To the Editor
In the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial, patients with heart failure and preserved ejection fraction were randomly assigned to receive either spironolactone or placebo. The investigators found that the incidence of the primary composite end point of death from cardiovascular causes, hospitalization for heart failure, or resuscitated cardiac arrest was not significantly lower in the spironolactone group than in the placebo group, but the incidence of hospitalization for heart failure was significantly lower in the spironolactone group.1 However, significant differences in the clinical profiles, event rates, and responses to spironolactone were identified between the patients who were enrolled in the trial in the Americas (United States, Canada, Brazil, and Argentina) and the patients who were enrolled in Russia and Georgia, and these differences have aroused concerns about study conduct at the Russian and Georgian sites.2
To further explore potential regional disparities in medication use,3 we measured concentrations of canrenone (an active metabolite of spironolactone4,5) in 366 patients in the TOPCAT trial (206 patients from the United States and Canada and 160 patients from Russia) who provided consent to participate in the TOPCAT biorepository and had sufficient serum samples available from the 12-month study visit (see the Supplementary Appendix, available with the full text of this letter at NEJM.org).
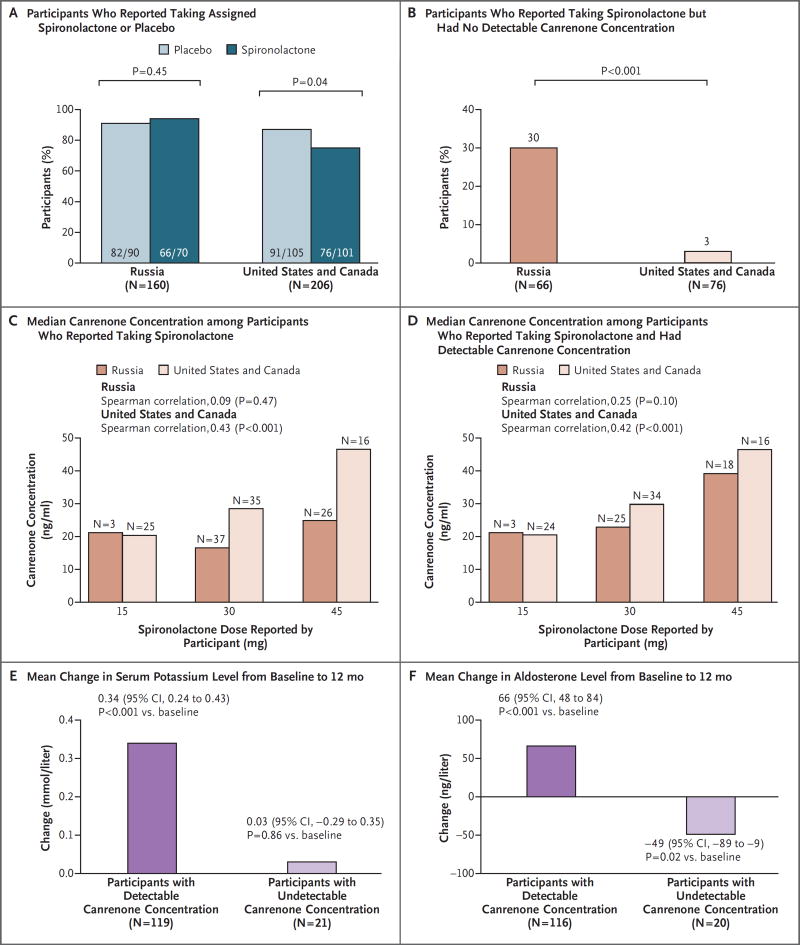
The clinical characteristics of the repository participants were generally representative of the overall TOPCAT trial population, although there were some differences in baseline characteristics (Table S1 in the Supplementary Appendix). As was the case in the overall trial population, regional disparities in reported study drug use and dosing were observed (Fig. 1A, and Table S2 in the Supplementary Appendix). Among the participants who were assigned to receive spironolactone and reported at the 12-month visit that they were taking the study drug (76 of 101 patients from the United States and Canada and 66 of 70 patients from Russia), canrenone concentrations were undetectable in a higher percentage of participants from Russia than from the United States and Canada (30% vs. 3%, P<0.001) (Fig. 1B). In an analysis of all the participants who reported taking the assigned doses of spironolactone, a significant correlation between the doses of spironolactone that the participants reported taking and canrenone concentrations was found among the participants from the United States and Canada, but not among the participants from Russia (Fig. 1C). In the subgroup of participants with detectable canrenone concentrations, the relationship between the doses of spironolactone that the participants reported taking and canrenone concentrations was consistent in the two regions (Fig. 1D). Regardless of the region, the participants with detectable canrenone concentrations had significant increases from baseline in potassium and aldosterone levels (a finding that is consistent with the expected effect of spironolactone), whereas the participants with undetectable canrenone concentrations did not (Fig. 1E and 1F).
Figure 1. Regional Discrepancies in the Reported Use and Actual Use of Spironolactone among Repository Participants in the TOPCAT Trial.
The repository participants included 366 patients in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial (206 patients from the United States or Canada and 160 patients from Russia) who provided consent to participate in the TOPCAT biorepository and had sufficient serum samples available from the 12-month study visit. Panel A shows the percentage of participants who reported at 12 months that they were taking the assigned study drug or placebo, and Panel B shows the percentage of participants assigned to spironolactone who reported taking the drug but did not have detectable canrenone concentrations at the 12-month visit. Panel C shows the median canrenone concentration, according to the spironolactone dose at 12 months reported by the participant, and Panel D shows the median canrenone concentration among the participants with detectable canrenone levels, according to the spironolactone dose at 12 months reported by the participant. The Spearman correlation coefficient represents the correlation between reported spironolactone doses and canrenone concentrations. Panel E shows the increases in serum potassium level from baseline to 12 months among the participants (from both regions) who reported taking spironolactone, according to whether they had detectable or undetectable canrenone concentrations. The between-group comparison was significant (P = 0.02). This difference was significant after adjustment for region (P = 0.04); region was not associated with changes in potassium level (P = 0.87). Panel F shows the changes in serum aldosterone level from baseline to 12 months among the participants (from both regions) who were assigned to spironolactone and reported taking the drug, according to whether they had detectable or undetectable canrenone concentrations. The between-group comparison was significant before and after adjustment for region (P<0.001 for both analyses); region was not associated with changes in aldosterone level (P = 0.53).
These findings show significant regional discrepancies in the reported use and the actual use of spironolactone, as assessed by measurement of metabolite concentrations. Taken together with other reported regional differences in the TOPCAT trial, the results of the current study arouse concerns regarding study conduct at some sites in Russia and, by implication, Georgia, where event rates and responses to spironolactone were also of concern (Table S3 in the Supplementary Appendix).2 Our findings suggest that the trial results obtained in Russia do not reflect the true therapeutic response to spironolactone.
Supplementary Material
Acknowledgments
Supported by a contract from the National Heart, Lung, and Blood Institute, National Institutes of Health (HHSN268200425207C).
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
Contributor Information
Simon de Denus, Montreal Heart Institute, Montreal, QC, Canada
Eileen O’Meara, Montreal Heart Institute, Montreal, QC, Canada
Akshay S. Desai, Brigham and Women’s Hospital, Boston, MA
Brian Claggett, Brigham and Women’s Hospital, Boston, MA
Eldrin F. Lewis, Brigham and Women’s Hospital, Boston, MA
Grégoire Leclair, Université de Montréal, Montreal, QC, Canada
Martin Jutras, Université de Montréal, Montreal, QC, Canada
Joël Lavoie, Montreal Heart Institute, Montreal, QC, Canada
Scott D. Solomon, Brigham and Women’s Hospital, Boston, MA
Bertram Pitt, University of Michigan School of Medicine, Ann Arbor, MI
Marc A. Pfeffer, Brigham and Women’s Hospital, Boston, MA
Jean L. Rouleau, Montreal Heart Institute, Montreal, QC, Canada
References
- 1.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–92. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 2.Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 3.Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart. 2014;100:855–61. doi: 10.1136/heartjnl-2013-305063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reilly RF, Jackson EK. Regulation of renal function and vascular volume. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman’s the pharmacological basis of therapeutics. 12. New York: McGraw-Hill Education; 2011. pp. 671–720. [Google Scholar]
- 5.Aldactone. New York: Pfizer; Oct, 2014. (package insert) ( http://labeling.pfizer.com/ShowLabeling.aspx?id=520) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.