Abstract
The generation of circularly polarized laser emission (CPLE) in photonic devices has attracted increasing attention due to the prospects of using CP light in displaying technologies or advanced microscopies. Organic systems excel as laser materials across the whole visible spectrum, and despite many of them displaying circularly polarized luminescence (CPL), none have been shown thus far to amplify their own CPL, let alone generate CPLE. Consequently, there is still a need to find alternative CPLE organic devices. Herein we demonstrate an effective strategy for achieving strong levels of CPLE (|glum| ~ 0.1–0.2) by using solutions of an achiral dye dissolved in optically active solvents to exploit the full potential of the dynamic birefringence induced by the intense and polarized laser pumping. The present approach enables changing the CPLE handedness by changing the handedness of the solvent optical activity, opening new avenues for developing cost-effective and easily processable chiro-photonic materials.
1. Introduction
The generation of circularly polarized (CP) emission in photonic devices, as opposed to conversion by means of wave-retardation, has attracted increasing attention due to the prospects of using CP light in displaying technologies or advanced microscopies. Many devices have been designed to emit CP light, including light emitting transistors,1 LEDs,2 OLEDs,3–5 plasmonic nano-antennas,6 or lasers (either inorganic7–9 or organic10–14). Whereas some of the aforementioned luminescence devices have been shown to emit directly circularly polarized luminescence (CPL) – i.e. the photons are emitted intrinsically with circular polarization – most CP lasers have relied thus far on intracavity conversion by means of optical retardation instead of amplification of inherent CPL.
Nevertheless, the direct generation and amplification of circularly polarized laser emission (CPLE) would be especially important in complex environments where optical retardation cannot be achieved. Fluorescent small molecular systems, either organic,15,16 inorganic,17–19 or hybrid,20–22 are especially appealing for these applications due to their huge processing and functionalization versatility, which enables incorporation into a wealth of host environments (laser cavities, fibres, biological tissues, etc.). In particular, organic systems such as molecular dyes or conjugated polymers excel as laser materials across the whole visible spectrum.16,22 Whereas CPL has been obtained in simple organic molecules,23 polymers,3,24,25 and supramolecular assemblies,26,27 only one CPL emitting system of many has been shown to lase so far, namely, a chiral dibromoBINOL-based substituted O-BODIPY.28 Unfortunately, its CPL level – comparable to those of similar CPL-simple organic molecules – was far too low to generate a detectable intrinsic CPLE signal. Hence, there is still a need to find alternative strategies to generate CPLE.
In a previous work, we showed that a laser induced birefringence (LIB), which is ubiquitous in many laser experiments involving strong and polarized laser pumping,29,30 might render non-vanishing CPLE levels in systems with silent CPL.14 Although the refractive index anisotropy generated in these experiments is usually strong,31 the resulting CPLE levels have tended to zero thus far, since the polarization planes of the emitted light and the LIB fast axis are parallel.14 These two previous magnitudes are intrinsically coupled, as both are determined by the orientation of the emission transition dipole moment, which in turn is fixed by the pump polarization plane.14 Thus, to exploit the full potential of the LIB anisotropy, a method should be envisaged to misalign, within the laser medium, the polarization plane of the emission and the anisotropy axes. A smart way of achieving this task would be to resort to hosts with optical rotation or activity (OA), a property that confers matter with the ability to rotate the polarization plane of light traversing it. In this sense, the polarized pump would determine the orientations of both the anisotropy axes and the initial polarization plane of the emission, but the OA would be responsible for the rotation of the latter upon propagation, in such a way that the optical retardation would be maximized. In other words, the interaction of a strong OA with LIB could serve as an effective strategy for achieving strong levels of net CPLE.
Besides, the understanding of OA/LIB interaction is of paramount importance for the characterization of future devices based on the amplification of pure CPL. In this sense, to have a real (free of optical retardation effects) and detectable CPLE signal, one should move to organic systems with stronger CPL levels by improving their helical (or pseudo-helical) character, and many groups are devoting huge efforts towards this end.23,26,27 It is expected, though, that the accomplishment of such a goal will necessarily entail stronger levels of OA due to the enhanced helical character. Hence, in the presence of both strong CPL, OA, and LIB, two different effects would contribute to the net CPLE; the “intrinsic” CPLE coming from the amplification of pure CPL, and the “extrinsic” CPLE induced by the combination of OA and LIB. Thus, the study of the sole effect of the OA on the extrinsic CPLE signal would help differentiating intrinsic CPLE signals from extrinsic ones in organic systems with high levels of CPL. And last but not least, from a fundamental and technological point of view, the understanding of the influence of OA on the performance and polarization state of conventional dye lasers, which has not been tackled so far, could have an impact on the improvement and optimization of commercial systems. Thus, to achieve this project, a medium with OA but without circular dichroism (CD) or CPL should be ideally utilized.
In this manuscript, we will study the polarization state of transversally pumped dye lasers with a conventional plane parallel cavity configuration containing an achiral commercial BODIPY dye (PM567), with silent CD and CPL, dissolved in both enantiomers of a chiral commercial solvent (limonene, inset Fig. 1a), responsible for the OA. The photophysical and chiroptical properties of the dye doped solutions will be comprehensively evaluated to assess whether it complies with the requirements to lase efficiently, and to quantify the levels of OA, CD and CPL. A complete polarimetric study of the laser emission will be performed as a function of pump energy and polarization, and the results obtained for each enantiomeric solution will be discussed in the framework of laser-induced birefringence. We will evidence that the inclusion of OA in laser dye solutions serves as an alternative strategy to obtain strong levels of net CPLE even in systems with no CPL. Finally, the consequences of having a system with OA for the detection of CPLE, and how to detect intrinsic CPLE signals, will be discussed.
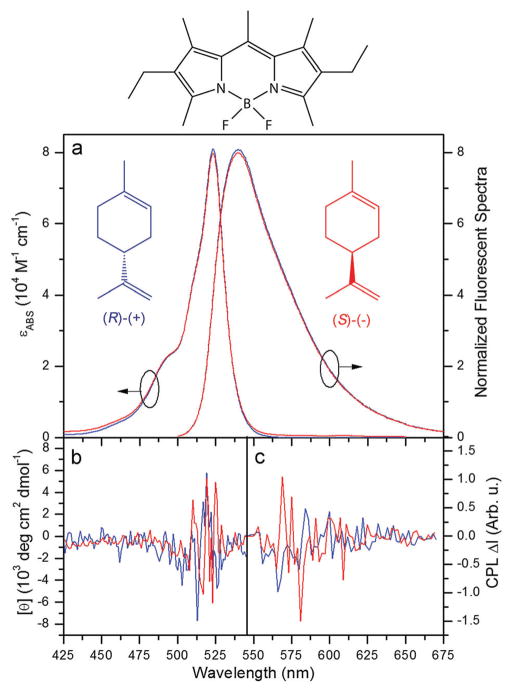
Fig. 1.
Photophysical and chiroptical spectra of PM567 in (R)-(+)- and (S)-(−)-limonene (blue and red curves, respectively). (a) Extinction coefficient εABS and normalized fluorescence spectra, (b) CD molar ellipticity [θ], and (c) CPL spectra. Inset and top: Chemical structures of dye PM567 and (R)-(+)- and (S)-(−)-limonene.
2. Experimental details
Materials
PM567 was laser grade and purchased from Exciton. (R)-(+)- and (S)-(−)-limonene (97% chemical purity) were purchased from Sigma-Aldrich and Alpha-Aeser, respectively. It must be noted here that, strictly speaking, limonene is a reagent more than a solvent. For example, it may act as a monomer in polymerization reactions,32 and, in a household environment, it has been shown to naturally undergo ozonolysis to afford formaldehyde,33 a compound known to be a human carcinogen. We have in fact observed that the dye can be partially degraded after a few weeks if dissolved in pure limonene. This reactivity explains why the as-purchased limonene usually contains additives to act as inhibitors (97% chemical purity). For the photophysical and chiroptical measurements, limonene was used without further purification. For the laser measurements, (R)-(+)-limonene solutions were passed through a pair of 0.2 μm pore size PTFE syringe filters, whereas (S)-(−)-limonene solutions had to be additionally distilled and passed through a silica gel (Scharlab; 0.04–0.06 mm (230–400 mesh ASTM)).
Optical activity specific rotation measurements
The measurement procedure was very similar to that for the calibration of the laser polarimeter.14 As is shown in Fig. S1 (ESI†), we placed a polarizer with a fast/slow axis transmission ratio at the laser wavelength of 1 : 100 000 between the dye laser cavity (containing PM567 5 × 10−4 M in ethyl acetate) and the polarimeter to ensure that the output beam was effectively linearly polarized (S3 ~ 0). Fitting eqn (S2) and (S3) of the ESI† (with S3 set to zero and leaving S0, S1 and S2 free) to the sets of data without and with the λ/4 retarder, respectively, will provide the polarizer fast axis orientation αpol (or 2ψ in our methodology) as a fitting parameter. Then, without removing the polarizer, a 1 cm optical path quartz cuvette containing the solutions of which OA is to be measured (dye + limonene in this case) is placed between the polarizer and the polarimeter, whereupon the polarization plane of the light exiting the polarizer will be rotated. Repeating the data acquisition and the fitting procedure (with S3 set to zero and leaving S0, S1 and S2 free) a new 2ψ value will be obtained. The net rotation α due to OA will be given by the difference in ψ (warning, not 2ψ!!!). Hence, . ρ is the solvent density (0.841 g ml−1) and L = 0.1 dm is the optical pathway.
Luminescence and laser measurements
The apparatus and procedures to obtain photophysical, chiroptical (circular dichroism and circularly polarized luminescence), and laser measurements, as well as the polarimetry formalism, can be found in the experimental section in the ESI.†
3. Results and discussion
Conventional steady-state and time-resolved UV-Vis spectroscopy (see Experimental section) reveals that the absorption and fluorescence photophysical parameters of PM567 dissolved in (R)-(+)- and (S)-(−)-limonene are identical to each other, and do not differ substantially from those in ethyl acetate, our reference achiral solvent (Fig. 1a and Table 1). These fluorescence figures of merit assure the excitation of efficient laser emission. The bathochromic shifts in the absorption and emission spectra, and the increase in the photoluminescence quantum yield of PM567 in limonene with respect to ethyl acetate, are very similar to those shown by PM567 in cyclohexane.34 This is not surprising, since both solvents, cyclohexane and limonene, are very similar low-polar hydrocarbons.
Table 1.
Photophysical and OA properties of PM567 in different solvents
| PM567 | λabs (nm) | εmax (104 M−1 cm−1) | λfl (nm) | ϕ | τ (ns) |
|
|
|---|---|---|---|---|---|---|---|
| (R)-(+)-Limonene | 523.5 | 8.1 | 539.5 | 0.88 | 5.91 | 134.6 | |
| (S)-(−)-Limonene | 523.5 | 8.0 | 540.0 | 0.87 | 5.91 | −105.9 | |
| Ethyl acetate | 517.0 | 7.6 | 533.0 | 0.84 | 5.78 | — |
The optical activity accounts for the difference in the phase velocity (or, alternatively, the difference in refractive index), experienced by the left and right circularly polarized components of the travelling polarized wave, and is manifested by a rotation in the polarization ellipse orientation. This rotation, quantified by the specific rotation (rotated angle normalized by compound concentration and travelled distance), is usually measured in a commercial polarimeter, but for some unknown reason, the measurements conducted in the dye-doped limonene solutions were not stable and the results were not reliable. Thus we resorted to the same polarimeter used for the laser measurements (see Experimental section). The thus measured specific rotation (Table 1) was recorded in solutions analogous to those used for the laser experiments (PM567 5 × 10−4 M in limonene) at 20 °C and at the laser emission wavelength shown by the PM567/limonene solutions (~575 nm). According to our measurements, which are in agreement with commercial specifications, (R)-(+)-limonene presents an OA stronger than (S)-(−)-limonene. The measurements in the pure solvent (no dye) rendered the same values. It is worth mentioning here that, as expected, no change in the polarization ellipticity was detected for the light traversing the dye doped limonene solution, indicating that the CPLE ellipticity reported in what follows comes only from the LIB + OA interaction.
Limonene has been used as a chiral template to confer chiroptical properties to achiral luminescent polymers,35 and the same could occur to our dye, either via a perturbation of its structure or molecular orbitals, or via the supramolecular assembly of various chromophores. Nevertheless, the supramolecular interaction between such molecules is expected to be highly weak and unspecific due to the lack of significant supra-molecular interactions (e.g., electrostatic interaction, hydrogen bonding, π–π stacking, etc.). To check the extent of this, we assessed the circular dicroism (CD) and circularly polarized luminescence (CPL) properties of both dye-doped enantiomeric solvents (see Experimental section). Whereas the OA provides information about the whole solution (dye + solvent), the CD and CPL measurements focus on the chiral character of the emissive system and, mainly, on its helically chiral character. CD and CPL account for the differential absorption and emission “strengths”, respectively, of the right and left circularly polarized light. The wavelength dependent molar absorption ellipticity [θ], which is the normalized parameter quantifying CD, shows a weak and noisy signal matching the absorption spectra of PM567 for both solvents (Fig. 1b). With peak values of ±4 × 103 deg cm2 dmol−1, it is at least 50-fold weaker than that obtained for chiral BODIPYs.28,36 Similarly, the CPL spectra for both solutions show a noisy signal that could overlap the fluorescence spectrum, but the results are inconclusive (Fig. 1c). It is not uncommon to have challenging results when one is considering an achiral dye with a chiral solvent. Hence, the OA, CD, and CPL levels suggest that the chiral nature of the solvation shell exerts a very weak, if any, chiral perturbation on the structure or molecular orbitals involved in the dispersion (OA), absorption (CD) and emission (CPL) properties. Nevertheless, the values are too low and noisy to be significant, and hence we can assume that our active medium is optically active but lacks CD and CPL.
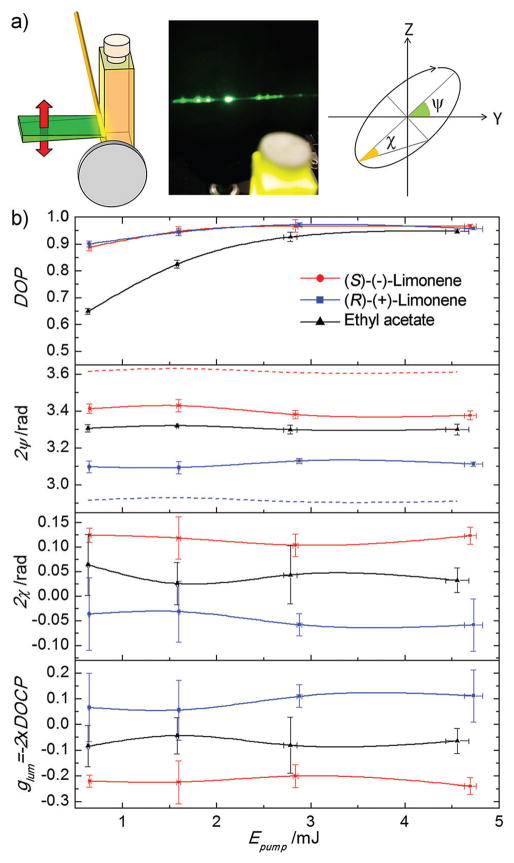
The laser wavelength and efficiency (output/input energy ratio) of PM567 in the used chiral solvents was evaluated in a transversally pumped cavity configuration (Fig. 2a and Fig. S2, of the ESI†). Nevertheless, adequate measurements could not be performed, as there were multiple laser spots (Fig. 2a), probably generated due to internal reflections in the cuvette walls, instead of the common single, nearly circular spot. These side spots “drained” the laser efficiency from the central spot, and affected its polarization state in an erratic way. Furthermore, at high levels of pump intensity, the cuvette wall acting as an output coupler was damaged due to the appearance of a hot-spot of unknown origin. Suspecting that the solvent additives (not specified by manufacturer) were responsible for this odd behaviour, we proceeded to purify them (see Experimental section). After purification, both the side spot and the cuvette wall damage were avoided, at least at pump energies below 5 mJ. We are not aware of the mechanisms involved in the appearence/disappearance of multiple spots, but it could be related to limonene oligomers32 as well as inhibitors and stabilizers present in these solvents. In fact, dynamic light scattering measurements of the unfiltered limonene (not shown) showed an unstable and non-well defined signal above 300 nm, which disappeared after solvent filtration.
Fig. 2.
(a) Sketch of cavity and pump (in green) configuration (from a point of view coincidental with that of the picture), picture showing multi spot laser emission, and polarization ellipse. (b) Laser emission polarization state parameters as a function of pump energy for linear vertical pump polarization. Each point and error bar in (b) represents the average and standard deviation, respectively, of the fitting parameters obtained over 4 sets of measurements. Lines are guides to the eye. Dye concentration: 0.5 mM.
Once the laser spot was “cleaned”, we undertook the polarimetric study of the OA effects on the polarization state of dye lasers. The global polarization state of a given arbitrary beam can be described in terms of the Stokes parameters Si (i = 0, 1, 2, 3),37 but it is physically more insightful to express it with the related parameters, beam energy (Eout), degree of polarization (DOP), degree of circular polarization (DOCP), orientation (ψ) and ellipticity (χ) of the polarization ellipse (Fig. 2a). Note that the DOCP calculated with this method, which in turn is related to χ, equals minus half the glum as-defined for CPL.14 We performed a complete polarimetric study of their laser emission with a home-made polarimeter14 (see Fig. S2 in ESI† for full details on the experimental set-up and methodology). The laser light polarization state parameters for PM567 in both limonenes and in ethyl acetate (for comparison purposes) were evaluated as a function of the pump energy and polarization.
Fig. 2b shows the results corresponding to a linear vertical pump polarization (those corresponding to a linear 45° pump polarization can be found in Fig. S3 of the ESI†). The DOP follows the expected behaviour for laser dyes with nearly parallel absorption and emission transition dipole moments;14 i.e., the emission is highly polarized. As explained elsewhere, this arises from the orientation anisotropy of the excited state dipole moments induced by the polarized pump (Fig. 3a and ref. 14). Note here that in both chiral solvents PM567 presents a laser emission, which is more polarized than that in ethyl acetate. The rationale of this increment can be found in the more viscous nature of the chiral solvents, nearly doubling the viscosity of ethyl acetate (0.923 cP 38 vs. 0.426 cP 39). In this sense, the rotational relaxation rate should be smaller, preventing the molecules to randomize their orientation before the photons are emitted. The very same DOP enhancement has been observed in a derivative of PM567 having a pendant dibromoBINOL group, whose rotational relaxation is slowed down due to its bulkier nature.28
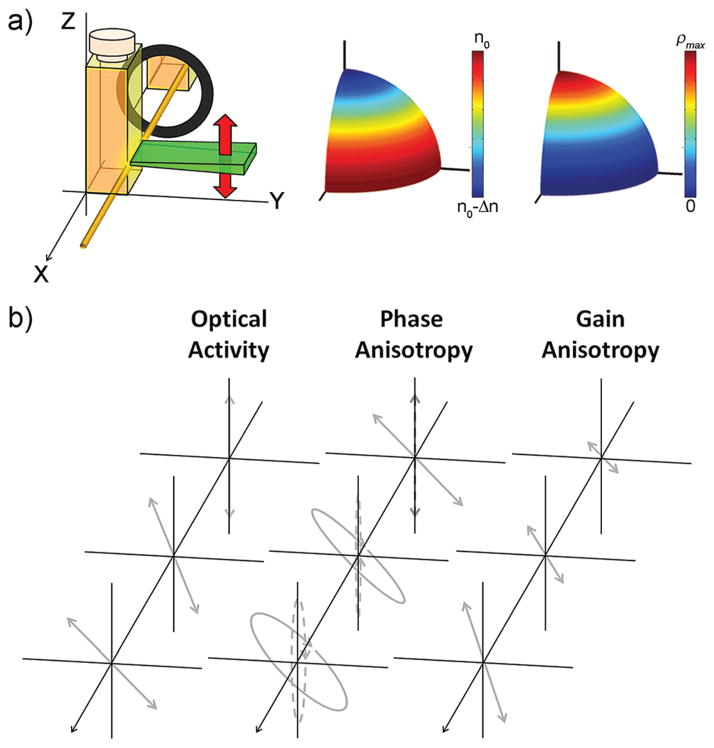
Fig. 3.
(a) Sketch of cavity and pump (in green) configuration (from a point of view coincidental with the rest of diagrams), and qualitative representation of the orientation dependant refractive index n and density of excited state molecules ρ upon excitation with vertically polarized light. (b) Schematic diagrams of the effects on the polarization state of optical activity, phase anisotropy (fast axis vertical), and gain anisotropy.
The polarization ellipse orientation 2ψ, which in ethyl acetate remains almost vertical with smooth oscillations, now shows a different behaviour for each chiral solvent. In (S)-(−)-limonene, the polarization plane is dextrorotated by nearly 0.1 rad, whereas in (R)-(+)-limonene, it is levorotated by around 0.2 rads (2ψ graph in Fig. 2b). These deviations, both direction and relative strength, are consistent with the rotation due to optical activity expected for both (S)-(−)- and (R)-(+)-limonene, with the former exerting a stronger rotation than the latter (Table 1). The absolute strength of the rotation, though, is significantly smaller than that expected for a light wave traversing a 1 cm optical path cuvette. According to the measured OA specific rotation [α] (Table 1), that deviation should be, respectively, around 0.39 rad (dextro) and 0.31 rad (levo) for (R)-(+)- and (S)-(−)-limonene (dashed lines in 2ψ graph of Fig. 2b). It is again the orientation anisotropy of the excited state dipole moments induced by the polarized pump that are ultimately responsible for the smaller rotation. Upon excitation with vertically polarized light, most of the molecules in the excited state will be aligned vertically (z-axis, Fig. 3a). By the same token, there will not be many excited molecules in the XY plane. On the other hand, the laser amplification (or gain) is directly proportional to the population in the excited state. Combining the previous facts, one can easily see that the polarized pump induces a gain/amplification anisotropy, which results in a significantly stronger amplification of the cavity mode aligned with the z-axis. In other words, in the presence of gain anisotropy, the polarization plane “effectively” tilts towards the axis of higher amplification, as is schematically depicted in Fig. 3b. In this sense, the gain anisotropy counterbalances the optical rotation due to OA.
Regarding the variation of the polarization ellipticity (2χ or glum) with the pump energy (Fig. 2b), the three solutions show the expected smooth oscillations that result from the combination of the complex nonlinear dependence of the induced anisotropy on the pump energy, position, and time,14 and the intrinsic measurement technique uncertainty. Nevertheless, there are significant differences in the “background” levels around which the different solutions oscillate. The chiral solvents shift the background levels by ~0.08 rad, with different signs or directions for each enantiomer. Whereas PM567 in ethyl acetate shows a weak level of right-handedness (2χ ~ 0.04 rad), when dissolved in (R)-(+)-limonene it acquires a clear left-handedness (2χ ~−0.04 rad). In contrast, when PM567 is dissolved in (S)-(−)-limonene, it strengthens its right-handedness (2χ ~0.12 rad). As a reminder, a fully circularly polarized wave has |2χ| = π/2 ~ 1.57 rad. Hence, the limonene solutions present a moderate level of ellipticity, as evidenced by the DOCP values of 0.11 and 0.04 (DOCP = 1 for circular polarization). In any case, the ellipticity of the CPLE is greatly enhanced with respect to those of ethyl acetate solutions of CPL-simple organic molecules.28
The origin of this enhancement and change of sign is the consequence of a combination of OA and phase anisotropy. As explained before,14 the intense and polarized pump radiation induces a refractive index anisotropy (Fig. 3a), turning the active medium into a wave-retarder. The fast axis, i.e., that with the lowest refractive index (highest propagation velocity), is always parallel to the electric field of the pump radiation. In the case of vertical pump polarization, it will be parallel to the z-axis. The orthogonal components of a polarized wave will be phase delayed as the wave propagates through a birefringent medium. Although the phase delay is constant, the ellipticity acquired by the wave depends on the orientation of its polarization plane and the fast axis of the medium. When they are parallel (or perpendicular) the circularization is negligible, and it is maximal when they subtend an angle, ±π/4 (Fig. 3b). In addition, opposite angles render opposite handedness. The laser light generated in the ethyl acetate solution is mostly vertically polarized and parallel to the fast axis, and it is kept vertical as it propagates, since there is no OA. Hence, it will gain a weak ellipticity (2χ graph in Fig. 2b). In contrast, when PM567 is dissolved in limonene, the generated light is still mostly vertical and parallel to the fast axis, but it will deviate from it due to OA, as discussed before. Hence, the light wave will gain a higher ellipticity upon propagation (2χ graph in Fig. 2b). Since (S)-(−)- and (R)-(+)-limonene tilt the polarization plane in different directions (2ψ graph in Fig. 2b), the ellipticity acquired by the laser emission from PM567 will have different signs (2χ graph in Fig. 2b).
4. Summary and conclusions
In summary, we have demonstrated for the first time that the interaction of LIB with the OA in CPL organic systems would lead to CPLE levels stronger than the intrinsic ones, even when the system has CPL level zeroing. A thorough investigation on the chiroptical properties of achiral dyes dissolved in chiral solvents has allowed establishing the influence of OA on the polarization state of dye lasers, which can be summarized as follows: it provides a higher DOP due to the more viscous nature of the solvent; the polarization ellipse orientation 2ψ is tilted in the direction corresponding to that of the OA, but the effect is counterbalanced by the gain anisotropy that tilts the polarization plane towards the axis with higher amplification; the polarization ellipticity 2χ is increased (in opposite directions for each enantiomeric solvent) due to the combined effect of OA and pump induced birefringence, since the latter makes the polarization plane (2ψ) subtend to a wider angle with the fast axis in the gain medium. Hence, the OA/LIB interaction could lead to misleading results in CPLE studies if not taken care of. Luckily, there are means of avoiding the artefacts due to OA and LIB in our current set-up. On the one hand, one could reduce the phase and gain anisotropy by pumping the system with horizontally polarized light (in a transversal pump configuration14) or with circular or non-polarized light (in a longitudinal pump configuration40). On the other hand, one could compensate for the rotation due to OA by adding chiral additives with opposite OA handedness, thus attaining specific rotations [α] approaching zero. Unfortunately, there are cases, such as in complex media (e.g., tissues), where this compensation may not be always attained, and thus the OA contribution must be taken into account.
Supplementary Material
Acknowledgments
Financial support from MICINN (grants MAT2014-51937-C3-1-P, MAT2014-51937-C3-2-P and MAT2015-68837-REDT) and the NIH Minority Biomedical Research Support (grant 1 SC3 GM089589-08) is acknowledged. G. M. acknowledges the Henry Dreyfus Teacher-Scholar Award for financial support. The authors thank Prof. J. Bañuelos (UPV-EHU) for his help with the time-resolved and steady-state photophysical measurements, and J. Jiménez (UCM) for limonene purification.
Footnotes
Electronic supplementary information (ESI) available: Photophysical, chiroptical, and laser characterization apparatus and procedures. Polarimetry formalism. Fig. S1–S3. See DOI: 10.1039/c7cp03303f
References
- 1.Zhang YJ, Oka T, Suzuki R, Ye JT, Iwasa Y. Science. 2014;344:725. doi: 10.1126/science.1251329. [DOI] [PubMed] [Google Scholar]
- 2.Fiederling R, Keim M, Reuscher G, Ossau W, Schmidt G, Waag A, Molenkamp LW. Nature. 1999;402:787. [Google Scholar]
- 3.Yang Y, Correa da Costa R, Smilgies D-M, Campbell AJ, Fuchter MJ. Adv Mater. 2013;25:2624–2628. doi: 10.1002/adma.201204961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li TY, Jing YM, Liu X, Zhao Y, Shi L, Tang Z, Zheng YZ, Zuo JL. Sci Rep. 2015;5:14912. doi: 10.1038/srep14912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zinna F, Pasini M, Galeotti F, Botta C, Di Bari L, Giovanella U. Adv Funct Mater. 2017;27:1603719. [Google Scholar]
- 6.Kruk SS, Decker M, Staude I, Schlecht S, Greppmair M, Neshev DN, Kivshar YS. ACS Photonics. 2014;1:1218. [Google Scholar]
- 7.Fujino H, Koh S, Iba S, Fujimoto T, Kawaguchi H. Appl Phys Lett. 2009;94:131108. [Google Scholar]
- 8.Demenev AA, Kulakovskii VD, Schneider C, Brodbeck S, Kamp M, Höfling S, Lobanov SV, Weiss T, Gippius NA, Tikhodeev SG. Appl Phys Lett. 2016;109:171106. [Google Scholar]
- 9.Liang G, Zeng Y, Hu X, Yu H, Liang H, Zhang Y, Li L, Giles Davies A, Linfield EH, Jie Wang Q. ACS Photonics. 2017;4:517. [Google Scholar]
- 10.Chen F, Gindre D, Nunzi JM. Opt Express. 2008;16:16746. doi: 10.1364/oe.16.016746. [DOI] [PubMed] [Google Scholar]
- 11.Furumi S. Chem Rec. 2010;10:394. doi: 10.1002/tcr.201000013. [DOI] [PubMed] [Google Scholar]
- 12.Gardiner DJ, Morris SM, Hands PJ, Mowatt C, Rutledge R, Wilkinson TD, Coles HJ. Opt Express. 2011;19:2432. doi: 10.1364/OE.19.002432. [DOI] [PubMed] [Google Scholar]
- 13.Knitter S, Kues M, Fallnich C. Opt Lett. 2012;37:3621. doi: 10.1364/OL.37.003621. [DOI] [PubMed] [Google Scholar]
- 14.Cerdán L, García-Moreno S, Costela A, García-Moreno I, de la Moya S. Sci Rep. 2016;6:28740. doi: 10.1038/srep28740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bañuelos J. Chem Rec. 2016;16:335. doi: 10.1002/tcr.201500238. [DOI] [PubMed] [Google Scholar]
- 16.Kuehne AJC, Gather MC. Chem Rev. 2016;116:12823. doi: 10.1021/acs.chemrev.6b00172. [DOI] [PubMed] [Google Scholar]
- 17.Dang C, Lee J, Breen C, Steckel JS, Coe-Sullivan S, Nurmikko A. Nat Nanotechnol. 2012;7:335. doi: 10.1038/nnano.2012.61. [DOI] [PubMed] [Google Scholar]
- 18.Cerdán L, Braborec J, García-Moreno I, Costela A, Londesborough MGS. Nat Commun. 2015;6:5958. doi: 10.1038/ncomms6958. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee S, Thilagar P. Chem Commun. 2016;52:1070. doi: 10.1039/c5cc08213g. [DOI] [PubMed] [Google Scholar]
- 20.Muller G. Dalton Trans. 2009:9692. doi: 10.1039/b909430j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armelao L, Quici S, Barigelletti F, Accorsi G, Bottaro G, Cavazzini M, Tondello E. Coord Chem Rev. 2010;254:487. [Google Scholar]
- 22.Grivas C, Pollnau M. Laser Photonics Rev. 2012;6:419. [Google Scholar]
- 23.Sánchez-Carnerero EM, Agarrabeitia AR, Moreno F, Maroto BL, Muller G, Ortiz MJ, de la Moya S. Chem – Eur J. 2015;21:13488. doi: 10.1002/chem.201501178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu JM, Sakamoto T, Watanabe K, Furumi S, Tamoaki N, Chen Y, Nakano T. Chem Commun. 2011;47:3799. doi: 10.1039/c0cc05564f. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Li Y, Liu S, Li F, Zhu C, Li S, Cheng Y. Macromolecules. 2016;49:5444. [Google Scholar]
- 26.Kumar J, Nakashima T, Kawai T. J Phys Chem Lett. 2015;6:3445. doi: 10.1021/acs.jpclett.5b01452. [DOI] [PubMed] [Google Scholar]
- 27.Roose J, Tang BZ, Wong KS. Small. 2016;12:6495. doi: 10.1002/smll.201601455. [DOI] [PubMed] [Google Scholar]
- 28.Jiménez J, Cerdán L, Moreno F, Maroto BL, García-Moreno I, Lunkley JL, Muller G, de la Moya S. J Phys Chem C. 2017;121:5287. doi: 10.1021/acs.jpcc.7b00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldeck D, Cross AJ, McDonald DB, Fleming GR. J Chem Phys. 1981;74:3381. [Google Scholar]
- 30.Haas RA, Rotter MD. Phys Rev A: At Mol Opt Phys. 1991;43:1573. doi: 10.1103/physreva.43.1573. [DOI] [PubMed] [Google Scholar]
- 31.Chernyavsky VA, Pikulik LG, Rudik KI, Grib AF. Quantum Semiclassical Opt. 1998;10:459. [Google Scholar]
- 32.Satoh K, Matsuda M, Nagai K, Kamigaito M. J Am Chem Soc. 2011;132:10003. doi: 10.1021/ja1042353. [DOI] [PubMed] [Google Scholar]
- 33.Carslaw N. Atmos Environ. 2013;80:507. [Google Scholar]
- 34.Durán-Sampedro G, Agarrabeitia AR, Cerdán L, Pérez-Ojeda ME, Costela A, García-Moreno I, Esnal I, Bañuelos J, López Arbeloa I, Ortiz MJ. Adv Funct Mater. 2013;23:4195. [Google Scholar]
- 35.Nakano Y, Liu Y, Fujiki M. Polym Chem. 2010;1:460. [Google Scholar]
- 36.Sánchez-Carnerero EM, Moreno F, Maroto BL, Agarrabeitia AR, Ortiz MJ, Vo BG, Muller G, de la Moya S. J Am Chem Soc. 2014;136:3346. doi: 10.1021/ja412294s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Born M, Wolf E. Principles of Optics. Pergamon Press; Oxford, UK: 1975. [Google Scholar]
- 38.Weast RC, editor. CRC Handbook of Chemistry and Physics. 69. CRC Press; Boca Raton, FL: 1988. [Google Scholar]
- 39.Speight J. Lange’s Handbook of Chemistry. 10. McGraw-Hill; New York, USA: 1967. pp. 1669–1674. [Google Scholar]
- 40.Gancheryonok I, Shapochkina I, Fujimura Y. Proc SPIE 3580, Photoconversion: Science and Technologies. 1998 Nov 6;:48. doi: 10.1117/12.330456. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.