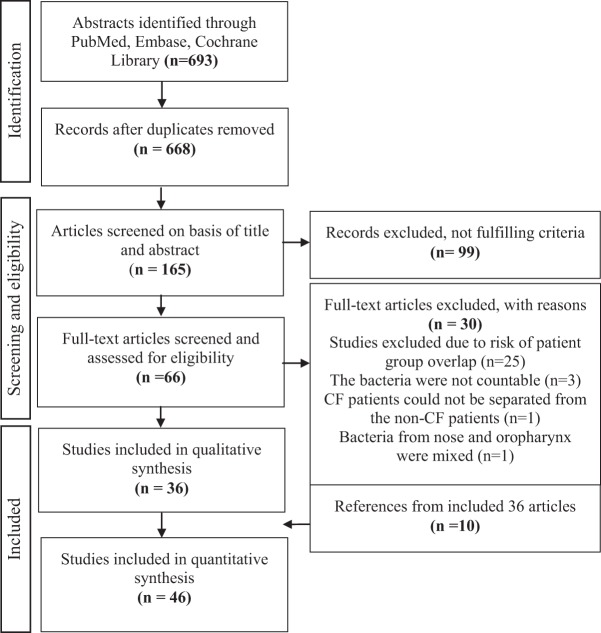
Overall, we included 46 studies with a total of 1823 patients (Fig. 1). The PCD group accounted for 3 studies,11,19,20 with a total of 105 patients, and the CF group accounted for 43 studies5,6,10,13,14,16,17,21–56 and 1718 patients. The origins of the studies are noted in the Online Supplemental Material.5,6,10,11,13,14,16,17,19–56 The most common bacteria isolated in the upper airways in CF were S. aureus (including methicillin resistant S. aureus, methicillin sensitive S. aureus, and glycopeptide intermediate S. aureus), which were identified in 513 patients (30%). The second most common bacterium was P. aeruginosa, which was isolated in 437 patients (25%). The third and fourth most common bacteria were coagulase negative staphylococci (CoNS) (5%) and Haemophilus influenza (4%). Overall, 55% of the patients with CF were either colonized in the nose or the sinuses with P. aeruginosa, S. aureus, or both (Table 1).
Figure 1.
Flowchart. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram: Study selection process (from Ref. 18).
Table 1.
The most common bacteria isolated from the nose and sinuses in patients with cystic fibrosis, including swab and blowing sample studies*
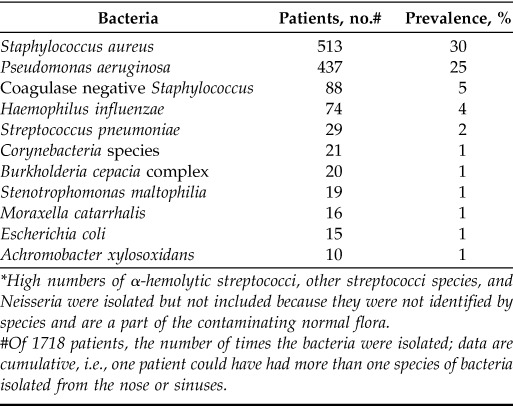
High numbers of α-hemolytic streptococci, other streptococci species, and Neisseria were isolated but not included because they were not identified by species and are a part of the contaminating normal flora.
#Of 1718 patients, the number of times the bacteria were isolated; data are cumulative, i.e., one patient could have had more than one species of bacteria isolated from the nose or sinuses.
In subanalyses based of the number of patients in the studies that investigated the occurrence of S. aureus (1449 patients), 35% of the patients were infected with S. aureus. In comparison, in studies that investigated the occurrence of P. aeruginosa (1383 patients), 32% were infected with P. aeruginosa. Based on these data, S. aureus was still more common in relation to the specific number of patients in studies in which it had been identified or specifically investigated. The most common upper airway pathogen in patients with PCD was H. influenzae, which was isolated in 29 patients (28%), followed by S. pneumoniae in 17%. P. aeruginosa and S. aureus were found in 14% and 3% of the patients, respectively. In total, 17% of the patients with PCD were colonized with one or both of these bacteria (Table 2). Sampling methods included sinus surgery with the use of endoscopy (54%), lavage and/or washouts (15%), and swabs (30%), which were sometimes done in combination with endoscopy or other procedures (26%) (Table 3). Sinus surgery with or without endoscopy was used to collect aspirate samples (22%), biopsy specimens and/or smears (11%), pus and/or secretions (7%), or crusts (4%). Nose blowing samples accounted for 2%. Data were cumulative, which meant that several methods could be used in one study.
Table 2.
The most common bacteria isolated from the nose and sinuses in patients with primary ciliary dyskinesia, including swab sample studies
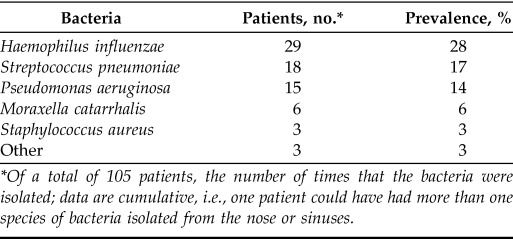
*Of a total of 105 patients, the number of times that the bacteria were isolated; data are cumulative, i.e., one patient could have had more than one species of bacteria isolated from the nose or sinuses.
Table 3.
Other procedures (that could be used to collect samples)

The most common sampling sites were the maxillary sinus (30%), middle meatus (24%), paranasal sinus unspecified (including the sinus ostia) (20%), nose specified (anterior nares, posterior nares, nostrils, inferior turbinate, and meatus) (20%), nose unspecified (17%), ethmoid sinuses (20%), frontal sinuses (9%), sphenoid sinuses (11%), and external nares (2%). Data presented were cumulative, which meant that samples could be obtained from more than one anatomic location per study. Forty-one percent of the studies13,17,20–22,24,26,27,29,30,38–42,45,50,52,53 provided information about the use of antibiotics before or during the sampling procedure. In four studies,14,16,25,33 it was unclear whether the patients received antibiotics before or during sampling. Fifty percent of the studies10,11,13,14,17,22–24,26–33,36,38,39,42,45,47,49 provided information about culture methods. Most studies10,11,14,17,22–24,27–32,38,39,42,45,47,49 used conventional culture methods (41%). In four studies,22,35,37,40 the method applied was unclear (referred to other articles, “microbiologic examination,” or “bacteriologic examination”). However, molecular methods (e.g., PCR) were applied in 26%.13,14,17,24,26–30,33,36,42
When excluding studies that used only swabs and blowing samples, nine studies13,19,20,24,27–31 were excluded, which left a total of 37 studies5,6,10,11,14,16,17,21–23,25,26,32–56 with 1139 patients. In CF, seven articles13,24,27–31 were excluded, which left 1131 patients with CF, and two PCD articles19,20 were excluded, which left eight patients with PCD in the study. In patients with CF, the most common bacteria were then P. aeruginosa found in 383 patients (34%), S. aureus (28%), and H. influenzae (5%). CoNS was found in 3.5% of the patients (Table 4). Among the seven excluded CF studies,13,24,27–31 three studies24,27,29 investigated only the presence of S. aureus and one study investigated exclusively for P. aeruginosa,30 and the last three were mixed.13,28,31 In the patients with PCD, the most commonly found bacteria were P. aeruginosa (found in four patients [50%]), S. aureus (12.5%), and H. influenza (12.5%) (Table 5).
Table 4.
The most common bacteria isolated from the nose and sinuses in patients with cystic fibrosis, without swab and blowing sample studies*
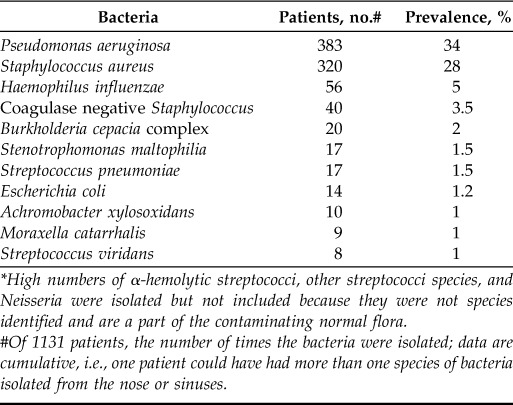
High numbers of α-hemolytic streptococci, other streptococci species, and Neisseria were isolated but not included because they were not species identified and are a part of the contaminating normal flora.
#Of 1131 patients, the number of times the bacteria were isolated; data are cumulative, i.e., one patient could have had more than one species of bacteria isolated from the nose or sinuses.
Table 5.
The most common bacteria isolated from the nose and sinuses in patients with primary ciliary dyskinesia, without swab sample studies

*Of a total of 8 patients, the number of times the bacteria were isolated; data are cumulative, i.e., one patient could have had more than one species of bacteria isolated from the nose or sinuses.
DISCUSSION
To our knowledge, we presented the first systematic review that addressed sinus and nose bacteriology in patients with PCD and in patients with CF. As expected, we found that S. aureus, P. aeruginosa, CoNS, and H. influenzae were the predominant bacteria that colonized the upper airways in patients with CF. In patients with PCD, H. influenzae, S. pneumoniae, and P. aeruginosa were the predominant bacteria. We found that the order of the bacteria changed when studies that included nonsurgically obtained swab samples as well as blowing samples were removed. The most common bacterium was then P. aeruginosa, followed by S. aureus, and H. influenza in both groups.
In a review by Brook,57 he indicated that, in healthy individuals, the normal flora in the nose consists of S. aureus, Staphylococcus epidermidis, α-streptococci and γ-streptococci, Propionibacterium acnes, and aerobic Diphtheroids. However, the listed bacteria exist as normal flora in the anterior nares due to contamination. Furthermore, CoNS is regarded as a contaminant and a part of the normal flora in the vestibulum nasi and the skin. Of healthy people, ∼30% carry S. aureus in vestibulum nasi.58 The resistance of S. aureus to antibiotics is reported to increase with exposure to a hospital environment.59 Methicillin resistant S. aureus is especially isolated in individuals with health care–associated exposure.60–62 Controversy remains as to whether there is a nonpathologic bacterial flora in the healthy sinus. Nevertheless, α-hemolytic streptococci and β-hemolytic streptococci, S. aureus, H. parainfluenzae, and anaerobes, have been isolated from nondiseased sinuses.63 A study by Cleland et al.64 found Acinetobacter johnsonii to be abundant in the healthy sinus and, furthermore, to increase in patients with CRS after surgery, which indicates a potential association between this bacterium and health.64
In acute bacterial rhinosinusitis in patients not suffering from CF or PCD, S. aureus, S. pneumoniae, S. pyogenes, H. influenza, and Moraxella catarrhalis are the most common bacteria.57 In CRS, S. aureus and anaerobic bacteria predominate,57 but CoNS are also is present in high numbers.65 Furthermore, P. aeruginosa is often isolated from patients with nosocomial CRS, immune deficiencies, and CF.22,57 P. aeruginosa, S. aureus, and H. influenzae are frequently isolated from the sinuses of patients with CF, and CoNS,6,13,21,32 as this survey also demonstrated in patients with CF. In patients with PCD, H. influenzae, M. catarrhalis, S. pneumoniae, and S. aureus are the most common bacteria isolated from the lungs.66 P. aeruginosa frequently colonizes the lower respiratory tract in patients with PCD, and the prevalence increases with age.66 Few reports on sinus bacteriology in patients with PCD have been published; however, a Danish study67 (not included due to patient group overlap with the study by Mygind and Pedersen19) found that H. influenzae was the most common bacteria in the nose and sinuses, followed by S. pneumoniae, P. aeruginosa, and S. aureus, which is in agreement with the results before the exclusion of the swab sample studies, which possibly indicates that P. aeruginosa becomes more abundant when the sample method involves surgery in this patient group.
In CF, patients are also infected in an age-dependent sequence. Several studies reported S. aureus and H. influenzae to be the most common bacteria in the upper airways in pediatric patients.32,68 With increasing age, P. aeruginosa was found more frequently, which is also characteristic for a more advanced stage of the disease.17,68 This is also the pattern seen in the lungs.23,69,70 There are no data on age variation in the sinus bacteriology in patients with PCD, but, in the lungs, it is reported that the majority of patients are colonized with H. influenzae and M. catarrhalis during childhood, whereas P. aeruginosa is seen later in life, with the incidence increasing with age.66 The bacteriology in the lungs of patients with PCD seemed to have similarities with non-CF bronchiectasis bacteriology.66
Goerke et al.24 reported that the prevalence of S. aureus among patients with CF is significantly higher in those who had not received antibiotics and higher than in healthy controls, which indicates that presample antibiotics can influence growth of S. aureus in the nose. Moreover, a study by Gitomer et al.71 showed that S. aureus transformed into small colony variants after exposure to gentamycin in vitro, which indicated that this resistant type of S. aureus increases with longtime exposure to antibiotics, which is the case in patients with CF and PCD. However, only 41% of the studies that comprise the present review provided information about the use of antibiotics, which is an obvious limitation. In addition, it was impossible in most cases to separate patients who received antibiotics from those who did not.
Use of culture and/or molecular methods can influence the variety, abundance, and type of bacteria found. A study by Rudkjøbing et al.72 indicated that a combination of molecular methods and culture-dependent routine diagnosis is an optimal way to find the greatest variety in detecting aerobes, anaerobes, and facultative anaerobes in the sinuses in patients with CF. However, the experience of patients with CF in our center indicates that classic culture methods sufficiently detects the clinically important bacteria.73 Moreover, PCR detects a broad variety of bacteria but may blur which bacteria are relevant to treat.
The sampling method may also influence the result. Sinus aspiration is the criterion standard for obtaining secretions from the sinuses, but it is invasive.25 Nasal lavages are suggested as a noninvasive alternative13 and were used in 15% of the studies10,13,20,33,35,37,54 in this review. However, cultures from nasal lavage may presumably not represent all the sinuses and the sample may be contaminated from the nasopharynx.74 In addition, the procedure may require some experience, and inadequate flushing may only rinse the vestibulum13 Furthermore, in a recent study by Kim et al.,26 S. aureus was shown to grow in intramucosal colonies in the sinuses in patients with CF. Intramucosal colonies may not be detected without invasive methods. Nasal swabs and blowing samples are both regarded as less sensitive and might not be an optimal method.13
Several articles were rather nonspecific with regard to information about the sampling site, e.g., writing “paranasal sinus,” which made it impossible to further localize the specific sampling site. We included studies from 1960 to 2016, which may have led to differences in the methods applied to sampling because the sinonasal endoscopic technique was introduced in the 1980s.75 Furthermore, patients admitted for endoscopic sinus surgery are a select group who probably have the most severe CRS, which we considered a serious limitation. Nevertheless, CRS is almost universal and underdiagnosed in CF.
Our study had other limitations. There was a lack of information and no standardization in the way that the findings in each article were measured, which made it difficult to provide a complete overview. Standardization and information on patients' age, use of antibiotics, culture methods, sample methods etc., are issues that are important when making an overview on CRS bacteriology.57 Factors such as genotype and lung infection status are important to include as well. In addition, it would have been valuable to analyze adult and pediatric data separately, but this was not possible because this further information was not provided in the majority of the articles. Neither was a division of patients based on lung infection status possible because too few of the studies had divided as such. Furthermore, many studies were not comparable because of the heterogeneity of the information and the variety of bacteria investigated. For example, a study that exclusively searches for P. aeruginosa or S. aureus will bias the overall result.
Moreover, there were a number of articles by the same authors or from the same centers, with a risk of inclusion of the same results from the same patients in the present review. In such cases, the article with the largest number of patients was included. Only the authors at the Copenhagen University Hospital were contacted regarding two articles with a wide range of years (i.e., 32 years) between the two publications. There was no overlap in patients between the two articles, which resulted in an inclusion of both articles. Generally, more exclusion criteria could have been applied to our search in an attempt to avoid or limit the number of bias and reduce the heterogenicity. Nevertheless, the heterogenicity in the articles affected the outcome of this paper. In our opinion, it would be impossible to avoid the heterogenicity completely, and, to apply more exclusion criteria, furthermore, would decrease a small study population. Bacterial cultures were not the main outcome in most of the articles included in our review; therefore, information about this may be present in full-text articles without being visible in the abstracts, which led to assessments of many less obvious full-text articles. Two conference abstracts were included because the bacterial isolates were relevant to include in the study. To the best of our knowledge, full-text articles from the same author or center have not been published afterward.
CONCLUSION
To our knowledge, we presented the first systematic review of upper airway pathogens in patients with CF or PCD. S. aureus, P. aeruginosa, CoNS, and H. influenzae seemed to be the most abundant bacteria in the nose and sinuses of patients with CF. H. influenzae, S. pneumoniae, and P. aeruginosa were the bacteria most frequently found in patients with PCD. P. aeruginosa seemed to be the most abundant in both patients with PCD and patients with CF after articles that included swab and blowing samples13,19,20,24,27–31 were removed. Only three studies11,19,20 in patients with PCD were included in the first analysis, and only one11 after swab sample studies were included. More studies that investigate the bacterial status in these patients are needed. Due to the heterogeneity with regard to methods and materials used in the included studies with the risk of bias made a firm conclusion difficult. A number of factors, such as the age of the patients, sampling methods, use of antibiotics, and culture methods, and, furthermore, lung infection status and use of a control study may have influenced the results.
Footnotes
Presented at the Danish Society of Otolaryngology, Head and Neck Surgery yearly meeting, Nyborg, Denmark, May 13, 2016, and the 26th Congress of the European Rhinologic Society (ERS) in conjunction with the 35th Congress of the International Society of Inflammation and Allergy of the Nose (ISIAN) & the 17th Congress of the International Rhinologic Society (IRS) (ERS-ISIAN-IRS), Stockholm, Sweden, July 3–6, 2016
C. Grønhøj is funded by Kræftfonden (The Cancer Foundation), and C. Grønhøj and M.C. Alanin are funded by Candys Foundation
The authors have no conflicts of interest to declare pertaining to this article
Supplemental data available at www.IngentaConnect.com
REFERENCES
- 1. Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 245:1066–1073, 1989. [DOI] [PubMed] [Google Scholar]
- 2. Gadsby DC, Vergani P, Csanády L. The ABC protein turned chloride channel whose failure causes cystic fibrosis. Nature 440:477–483, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramsey B, Richardson M. Impact of sinusitis in cystic fibrosis. J Allergy Clin Immunol 90:547–552, 1992. [DOI] [PubMed] [Google Scholar]
- 4. Boucher RC. New concepts of the pathogenesis of cystic fibrosis lung disease. Eur Respir J 23:146–158, 2004. [DOI] [PubMed] [Google Scholar]
- 5. Krzeski A, Kapiszewska-Dzedzej D, Gorski NP, Jakubczyk I. Cystic fibrosis in rhinologic practice. Am J Rhinol 16:155–160, 2002. [PubMed] [Google Scholar]
- 6. Wise SK, Kingdom TT, McKean L, et al. Presence of fungus in sinus cultures of cystic fibrosis patients. Am J Rhinol 19:47–51, 2005. [PubMed] [Google Scholar]
- 7. Afzelius B. A human syndrome caused by immotile cilia. Science 193:317–319, 1976. [DOI] [PubMed] [Google Scholar]
- 8. Bush A, Cole P, Hariri M, et al. Primary ciliary dyskinesia: Diagnosis and standards of care. Eur Respir J 12:982–988, 1998. [DOI] [PubMed] [Google Scholar]
- 9. Knowles MR, Daniels LA, Davis SD, et al. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med 188:913–922, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berkhout MC, Rijntjes E, El Bouazzaoui LH, et al. Importance of bacteriology in upper airways of patients with cystic fibrosis. J Cyst Fibros 12:525–529, 2013. [DOI] [PubMed] [Google Scholar]
- 11. Alanin MC, Johansen HK, Aanaes K, et al. Simultaneous sinus and lung infections in patients with primary ciliary dyskinesia. Acta Otolaryngol 135:58–63, 2015. [DOI] [PubMed] [Google Scholar]
- 12. Hansen SK, Rau MH, Johansen HK, et al. Evolution and diversification of Pseudomonas aeruginosa in the paranasal sinuses of cystic fibrosis children have implications for chronic lung infection. ISME J 6:31–45, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mainz JG, Naehrlich L, Schien M, et al. Concordant genotype of upper and lower airways P aeruginosa and S aureus isolates in cystic fibrosis. Thorax 64:535–540, 2009. [DOI] [PubMed] [Google Scholar]
- 14. Johansen HK, Aanaes K, Pressler T, et al. Colonisation and infection of the paranasal sinuses in cystic fibrosis patients is accompanied by a reduced PMN response. J Cyst Fibros 11:525–531, 2012. [DOI] [PubMed] [Google Scholar]
- 15. Moss RB, King VV. Management of sinusitis in cystic fibrosis by endoscopic surgery and serial antimicrobial lavage. Reduction in recurrence requiring surgery. Arch Otolaryngol Head Neck Surg 121:566–572, 1995. [DOI] [PubMed] [Google Scholar]
- 16. Halvorson DJ, Dupree JR, Porubsky ES. Management of chronic sinusitis in the adult cystic fibrosis patient. Ann Otol Rhinol Laryngol 107:946–952, 1998. [DOI] [PubMed] [Google Scholar]
- 17. Muhlebach MS, Miller MB, Moore C, et al. Are lower airway or throat cultures predictive of sinus bacteriology in cystic fibrosis? Pediatr Pulmonol 41:445–451, 2006. [DOI] [PubMed] [Google Scholar]
- 18. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 8:336–341, 2010. [DOI] [PubMed] [Google Scholar]
- 19. Mygind N, Pedersen M. Nose-, sinus- and ear-symptoms in 27 patients with primary ciliary dyskinesia. Eur J Respir Dis Suppl 127:96–101, 1983. [PubMed] [Google Scholar]
- 20. Marsh GS, Collins NL, Bush A, et al. Do children with primary ciliary dyskinesia harbour the same pathogens in the upper and lower airways? Thorax 69:A122, 2014(Abs). [Google Scholar]
- 21. Roby BB, McNamara J, Finkelstein M, Sidman J. Sinus surgery in cystic fibrosis patients: Comparison of sinus and lower airway cultures. Int J Pediatr Otorhinolaryngol 72:1365–1369, 2008. [DOI] [PubMed] [Google Scholar]
- 22. Shapiro ED, Milmoe GJ, Wald ER, et al. Bacteriology of the maxillary sinuses in patients with cystic fibrosis. J Infect Dis 146:589–593, 1982. [DOI] [PubMed] [Google Scholar]
- 23. Bonestroo HJ, de Winter-de Groot KM, van der Ent CK, et al. Upper and lower airway cultures in children with cystic fibrosis: Do not neglect the upper airways. J Cyst Fibros 9:130–134, 2010. [DOI] [PubMed] [Google Scholar]
- 24. Goerke C, Kraning K, Stern M, et al. Molecular epidemiology of community-acquired Staphylococcus aureus in families with and without cystic fibrosis patients. J Infect Dis 181:984–989, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Lavin J, Bhushan B, Schroeder JW. Correlation between respiratory cultures and sinus cultures in children with cystic fibrosis. Int J Pediatr Otorhinolaryngol 77:686–689, 2013. [DOI] [PubMed] [Google Scholar]
- 26. Kim RJ, Park L, Wood AJ, et al. Chronic rhinosinusitis and cystic fibrosis: The interaction between sinus bacteria and mucosal immunity. Int Forum Allergy Rhinol 5:380–385, 2015. [DOI] [PubMed] [Google Scholar]
- 27. Ridder-Schaphorn S, Ratjen F, Dubbers A, et al. Nasal Staphylococcus aureus carriage is not a risk factor for lower-airway infection in young cystic fibrosis patients. J Clin Microbiol 45:2979–2984, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stelzer-Braid S, Johal H, Skilbeck K, et al. Detection of viral and bacterial respiratory pathogens in patients with cystic fibrosis. J Virol Methods 186:109–112, 2012. [DOI] [PubMed] [Google Scholar]
- 29. Stone A, Quittell L, Zhou J, et al. Staphylococcus aureus nasal colonization among pediatric cystic fibrosis patients and their household contacts. Pediatr Infect Dis J 28:895–899, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Taylor RF, Morgan DW, Nicholson PS, et al. Extrapulmonary sites of Pseudomonas aeruginosa in adults with cystic fibrosis. Thorax 47:426–428, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Taylor CJ, McGaw J, Howden R, et al. Bacterial reservoirs in cystic fibrosis. Arch Dis Child 65:175–177, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Digoy GP, Dunn JD, Stoner JA, et al. Bacteriology of the paranasal sinuses in pediatric cystic fibrosis patients. Int J Pediatr Otorhinolaryngol 76:934–938, 2012. [DOI] [PubMed] [Google Scholar]
- 33. Linnane B, Kearse L, O'Connell NH, et al. A case of failed eradication of cystic fibrosis-related sinus colonisation by Pseudomonas aeruginosa. BMC Pulm Med 15:114, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Holdsworth SM. A case study of medical and surgical management of a cystic fibrosis patient. ORL Head Neck Nurs 33:20, 22, 2015. [PubMed] [Google Scholar]
- 35. Rubira L, Farel M, Nassibou S, et al. Longitudinal survey of decreased susceptibility to glycopeptides in Staphylococcus aureus chronically colonized CF patients. 2015 Cyst Fibros Conf 315, 2015. [Google Scholar]
- 36. Chalermwatanachai T, Zhang N, Holtappels G, Bachert C. Association of mucosal organisms with patterns of inflammation in chronic rhinosinusitis. PLoS One 10:e0136068, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson P, Lambert C, Carr SB, Pao C. Paranasal sinus pathogens in children with cystic fibrosis: Do they relate to lower respiratory tract pathogens and is eradication successful? J Cyst Fibros 13:449–454, 2014. [DOI] [PubMed] [Google Scholar]
- 38. Di Cicco M, Alicandro G, Claut L, et al. Efficacy and tolerability of a new nasal spray formulation containing hyaluronate and tobramycin in cystic fibrosis patients with bacterial rhinosinusitis. J Cyst Fibros 13:455–460, 2014. [DOI] [PubMed] [Google Scholar]
- 39. Tunes A, Reis JN, Terse R, et al. Microbiology of the middle meatus compared to sputum in young patients with cystic fibrosis from Bahia—Brazil. Braz J Infect Dis 18:215–219, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vital D, Hofer M, Boehler A, Holzmann D. Posttransplant sinus surgery in lung transplant recipients with cystic fibrosis: A single institutional experience. Eur Arch Otorhinolaryngol 270:135–139, 2013. [DOI] [PubMed] [Google Scholar]
- 41. Virgin FW, Rowe SM, Wade MB, et al. Extensive surgical and comprehensive postoperative medical management for cystic fibrosis chronic rhinosinusitis. Am J Rhinol Allergy 26:70–75, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Godoy JM, Godoy AN, Ribalta G, Largo I. Bacterial pattern in chronic sinusitis and cystic fibrosis. Otolaryngol Head Neck Surg 145:673–676, 2011. [DOI] [PubMed] [Google Scholar]
- 43. Ghegan MD, Wise SK, White DR, et al. Cost-effective airway cultures in the cystic fibrosis patient. Am J Otolaryngol 30:150–152, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Leung MK, Rachakonda L, Weill D, Hwang PH. Effects of sinus surgery on lung transplantation outcomes in cystic fibrosis. Am J Rhinol 22:192–196, 2008. [DOI] [PubMed] [Google Scholar]
- 45. Franche GL, Abreu e Silva F, Saleh Cde S. Bacteriology of the middle meatus aspirate in patients with cystic fibrosis. Braz J Otorhinolaryngol 73:494–499, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cimmino M, Cavaliere M, Nardone M, et al. Clinical characteristics and genotype analysis of patients with cystic fibrosis and nasal polyposis. Clin Otolaryngol Allied Sci 28:125–132, 2003. [DOI] [PubMed] [Google Scholar]
- 47. Eggesbo HB, Sovik S, Dolvik S, Kolmannskog F. CT characterization of inflammatory paranasal sinus disease in cystic fibrosis. Acta Radiol 43:21–28, 2002. [DOI] [PubMed] [Google Scholar]
- 48. Marks SC, Kissner DG. Management of sinusitis in adult cystic fibrosis. Am J Rhinol 11:11–14, 1997. [DOI] [PubMed] [Google Scholar]
- 49. Gold SM, Tami TA. Role of middle meatus aspiration culture in the diagnosis of chronic sinusitis. Laryngoscope 107:1586–1589, 1997. [DOI] [PubMed] [Google Scholar]
- 50. Kurlandsky LE. Recognition of a paranasal sinus mucocele in a child with cystic fibrosis. Clin Pediatr (Phila) 36:595–597, 1997. [DOI] [PubMed] [Google Scholar]
- 51. Coste A, Gilain L, Roger G, et al. Endoscopic and CT-scan evaluation of rhinosinusitis in cystic fibrosis. Rhinology 33:152–156, 1995. [PubMed] [Google Scholar]
- 52. Kaplan DM, Niv A, Aviram M, et al. The 3849 + 10 kB C–>T mutation in a 21-year-old patient with cystic fibrosis. Ear Nose Throat J 75:793–795, 1996. [PubMed] [Google Scholar]
- 53. Duplechain JK, White JA, Miller RH. Pediatric sinusitis. The role of endoscopic sinus surgery in cystic fibrosis and other forms of sinonasal disease. Ann Otolaryngol Head Neck Surg 117:422–426, 1991. [DOI] [PubMed] [Google Scholar]
- 54. Drake-Lee AB, Morgan DW. Nasal polyps and sinusitis in children with cystic fibrosis. J Laryngol Otol 103:753–755, 1989. [DOI] [PubMed] [Google Scholar]
- 55. Jaffe BF, Strome M, Khaw KT, Shwachman H. Nasal polypectomy and sinus surgery for cystic fibrosis—A 10 year review. Otolaryngol Clin North Am 10:81–90, 1977. [PubMed] [Google Scholar]
- 56. Cunningham DG, Gatti WM, Eitenmiller AM, et al. Cystic fibrosis: Involvement of the ear, nose, and paranasal sinuses. IMJ Ill Med J 148:470–471, 474, 1975. [PubMed] [Google Scholar]
- 57. Brook I. Microbiology of sinusitis. Proc Am Thorac Soc 8:90–100, 2011. [DOI] [PubMed] [Google Scholar]
- 58. Bischoff WE, Wallis ML, Tucker KB, et al. Staphylococcus aureus nasal carriage in a student community: Prevalence, clonal relationships, and risk factors. Infect Control Hosp Epidemiol 25:485–491, 2004. [DOI] [PubMed] [Google Scholar]
- 59. Stubbs E, Pegler M, Vickery A, Harbour C. Nasal carriage of Staphylococcus aureus in Australian (pre-clinical and clinical) medical students. J Hosp Infect 27:127–134, 1994. [DOI] [PubMed] [Google Scholar]
- 60. McKinnell JA, Miller LG, Eells SJ, et al. A systematic literature review and meta-analysis of factors associated with methicillin-resistant Staphylococcus aureus colonization at time of hospital or intensive care unit admission. Infect Control Hosp Epidemiol 34:1077–1086, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brans R, Kolomanski K, Mentzel F, et al. Colonisation with methicillin-resistant Staphylococcus aureus and associated factors among nurses with occupational skin diseases. Occup Environ Med 73:670–675, 2016. [DOI] [PubMed] [Google Scholar]
- 62. Opal SM, Mayer KH, Stenberg MJ, et al. Frequent acquisition of multiple strains of methicillin-resistant Staphylococcus aureus by healthcare workers in an endemic hospital environment. Infect Control Hosp Epidemiol 11:479–485, 1990. [DOI] [PubMed] [Google Scholar]
- 63. Brook I. Aerobic and anaerobic bacterial flora of normal maxillary sinuses. Laryngoscope 91:372–376, 1981. [PubMed] [Google Scholar]
- 64. Cleland EJ, Bassiouni A, Vreugde S, Wormald PJ. The bacterial microbiome in chronic rhinosinositis: Richness, diversity, postoperative changes and patient outcomes. Am J Rhinol Allergy 30:37–43, 2016. [DOI] [PubMed] [Google Scholar]
- 65. Zhang Z, Adappa ND, Lautenbach E, et al. Coagulase-negative Staphylococcus culture in chronic rhinosinusitis. Int Forum Allergy Rhinol 5:204–213, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Alanin MC, Nielsen KG, von Buchwald C, et al. A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect 21:1093.e1–7, 2015. [DOI] [PubMed] [Google Scholar]
- 67. Pedersen M, Mygind N. Rhinitis, sinusitis and otitis media in Kartagener's syndrome (primary ciliary dyskinesia). Clin Otolaryngol Allied Sci 7:373–380, 1982. [DOI] [PubMed] [Google Scholar]
- 68. Robertson JM, Friedman EM, Rubin BK. Nasal and sinus disease in cystic fibrosis. Paediatr Respir Rev 9:213–219, 2008. [DOI] [PubMed] [Google Scholar]
- 69. FitzSimmons S. The changing epidemiology of cystic fibrosis. J Pediatr 122:1–9, 1993. [DOI] [PubMed] [Google Scholar]
- 70. Cystic Fibrosis Foundation Patient Registry. 2015 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation, 2016. [Google Scholar]
- 71. Gitomer SA, Ramakrishnan VR, Malcolm KC, et al. Initial investigation of small colony variants of Staphylococcus aureus in chronic rhinosinusitis. Am J Rhinol Allergy 29:29–34, 2015. [DOI] [PubMed] [Google Scholar]
- 72. Rudkjøbing VB, Aanaes K, Wolff TY, et al. An exploratory study of microbial diversity in sinus infections of cystic fibrosis patients by molecular methods. J Cyst Fibros 13:645–652, 2014. [DOI] [PubMed] [Google Scholar]
- 73. Rudkjøbing VB, Thomsen TR, Alhede M, et al. True microbiota involved in chronic lung infection of cystic fibrosis patients found by culturing and 16S rRNA gene analysis. J Clin Microbiol 49:4352–4355, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Aanæs K. Bacterial sinusitis can be a focus for initial lung colonisation and chronic lung infection in patients with cystic fibrosis. J Cyst Fibros 12(suppl.):S1–S20, 2013. [DOI] [PubMed] [Google Scholar]
- 75. Sakano E, Ribeiro AF, Barth L, et al. Nasal and paranasal sinus endoscopy, computed tomography and microbiology of upper airways and the correlations with genotype and severity of cystic fibrosis. Int J Pediatr Otorhinolaryngol 71:41–50, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.