Abstract
Introduction
Clinically, patients with significant reductions in renal function present with cardiovascular dysfunction typically termed, uremic cardiomyopathy. It is a progressive series of cardiac pathophysiological changes, including left ventricular diastolic dysfunction and hypertrophy (LVH) which sometimes progress to left ventricular dilation (LVD) and systolic dysfunction in the setting of chronic kidney disease (CKD). Uremic cardiomyopathy is almost ubiquitous in patients afflicted with end stage renal disease (ESRD).
Areas covered
This article reviews recent epidemiology, pathophysiology of uremic cardiomyopathy and provide a board overview of Na/K-ATPase research with detailed discussion on the mechanisms of Na/K-ATPase/Src/ROS amplification loop. We also present clinical and preclinical evidences as well as molecular mechanism of this amplification loop in the development of uremic cardiomyopathy. A potential therapeutic peptide that targets on this loop is discussed.
Expert opinion
Current clinical treatment for uremic cardiomyopathy remains disappointing. Targeting the ROS amplification loop mediated by the Na/K-ATPase signaling function may provide a novel therapeutic target for uremic cardiomyopathy and related diseases. Additional studies of Na/K-ATPase and other strategies that regulate this loop will lead to new therapeutics.
Keywords: Na/K-ATPase, ROS, cell signaling, uremic cardiomyopathy
1. Epidemiology of uremic cardiomyopathy
Based on data from the 2015 US Renal Data System (USRDS) [1], the prevalence of CKD in the general population was approximately 14%, with Stage 3 kidney disease showing the largest growth over the past 20 years. This is not unexpected as older age, diabetes, hypertension, cardiovascular diseases and higher body mass index (BMI) are associated with the development of CKD, and the prevalence of these conditions are also on the rise. Although most cases of CKD do not progress to ESRD, the prevalence of ESRD has increased to approximately 2 cases per thousand people in the USA Mortality rates are significantly increased in patients with CKD and ESRD and cardiovascular disease accounts for a substantial fraction of this increase. Despite trending decreases in mortality rates for dialysis and transplant patients from 1996 to 2013 which may be related to improvements in treatments, the adjusted mortality rate for ESRD patients are still several fold higher than in populations without CKD, and these mortality rate differences persist across the continuum of age[1].
Clear evidence supports the existence of a ‘cardio-renal’ axis in which injury or dysfunction of either the heart or the kidneys leads to pathological changes in both organ systems. For example, the presence of renal insufficiency significantly worsens outcomes in congestive heart failure (CHF) patients [2–5]. Mortality due to cardiovascular disease (CVD) is 10–30 times higher in dialysis patients than in the general population [6]. Among all major causes of death in patients with CKD or ESRD, CVD accounts for 69.6% in patients with CKD compared to 34.7% without. The 2-year survival rate of patients with both CKD and CHF is around 75%, compared with 90 and 82% for patients with CKD or CHF alone [1]. Cardiovascular comorbidities of CKD include coronary artery disease (CAD), CHF, valvular heart disease, stroke, peripheral artery disease and cardiac arrest and arrhythmias [7–10].
Although the relationship between CVD and CKD is still under investigation, some common risk factors including diabetes mellitus, hypertension, obesity, aging, metabolic syndrome and smoking, have been identified that correlate with the development of both CVD and renal disorders [9,11,12]. Moreover, CVDs and renal disorders have certain molecular changes in common, in particular, markers of inflammation and oxidant stress (e.g. elevated reactive oxygen species (ROS)) [9,11–14].
2. Pathophysiology of uremic cardiomyopathy
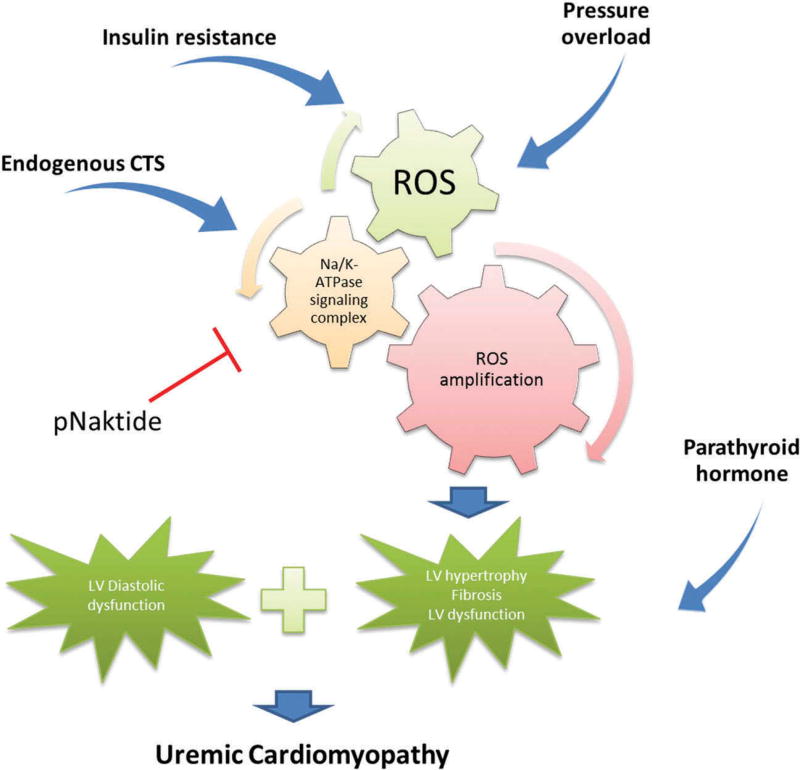
A number of factors are present in CKD and especially ESRD patients that can lead to cardiomyopathy (Figure 1). Hypertension is one of the main factors present which can lead to diastolic dysfunction and LVH [7,8]. Also, volume overload due to salt and water retention along with anemia can lead to LV dilation. Sustained increase in preload and afterload activates a series of intracellular signaling cascades that lead to maladaptive cardiac cell responses like apoptosis of myocytes, initiation of fibrosis via cardiac fibroblasts, and possible cardiac cell regeneration [15–19].
Figure 1.
Schematic showing central role for Na/K-ATPase alpha1 subunit in the amplification of ROS in chronic kidney disease, ultimately leading to uremic cardiomyopathy.
That said, it is very clear that there is something about the CKD milieu that facilitates the development of cardiomyopathy. In particular, neither intensive blood pressure control nor reduced preload through hemodialysis and aggressive ultra-filtration are able to ameliorate the pathological alterations found in patients who have developed uremic cardiomyopathy [13,20,21]. Over the past 20 years, other factors have been identified be important for the development of LV hypertrophy, fibrosis, and apoptosis independent of pressure and volume overload.
Insulin resistance is known to be involved in the development of atherosclerosis, hypertension, dyslipidemia, and LV ventricle hypertrophy. Multiple laboratories have identified insulin resistance as an independent factor for the development of uremic cardiomyopathy as well as a strong predictor of cardiovascular death in chronic renal failure [22– 25]. Specifically, in 227 nondiabetic renal dysfunction patients, insulin resistance, which was evaluated by the homeostasis model assessment method (HOMA-IR), appeared to be significantly elevated and had an elevated association with cardiovascular events than those observed in healthy subjects [26]. Although the exact mechanism of insulin-induced protein kinase B (AKT) signaling in the uremic cardiomyopathy is not fully understood, it is clear that insulin resistance during the CKD altered AKT signaling is involved in uremic cardiomyopathy.
Parathyroid hormone (PTH) has been known as an important indicator of cardiac dysfunction in uremia since early 1960s [27,28]. A study evaluating hemodialysis patients without hypertension showed that left ventricle hypertrophy and diastolic dysfunction were correlated with secondary hyper-parathyroidism [29]. Furthermore, administration of PTH causes more cardiac lesions in uremic rats, while parathyroidectomy could attenuate the development of cardiac lesions and fibrosis. Although uremia-related PTH increase plays an important role in the development of uremic cardiomyopathy, the mechanism is still not elucidated. It is possible that elevated PTH which promotes Ca2+/PKC downstream signaling is involved in the development of left ventricle hypertrophy.
Additionally, the uremic state itself appears to result in the transactivation of downstream signaling events that induce cardiomyocyte enlargement and cardiac fibroblast proliferation which account for the process of cardiac [11,30,31].
Furthermore, increase in ROS stress has also been well documented as an important indicator for CKD in both patients and animal models. Specifically, Tepel et al., have shown that the spontaneous production of ROS was significantly increased in lymphocytes from patients with ESRD compared with healthy volunteers [32]. Another clinical study with 60 CKD patients finished by Oberg et al., also presents elevated oxidative stress biomarkers compared with healthy subjects [33]. Additionally, mitochondrial dysfunction and increased oxidative stress were reported in uremic cardiomyopathy murine model [34]. Therefore, the medical hypothesis of ROS generation/oxidative stress as a therapeutic target for CKD patients with uremic cardiomyopathy is one of our focuses in this review.
More importantly, a number of laboratories including our own have identified elevated concentrations of cardiotonic steroids (CTS) in patients with CKD and or CVDs (Table 1) [35–39]. We have demonstrated the involvement of these cardiac glycosides-specific receptor Na/K-ATPase and ROS amplification, in mediating the development of LV hypertrophy, cardiac cell apoptosis and fibrosis in the process of uremic cardiomyopathy [11,19,40,41]. For the remainder of this review, we will focus on the mechanisms by which this appears to occur.
Table 1.
Plasma level of related endogenous digitalis in different diseases.
| Disease | Patients | Healthy volunteers/control | Ref. |
|---|---|---|---|
| Essential hypertension | EO: 377 ± 19 pmol/L* | EO: 253 ± 53pmol/L | [37] |
| Heart failure | Median MBG: 583 pmol/L* | Median MBG: 241pmol/L | [38] |
| End-stage renal disease | MBG: 3.81 ± 1.92 ng/ml* | MBG: 0.94 ± 0.28 ng/ml | [36] |
| First Day AMI | MBG: 1850 ± 380 pmol/L* | MBG: 500 ± 70 pmol/L | [39] |
| Chronic renal failure | MBG: 860 ± 70 pmol/L* | MBG: 280 ± 20 pmol/L | [35] |
Significant difference compare to healthy volunteer.
EO: endogenous ouabain-like factor; AMI: acute myocardial infraction.
3. Na/K-ATPase – an ion pump, a signaling receptor and a signaling scaffold
3.1. Na/K-ATPase as an ion pump
The Na/K-ATPase, first discovered by Skou [42], belongs to the family of P-type ATPases and transports Na+ and K+ across the plasma membrane in animals in an ATP-dependent process [43–46]. It consists of two noncovalently linked polypeptides, an α-subunit and β-subunit as well as other subunits which are inconsistently expressed in different tissues.
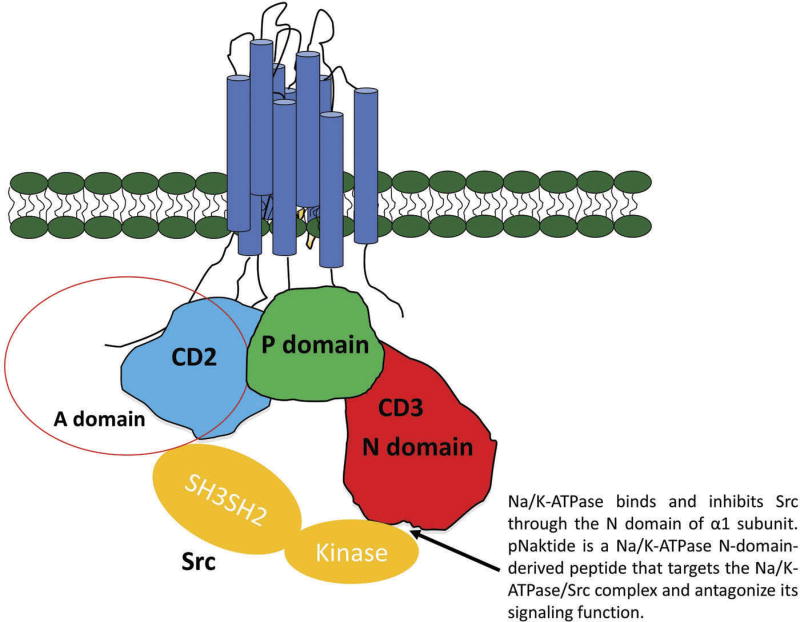
The α-subunit contains the binding sites for ATP, CTS, and other ligands, and plays the central role in the ion pumping function. The intracellular part of this transmembrane subunit is divided into three well-characterized regions according to their distinct functions (Figure 2): the actuator (A) domain is composed of the N-terminus and the second cytosolic domain (CD2) connecting the M2 and M3 transmembrane helices; the phosphorylation (P) domain and the nucleotide binding (N) domain are located in the loop between M4 and M5 transmembrane helices [47–49]. The β subunit is important for the assembly of the functional enzyme on the plasma membrane. Several α and β subunits have been identified and functionally characterized [43,44]. In addition, isoforms of α subunit are expressed in a tissue-specific manner [50]. The α1 isoform is found in all kinds of cell types. The α2 and α3 isoforms are expressed in skeletal muscle, neuronal tissue, and cardiac myocytes. The α4 isoform is expressed exclusively in spermatozoids and regulates sperm motility [51,52].
Figure 2.
Schematic of Na/K-ATPase alpha1 subunit A, N and P domains on the cytosolic aspect of the membrand its interaction with Src kinase.
3.2. Receptor function of Na/K-ATPase
3.2.1. Endogenous CTS
CTS include plant-derived digitalis drugs such as digoxin and ouabain, and vertebrate-derived aglycones such as bufalin and MBG [53–55]. They are specific inhibitors of Na/K-ATPase with vast difference in affinity between them [56,57]. On the other hand, the affinity of Na/K-ATPase to CTS are highly dependent on the type of CTS, the type of the α1subunit, the combination of α/β subunit, as well as the species studied. For instance, the IC50 of the human α1 is about 50 nM to ouabain, whereas the IC50 of rat α1 is 48,000 nM [58–61]. Depending on the methodology and experimental condition, the values of IC50 may also be vastly varied. CTS have largely only been considered for their use as drugs since their discovery, recent studies have identified both ouabain and MBG as endogenous hormones whose production and secretion are regulated by multiple physiological stimuli including ACTH and angiotensin II [62,63]. Evidence for this assertion was generated by specific identification of subnanomolar concentrations of endogenous CTS in both human and experimental animals, and raised questions about their roles in human physiology as a pump inhibitor [55,58,59,64–66]. These CTS have mostly been measured using antibody based assays [67,68]. However, some studies have confirmed the presence of some CTS with mass spectroscopy and NMR and found differences in humans with diseases such as kidney failure and heart failure [68–71]. Although the exact pathophysiological role of increased endogenous CTS in these diseases continues to be debated [72–74], a study from Lingrel’s laboratory using gene replacement technology demonstrated that endogenous CTS, do play a measurable role in the regulation of blood pressure, cardiac function and muscle physiology [75,76]. Under normal conditions, the rodent Na/K-ATPase α1 isoform has a very low sensitivity to ouabain compared with human α1, whereas the rodent α2 isoform is sensitive. By manipulations of the Na/K-ATPase genetic code, this group created mice with mutant Na/K-ATPase (ouabain-sensitive α1 isoform and ouabain-resistant α2 isoform). Using these genetically modified animals, they have shown that mutant Na/K-ATPase animal appeared to possess remarkably increased effects of low concentration of CTS (0.48 nmol/g body weight) resulting in threefold elevated cardiac contractility compared with wild-type mice [77]. Another group using these genetically modified animals has identified dramatically increased cardiac hypertrophy and fibrosis as well as cardiac dysfunction in terms of left ventricular dilatation and systolic dysfunction in α1-sensitive mice than wild-type control mice after four-weeks of pressure overload induced by transverse aortic constriction (TAC) [78]. Taken together, these studies indicate that Na/K-ATPase plays a significant role in regulation of cardiac function and development of heart diseases. It is reasonable to propose the potential involvement of endogenous CTS and Na/K-ATPase signaling function (please see section 4.3.1. for further discussion). In addition, Aperia et al. and Lichtstein et al. have suggested a role for endogenous CTS in fetal growth in general and kidney development specifically [79,80]. Although we cannot ignore the effect of endogenous CTS on the inhibition of Na/K-ATPase and the subsequent elevation of intracellular Ca2+ levels, recent studies mentioned above have demonstrated that binding of CTS to the α-subunit of the Na/K-ATPase may also directly initiate a variety of intracellular signaling cascades, independent of changes in transport activity.
3.2.2. Na/K-ATPase as a signaling receptor
The identification of endogenous CTS along with the evidences that low concentrations of CTS, like ouabain or marinobufagenin (MBG), in concentrations which do not affect ion pumping function, can activate downstream signaling cascades through tyrosine phosphorylation, ultimately altering gene expression, suggest the possible receptor function of Na/K-ATPase rather than its canonic pumping function. Like other cytokine receptors and GPCRs, Na/K-ATPase does not have intrinsic activity. Therefore, it requires coupling with non-receptor kinase to fulfill the phosphorylation capability. Based on the previous studies from Xie’s lab, Src kinase turns out to be one of the most proximal kinase associating with Na/K-ATPase. First, researchers from this lab and many other labs have reported the activation of Src by ouabain in different types of cells [81–83]. Follow-up studies suggest that the receptor function of Na/K-ATPase require Src-mediated trans-activation of EGF receptor, and subsequent recruitment and assembly of signaling proteins for the activation of protein/ lipid kinase cascades [84]. Second, using a combination of several different methods including coimmunoprecipitation, confocal imaging, FRET and GST pull-down assay [84], we have identified two potential binding sites between α1 Na/K-ATPase and Src. One is between A domain of Na/K-ATPase α1 subunit and Src homology2 (SH2) domain. The second site involved N domain of Na/K-ATPase α1 subunit and Src kinase domain. The latter interaction between Na/K-ATPase and Src keeps Src in an inactivate state by inhibiting Try 418 phosphorylation without affecting the phosphorylation of Tyr 529. Ouabain binding releases Src kinase domain from Na/K-ATPase/Src receptor complex and activates the Na/K-ATPase-associated Src [84]. The activation of Src results in transactivation of multiple downstream effectors, and regulates cellular activities such as clathrin-mediated endocytosis of a1 Na/K-ATPase [85]. Third, genetic modification of Na/K-ATPase expression in live cells show that knockdown of endogenous Na/K-ATPase α1 subunit using siRNA significantly elevated Src activity as well as abolished ouabain-induced downstream signaling, whereas restoring Na/K-ATPase inhibited Src activity and receptor function of Na/K-ATPase. Although the involvement of Src and EGF receptor in the Na/K-ATPase α1-mediated signal transduction and the interaction between Na/K-ATPase and Src have reaffirmed and accepted by many laboratories (Figure 2). There are some data that are opposite with our model. It has been shown that c-Src activation was not involved in ouabain induced Na/K-ATPase endocytosis in non-small cell lung cancer cells [86] and no stable interactions were detected between purified recombinant Na/K-ATPase and purified human Src kinase [87]. We would like to point out that these observations are different from what we have reported using purified pig kidney Na/K-ATPase, LLC-PK1 cell, human HK-2, human dermal fibroblasts, renal fibroblast cell line, and rat cardiac fibroblasts cell line [84,88,89]. Therefore, the model that Na/K-ATPase signal from interaction with Src is reliable and worthy to investigate. Taken together, these evidences indicate that besides its classic pumping function, Na/ K-ATPase is capable of associating with Src kinase to form a receptor complex for CTS to activate protein kinases in live cells.
3.3. Na/K-ATPase as signaling scaffold
Based on the characterization of the A and N domains of Na/K-ATPase α1 subunit, both are highly exposed and enable interacting with other intracellular proteins. Work in our and other labs have identified that the α1 subunit is capable of interacting with various proteins, which play important roles in protein trafficking and signal transduction processes. Depending of the binding site on the α1 subunit, they can be divided into two groups, The first group contains proteins bind to the A domain of a1 subunit including 14-3-3 [90], phosphatidylinositol-3-kinases (PI3 K) [91], inositol trisphosphate receptor (IP3R)[92], Src kinase[84] and caveolin-1 [93]. The second group includes those interact with N domain of a1 subunit such as arrestin, spinophilin, GPCR kinase, 14-3-3 epsilon [94], adapter protein-1/2 [95,96], ankyrin [97], and Src, On the other hand, depending on the function of Na/K-ATPase a1-association, they can be separated into three groups. The first group contains those bind to Na/K-ATPase α1 subunit and modulate its trafficking. For instance, in response to dopamine-induced GPCR signals, several proteins were recruited to the α1 subunit including arrestin, spinophilin, 14-3-3 epsilon, and GPCR kinase, which mediated endocytosis of Na/K-ATPase [94]. Moreover, the interaction between N terminal Na/K-ATPase α1 subunit and caveolin-1 appears to be important for ouabain-induced endocytosis of Na/K-ATPase [85]. The second group includes those that dynamically interact with Na/K-ATPase α1 subunit, which affect Ca2+ signaling events or kinase activity. For example, binding of IP3R and phospholipase C-γ (PLC-γ) to α1 formed the signaling complex that regulated intracellular Ca2+ signal [92,98]. Additionally, as we mentioned above, interaction of α1 subunit with Src kinase inhibited its kinase activity. Binding of ouabain to the α1 subunit released Src and activated its kinase activity, which led to activation of mitogen-activated protein kinase (MAPK) pathway [84]. The third group consists of those directly enhance the Na/K-ATPase activity such as cofilin and polycystin-1 [99,100].
Further studies by Xie’s laboratory have demonstrated that cells appear to contain two functionally separable pools of Na/ K-ATPase i.e. the so called ion pumping pool and the non-pumping pool [101]. The non-pumping Na/K-ATPase apparently resides in caveolae and interacts directly with multiple proteins including Src, ion transporters, and caveolin to fulfill its signal transducer function [85,102,103]. Caveolae were first identified as flask-shaped vesicular invaginations of cellular plasma membranes enriched in cholesterol, glycosphingolipids and sphingomyelin [104,105]. Now, caveolae have been implicated in endocytosis, transcytosis, calcium signaling, and many other signaling processes [105,106]. Additionally, many signaling proteins have been discovered to be localized and concentrated within caveolae [93,107], which indicates that caveolae may serve as a micro domain for compartmentalization of signal transduction and facilitate the regulation of different signaling processes. Interestingly, previous work from our and Xie’s lab have shown that Na/K-ATPase α1 sub-unit interact with caveolin-1 and highly concentrated in caveolae [93]. The signaling Na/K-ATPase mainly resides in caveolae. Disruption of the caveolae structure by either removal of cholesterol or knockdown of caveolin-1, leads to disassembly of the Na/K-ATPase/Src signaling complex, abolishes the activation of Na/K-ATPase-mediated signal transduction by ouabain and interrupts endocytosis of Na/K-ATPase [93,102,108]. Taken together, it is clear that Na/K-ATPase, mainly localized in caveolae, could also serve as a signaling scaffold through interaction with other proteins or receptors.
4. Na/K-ATPase and ROS in uremic cardiomyopathy
4.1. Reactive oxygen species
Over the past five decades, oxygen-derived free radicals called ROS have been identified in both intracellular and extracellular locations and have been shown to play important roles in physiology and pathology. Common forms of ROS include superoxide (.O2−), hydrogen peroxide (H2O2), and hydroxyl radical (.OH) [109]. ROS was initially thought to exclusively induce cellular damage but not physiological function [110,111]. However, evidence has accumulated linking ROS to multiple pathophysiological events, such as like diabetes, cancer, CVD, obesity, CKD, ESRD, aging, and neuronal disorder [112–117]. Furthermore, many publications have indicated that H2O2 induces tyrosine phosphorylation and affects cell signaling pathways [118].
4.2. Na/K-ATPase/ROS amplification loop
Interestingly, Liu et al. made the connection of Na/K-ATPase-mediated signal transduction to the generation of ROS, and provided strong evidence that this regulation occurs independent of changes in intracellular calcium and sodium concentrations [88,119]. Specifically, ouabain caused no change in calcium concentration when cardiac myocytes were incubated in calcium-free medium, whereas it did induce ROS generation under the same conditions. Moreover, ouabain-induced protein tyrosine phosphorylation was also independent of changes in intracellular calcium concentration, but can be blocked by Ras inhibitors. This finding again emphasizes that Na/K-ATPase as a signaling scaffold in cell physiology. Our early studies suggest that ouabain could stimulates ROS generation in a Ras-dependent way through Na/K-ATPase/Src signaling complex [88,119]. Further studies by Xie’s lab show that disruption of Na/K-ATPase/Src signaling by either pNaktide (an antagonist of Na/K-ATPase/Src signaling) or by the expression of Src-binding sites null Na/K-ATPase (A420P) abolishes ROS-induced Src/ERK/MAPK activation and protein carboxylation [120]. We also observed that ouabain is able to induce direct carbonylation on Pro 222 and Thr 224 of pig Na/K-ATPase a1 subunit, and that inhibition of the carbonylation by antioxidants or mutation of Pro222 attenuates ouabain-induced activation of protein kinase cascades [121].
Conversely, oxidative stress can also induce Na/K-ATPase downstream signaling. First, increases in ROS generation could oxidize the Na/K-ATPase α and β subunits as well as its independent regulator FXYD proteins. Oxidation of Na/K-ATPase inhibits its activity and promotes its sensitivity to protein endocytosis pathway [122–126]. For instance, oxidative stress could induce glutathionylation of Na/K-ATPase β1 subunits in pig kidney and cardiomyocytes. Peroxynitrite-mediated oxidation of Na/K-ATPase in purified pig kidney inhibits its activity by stabilizing the enzyme in an E2-P prone conformation. FXYD proteins could reverse oxidative stress induced inhibition of Na/K-ATPase by deglutathionylation of β1 subunit [124]. On the other hand, administration of either H2O2 or glucose oxidase in cardiac myocytes activates Na/K-ATPase signaling in cardiomyocytes [127], whereas pretreatment with antioxidant N-acetyl cysteine (NAC) prevents ouabain-induced Na/K-ATPase downstream signaling [88,128]. Thus, we propose that Na/K-ATPase/Src signaling complex and ROS form a signal amplification loop allowing not only CTS but also ROS to generate signaling from the Na/K-ATPase.
4.3. Na/K-ATPase/ROS amplification loop in diseases
In view of the well-established role of oxidative stress in the development of many chronic disease, Chen et al. and Kennedy et al. have recently explored whether the newly appreciated Na/K-ATPase/Src/ROS amplification loop is critical for ROS signaling during diseases. Using gene modified animal, Chen el al. indicated the role of Na/K-ATPase in participating oxidative stress induced atherosclerosis [129]. Whereas Kennedy et al. suggested the involvement of this signaling loop in hyperlipidemic states induced renal inflammation and tissue damage [130]. Furthermore, our recent animal studies directly point the role for Na/K-ATPase/Src-ROS amplification in high-fat diet induced adipogenesis [131]. Specifically, it is well known that elevated oxidative stress due to high-fat diet is an important pathological mechanism in obesity and metabolic imbalance in adipocytes, which makes the redox state of adipocyte become a therapeutic target for obesity and metabolic syndrome [115,132,133]. C57/B6 mice fed high-fat chow for 12 weeks presented with obesity and metabolic imbalance due to the activation of ROS. Using pNaktide, we observed significant attenuation of obesity and development of metabolic syndrome. More importantly, our results also showed that targeting on Na/K-ATPase/Src/ROS amplification loop could have decreased visceral and subcutaneous fat mass potentially due to attenuated oxidative stress and insulin resistance with an increase in adiponectin level in adipocytes.
4.3.1. Na/k-ATPase/ROS amplification loop in uremic cardiomyopathy
Uremic cardiomyopathy is complicated by diastolic dysfunction in its earliest stages followed by significant ventricular hypertrophy and ultimately, systolic dysfunction [13]. Among many factors, cardiac hypertrophy and sequential replacement of cardiomyocytes with fibrosis seem to attribute more to cardiac dysfunction than other mechanisms [7,8,134]. Although many molecular signals induce fibrosis in uremic cardiomyopathy, oxidative stress is certainly a significant contributory mechanism [34,41,135]. In CKD, elevated ACE and endogenous CTS target cardiac cells and upon binding induce the generation of ROS through different signaling pathways. Increased ROS generation stimulates downstream signaling that induces cell proliferation, markers for cardiac hypertrophy, as well as collagen synthesis [40,88,136]. One the other hand, many laboratories including ours have shown that elevated CTS levels or H2O2, can induce cardiomyocyte hypertrophy as well as cardiovascular remodeling both in vitro and in vivo [19,40,137,138]. The first clue that link Na/K-ATPase and CTS with cardiac hypertrophy based on the observation that cultured cardiomyocytes appeared to display increased cellular survival signaling instead of activation of apoptosis following CTS treatment [88]. Following studies by Xie et al. demonstrated that CTS treatment in cardiac myocytes stimulated ROS generation and NF-kappaB activation which could be blocked by pretreatment with antioxidants such as N-acetylcysteine (NAC) or vitamin E [88]. These in vitro studies not only reveal the signaling receptor function of Na/K-ATPase aforementioned, but also suggest that Na/K-ATPase/Src/ROS feed-forward amplification loop that activates downstream signaling cascades responsible for cardiac hypertrophy. Clinically, many lab including ours have reported an increase in endogenous CTS in CKD patients [35,36], as well as a decrease in Na/K-ATPase expression in the heart of dilated cardiomyopathy patients [139,140], and experimental animal model [40,141]. In addition, MBG infusion through mini-pump was shown to produce the same uremic cardiomyopathy phenotype as PNx surgery in rats, in terms of elevated circulating MBG, cardiac hypertrophy, impaired cardiac function, and cardiac fibrosis [136]. Subsequently, antagonizing CTS by spironolactone or targeting mTOR pathway with rapamycin or neutralization of CTS attenuates pathological remodeling characterized by cardiomyocyte hypertrophy, cardiac fibrosis and diastolic dysfunction in experimental uremic cardiomyopathy animal models [14,134].
Importantly, in our recently in vivo study, we not only first demonstrate the essential role of Na/K-ATPase/Src/ROS signaling amplification loop in the development of uremic cardiomyopathy in vivo, but also provide a potential drug candidate for uremic cardiomyopathy [41]. Specifically, using a 5/6th partial nephrectomy (PNx) mouse model, we observed increased Src/ERK phosphorylation along with elevated expression of heme oxygenase-1 (HO-1), collagen-1 and protein carbonylation in PNx mouse left ventricle. The Na/K-ATPase/Src signaling complex antagonist, pNaktide, could attenuate physiological, morphological, and biochemical alterations of uremic cardiomyopathy, which including reduced left ventricle hypertrophy and improved diastolic function, decreased cardiac fibrosis, and decreased Src/ERK phosphorylation as well as protein carbonylation. Surprisingly, pNaktide but not induction of HO-1 appeared to ameliorate anemia in renal dysfunction animal. More interestingly, in reversal study, pNaktide was found to have a dose-dependent effect in developed cardiac hypertrophy, fibrosis and anemia. Taken together, it is clear that Na/K-ATPase/Src/ ROS signaling amplification loop is important for the development of uremic cardiomyopathy. Therapeutic targeting on this signaling pathway may provide a new direction for this disease.
5. Development of pNaktide
As we mentioned above, pNaktide a novel peptide that was developed in our lab, could attenuate uremic cardiomyopathy and high-fat diet induced obesity [41,131]. The idea for the development of pNaktide arose from the observation that Na/ K-ATPase allosterically interacts with Src kinase, keeping it inactive; however, when CTS-bind to the Na/K-ATPase α1 sub-unit, a conformational change occurs in this subunit which frees the allosteric inhibition of Src kinase allowing it to become activated [84]. The first step in this process was afforded by the studies in LLC-PK1 cells mentioned above which demonstrated that downregulation of Na/K-ATPase with siRNA lead to significantly elevated Src activity [89]. Detailed sequencing analysis showed that a 20-amino acid sequence on the N domain of Na/K-ATPase α1 subunit is essential for Src inhibition [142]. Based on this sequence (Figure 2), we synthesized a patented 33-amino acid peptide (pNaktide-GRKKR RQRRR PPQSA TWLAL SRIAG LCNRA VFQ) the extra 13 amino acids correspond to an HIV-TAT leading sequence which facilitates cellular permeation. This leading sequence renders pNaktide permeable to the cell membrane and leads to localization of pNaktide to the intracellular face of plasma membrane, limiting the effects of this peptide to cellular membrane-associated Src thus preventing inhibition of cytosolic Src activity a feature of its specificity [142]. pNaktide induces Src inhibition at an IC50 = 70 nM in LLC-PK1 cells and cardiomyocytes, importantly pNaktide is a specific inhibitor of Na/K-ATPase/Src complex signaling and appears to have no effect on Na/K-ATPase ion pumping function nor IGF1 induced Src activity [142]. In cultured neonatal cardiac myocytes, pNaktide has the capability to abolish CTS-induced hypertrophy in a dose-dependent manner. Furthermore, based on the observation that expression of Na/K-ATPase α1 is significantly reduced with accompanying elevated Src activity in human prostate carcinoma, we have shown that administration of pNaktide to NOD/SCID mice with this tumor xenograft potently inhibit tumor growth likely due to attenuation of overactive Src and angiogenesis [143,144].
6. Conclusion
In accord with the focus of the current review, we suggest that increased endogenous CTS or oxidative stress-induced Na.K-ATPase signaling complex activation and/or the subsequent oxidant amplification loops are well known to occur in CKD patients with cardiac dysfunction. As such we propose that Na/ K-ATPase/Src/ROS amplification loop is a suitable target to regulate the development of uremic cardiomyopathy and that work in our lab and others specifically focused on inhibition of this signaling cascade, like the development of pNaktide will function to prevent or reverse uremic cardiomyopathy and act as a therapeutic agent by inhibiting Na/K-ATPase signaling and oxidant amplification (Figure 1).
7. Expert opinion
Over the past 20 years, many molecular mechanisms leading to uremic cardiomyopathy have been proposed and explored [8,13,34,40]. However, clinical treatment targeting these mechanisms remains disappointing.
We would suggest that this is probably due to two factors. First, the cardiac abnormalities are usually present when dialysis treatment is initiated, and reversal is almost always much, much harder to achieve than prevention. This indicates the importance of recognizing and correcting cardiac alterations and/or their risk factors in the patients before the cardiomyopathy becomes advanced. Second and of greater relevance to this review, oxidative stress is clearly a contributor, but our clinical approaches have been inadequate. To date, most strategies to address oxidant stress employ the use of ‘antioxidants’ or free radical ‘scavengers.’ As these do not impact the production of oxidants, we think they will eventually become overwhelmed. Whether it is through this mechanism or others, they have not been effective clinically. We believe that targeting the amplification of ROS generation will be effective clinically as we have shown for experimental animals. However, it is still very clear that we have a very long way to go before this can be proven.
As discussed, we believe that the Na/K-ATPase/Src/ROS signaling amplification loop is important in the development of uremic cardiomyopathy, and thus, may become a fertile therapeutic target for the prevention and treatment of uremic cardiomyopathy as well as other medical issues that are derived from the oxidant stress seen with CKD. Both in vitro and in vivo studies have demonstrated the effectiveness of pNakitde in blocking this signaling loop. As such, we and others have shown that administration of pNaKtide attenuates/reverses cardiac hypertrophy and fibrosis in animal models of uremic cardiomyopathy. Although the role of Na/K-ATPase in cellular signal transduction has been well documented in the literature, it should be noted that Karlish and his colleagues have recently questioned whether there is a direct interaction between Na/K-ATPase and Src. Needless to say, further investigation is needed to resolve this important issue. However, it is equally worth noting that the concept of direct interaction between the α1Na/K-ATPase and Src has been evolved from multiple studies from several independent laboratories. Moreover, our recent success in the use of pNaKtide in several disease models has re-enforced the concept of direct protein interaction. On the other hand, the concept that increase in ROS generation can occur through the pathophysiology of CKD and uremic cardiomyopathy is well characterized, suggests that targeting on ROS amplification may serve as a newly potential therapeutic target in a number of conditions characterized by oxidative stress. For instances, elevated oxidative stress has been documented as important mechanism for many risk factors of CKD such as diabetes, hypertension, and obesity [145–147]. Based on these observation, we believe that further investigation targeting on ROS amplification would benefit not only CKD and uremic cardiomyopathy, but also its risk factors and provide better therapeutic effect. In this case, Na/K-ATPase/Src/ROS amplification loop based on this review provides a new direction and a good start point for this hypothesis. Although a novel, well-characterized anti-ROS generation agent would be a welcome addition to our clinical armamentarium, much work is necessary to fill the gap between rodents and humans. Furthermore, additional studies on pNaktide and other strategies that target this loop focusing on the tissue-specific and time-specific manner will be necessary to test this hypothesis.
Article highlights.
Epidemiology and pathophysiology of uremic cardiomyopathy
Na/K-ATPase and its pumping function, receptor function and signaling scaffold
The role of ROS and the newly discovered Na/K-ATPase/Src/ROS amplification loop in uremic cardiomyopathy
The development of a potential therapeutic peptide targeting on this amplification loop
Expert opinion of current study and future direction
This box summarizes key points contained in the article.
Acknowledgments
Funding
This manuscript was supported by NIH grants HL105649, HL071556, HL109085 and NIDDK 1R15DK106666-01A1; grants from the Huntington Foundation and Brickstreet Insurance.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.United States Renal Data System. 2015 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the U.S. government. 2015. [Google Scholar]
- 2.Saltzman HE, Sharma K, Mather PJ, et al. Renal dysfunction in heart failure patients: what is the evidence? Heart Fail Rev. 2007;12:37–47. doi: 10.1007/s10741-007-9006-5. Epub 2007/03/30. [DOI] [PubMed] [Google Scholar]
- 3.McClellan WM, Flanders WD, Langston RD, et al. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. Jasn. 2002;13:1928–1936. doi: 10.1097/01.asn.0000018409.45834.fa. Epub 2002/06/29. [DOI] [PubMed] [Google Scholar]
- 4.Dries DL, Exner DV, Domanski MJ, et al. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. Epub 2000/03/15. [DOI] [PubMed] [Google Scholar]
- 5.Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J Am Coll Cardiol. 2004;44:1587–1592. doi: 10.1016/j.jacc.2004.06.072. Epub 2004/10/19. [DOI] [PubMed] [Google Scholar]
- 6.Levey AS, Beto JA, Coronado BE, et al. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? what do we need to learn? where do we go from here? National Kidney Foundation Task Force on cardiovascular disease. Am J Kidney Dis. 1998;32:853–906. doi: 10.1016/s0272-6386(98)70145-3. Epub 1998/11/20. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Parfrey PS, Harnett JD, et al. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5:2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 8.London GM, Fabiani F, Marchais SJ, et al. Uremic cardiomyopathy: an inadequate left ventricular hypertrophy. Kidney Int. 1987;31:973–980. doi: 10.1038/ki.1987.94. Epub 1987/04/01. [DOI] [PubMed] [Google Scholar]
- 9.London GM, Parfrey PS. Cardiac disease in chronic uremia: pathogenesis. Adv Ren Replace Ther. 1997;4:194–211. doi: 10.1016/s1073-4449(97)70029-3. Epub 1997/07/01. [DOI] [PubMed] [Google Scholar]
- 10.Tsuzuki K. Uremic cardiomyopathy. Ryoikibetsu Shokogun Shirizu. 1997:56–59. Epub 1997/01/01. [PubMed] [Google Scholar]
- 11.Mohmand B, Malhotra DK, Shapiro JI. Uremic cardiomyopathy: role of circulating digitalis like substances. Front Biosci. 2005;10:2036–2044. doi: 10.2741/1679. Epub 2005/06/23. [DOI] [PubMed] [Google Scholar]
- 12.Ookawara S, Tabei K, Asano Y. [Uremic cardiomyopathy] Ryoikibetsu Shokogun Shirizu. 1997:98–101. Epub 1997/01/01. [PubMed] [Google Scholar]
- 13.Alhaj E, Alhaj N, Rahman I, et al. Uremic cardiomyopathy: an under-diagnosed disease. Congest Heart Fail. 2013;19:E40–5. doi: 10.1111/chf.12030. [DOI] [PubMed] [Google Scholar]
- 14.Tian J, Shidyak A, Periyasamy SM, et al. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54:1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. 2009;75:771–773. doi: 10.1038/ki.2009.35. Epub 2009/04/02. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay RM, Leitch R, Heidenheim AP, et al. The London Daily/ Nocturnal Hemodialysis Study-study design, morbidity, and mortality results. Am J Kidney Dis. 2003;42:5–12. doi: 10.1016/s0272-6386(03)00531-6. Epub 2003/06/28. [DOI] [PubMed] [Google Scholar]
- 17.Kayikcioglu M, Tumuklu M, Ozkahya M, et al. The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant. 2009;24:956–962. doi: 10.1093/ndt/gfn599. Epub 2008/11/14. [DOI] [PubMed] [Google Scholar]
- 18.Shi H, Drummond CA, Fan X, et al. Hiding inside? Intracellular expression of non-glycosylated c-kit protein in cardiac progenitor cells. Stem Cell Res. 2016;16:795–806. doi: 10.1016/j.scr.2016.04.017. Epub 2016/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drummond CA, Sayed M, Evans KL, et al. Reduction of Na/K-ATPase affects cardiac remodeling and increases c-kit cell abundance in partial nephrectomized mice. Am J Physiol Heart Circ Physiol. 2014;306:H1631–43. doi: 10.1152/ajpheart.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raev DC. Which left ventricular function is impaired earlier in the evolution of diabetic cardiomyopathy? An echocardiographic study of young type I diabetic patients. Diabetes Care. 1994;17:633–639. doi: 10.2337/diacare.17.7.633. Epub 1994/07/01. [DOI] [PubMed] [Google Scholar]
- 21.Wolf WC, Yoshida H, Agata J, et al. Human tissue kallikrein gene delivery attenuates hypertension, renal injury, and cardiac remodeling in chronic renal failure. Kidney Int. 2000;58:730–739. doi: 10.1046/j.1523-1755.2000.00219.x. Epub 2000/08/01. [DOI] [PubMed] [Google Scholar]
- 22.Shinohara K, Shoji T, Emoto M, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. Jasn. 2002;13:1894–1900. doi: 10.1097/01.asn.0000019900.87535.43. Epub 2002/06/29. [DOI] [PubMed] [Google Scholar]
- 23.Dogra G, Irish A, Chan D, et al. Insulin resistance, inflammation, and blood pressure determine vascular dysfunction in CKD. Am J Kidney Dis. 2006;48:926–934. doi: 10.1053/j.ajkd.2006.08.008. Epub 2006/12/13. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura M, Murase M, Hashimoto T, et al. Insulin resistance and impaired myocardial fatty acid metabolism in dialysis patients with normal coronary arteries. Kidney Int. 2006;69:553–559. doi: 10.1038/sj.ki.5000100. Epub 2006/01/06. [DOI] [PubMed] [Google Scholar]
- 25.Semple D, Smith K, Bhandari S, et al. Uremic cardiomyopathy and insulin resistance: a critical role for akt? Jasn. 2011;22:207–215. doi: 10.1681/ASN.2009090900. Epub 2010/07/17. [DOI] [PubMed] [Google Scholar]
- 26.Becker B, Kronenberg F, Kielstein JT, et al. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. Jasn. 2005;16:1091–1098. doi: 10.1681/ASN.2004090742. Epub 2005/03/04. [DOI] [PubMed] [Google Scholar]
- 27.McGonigle RJ, Fowler MB, Timmis AB, et al. Uremic cardiomyopathy: potential role of vitamin D and parathyroid hormone. Nephron. 1984;36:94–100. doi: 10.1159/000183125. Epub 1984/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Smogorzewski M, Massry SG. Uremic cardiomyopathy: role of parathyroid hormone. Kidney Int Suppl. 1997;62:S12–4. Epub 1997/11/14. [PubMed] [Google Scholar]
- 29.London GM, De Vernejoul MC, Fabiani F, et al. Secondary hyperparathyroidism and cardiac hypertrophy in hemodialysis patients. Kidney Int. 1987;32:900–907. doi: 10.1038/ki.1987.293. Epub 1987/12/01. [DOI] [PubMed] [Google Scholar]
- 30.Winchester JF, Audia PF. Extracorporeal strategies for the removal of middle molecules. Semin Dial. 2006;19:110–114. doi: 10.1111/j.1525-139X.2006.00135.x. Epub 2006/03/23. [DOI] [PubMed] [Google Scholar]
- 31.Lekawanvijit S, Kompa AR, Manabe M, et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulfate. PLoS One. 2012;7:e41281. doi: 10.1371/journal.pone.0041281. Epub 2012/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Tepel M, Echelmeyer M, Orie NN, et al. Increased intracellular reactive oxygen species in patients with end-stage renal failure: effect of hemodialysis. Kidney Int. 2000;58:867–872. doi: 10.1046/j.1523-1755.2000.00236.x. Epub 2000/08/01. This study provided clinical evidence that link ROS generation with CKD. [DOI] [PubMed] [Google Scholar]
- 33.Oberg BP, McMenamin E, Lucas FL, et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004;65:1009–1016. doi: 10.1111/j.1523-1755.2004.00465.x. Epub 2004/02/12. [DOI] [PubMed] [Google Scholar]
- 34.Taylor D, Bhandari S, Seymour AM. Mitochondrial dysfunction in uremic cardiomyopathy. Am J Physiol Renal Physiol. 2015;308:F579–87. doi: 10.1152/ajprenal.00442.2014. Epub 2015/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolmakova EV, Haller ST, Kennedy DJ, et al. Endogenous cardiotonic steroids in chronic renal failure. Nephrol Dial Transplant. 2011;26:2912–2919. doi: 10.1093/ndt/gfq772. Epub 2011/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komiyama Y, Dong XH, Nishimura N, et al. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. Epub 2004/12/21. [DOI] [PubMed] [Google Scholar]
- 37.Manunta P, Stella P, Rivera R, et al. Left ventricular mass, stroke volume, and ouabain-like factor in essential hypertension. Hypertension (Dallas, Tex: 1979) 1999;34:450–456. doi: 10.1161/01.hyp.34.3.450. Epub 1999/09/18. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy DJ, Shrestha K, Sheehey B, et al. Elevated plasma marinobufagenin, an endogenous cardiotonic steroid, is associated with right ventricular dysfunction and nitrative stress in heart failure. Circ Heart Fail. 2015;8:1068–1076. doi: 10.1161/CIRCHEARTFAILURE.114.001976. Epub 2015/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagrov AY, Fedorova OV, Dmitrieva RI, et al. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension (Dallas, Tex: 1979) 1998;31:1097–1103. doi: 10.1161/01.hyp.31.5.1097. Epub 1998/05/12. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy DJ, Vetteth S, Periyasamy SM, et al. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension (Dallas, Tex: 1979) 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. Epub 2006/02/01. [DOI] [PubMed] [Google Scholar]
- 41••.Liu J, Tian J, Chaudhry M, et al. Attenuation of Na/K-ATPase mediated oxidant amplification with pnaktide ameliorates experimental uremic cardiomyopathy. Sci Rep. 2016;6:34592. doi: 10.1038/srep34592. Epub 2016/10/05. The important evidence that pNaktide could attenuate uremic cardiomyopathy by attenuation of Na/K-ATPase/Src/ROS amplification loop. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. 1957. Biochim Biophys Acta. 1989;1000:439–446. Epub 1989/01/01. The discovery of Na/K-ATPase. [PubMed] [Google Scholar]
- 43.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. Epub 2002/06/05. [DOI] [PubMed] [Google Scholar]
- 44.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. Epub 1989/05/09. [DOI] [PubMed] [Google Scholar]
- 45.Skou JC. The fourth Datta lecture. The energy coupled exchange of Na+ for K+ across the cell membrane. The Na+, K(+)-pump. FEBS Lett. 1990;268:314–324. doi: 10.1016/0014-5793(90)81278-v. Epub 1990/08/01. [DOI] [PubMed] [Google Scholar]
- 46.Skou JC, Esmann M. The Na,K-ATPase. J Bioenerg Biomembr. 1992;24:249–261. doi: 10.1007/BF00768846. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 47.Toyoshima C, Nakasako M, Nomura H, et al. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. Epub 2000/06/23. [DOI] [PubMed] [Google Scholar]
- 48.Sweadner KJ, Donnet C. Structural similarities of Na,K-ATPase and SERCA, the Ca(2+)-ATPase of the sarcoplasmic reticulum. Biochem J. 2001;356:685–704. doi: 10.1042/0264-6021:3560685. Epub 2001/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morth JP, Pedersen BP, Toustrup-Jensen MS, et al. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. Epub 2007/12/14. [DOI] [PubMed] [Google Scholar]
- 50.Woo AL, James PF, Lingrel JB. Characterization of the fourth alpha isoform of the Na,K-ATPase. J Membr Biol. 1999;169:39–44. doi: 10.1007/pl00005899. Epub 1999/05/05. [DOI] [PubMed] [Google Scholar]
- 51.Woo AL, James PF, Lingrel JB. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J Biol Chem. 2000;275:20693–20699. doi: 10.1074/jbc.M002323200. Epub 2000/04/15. [DOI] [PubMed] [Google Scholar]
- 52.Blanco G, Sanchez G, Melton RJ, et al. The alpha4 isoform of the Na, K-ATPase is expressed in the germ cells of the testes. J Histochem Cytochem. 2000;48:1023–1032. doi: 10.1177/002215540004800801. Epub 2000/07/18. [DOI] [PubMed] [Google Scholar]
- 53.Akera T, Brody TM. Inotropic action of digitalis and ion transport. Life Sci. 1976;18:135–144. doi: 10.1016/0024-3205(76)90017-5. Epub 1976/01/15. [DOI] [PubMed] [Google Scholar]
- 54.Barry WH, Hasin Y, Smith TW. Sodium pump inhibition, enhanced calcium influx via sodium-calcium exchange, and positive inotropic response in cultured heart cells. Circ Res. 1985;56:231–241. doi: 10.1161/01.res.56.2.231. Epub 1985/02/01. [DOI] [PubMed] [Google Scholar]
- 55.Schoner W. Endogenous cardiac glycosides, a new class of steroid hormones. Eur J Biochem. 2002;269:2440–2448. doi: 10.1046/j.1432-1033.2002.02911.x. Epub 2002/05/25. [DOI] [PubMed] [Google Scholar]
- 56.Katz A, Lifshitz Y, Bab-Dinitz E, et al. Selectivity of digitalis glycosides for isoforms of human Na,K-ATPase. J Biol Chem. 2010;285:19582–19592. doi: 10.1074/jbc.M110.119248. Epub 2010/04/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touza NA, Pocas ES, Quintas LE, et al. Inhibitory effect of combinations of digoxin and endogenous cardiotonic steroids on Na+/K+-ATPase activity in human kidney membrane preparation. Life Sci. 2011;88:39–42. doi: 10.1016/j.lfs.2010.10.027. Epub 2010/11/05. [DOI] [PubMed] [Google Scholar]
- 58.Dostanic I, Lorenz JN, Schultz Jel J, et al. The alpha2 isoform of Na, K-ATPase mediates ouabain-induced cardiac inotropy in mice. J Biol Chem. 2003;278:53026–53034. doi: 10.1074/jbc.M308547200. Epub 2003/10/16. [DOI] [PubMed] [Google Scholar]
- 59.Lingrel JB. The physiological significance of the cardiotonic steroid/ ouabain-binding site of the Na,K-ATPase. Annu Rev Physiol. 2010;72:395–412. doi: 10.1146/annurev-physiol-021909-135725. Epub 2010/02/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paula S, Tabet MR, Ball WJ., Jr Interactions between cardiac glycossides and sodium/potassium-ATPase: three-dimensional structure-activity relationship models for ligand binding to the E2-Pi form of the enzyme versus activity inhibition. Biochemistry. 2005;44:498–510. doi: 10.1021/bi048680w. Epub 2005/01/12. [DOI] [PubMed] [Google Scholar]
- 61.Periyasamy SM, Lane LK, Askari A. Ouabain-insensitivity of highly active Na+, K+-dependent adenosinetriphosphatase from rat kidney. Biochem Biophys Res Commun. 1979;86:742–747. doi: 10.1016/0006-291x(79)91775-3. Epub 1979/02/14. [DOI] [PubMed] [Google Scholar]
- 62.Lorenz JN, Loreaux EL, Dostanic-Larson I, et al. ACTH-induced hypertension is dependent on the ouabain-binding site of the alpha2-Na+-K+-ATPase subunit. Am J Physiol Heart Circ Physiol. 2008;295:H273–80. doi: 10.1152/ajpheart.00183.2008. Epub 2008/05/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fedorova OV, Agalakova NI, Talan MI, et al. Brain ouabain stimulates peripheral marinobufagenin via angiotensin II signalling in NaCl-loaded Dahl-S rats. J Hypertens. 2005;23:1515–1523. doi: 10.1097/01.hjh.0000174969.79836.8b. Epub 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 64.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20:581–591. doi: 10.3109/10641969809053236. Epub 1998/07/31. [DOI] [PubMed] [Google Scholar]
- 65.Hamlyn JM, Blaustein MP, Bova S, et al. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991;88:6259–6263. doi: 10.1073/pnas.88.14.6259. Epub 1991/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laredo J, Shah JR, Lu ZR, et al. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angio-tensin type 2 receptors. Hypertension (Dallas, Tex: 1979) 1997;29:401–407. doi: 10.1161/01.hyp.29.1.401. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 67.Fedorova OV, Kolodkin NI, Agalakova NI, et al. Marinobufagenin, an endogenous alpha-1 sodium pump ligand, in hypertensive Dahl salt-sensitive rats. Hypertension (Dallas, Tex: 1979) 2001;37:462–466. doi: 10.1161/01.hyp.37.2.462. Epub 2001/03/07. [DOI] [PubMed] [Google Scholar]
- 68.Lopatin DA, Ailamazian EK, Dmitrieva RI, et al. Circulating bufodie-nolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. Epub 1999/08/31. [DOI] [PubMed] [Google Scholar]
- 69.Fedorova OV, Talan MI, Agalakova NI, et al. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride-dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. Epub 2002/03/06. [DOI] [PubMed] [Google Scholar]
- 70.Hamlyn JM, Manunta MP. Ouabain, digitalis-like factors and hypertension. J Hypertens Suppl. 1992;10:S99–111. Epub 1992/12/01. [PubMed] [Google Scholar]
- 71.Takahashi H, Komiyama Y. [Endogenous digitalis-like factor] Nihon Rinsho. 2006;64(Suppl 5):177–181. Epub 2006/08/10. [PubMed] [Google Scholar]
- 72.Baecher S, Kroiss M, Fassnacht M, et al. No endogenous ouabain is detectable in human plasma by ultra-sensitive UPLC-MS/MS. Clin Chim Acta. 2014;431:87–92. doi: 10.1016/j.cca.2014.01.038. Epub 2014/02/11. [DOI] [PubMed] [Google Scholar]
- 73.Blaustein MP. Why isn’t endogenous ouabain more widely accepted? Am J Physiol Heart Circ Physiol. 2014;307:H635–9. doi: 10.1152/ajpheart.00404.2014. Epub 2014/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hamlyn JM, Blaustein MP. Endogenous ouabain: recent advances and controversies. Hypertension (Dallas, Tex: 1979) 2016;68:526–532. doi: 10.1161/HYPERTENSIONAHA.116.06599. Epub 2016/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dostanic-Larson I, Lorenzs JN, van Huysse JW, et al. Physiological role of the alpha1- and alpha2-isoforms of the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol. 2006;290:R524–8. doi: 10.1152/ajpregu.00838.2005. Epub 2006/02/10. [DOI] [PubMed] [Google Scholar]
- 76.Dostanic-Larson I, Van Huysse JW, Lorenz JN, et al. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci U S A. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. Epub 2005/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dostanic I, Schultz Jel J, Lorenz JN, et al. The alpha 1 isoform of Na, K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. Epub 2004/10/16. [DOI] [PubMed] [Google Scholar]
- 78.Wansapura AN, Lasko VM, Lingrel JB, et al. Mice expressing ouabain-sensitive alpha1-Na,K-ATPase have increased susceptibility to pressure overload-induced cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2011;300:H347–55. doi: 10.1152/ajpheart.00625.2010. Epub 2010/10/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dvela-Levitt M, Cohen-Ben Ami H, Rosen H, et al. Reduction in maternal circulating ouabain impairs offspring growth and kidney development. Jasn. 2015;26:1103–1114. doi: 10.1681/ASN.2014020130. Epub 2014/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J, Khodus GR, Kruusmagi M, et al. Ouabain protects against adverse developmental programming of the kidney. Nat Commun. 2010;1:1–7. doi: 10.1038/ncomms1043. Epub 2010/10/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–27837. doi: 10.1074/jbc.M002951200. Epub 2000/06/30. [DOI] [PubMed] [Google Scholar]
- 82.Kometiani P, Li J, Gnudi L, et al. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–15256. doi: 10.1074/jbc.273.24.15249. Epub 1998/06/17. [DOI] [PubMed] [Google Scholar]
- 83.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. Jasn. 2007;18:46–57. doi: 10.1681/ASN.2006010086. Epub 2006/12/08. [DOI] [PubMed] [Google Scholar]
- 84•.Tian J, Cai T, Yuan Z, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. Epub 2005/11/04. First evidence indicating Na/K-ATPase/Src signaling complex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J, Liang M, Liu L, et al. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 86.Cherniavsky-Lev M, Golani O, Karlish SJ, et al. Ouabain-induced internalization and lysosomal degradation of the Na+/K+-ATPase. J Biol Chem. 2014;289:1049–1059. doi: 10.1074/jbc.M113.517003. Epub 2013/11/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yosef E, Katz A, Peleg Y, et al. Do Src kinase and caveolin interact directly with Na,K-ATPase? J Biol Chem. 2016;291:11736–11750. doi: 10.1074/jbc.M116.721084. Epub 2016/03/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88••.Xie Z, Kometiani P, Liu J, et al. Intracellular reactive oxygen species mediate the linkage of Na+/K+-ATPase to hypertrophy and its marker genes in cardiac myocytes. J Biol Chem. 1999;274:19323–19328. doi: 10.1074/jbc.274.27.19323. Epub 1999/06/26. This paper provides evidence of Na/K-ATPase/ROS signaling in cardiac myocytes. [DOI] [PubMed] [Google Scholar]
- 89.Liang M, Cai T, Tian J, et al. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem. 2006;281:19709–19719. doi: 10.1074/jbc.M512240200. Epub 2006/05/16. [DOI] [PubMed] [Google Scholar]
- 90.Efendiev R, Chen Z, Krmar RT, et al. The 14-3-3 protein translates the NA+,K+-ATPase {alpha}1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem. 2005;280:16272–16277. doi: 10.1074/jbc.M500486200. Epub 2005/02/22. [DOI] [PubMed] [Google Scholar]
- 91.Yudowski GA, Efendiev R, Pedemonte CH, et al. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A. 2000;97:6556–6561. doi: 10.1073/pnas.100128297. Epub 2000/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang S, Malmersjo S, Li J, et al. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281:21954–21962. doi: 10.1074/jbc.M601578200. Epub 2006/05/26. [DOI] [PubMed] [Google Scholar]
- 93.Wang H, Haas M, Liang M, et al. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. Epub 2004/02/14. [DOI] [PubMed] [Google Scholar]
- 94.Kimura T, Allen PB, Nairn AC, et al. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell. 2007;18:4508–4518. doi: 10.1091/mbc.E06-08-0711. Epub 2007/09/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Efendiev R, Budu CE, Bertorello AM, et al. G-protein-coupled receptor-mediated traffic of Na,K-ATPase to the plasma membrane requires the binding of adaptor protein 1 to a Tyr-255-based sequence in the alpha-subunit. J Biol Chem. 2008;283:17561–17567. doi: 10.1074/jbc.M709260200. Epub 2008/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Done SC, Leibiger IB, Efendiev R, et al. Tyrosine 537 within the Na+,K+-ATPase alpha-subunit is essential for AP-2 binding and clathrin-dependent endocytosis. J Biol Chem. 2002;277:17108–17111. doi: 10.1074/jbc.M201326200. Epub 2002/02/23. [DOI] [PubMed] [Google Scholar]
- 97.Devarajan P, Scaramuzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase alpha subunit. Proc Natl Acad Sci U S A. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. Epub 1994/04/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan Z, Cai T, Tian J, et al. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. Epub 2005/06/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee K, Jung J, Kim M, et al. Interaction of the alpha subunit of Na,K-ATPase with cofilin. Biochem J. 2001;353:377–385. doi: 10.1042/0264-6021:3530377. Epub 2001/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zatti A, Chauvet V, Rajendran V, et al. The C-terminal tail of the polycystin-1 protein interacts with the Na,K-ATPase alpha-subunit. Mol Biol Cell. 2005;16:5087–5093. doi: 10.1091/mbc.E05-03-0200. Epub 2005/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liang M, Tian J, Liu L, et al. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. Epub 2007/02/14. [DOI] [PubMed] [Google Scholar]
- 102.Liu L, Mohammadi K, Aynafshar B, et al. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–60. doi: 10.1152/ajpcell.00555.2002. Epub 2003/02/28. [DOI] [PubMed] [Google Scholar]
- 103.Quintas LE, Pierre SV, Liu L, et al. Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J Mol Cell Cardiol. 2010;49:525–531. doi: 10.1016/j.yjmcc.2010.04.015. Epub 2010/05/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. Epub 1998/10/06. [DOI] [PubMed] [Google Scholar]
- 105.Razani B, Woodman SE, Lisanti MP. Caveolae: from cell biology to animal physiology. Pharmacol Rev. 2002;54:431–467. doi: 10.1124/pr.54.3.431. Epub 2002/09/12. [DOI] [PubMed] [Google Scholar]
- 106.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr Opin Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. Epub 1999/08/17. [DOI] [PubMed] [Google Scholar]
- 107.Lisanti MP, Scherer PE, Vidugiriene J, et al. Characterization of caveolin-rich membrane domains isolated from an endothelialrich source: implications for human disease. J Cell Biol. 1994;126:111–126. doi: 10.1083/jcb.126.1.111. Epub 1994/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen Y, Li X, Ye Q, et al. Regulation of alpha1 Na/K-ATPase expression by cholesterol. J Biol Chem. 2011;286:15517–15524. doi: 10.1074/jbc.M110.204396. Epub 2011/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. Epub 2003/10/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.D’Autreaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. Epub 2007/09/13. [DOI] [PubMed] [Google Scholar]
- 111.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. Epub 2008/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. Epub 2012/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. Epub 1998/04/30. [DOI] [PubMed] [Google Scholar]
- 114.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. Epub 2006/04/14. [DOI] [PubMed] [Google Scholar]
- 115.McMurray F, Patten DA, Harper ME. Reactive oxygen species and oxidative stress in obesity-recent findings and empirical approaches. Obesity (Silver Spring, Md) 2016;24:2301–2310. doi: 10.1002/oby.21654. Epub 2016/11/03. [DOI] [PubMed] [Google Scholar]
- 116.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. Epub 2013/02/26. [DOI] [PubMed] [Google Scholar]
- 117.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. Epub 2009/05/30. [DOI] [PubMed] [Google Scholar]
- 118.Rhee SG, Bae YS, Lee SR, et al. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. Epub 2001/12/26. [DOI] [PubMed] [Google Scholar]
- 119••.Liu J, Tian J, Haas M, et al. Ouabain interaction with cardiac Na+/K+-ATPase initiates signal cascades independent of changes in intracellular Na+ and Ca2+ concentrations. J Biol Chem. 2000;275:27838–27844. doi: 10.1074/jbc.M002950200. Epub 2000/06/30. First publication that linked Na/K-ATPase signaling function with ROS. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Ye Q, Liu C, et al. Involvement of Na/K-ATPase in hydrogen peroxide-induced activation of the Src/ERK pathway in LLC-PK1 cells. Free Radic Biol Med. 2014;71:415–426. doi: 10.1016/j.freeradbiomed.2014.03.036. Epub 2014/04/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yan Y, Shapiro AP, Mopidevi BR, et al. Protein carbonylation of an amino acid residue of the Na/K-ATPase alpha1 subunit determines Na/K-ATPase signaling and sodium transport in renal proximal tubular cells. J Am Heart Assoc. 2016;5:e003675. doi: 10.1161/JAHA.116.003675. Epub 2016/09/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ellis DZ, Rabe J, Sweadner KJ. Global loss of Na,K-ATPase and its nitric oxide-mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2003;23:43–51. doi: 10.1523/JNEUROSCI.23-01-00043.2003. Epub 2003/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim MS, Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol. 1987;252:H252–7. doi: 10.1152/ajpheart.1987.252.2.H252. Epub 1987/02/01. [DOI] [PubMed] [Google Scholar]
- 124.Bibert S, Liu CC, Figtree GA, et al. FXYD proteins reverse inhibition of the Na+-K+ pump mediated by glutathionylation of its beta1 subunit. J Biol Chem. 2011;286:18562–18572. doi: 10.1074/jbc.M110.184101. Epub 2011/04/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Figtree GA, Liu CC, Bibert S, et al. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ Res. 2009;105:185–193. doi: 10.1161/CIRCRESAHA.109.199547. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 126.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol. 2006;290:F241–50. doi: 10.1152/ajprenal.00126.2005. Epub 2006/01/13. [DOI] [PubMed] [Google Scholar]
- 127.Liu L, Li J, Liu J, et al. Involvement of Na+/K+-ATPase in hydrogen peroxide-induced hypertrophy in cardiac myocytes. Free Radic Biol Med. 2006;41:1548–1556. doi: 10.1016/j.freeradbiomed.2006.08.018. Epub 2006/10/19. [DOI] [PubMed] [Google Scholar]
- 128.Kennedy DJ, Vetteth S, Xie M, et al. Ouabain decreases sarco(endo) plasmic reticulum calcium ATPase activity in rat hearts by a process involving protein oxidation. Am J Physiol Heart Circ Physiol. 2006;291:H3003–11. doi: 10.1152/ajpheart.00603.2006. Epub 2006/07/25. [DOI] [PubMed] [Google Scholar]
- 129.Chen Y, Kennedy DJ, Ramakrishnan DP, et al. Oxidized LDL-bound CD36 recruits an Na(+)/K(+)-ATPase-Lyn complex in macrophages that promotes atherosclerosis. Sci Signal. 2015;8:ra91. doi: 10.1126/scisignal.aaa9623. Epub 2015/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kennedy DJ, Chen Y, Huang W, et al. CD36 and Na/K-ATPase-alpha1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216–224. doi: 10.1161/HYPERTENSIONAHA.112.198770. Epub 2012/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131•.Sodhi K, Maxwell K, Yan Y, et al. pNaKtide inhibits Na/K-ATPase reactive oxygen species amplification and attenuates adipogenesis. Sci Adv. 2015;1:e1500781. doi: 10.1126/sciadv.1500781. Epub 2015/11/26. The first in vivo study of pNaktide in attenuation of ROS amplification. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 132.Diano S, Liu ZW, Jeong JK, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. Epub 2011/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–1761. doi: 10.1172/JCI21625. Epub 2004/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haller ST, Yan Y, Drummond CA, et al. Rapamycin attenuates cardiac fibrosis in experimental uremic cardiomyopathy by reducing marinobufagenin levels and inhibiting downstream pro-fibrotic signaling. J Am Heart Assoc. 2016;5:e004106. doi: 10.1161/JAHA.116.004106. Epub 2016/10/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Small DM, Coombes JS, Bennett N, et al. Oxidative stress, antioxidant therapies and chronic kidney disease. Nephrology (Carlton, Vic) 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. Epub 2012/02/01. [DOI] [PubMed] [Google Scholar]
- 136.Elkareh J, Kennedy DJ, Yashaswi B, et al. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–224. doi: 10.1161/01.HYP.0000252409.36927.05. Epub 2006/12/06. [DOI] [PubMed] [Google Scholar]
- 137.Ferrandi M, Molinari I, Barassi P, et al. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279:33306–33314. doi: 10.1074/jbc.M402187200. Epub 2004/05/27. [DOI] [PubMed] [Google Scholar]
- 138.Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol. 1997;29:429–437. doi: 10.1006/jmcc.1996.0320. Epub 1997/02/01. [DOI] [PubMed] [Google Scholar]
- 139.Norgaard A, Bagger JP, Bjerregaard P, et al. Relation of left ventricular function and Na,K-pump concentration in suspected idiopathic dilated cardiomyopathy. Am J Cardiol. 1988;61:1312–1315. doi: 10.1016/0002-9149(88)91175-7. Epub 1988/06/01. [DOI] [PubMed] [Google Scholar]
- 140.Schwinger RH, Bundgaard H, Muller-Ehmsen J, et al. The Na, K-ATPase in the failing human heart. Cardiovasc Res. 2003;57:913–920. doi: 10.1016/s0008-6363(02)00767-8. Epub 2003/03/26. [DOI] [PubMed] [Google Scholar]
- 141.Kennedy D, Omran E, Periyasamy SM, et al. Effect of chronic renal failure on cardiac contractile function, calcium cycling, and gene expression of proteins important for calcium homeostasis in the rat. Jasn. 2003;14:90–97. doi: 10.1097/01.asn.0000037403.95126.03. Epub 2002/12/31. [DOI] [PubMed] [Google Scholar]
- 142•.Li Z, Cai T, Tian J, et al. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284:21066–21076. doi: 10.1074/jbc.M109.013821. Epub 2009/06/10. The first publication to present the development of pNaktide. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2009;457:635–644. doi: 10.1007/s00424-008-0470-0. Epub 2008/02/20. [DOI] [PubMed] [Google Scholar]
- 144•.Li Z, Zhang Z, Xie JX, et al. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem. 2011;286:32394–32403. doi: 10.1074/jbc.M110.207597. Epub 2011/07/26. The effectiveness of pNaktide in an animal model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Matsuda M, Shimomura I. Increased oxidative stress in obesity: implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancer. Obes Res Clin Pract. 2013;7:e330–e341. doi: 10.1016/j.orcp.2013.05.004. Epub 2014/01/24. [DOI] [PubMed] [Google Scholar]
- 146.Kashihara N, Haruna Y, Kondeti VK, et al. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–4269. doi: 10.2174/092986710793348581. Epub 2010/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Datla SR, Griendling KK. Reactive oxygen species, NADPH oxidases, and hypertension. Hypertension. 2010;56:325–330. doi: 10.1161/HYPERTENSIONAHA.109.142422. Epub 2010/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]