Abstract
Human herpesviruses (HHVs) and Human papillomaviruses (HPV) are common in the general population and, in immunocompetent people, are mostly carried asymptomatically. However, once an individual becomes immunocompromised by age, illness, or HIV infection these dormant viruses can manifest themselves and produce disease. In HIV-positive patients there is an increased risk of disease caused by HHVs and HPV infections and cancers caused by the oncoviruses EBV, HHV-8, and HPV. This workshop examined four questions regarding the viruses associated with oral cancers disease in the HIV-positive and -negative populations, the immune response, and biomarkers useful for accurate diagnostics of these infections and their sequalae. Each presenter identified a number of key areas where further research is required.
Keywords: oral cancer, oncoviruses, Human Herpesvirus, Human Papillomavirus, HIV infection, biomarkers, immune response
The role of viral infections associated with oral cancers and disease in the context of human immunodeficiency virus (HIV) was the focus of Workshop 3B at the Seventh World Workshop on Oral Health and Disease in AIDS (November 2014, Hyderabad, India), which identified areas in need of further research (Tappuni & Shiboski, 2016). Whilst this area has been well covered by extensive reviews and ongoing research, such as that conducted by the International Agency for Research on Cancer, and was reviewed at the Sixth World Workshop on Oral Health and Disease in AIDS (April 2009, Beijing, China) much remains unsolved (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2009, Patton et al., 2011). This workshop underscored that the defined oral cancers should include those cancers of the whole head and neck region regardless of anatomical location. Oral cancers are not only those of the oral cavity, tongue and tonsils, (i.e. those visible upon a standard oral exam), but should include cancers of the entire head and neck region, including salivary glands, sublingual lymph nodes, as well as cancers that do not occur exclusively in the oral cavity. Therefore, the detection and management of opportunistic viral infections associated with oral cancers is the responsibility of the practicing dentist, especially in the context of HIV infection.
The majority of oral viral infections manifesting as oral diseases or cancers are caused by human herpesviruses (HHVs) and human papillomavirus (HPV). These diseases include herpes labialis [HSV-1 & 2], herpes zoster or shingles [VZV; HHV-3] infections, oral ulcers and retinitis/uveítis [HCMV; HHV-5], oral hairy leukoplakia (OHL; EBV) and oral cancers caused by the oncoviruses Epstein-Barr virus [EBV; HHV-4], Kaposi’s sarcoma-associated herpesvirus [KSHV; HHV-8] and “high-risk” HPV’s (especially HPV-16, 18, 31, and 33). Oral cancers include Burkitt’s lymphoma (BL), Kaposi’s sarcoma (KS), and a subset of upper aerodigestive tract cancers respectively (Johnson, 2010) (Table 1). Whilst the prevalence of these oncoviruses varies greatly, and in some regions can exceed 50%, only a small percentage of infected individuals will develop disease possibly due to the import role of the host cofactors. Many of these oncoviruses are transmitted through oral-oral contact and are readily detected in saliva. The exception is HPV where its detection is primarily in samples reflective of tonsillar tissue (tonsillar samples or gargles) (Duray et al., 2011). In addition, HPV is also likely transmitted through oral-genital contact (Pickard et al., 2012). These oncoviruses also work directly through several mechanisms, which include induction of cell proliferation, genomic instability, cell migration, and the inhibition of apoptosis (Table 2). On the other hand, hepatitis B virus (HBV) and hepatitis C virus (HCV) work indirectly through chronic inflammation.
Table 1.
Cancers caused by oncoviruses with sufficient and limited evidence according to the IARC criteria.
| Virus | Cancers with sufficient evidence | Cancers with limited evidence |
|---|---|---|
| EBV | Nasopharyngeal carcinoma Burkitt’s Lymphoma Immunesuppression-related non- Hodgkin lymphoma, Extranodal NK/T cell lymphoma (nasal type), Hodgkin’s lymphoma |
Gastric carcinoma Lympho-epithelioma-like carcinoma Plasmablastic lymphoma Diffuse large B-cell/immunoblastic lymphoma (DLBCL) |
| HHV-8 | Kaposi’s sarcoma Primary Effusion Lymphoma (classic and solid variants) Plasmablastic lymphomas |
Extracavitary KSHV positive solid lymphoma Early PEL Germinotrophic lymphoproliferation |
| HPV-16 | Cancers of the cervix, vulva, vagina, penis, anus, oral cavity, oropharynx, and tonsil | Cancer of the larynx |
| HPV-18, 31, 33,35, 39, 45, 51, 52, 56, 58, and 59 | Cervical cancer |
Table 2.
Established carcinogenic mechanisms of oncogenic viruses.
| Mechanism | Oncovirus | Carcinogenic Properties |
|---|---|---|
|
| ||
| Direct | EBV | Cell proliferation, Inhibition of apoptosis, Genomic instability, Cell migration |
| HHV-8 | Cell proliferation, Inhibition of apoptosis, Genomic instability, Cell migration | |
| HPV | Immortalization, Genomic instability, Inhibition of DNA damage response, Anti-apoptotic activity | |
| HTLV-1 | Immortalization and transformation of T cells | |
| HIV | Induce B-cell activation | |
|
| ||
| Indirect through chronic inflammation | HBV | Inflammation, Liver cirrhosis, Chronic hepatitis |
| HCV | Inflammation, Liver cirrhosis, Liver fibrosis | |
|
| ||
| Indirect through immunosuppression | HIV-1 | Immunosuppression |
Whilst some consider HIV to be an “oncovirus”, its role is mainly indirect through increasing immunosuppression. HIV is also associated with immune activation, which can result in chronic inflammation and subsequent carcinogenic effects. Subsequently, this may result in an altered oral microbiome and loss of local immune surveillance and/or chronic inflammation. HIV may also directly induce B-cell activation by up-regulating activation-induced cytidine deaminase (AID) (He et al., 2006). Complicating the association with HIV are other co-factors associated with head and neck cancers (HNC). These include smoking (both cigarettes and smokeless tobacco), chewing betel quid, the use of alcohol and illicit drugs, as well as viral co-infections (Gupta & Johnson, 2014). Through increased immunosuppression HIV increases the risk of KS, non-Hodgkin’s lymphomas, Hodgkin’s lymphoma, anal and cervical cancer, oral cavity/pharyngeal cancer and liver cancer (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2009).
In the HIV-positive individual, oral diseases may result from an increased risk of acquisition of virus or the emergence of disease due to reactivation from a pre-existing latent infection. Oral disease progression can be defined as a transition from asymptomatic to symptomatic chronic infection or a symptomatic disease that worsens over an accelerated time course (e.g. HPV-associated dysplasia to invasive carcinoma). Understanding these distinctions remains a challenge as the natural history of these viral infections in the oral cavity in general is not well understood.
Questions
Questions addressed by the workshop include the following:
Question 1: What are the viral associations with cancer and the co-infections EBV, HPV and HHV-8 in the head and neck?
Question 2: Are HPV-associated oral lesions including oral-pharyngeal cancers higher among HIV-infected men, women, and children?
Question 3: What alterations in immune responses to viral co-infections including EBV HPV and HHV8 are HIV specific?
Question 4: Are there biomarkers for oral clinical disease associated with viral co-infections in HIV infected persons?
Question 1: What are the viral associations with cancer and the co-infections EBV, HPV and HHV8 in the head and neck?
Presenter: Professor Marc Goodman.
Cancers of the head and neck arise from the lining of the oral cavity, oropharynx, hypopharynx, larynx, sinonasal tract and nasopharynx. Globally the incidence and mortality of HNC is increasing. Based on the GLOBOCAN estimates for 2012 there were 686,328 new cases with 375,665 deaths worldwide (Ferlay et al., 2012). Of these, 300,373 are from the oral cavity, 156,877 from the larynx, 142,387 from other pharynx, and 86,691 from the nasopharynx (Figure 1). Head and neck malignancies represent 6.9% of cancers in men and 2.6% of cancers in women.
Figure 1.
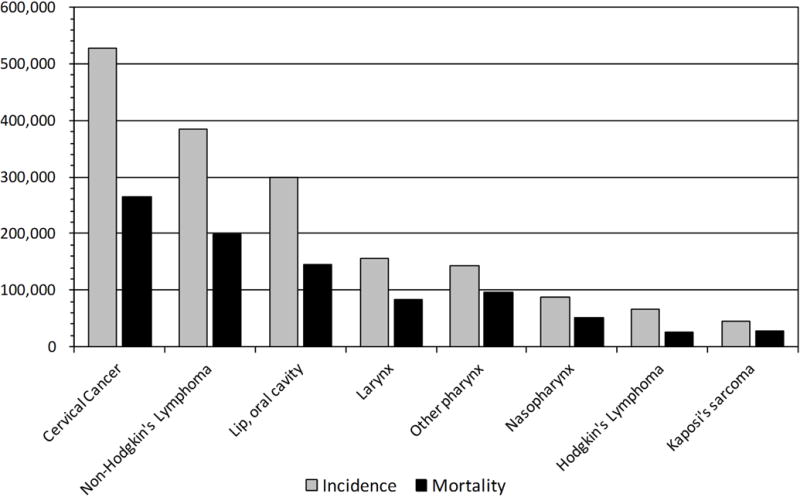
Incidence and mortality of cancers caused EBV, HHV-8, and HPV in both genders worldwide (Globocan 2012).
Kaposi sarcoma-associated herpesvirus (HHV-8)
HHV-8 is the aetiological agent of KS, multicentric Castleman’s disease and primary effusion lymphoma. There are four clinical-epidemiological variants of KS: classic, endemic (African), iatrogenic (transplant-associated) and HIV/AIDS-associated (epidemic). These variants can be distinguished by the severity and presentation of clinical symptoms, manifestations that vary by (i) the extent of anatomical involvement, (ii) the aggressiveness of lesion formation and progression, (iii) patient risk factors (i.e. ethno-geographic origin, age of onset, and gender), and (iv) the association with patient morbidity and mortality. However, all epidemiological forms of KS are histopathologically identical (Ablashi et al., 2002). KS is AIDS defining and the most frequent AIDS-associated neoplasm worldwide due to the underlying HHV-8 prevalence, limited access to antiretroviral therapy (ART) and the health delivery infrastructure in many developing nations (Dittmer & Damania, 2013).
Globally, the prevalence of KS usually mirrors the seroprevalence of HHV-8 and HIV, except for in the Amazon Amerindians populations of South America and in India. In the HIV-negative Amazon Amerindians populations of Brazil, Ecuador and French Guiana HHV-8 is endemic (i.e. 75.4% seroprevalence) but clinical KS is rare (Borges et al., 2012). In 2012 the Government of India estimated that 2.40 million Indians are living with HIV with an adult prevalence of 0.31% (Joint United Nations Programme on HIV/AIDS (UNAIDS), 2014). However, KS has only been documented a total of 7 times in the literature (Vaishnani et al., 2010, Dongre & Montaldo, 2009, Kharkar et al., 2009, Kura et al., 2008, Soufiane et al., 2010) with the first recorded case from YRG CARE (Kumarasamy et al., 1996). In 1999, Ablashi et al. found a 4% HHV-8 seropositivity rate in blood collected from 108 healthy individuals at blood bank facilities in Bombay, Chennai, and New Delhi (Ablashi et al., 1999). These results are comparable to those found in blood donors in the USA (5.2%; 7/135) as reported in the same study. Further studies have shown that the seroprevalence of HHV-8 in the HIV-positive ART-naive population in India ranges between 10.64% (Speicher et al., 2014a) and 26% (Munawwar et al., 2014). These results suggest that HHV-8 is present in India but it may not express itself as KS.
In the USA, one third of KS cases develop in patients (mostly older men) on suppressive ART (Krown et al., 2008). While cutaneous KS is more common, oral KS is present in up to 60% of KS cases with up to 45% of cases involving both oral and cutaneous lesions. Oral KS is associated with a higher death rate with mortality occurring 24 months after diagnosis, compared to 72 months as seen in cutaneous lesions (Jindal et al., 1995). Molecularly and histologically indistinguishable iatrogenic KS can also develop in solid organ allograph recipients irrespective of HIV status. HIV is not likely to contribute directly to the pathology of the disease above inducing immunosuppression and thus increasing the rate of disease progression.
Treatment regimens of HIV/AIDS play a huge role in KS pathology. The use of ART causes remission of many, but not all KS lesions. Following the release of ART, the incidence of KS was reduced in the USA from 333 cases/million in 1987 to 28 cases/million by 1998 (Ziegler et al., 1997). Since 2000 no further decline in KS was observed and the number of new cases plateaued. ART was so effective that 90% of AIDS-KS cases displayed complete remission of cutaneous lesions within six months as well as an 81% reduction in mortality (Ziegler et al., 1997). However, in a small subset of patients, the initiation of ART can cause an intense rebound of inflammatory responses including those to non-HIV viral infections, a situation now widely recognised as immune reconstitution inflammatory syndrome (IRIS). IRIS occurs in two forms. If an opportunistic infection worsens despite successful treatment of HIV this is called paradoxical IRIS, whereas the emergence of a previously absent infection is called unmasking IRIS (Tappuni, 2011). When KS is associated with IRIS, this is called KS-IRIS. KS-IRIS can occur in up to 11% of newly diagnosed HIV-positive patients initiating ART (Speicher et al., 2013, Achenbach et al., 2012). The appearance of KS-IRIS should be treated by maintaining ART and treating the KS with liposomal doxorubicin.
In summary, HHV-8 is associated with all epidemiological variants of KS, multicentric Castleman’s disease and primary effusion lymphomas. AIDS-KS commonly affects the oral cavity with the oral mucosa being the initial site of clinical disease in ~22% of patients (Mesri et al., 2010). Whilst ART has successfully decreased the prevalence and incidence of AIDS-KS worldwide, in parts of Sub-Saharan Africa clinical KS and HHV-8 infection remain endemic (Dittmer & Damania, 2013, Butt et al., 2008). The use of ART may also cause KS to either regress or flare up as either unmasking or paradoxical KS-IRIS (Speicher et al., 2013, Achenbach et al., 2012).
Epstein Barr Virus (EBV)
EBV can manifest in the oral cavity and/or head and neck region as BL, mononucleosis and OHL with disease severity and prevalence increased in individuals co-infected with HIV. Mononucleosis is common irrespective of HIV status and is associated with a primary EBV infection during adolescents and young adulthood. However, age of primary infection varies greatly worldwide. OHL is a manifestation EBV reactivation detected in association with immunosuppression. OHL is often asymptomatic, and is the only pathologic manifestation of permissive EBV infection. OHL is not confined to HIV infected people, but was detected in solid organ transplants and bone marrow transplant recipients (Itin et al., 1988, Epstein et al., 1991, King et al., 1994). OHL serves as a clinical biomarker for HIV infection and progression to AIDS. Even in the HAART era, the prevalence of OHL is 12% of the HIV population (Shiboski et al., 2015). This is of particular importance because OHL may serve as an oral indicator, not only of new HIV infections, but of ineffective ART. OHL demonstrates an interesting pattern of gene expression with a combination of lytic and transforming genes (Webster-Cyriaque et al., 2000). While transforming genes are expressed in OHL, the lesion has not been associated with malignancy in the context of HIV. Mutalima et al. (2008) found that HIV status increased the risk of BL by 12-fold (Mutalima et al., 2008). Among HIV-negative individuals, high levels of antibodies for EBV and malaria increased the risk of BL by 12 and 2.5 fold respectively. These EBV-positive Hodgkin’s and non-Hodgkin’s lymphomas may manifest in the head and neck. Nasopharyngeal cancers (NPC) are also head and neck cancers associated with an EBV infection. NPC, however, is not typically detected as an EBV-associated AIDS defining cancer. Hazard ratios for developing NPC associated with anti–EBV viral capsid antigen immunoglobulin A: low antibody levels have elevated odds ratio (OR) of 9.5 (range: 2.2 to 40.1) and high antibody levels have an OR of 21.4 (range: 2.8 to 161.7) (Hsu et al., 2009). Transmission of HIV during pregnancy or birth adds complexity to the aetiology of EBV-associated cancers and virus-associated oral transmission. In many developing countries HIV is acquired prior to the first infection with a human oncovirus and increases the risk of disease. Whether HIV has direct biochemical effects on EBV-associated disease beyond modulation of transmission and “seeding” of the latent reservoir of EBV remains a subject of debate.
Human Papillomavirus (HPV)
In many countries the incidence of HNC is increasing mostly due to the increase of HPV-associated oropharyngeal cancers (e.g. tonsils, base of tongue and other parts of the oropharynx) (Marur et al., 2010). HPV-associated HNC also tend to respond better to standard therapy than HPV-negative HNC. HIV infection can increase incidence and alter pathology by three mechanisms. Firstly, HPV acquisition is increased by high-risk sexual behaviour in those populations considered at higher risk of HIV acquisition. Secondly, HPV persistence is increased in HIV-positive individuals due to immune dysfunction; even in individuals on long-term, successful ART. Lastly, the direct biochemical effects of HIV proteins on the oral epithelial cell physiology may enhance, sustain or modulate HPV infection in the oral cavity (Tugizov et al., 2013).
The prevalence of HPV DNA detection orally can be up to 31% in the HIV-positive population compared to 6% in the general population (Beachler et al., 2014, Antonsson et al., 2014, Parisi et al., 2011). Several prevalence studies have shown that the number of oral sex partners is directly proportional to the risk of HPV infection irrespective of HIV status. In the HIV-positive population Kreimer et al., reported that the odds of having an oral HPV infection increased greatly with more than two recent HIV-seropositive oral sex partners (OR: 12.8; 95% CI; 3.1–52.7) compared to having one or fewer (OR: 1.0) (Kreimer et al., 2004). In the HIV-negative population, D’Souza et al. reported a similar pattern in that the risk of oral HIV infection increased from one or fewer (OR: 1.0) to eleven or more (OR: 5.20; 95% CI; 1.13–24.7) lifetime number of oral sex partners (D’Souza et al., 2009). This correlation was confirmed by Pickard et al. who reported that five or more lifetime open-mouth kissing (OR, 4.0; 95% CI: 1.1–14.8) or lifetime oral sex (OR, 4.0; 95% CI: 1.3–11.9) partners were associated with infection (Pickard et al., 2012). Prospective studies have been inconsistent in determining an association between oral sex behaviours and oral acquisition of HPV (Kreimer et al., 2013b, Edelstein et al., 2012). Further studies are needed to determine (1) the mechanistic interactions between oncoviruses and HIV, (2) the cost-effectiveness of current HPV vaccines towards HNC in population–based studies, and (3) the effect of smoking and drinking on the risk of HPV-associated HNC.
Question 2: Are HPV-associated oral lesions including oral-pharyngeal cancers higher among HIV-positive men, women, and children?
Presenter: Dr. Velia A. Ramírez-Amador.
HPV-associated oral lesions
HPV-associated oral lesions have been called HPV-associated oral warts (HPV-OW) in different studies (King et al., 2002, Greenspan et al., 2001, Patton et al., 2000). HPV-OW is a nonspecific generic term that refers to a group of benign lesions that includes: squamous cell papilloma (SCP), verruca vulgaris (VV), condyloma acuminatum (CA), and focal epithelial hyperplasia (FEH) (Syrjanen, 2003). In 2001, an increase in oral warts was described in adult HIV-infected individuals from 5% in individuals without ART, to 15% in patients on ART other than highly active antiretroviral therapy (HAART), and to 23% in those on HAART (Greenspan et al., 2001). Similarly, other studies have mentioned an augmented incidence of these lesions in HIV-infected population (King et al., 2002, Patton et al., 2000).
Currently, in the context of HIV, information related to oral HPV-OW frequency and behaviour is scarce. Studies published during 2008–2012 reported a prevalence of oral HPV-OW from 0.5% (8/1,595) to 6.9% (55/787) in HIV-positive patients from Brazil (Ortega et al., 2009) and Mexico (Anaya-Saavedra et al., 2013) respectively. In the HIV-negative population most data on HPV-OW comes from case reports (Nagaraj, 2013, Liu et al., 2012, Falaki et al., 2009). However, three large population-based studies reported a prevalence of ≤0.3% in Swedish adults (Robledo-Sierra et al., 2013, Salonen et al., 1990, Axell, 1976), which is similar to a 0.37% (89/23,789) prevalence reported in the Mexican HIV-negative adult population (Castellanos & Diaz-Guzman, 2008). Due to immune dysfunction the risk of developing HPV-OW is higher in the HIV-positive population compared to the general population (Table 3). In HIV-positive adults the prevalence of oral HPV infection ranges from 16% (Videla et al., 2013) to 40% (Beachler et al., 2012), which is higher than in the general population. Population studies have also shown that the prevalence of HPV-OW in HIV-positive children (1.6%) is also slightly higher than in HIV-negative children (0.51%) (Nabbanja et al., 2013).
Table 3.
HPV-related oral lesions in adult patients
| Authors & year | N | Gender (%) |
Age (years-old) |
HAART (%) |
HPV-OL | |
|---|---|---|---|---|---|---|
| Prevalence % | Type | |||||
| HIV/AIDS adult patients | ||||||
| (Estrella, 2015) | 29 | Male (93.1) | 32.5–44 | (89.6) | 3.4 | SCP, MEH, VV, CA |
| (Anaya-Saavedra et al., 2013) | 787 | Male (93.4) | 27–40 | (30.9) | 6.9 | SCP, MEH, VV |
| (Lourenco et al., 2011) | 388 | Male (61.6) | Mean: 38 | (79.9) | 0.6 | MEH, CA |
| (Ortega et al., 2009) | 1595 | ND | ND | (57.9) | 0.5 | CA |
| (Giuliani et al., 2008) | 130 | Male (54.6) | Mean: 39.6 | (79.2) | 4.6 | HPV-OL |
| (Kakabadze et al., 2008) | 732 | Male (82.2) | ND | ND | 5.0 | Oral warts |
| (Nunes Mde et al., 2008) | 129 | Male (100) | 31–50 | (77.8) | 2.3 | 2 CA, 1 VV |
| NON HIV/AIDS adult patients | ||||||
| (Robledo-Sierra et al., 2013) | 6,448 | Male & female | Adults | ND | <0.1 | SCP |
| (Castellanos & Diaz-Guzman, 2008) | 23,785 | Male (31.2) | 15–97 | ND | 0.29 | SCP |
SCP = squamous cell papilloma; MEH = multifocal epithelial hyperplasia; VV = verruca vulgaris; CA = condyloma accuminatum; ND = no data; HPV-OL = HPV-associated oral lesions.
For the purpose of this review, studies on the frequency of FEH in children have not been considered because FEH may be present only in specific ethnic groups and geographical regions, particularly in childhood, independently of the HIV status (Bennett & Hinshaw, 2009, Said et al., 2013, Akoglu et al., 2015).
While the majority of studies have emphasized the benign nature of HPV-OL in HIV-positive patients, others have shown a range of high-risk HPV types (HR-HPV) in these lesions. Some studies that have shown HR-HPV mainly HPV-16, 18 and 31, have been also identified in these lesions (Estrella, 2015, Anaya-Saavedra et al., 2013, Ma et al., 2004). The prevalence of HR-HPV found in HPV-OL displays the potential for a malignant change in these lesions, particularly considering the increased risk of HPV-associated malignancy among HIV patients (Beachler et al., 2014). Consequently, longitudinal studies based on viral transcriptional activity are warranted.
Oral-pharyngeal Cancers
Based on standardized incidence ratios (SIRs) the incidence of oral-pharyngeal cancer in HIV-infected individuals is higher in HIV-positive individuals than that of the age-and gender matched general population. One study in Italy showed an increased risk of tonsil cancer (SIR = 10.9, 95% CI: 1.2–39.4) in HIV-infected men (Franzetti et al., 2013). Another study from Puerto Rico found an increased risk of oral-pharyngeal cancer in HIV-infected women (SIR = 10.9, 95% CI: 1.26–37.6) (Ortiz et al., 2014). Other studies have reported significant albeit lower risks than these two studies –ranging from an SIR of 1.6 (95% CI: 1.2–2.1) in the United States (Chaturvedi et al., 2009) to 1.9 (95% CI: 1.8–2.1) in Northern Italy (Calabresi et al., 2013). Engsis et al reported an incidence rate ratio (IRR) of 5.1 (95% CI: 2.28–11.44) in a Danish HIV-cohort study (Engsig et al., 2011). In the USA a prospective study determined that the standardized incidence of HPV-related and -unrelated head and neck squamous cell cancers (HNSCC) were both 3-fold higher in HIV-positive individuals than the general population (Beachler et al., 2014).
Data must be taken with caution as most studies either lack information or could not be adjusted for the effect of confounders such as smoking, alcohol use and viral co-infections. A large non-registry study has shown that the risk factors for developing HNSCC are similar between the HIV-positive and –negative populations for both HPV-related and tobacco/alcohol-related HNSCC (D’Souza et al., 2014). In order to elucidate the incidence and risk of HPV-associated oral lesions in the HIV-positive population further studies are needed (i) to develop detailed case definitions, which would constitute a starting point for clinical studies to arrive at true population-specific incidence rates, (ii) to determine if the increased incidence in these benign oral lesions is associated with greater risk or faster progression to oral cancer, (iii) to identify the HPV types involved in oral lesions and determine the role and importance of high-risk and atypical types, and (iv) to develop collaborative and multicentric studies to perform randomized controlled trials for HPV-OL treatment.
Question 3: What alterations in immune responses to viral co-infections including EBV, HPV, and HHV8 are HIV specific?
Presenter: Associate Professor Jennifer Webster-Cyriaque.
It is not well understood what different immune modulating events contribute to an individual’s risk of developing oral lesions, how they change the pathophysiology of oral lesions, their progression to cancer, response to intervention or how immune modulation affects transmission via the oral cavity. Whilst it is know that acute HIV infection or end stage AIDS contribute to transmission rates, disease incidence, and outcome, currently there is insufficient evidence to support the notion that, on a molecular and pathophysiological level, oral cancer, HNC, or any cancer differs from the same cancer that develops in an HIV-negative person.
Modulation of the immune response by HIV and its associated therapies occurs at many different levels. HIV itself disarms lymphoid and macrophage subsets and their effectors, which are critical to control other viral infections. Furthermore, innate immune responses to viral co-infections are compromised by both HIV infection and ART. Type I interferon (IFN) is critical to control viral infections. A viral infection results in the downstream activation of type 1 IFN with rapid induction of IFN stimulated genes (ISGs) that encode proteins with direct antiviral and immunomodulatory activities (Acchioni et al., 2015, Gibbert et al., 2013). However, persistent HIV-1 infection disables this response such that IFN, although produced, cannot block infection facilitating chronic IFN-mediated immune activation and inflammation (Zheng et al., 2014). IFN responses against opportunists are then compromised and the chronic immune activation characteristic of HIV infection may facilitate oncoviruses like EBV, KSHV, and HPV. HIV-associated therapies may further alter immune responses that affect viral co-infections as seen in HIV-positive individuals treated successfully with ART who succumb to IRIS. IRIS is characterized by heightened HIV specific B and T cell responses and enhanced chronic immune activation (Tappuni, 2011). It is thought that chronic immune activation facilitates these opportunistic infections. IRIS typically occurs in the first few months of ART and is accompanied by worsening of clinical status in spite of improved CD4+ T cell counts. The accompanying proinflammatory response may result in the pathologic manifestations of these opportunistic infections. These IRIS associated oral manifestations have been described in the context of DNA oncoviral infections. KS-IRIS is well described in both the mouth and on the skin. Oral KS-IRIS has been described in a series of case reports (Speicher et al., 2013, Papagatsia et al., 2009, Feller et al., 2008, Feller & Lemmer, 2008, Englert et al., 2014). EBV-associated IRIS has been described both in the context of permissive infection and of EBV-associated malignancy. There has been one report reviewing oral manifestations in the context of IRIS that determined three of eight patients with IRIS-associated oral disease had hairy leukoplakia (Ramirez-Amador et al., 2009). Hodgkin’s Lymphoma, is associated with EBV infection and its risk was higher in the first 12 months (IRR=2.02, 95%CI=1.32–3.10) after cART initiation (Kowalkowski et al., 2013). In a recent study of HIV subjects undergoing IRIS, the most common oral manifestation was parotid enlargement (57.14%) (P = 0.019) (Ortega et al., 2008). This parotid enlargement has recently been associated with permissive human polyomavirus BK (BKPyV) infections; an oncovirus that is a well described opportunist in the kidney transplant setting (Jeffers et al., 2009, Burger-Calderon et al., 2014). The existence of HPV-associated IRIS is not clear. Cervical (Rositch et al., 2013, Shrestha et al., 2010) and skin HPV infections (Lowe et al., 2012) were not affected by IRIS. The increase in oral warts subsequent to the inception of ART suggested a role for IRIS (Greenspan et al., 2001, King et al., 2002). The OHARA trial A5272 asked this question and determined that while warts were not associated with rising CD4+ counts, oral HPV shedding did increase post-ART (Shiboski, personal communication). Hence it appeared that while there may not be an HPV pathologic manifestation, associated with IRIS in the skin, cervix or oral cavity – HPV shedding may be associated with immune reconstitution.
Few studies discuss the relationship between oral innate immunity and HPV, EBV or KSHV infection. However, human natural killer (NK) cells have been suggested to restrict viral infections. Recent studies show that early differentiated human NK cells limit lytic EBV replication (Munz, 2014). In the context of HIV, regardless of ART therapy, there are functional defects and numeric shifts in NK cell subsets that lead to decreased frequencies IFN-γ-producing NK (Dillon et al., 2014). Furthermore, AIDS patients show lowered ratios between fold increase in ligand expression on CD4+ T cells upon in vitro stimulation and respective NK cell receptor expression (Bisio et al., 2013). Cellular pathogen sensing is also critical to innate control of opportunistic viral co-infections. Abnormal expression and function of cytosolic IFI16 DNA sensors was detected in HIV-positive patients. IFI16 expression was correlated with CD38 a marker of immune activation as well as with a high viral load and low CD4 (Nissen et al., 2014).
Both innate and humoral responses are compromised in the setting of HIV. Cellular editing enzymes like AID/APOBEC are important to the innate immune responses to viral infection. Cytidine induced deaminases have recently been shown to be important to control of KSHV, EBV and HPV (Vieira & Soares, 2013, Bekerman et al., 2013). Cytidine induced deaminases hypermutate and deaminate viral genomes resulting in compromised infection by upregulation of NK ligands and formation of misfolded/truncated viral proteins that initiate CTL responses. However, HIV targets AID/APOBEC for proteosomal degradation further compromising responses against opportunistic viral co-infections. HIV may also diminish viral co-infection specific humoral responses. Antibody function depends on somatic hypermutation of variable regions of immunoglobulin heavy chain genes. AID is specifically induced in germinal center B cells to perform somatic hypermutation and class-switch recombination. The antigen-binding hypervariable regions and hypervariable complementarity determining regions of IgG class-switched and antigen-binding VH3 CDR genes, from B cells of HIV-1-infected patients demonstrated decreased mutation frequencies (Bowers et al., 2014). Lower levels of somatic hypermutation in IgG class-switched B cells from HIV-1-infected patients may contribute to the increased risk of viral opportunistic infections. In order to elucidate the immune response to viral co-infections further studies are needed (i) to determine the oral innate immune responses in both the oral mucosa and in saliva, and their relationship to HPV, KSHV and EBV, (ii) to determine oral IgA associated humoral responses to oral viral co-infection, and (iii) to clearly define oral IRIS.
Question 4: Are there biomarkers for oral clinical disease associated with viral co-infections in HIV infected persons?
Presenter: Dr David J. Speicher.
Viral biomarkers are measurable substances indicative of a viral infection and usually predictive of disease such as cancer. Biomarkers can identify active disease, effective immunity or increased risk of disease. Active disease is often identified by histological or molecular diagnostic biomarkers, whereas effective immunity has been measured traditionally by serological biomarkers. Increased risk of disease is associated with a variety of markers usually associated with pathogenesis. Useful biomarkers must have clinical relevance and be cost-effective.
Both histopathology, employing both H&E and IHC stating, and molecular assays are useful for identifying HHVs and HPV from lesional tissue and assigning causality. Whilst, in most HHV infections, viraemia will precede disease and viral shedding can be detected, this is not always true. Asymptomatic shedding is common in individuals with HSV-1 or -2 making it essential to detect the virus in anogenital and oral swabs (Mertz, 2008). It is also unwise to diagnose oral lesions solely upon clinical appearance and tissue morphology revealed by H&E staining. IHC stains are available for all HHVs and HPV (Table 4). Whilst IHC, in-situ hybridization and molecular assays are available for HPV, studies have shown that p16 immunohistochemistry is the best test for the identification of high-risk HPV associated high grade lesions (Lewis et al., 2010, Wittekindt et al., 2005).
Table 4.
Histological antibodies and molecular targets and bodily fluids useful for the diagnosis of viral co-infections
| Histological Assays | Molecular Assays | ||
|---|---|---|---|
| Virus | Immunohistochemical Markers | Target | Specimen |
| HSV-1 | anti-HSV-1/2 pAb (CP108) | US5 Region | WMF, Plasma |
| HSV-2 | anti-HSV-1/2 pAb (CP108) | Glycoprotein D (US6) | Plasma |
| VZV | anti-VZV gE mAb (ORF68, C90.2.8) | MBP (ORF29) / gpI (ORF67) | PBMC, Plasma |
| EBV | EBV-EBER and EBV-LMP | EBNA-1 / BALF5 | WMF, PBMC |
| HCMV | anti-HCMV clone DDG9 + CCH2 mAb | UL83 gene / MIE region | WMF, PBMC, Plasma |
| HHV-8 | NCL-HHV8-LNA (13B10) | ORF26 & ORF73 | WMF, PBMC, Plasma |
| HPV | P16 IHC (p16INK4A); HPV16 IHC | Latent Protein 1 | WMF, PBMC* |
PBMC = peripheral blood mononuclear cells
HPV is detectable in PBMC’s but is controversial.
In developed countries, these biomarkers are common practice. On the other hand, in many resource-constrained settings lesions are often diagnosed solely by clinical appearance and H&E staining thus increasing the risk of misdiagnosis other reactive and neoplastic vascular proliferations. Two examples where a misdiagnosis is possible are Sub-Saharan Africa where KS is endemic, and India where clinical KS is very rare. In one study, Speicher et al found that in Nairobi, Kenya (where KS is endemic), whilst all cutaneous KS were correctly diagnosed, 12/28 oral KS lesions were misdiagnosed and were re-diagnosed as either pyogenic granulomata (n=6), deep mycosis (n=1), inflamed mucosa (n=2) or “uncertain but not KS” (n=3) (Speicher et al., 2015b). Therefore, even where molecular assays are not available it is essential that HHV-8 immunohistochemistry be used for the correct diagnosis of oral KS.
In India, the presence of KS has only been documented a total of 7 times (Vaishnani et al., 2010, Dongre & Montaldo, 2009, Kharkar et al., 2009, Kura et al., 2008, Soufiane et al., 2010, Kumarasamy et al., 1996). However, caution must be given as all but one of the reported cases of KS were diagnosed solely on the H&E staining. Thus the presence/absence of HHV-8 in India cannot be confirmed nor denied and work is needed to determine the true status of HHV-8 in India.
Molecular biomarkers for the presence of HHVs typically focus on the identification of states of viraemia and/or confirm histological findings. As HHVs establish undetectable latent infections it is the presence of viraemia that appears immediately prior to clinical disease thus molecular assays (i.e. PCR assays) are useful for monitoring disease progression and treatment efficacy (Speicher et al., 2014b). Viraemia can be determined from either plasma or peripheral blood mononuclear cells depending on whether the virus circulates as cell-free intact virion or cell-associated virus (Table 4). All diagnostic molecular assays should use two genomic targets to rule out false positives and be designed to conserved regions to rule out false negatives (Whiley et al., 2008). This is especially true when detecting HCMV due to two glycoprotein B splice variants (Nye et al., 2005).
The molecular detection of HPV has been primarily from cell scrapings collected from mucosal surfaces with DNA detected predominately by L1 general or consensus (i.e. GP5+/6+ or MY09/MY11) primers and “high-risk” and “low-risk” types determined by sequencing the amplicon (de Roda Husman et al., 1995). Today, molecular testing for HPV DNA or RNA in conjunction with cytology are commonly used in cervical cancer screening starting at age 30 years Recently the FDA approved the HPV DNA test, Roche Cobas, for primary screening starting at 25 years of age. HPV genome testing is also used in a variety of algorithms for management of abnormal cytology. HPV infections do not normally produce viraemia. However, whilst still controversial with uncertain clinical relevance, HPV can be detected in blood where the virus is attached to the outside of blood cells and can also be isolated from B-cells, dendritic cells, NK cells and neutrophils (Chen et al., 2009). As mentioned earlier, p16 staining is now used routinely to define high grade disease since CIN 2 is often over or under called. P16 INK4 positive staining is considered the hallmark of a significant high grade lesion. More recently the combination of p16 INK4 and Ki67 is being used with excellent sensitivities and specificities—both are commercially available (Wentzensen et al., 2012, Ikenberg et al., 2013). P16 and HPV DNA are not used routinely in cancer diagnoses. However, it is often used to define HPV type causality using laser capture microdissection. Currently oral HPV DNA testing is not used for the detection of oral cancers clinically but is now of great interest in associating causality.
Saliva is a diagnostic fluid with great potential: it is abundant, collection is easy and non-invasive, and represent a much lower risk of exposure of the health care worker to blood-borne infectious agents. Saliva is ideal for the detection of HHV-8, especially for screening patients prior to disease development. Not only is HHV-8 transmitted via saliva, but the epithelium of the oropharynx is the site of primary infection with the tonsils and adenoids being a reservoir of infection (Mbulaiteye et al., 2004, Taylor et al., 2004, Chagas et al., 2006). However, in saliva biomarkers are present in lower concentration than in blood, and the presence of various nucleolytic enzymes, such as endonucleases, ribonucleases, and bacterial proteases, are detrimental (Park et al., 2006, Bardon & Shugar, 1980). Traditionally, unstimulated WMF has been collected by dribbling into a chilled collection cup (Al-Otaibi et al., 2009), or by collecting throat-gargles (TG) with either phosphate buffered saline (Webster-Cyriaque et al., 2006) or commercial mouthwashes (Marshall et al., 2007). While these methods work well, samples must immediately be stored at −80°C to slow enzymatic activity, in resource-constrained settings this is usually not possible. It has also been shown that after storage for 14 months at −80°C DNA extracted from saliva is highly degraded (Speicher et al., 2015a). The DNA Genotek OMNIgene™·DISCOVER OM-501 and OM-505 kits have been designed for the collection and stabilization of microbial DNA and RNA from saliva stored at room temperature. Whilst DNA Genotek claim RNA is stable for up to 3 months, DNA is stable much longer. After 14 months of storage at room temperature extracted DNA showed little or no degradation (Speicher et al., 2015a). The OMNIgene™·DISCOVER kits are also useful for the accurate quantitation of HHV-8 in saliva and should be useful for any virus shed orally (Speicher & Johnson, 2014).
Biomarkers of effective immunity have traditionally been detected via the serological measurement of antibodies (i.e. IgM for current infections and IgG for previous exposure via infection or vaccination). Whilst serological assays are usually relevant for epidemiological studies, their clinical interpretation is more complex. One problem with serology is that asymptomatic patients will exhibit seropositivity due to a past infection, but this reactive result doesn’t correlated well with disease. This is seen globally as the IgG seroprevalence of EBV, HCMV and VZV in adults is between 80–100%, thus making any clinical interpretation impossible. Regarding HHV-8, seroconversion precedes and often predicts disease and ranges from 3–6% to 20–40% in the general population in the USA and Sub-Saharan Africa respectively (Ablashi et al., 1999, Dollard et al., 2010). In the HIV-positive community the HHV-8 seroprevalence is much higher. Whilst there are no standardized HHV-8 serological algorithm both the CDC and NIH algorithms work (Dollard et al., 2010, Mbisa et al., 2010).
HPV serological tests are available, and cervical cancer patients often have serum antibodies against the HPV genotype found in the tumour. Those who have been vaccinated will be seropositive for those vaccine types. Therefore, most research assays examine antibodies to HPV L1, E2 E6 or E7, but prevalence ranges widely to each of these antigens in different populations. The majority of individuals with cervical HPV infections other than HPV-16 do not seroconvert (Marais et al., 2008, Giuliano et al., 2015). Approximately 75% of women with HPV-16 will seroconvert (positive anti-HPV-16 L1) within 12 months – other HPV types have much lower seroconversion rates ranging from 10–35% (Giuliano et al., 2015). Interestingly seroconversion is more common in women than in men suggesting that men have superficial infections resulting in poor immune responses. This may be one explanation as to why HPV-associated HNC are much higher in men than women. Recent data show that anti-HPV-16 E6 is highly predictive of HPV-associated oral cancers (Kreimer et al., 2013a). There is a great need for better, properly validated, biomarkers indicating the risk of pre-cancer and HPV-HNC, which are not affected by seropositivity due to the vaccination.
Biomarkers that show increased risk of disease include immunosuppression, poor health, other opportunistic infections and low humoral immunity. A patient’s HIV status and/or age is superfluous for the presence/absence of viral biomarkers for active disease and effective immunity as these are virus specific, but the presence of HIV does suggest immunosuppression and thus an increased risk of viral co-infections. Poor health due to an inactive (lack of exercise) and/or unhealthy lifestyle (excessive smoking and alcohol use) also lowers the immune system and increases the risk of disease. Other diseases, such as diabetes and cardiovascular disease, as well as low humoral immunity suggest a weakened immune system and thus an increased risk of disease.
In summary, diagnostic biomarkers usually focus on measuring viral nucleic acids and antibodies. Unfortunately, these tests often lack sensitivity and specificity to identify oral cancers. Challenges include the lack of standardized endpoints, limitations of disease ascertainment, impact on risk stratification. Whilst saliva as a diagnostic field is emerging, more work is needed to standardize collection, storage and testing of saliva. Once standardized these assays may assist in understanding the salivary transmission of viruses and be used to predict disease. There is also a need for better, properly validated biomarkers that indicate the risk of pre-cancer and HPV-HNC, which are not affected by seropositivity due to the vaccination. Regardless of the diagnostic platform the assays must be available to those who need them the most. Therefore, low cost diagnostic assays are urgently needed for resource-constrained countries. Finally, there is a need for studies that show how viral biomarkers are affected by immunosuppression caused by HIV infection. Long-term efficacy studies in HIV infected persons are important.
Acknowledgments
This work was supported by Public Health Service grants DE018304, DE023946 to D.P.D. from the US National Institute for Dental and Craniofacial Research (NIDCR).
Footnotes
There are no conflicts of interests for the authors to report.
References
- Ablashi D, Chatlynne L, Cooper H, Thomas D, Yadav M, Norhanom AW, Chandana AK, Churdboonchart V, Kulpradist SA, Patnaik M, Liegmann K, Masood R, Reitz M, Cleghorn F, Manns A, Levine PH, Rabkin C, Biggar R, Jensen F, Gill P, Jack N, Edwards J, Whitman J, Boshoff C. Seroprevalence of human herpesvirus-8 (HHV-8) in countries of Southeast Asia compared to the USA, the Caribbean and Africa. British journal of cancer. 1999;81:893–7. doi: 10.1038/sj.bjc.6690782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ablashi DV, Chatlynne LG, Whitman JE, Jr, Cesarman E. Spectrum of Kaposi’s sarcoma-associated herpesvirus, or human herpesvirus 8, diseases. Clinical microbiology reviews. 2002;15:439–64. doi: 10.1128/CMR.15.3.439-464.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acchioni C, Marsili G, Perrotti E, Remoli AL, Sgarbanti M, Battistini A. Type I IFN–a blunt spear in fighting HIV-1 infection. Cytokine & growth factor reviews. 2015;26:143–58. doi: 10.1016/j.cytogfr.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Achenbach CJ, Harrington RD, Dhanireddy S, Crane HM, Casper C, Kitahata MM. Paradoxical immune reconstitution inflammatory syndrome in HIV-infected patients treated with combination antiretroviral therapy after AIDS-defining opportunistic infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;54:424–33. doi: 10.1093/cid/cir802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akoglu G, Metin A, Ceylan GG, Emre S, Akpolat D, Sungu N. Focal epithelial hyperplasia associated with human papillomavirus 13 and common human leukocyte antigen alleles in a Turkish family. International journal of dermatology. 2015;54:174–8. doi: 10.1111/ijd.12538. [DOI] [PubMed] [Google Scholar]
- Al-Otaibi LM, Al-Sulaiman MH, Teo CG, Porter SR. Extensive oral shedding of human herpesvirus 8 in a renal allograft recipient. Oral microbiology and immunology. 2009;24:109–15. doi: 10.1111/j.1399-302X.2008.00481.x. [DOI] [PubMed] [Google Scholar]
- Anaya-Saavedra G, Flores-Moreno B, Garcia-Carranca A, Irigoyen-Camacho E, Guido-Jimenez M, Ramirez-Amador V. HPV oral lesions in HIV-infected patients: the impact of long-term HAART. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2013;42:443–9. doi: 10.1111/jop.12032. [DOI] [PubMed] [Google Scholar]
- Antonsson A, Cornford M, Perry S, Davis M, Dunne MP, Whiteman DC. Prevalence and risk factors for oral HPV infection in young Australians. PloS one. 2014;9:e91761. doi: 10.1371/journal.pone.0091761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axell T. A prevalence study of oral mucosal lesions in an adult Swedish population. Odontologisk revy. Supplement. 1976;36:1–103. [PubMed] [Google Scholar]
- Bardon A, Shugar D. Properties of purified salivary ribonuclease, and salivary ribonuclease levels in children with cystic fibrosis and in heterozygous carriers. Clin Chim Acta. 1980;101:17–24. doi: 10.1016/0009-8981(80)90051-0. [DOI] [PubMed] [Google Scholar]
- Beachler DC, Abraham AG, Silverberg MJ, Jing Y, Fakhry C, Gill MJ, Dubrow R, Kitahata MM, Klein MB, Burchell AN, Korthuis PT, Moore RD, D’Souza G, North American ACCoR and Design of Ie DEA Incidence and risk factors of HPV-related and HPV-unrelated Head and Neck Squamous Cell Carcinoma in HIV-infected individuals. Oral oncology. 2014;50:1169–76. doi: 10.1016/j.oraloncology.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachler DC, Weber KM, Margolick JB, Strickler HD, Cranston RD, Burk RD, Wiley DJ, Minkoff H, Reddy S, Stammer EE, Gillison ML, D’Souza G. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2012;21:122–33. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekerman E, Jeon D, Ardolino M, Coscoy L. A role for host activation-induced cytidine deaminase in innate immune defense against KSHV. PLoS pathogens. 2013;9:e1003748. doi: 10.1371/journal.ppat.1003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett LK, Hinshaw M. Heck’s disease: diagnosis and susceptibility. Pediatric dermatology. 2009;26:87–9. doi: 10.1111/j.1525-1470.2008.00830.x. [DOI] [PubMed] [Google Scholar]
- Bisio F, Bozzano F, Marras F, Di Biagio A, Moretta L, De Maria A. Successfully treated HIV-infected patients have differential expression of NK cell receptors (NKp46 and NKp30) according to AIDS status at presentation. Immunology letters. 2013;152:16–24. doi: 10.1016/j.imlet.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Borges JD, Souza VA, Giambartolomei C, Dudbridge F, Freire WS, Gregorio SA, Torrez PP, Quiroga M, Mayaud P, Pannuti CS, Nascimento MC. Transmission of human herpesvirus type 8 infection within families in american indigenous populations from the Brazilian Amazon. J Infect Dis. 2012;205:1869–76. doi: 10.1093/infdis/jis278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers E, Scamurra RW, Asrani A, Beniguel L, MaWhinney S, Keays KM, Thurn JR, Janoff EN. Decreased mutation frequencies among immunoglobulin G variable region genes during viremic HIV-1 infection. PloS one. 2014;9:e81913. doi: 10.1371/journal.pone.0081913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger-Calderon R, Madden V, Hallett RA, Gingerich AD, Nickeleit V, Webster-Cyriaque J. Replication of oral BK virus in human salivary gland cells. Journal of virology. 2014;88:559–73. doi: 10.1128/JVI.02777-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt FM, Chindia ML, Rana F, Machigo FG. Pattern of head and neck malignant neoplasms in HIV-infected patients in Kenya. Int J Oral Maxillofac Surg. 2008;37:907–11. doi: 10.1016/j.ijom.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Calabresi A, Ferraresi A, Festa A, Scarcella C, Donato F, Vassallo F, Limina R, Castelli F, Quiros-Roldan E, Brescia HIVCSG Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of Northern Italy, 1999–2009. HIV medicine. 2013;14:481–90. doi: 10.1111/hiv.12034. [DOI] [PubMed] [Google Scholar]
- Castellanos JL, Diaz-Guzman L. Lesions of the oral mucosa: an epidemiological study of 23785 Mexican patients. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2008;105:79–85. doi: 10.1016/j.tripleo.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Chagas CA, Endo LH, Sakano E, Pinto GA, Brousset P, Vassallo J. Detection of herpesvirus type 8 (HHV8) in children’s tonsils and adenoids by immunohistochemistry and in situ hybridization. Int J Pediatr Otorhinolaryngol. 2006;70:65–72. doi: 10.1016/j.ijporl.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. Journal of the National Cancer Institute. 2009;101:1120–30. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Keleher A, Kedda MA, Spurdle AB, McMillan NA, Antonsson A. Human papillomavirus DNA detected in peripheral blood samples from healthy Australian male blood donors. Journal of medical virology. 2009;81:1792–6. doi: 10.1002/jmv.21592. [DOI] [PubMed] [Google Scholar]
- D’Souza G, Agrawal Y, Halpern J, Bodison S, Gillison ML. Oral sexual behaviors associated with prevalent oral human papillomavirus infection. The Journal of infectious diseases. 2009;199:1263–9. doi: 10.1086/597755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza G, Carey TE, William WN, Jr, Nguyen ML, Ko EC, Riddell Jt, Pai SI, Gupta V, Walline HM, Lee JJ, Wolf GT, Shin DM, Grandis JR, Ferris RL. Epidemiology of head and neck squamous cell cancer among HIV-infected patients. Journal of acquired immune deficiency syndromes. 2014;65:603–10. doi: 10.1097/QAI.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roda Husman AM, Walboomers JM, van den Brule AJ, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. The Journal of general virology. 1995;76(Pt 4):1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- Dillon SM, Lee EJ, Bramante JM, Barker E, Wilson CC. The natural killer cell interferon-gamma response to bacteria is diminished in untreated HIV-1 infection and defects persist despite viral suppression. Journal of acquired immune deficiency syndromes. 2014;65:259–67. doi: 10.1097/01.qai.0000435603.50598.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer DP, Damania B. Kaposi sarcoma associated herpesvirus pathogenesis (KSHV)–an update. Current opinion in virology. 2013;3:238–44. doi: 10.1016/j.coviro.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollard SC, Butler LM, Jones AM, Mermin JH, Chidzonga M, Chipato T, Shiboski CH, Brander C, Mosam A, Kiepiela P, Hladik W, Martin JN. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-Saharan Africa: insights on the origin of the “Kaposi’s sarcoma belt”. International journal of cancer. Journal international du cancer. 2010;127:2395–401. doi: 10.1002/ijc.25235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dongre A, Montaldo C. Kaposi’s sarcoma in an HIV-positive person successfully treated with paclitaxel. Indian J Dermatol Venereol Leprol. 2009;75:290–2. doi: 10.4103/0378-6323.51254. [DOI] [PubMed] [Google Scholar]
- Duray A, Descamps G, Bettonville M, Sirtaine N, Ernoux-Neufcoeur P, Guenin S, Mouallif M, Depuydt CE, Delvenne P, Saussez S. High prevalence of high-risk human papillomavirus in palatine tonsils from healthy children and adults. Otolaryngology–head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2011;145:230–5. doi: 10.1177/0194599811402944. [DOI] [PubMed] [Google Scholar]
- Edelstein ZR, Schwartz SM, Hawes S, Hughes JP, Feng Q, Stern ME, O’Reilly S, Lee SK, Fu Xi L, Koutsky LA. Rates and determinants of oral human papillomavirus infection in young men. Sexually transmitted diseases. 2012;39:860–7. doi: 10.1097/OLQ.0b013e318269d098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert D, Seal P, Parsons C, Arbour A, Roberts E, 3rd, Lopez FA. Clinical case of the month: a 22-year-old man with AIDS presenting with shortness of breath and an oral lesion. The Journal of the Louisiana State Medical Society : official organ of the Louisiana State Medical Society. 2014;166:224–30. [PubMed] [Google Scholar]
- Engsig FN, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Obel N. Head and neck cancer in HIV patients and their parents: a Danish cohort study. Clinical epidemiology. 2011;3:217–27. doi: 10.2147/CLEP.S19875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JB, Sherlock CH, Greenspan JS. Hairy leukoplakia-like lesions following bone-marrow transplantation. AIDS. 1991;5:101–2. doi: 10.1097/00002030-199101000-00016. [DOI] [PubMed] [Google Scholar]
- Estrella RT. P16ink4a Immunoexpression Profile in HPV-Oral Lesions from HIV-Infected Patients. Journal of AIDS & Clinical Research. 2015;06 [Google Scholar]
- Falaki F, Amir Chaghmaghi M, Pakfetrat A, Delavarian Z, Mozaffari PM, Pazooki N. Detection of human papilloma virus DNA in seven cases of focal epithelial hyperplasia in Iran. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2009;38:773–6. doi: 10.1111/j.1600-0714.2009.00784.x. [DOI] [PubMed] [Google Scholar]
- Feller L, Anagnostopoulos C, Wood NH, Bouckaert M, Raubenheimer EJ, Lemmer J. Human immunodeficiency virus-associated Kaposi sarcoma as an immune reconstitution inflammatory syndrome: a literature review and case report. J Periodontol. 2008;79:362–8. doi: 10.1902/jop.2008.070225. [DOI] [PubMed] [Google Scholar]
- Feller L, Lemmer J. Insights into pathogenic events of HIV-associated Kaposi sarcoma and immune reconstitution syndrome related Kaposi sarcoma. Infect Agent Cancer. 2008;3:1. doi: 10.1186/1750-9378-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin D, Forman D, Bray F. GLOBOCAN 2012 v1.0: Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer; Lyon, France: 2012. 2013. http://globocan.iarc.fr. [Google Scholar]
- Franzetti M, Adorni F, Parravicini C, Vergani B, Antinori S, Milazzo L, Galli M, Ridolfo AL. Trends and predictors of non-AIDS-defining cancers in men and women with HIV infection: a single-institution retrospective study before and after the introduction of HAART. Journal of acquired immune deficiency syndromes. 2013;62:414–20. doi: 10.1097/QAI.0b013e318282a189. [DOI] [PubMed] [Google Scholar]
- Gibbert K, Schlaak JF, Yang D, Dittmer U. IFN-alpha subtypes: distinct biological activities in anti-viral therapy. British journal of pharmacology. 2013;168:1048–58. doi: 10.1111/bph.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani M, Lajolo C, Sartorio A, Ammassari A, Lacaita MG, Scivetti M, Tamburrini E, Tumbarello M. Oral lesions in HIV and HCV co-infected individuals in HAART era. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2008;37:468–74. doi: 10.1111/j.1600-0714.2008.00647.x. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Viscidi R, Torres BN, Ingles DJ, Sudenga SL, Villa LL, Baggio ML, Abrahamsen M, Quiterio M, Salmeron J. Seroconversion following anal and genital HPV infection in men: The HIM study. Papillomavirus Research. 2015 doi: 10.1016/j.pvr.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan D, Canchola AJ, MacPhail LA, Cheikh B, Greenspan JS. Effect of highly active antiretroviral therapy on frequency of oral warts. Lancet. 2001;357:1411–2. doi: 10.1016/S0140-6736(00)04578-5. [DOI] [PubMed] [Google Scholar]
- Gupta B, Johnson NW. Systematic review and meta-analysis of association of smokeless tobacco and of betel quid without tobacco with incidence of oral cancer in South Asia and the Pacific. PloS one. 2014;9:e113385. doi: 10.1371/journal.pone.0113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Qiao X, Klasse PJ, Chiu A, Chadburn A, Knowles DM, Moore JP, Cerutti A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. Journal of immunology. 2006;176:3931–41. doi: 10.4049/jimmunol.176.7.3931. [DOI] [PubMed] [Google Scholar]
- Hsu WL, Chen JY, Chien YC, Liu MY, You SL, Hsu MM, Yang CS, Chen CJ. Independent effect of EBV and cigarette smoking on nasopharyngeal carcinoma: a 20-year follow-up study on 9,622 males without family history in Taiwan. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:1218–26. doi: 10.1158/1055-9965.EPI-08-1175. [DOI] [PubMed] [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. A review of human carcinogens Part B: Biological agents. International Agency for Research on Cancer; Lyon France: 2009. [Google Scholar]
- Ikenberg H, Bergeron C, Schmidt D, Griesser H, Alameda F, Angeloni C, Bogers J, Dachez R, Denton K, Hariri J, Keller T, von Knebel Doeberitz M, Neumann HH, Puig-Tintore LM, Sideri M, Rehm S, Ridder R, Group PS Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. Journal of the National Cancer Institute. 2013;105:1550–7. doi: 10.1093/jnci/djt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itin P, Rufli T, Rudlinger R, Cathomas G, Huser B, Podvinec M, Gudat F. Oral hairy leukoplakia in a HIV-negative renal transplant patient: a marker for immunosuppression? Dermatologica. 1988;177:126–8. doi: 10.1159/000248529. [DOI] [PubMed] [Google Scholar]
- Jeffers LK, Madden V, Webster-Cyriaque J. BK virus has tropism for human salivary gland cells in vitro: implications for transmission. Virology. 2009;394:183–93. doi: 10.1016/j.virol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Jindal JR, Campbell BH, Ward TO, Almagro US. Kaposi’s sarcoma of the oral cavity in a non-AIDS patient: case report and review of the literature. Head Neck. 1995;17:64–8. doi: 10.1002/hed.2880170114. [DOI] [PubMed] [Google Scholar]
- Johnson NW. The mouth in HIV/AIDS: markers of disease status and management challenges for the dental profession. Aust Dent J. 2010;55(Suppl 1):85–102. doi: 10.1111/j.1834-7819.2010.01203.x. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) The gap report. Geneva: 2014. [PubMed] [Google Scholar]
- Kakabadze T, Rukhadze N, Mshvidobadze K, Lomtadze M, Kandelaki G. Oral lesions in HIV-positive patients in Georgia. Georgian medical news. 2008:60–5. [PubMed] [Google Scholar]
- Kharkar V, Gutte RM, Khopkar U, Mahajan S, Chikhalkar S. Kaposi’s sarcoma: a presenting manifestation of HIV infection in an Indian. Indian J Dermatol Venereol Leprol. 2009;75:391–3. doi: 10.4103/0378-6323.53137. [DOI] [PubMed] [Google Scholar]
- King GN, Healy CM, Glover MT, Kwan JT, Williams DM, Leigh IM, Thornhill MH. Prevalence and risk factors associated with leukoplakia, hairy leukoplakia, erythematous candidiasis, and gingival hyperplasia in renal transplant recipients. Oral surgery, oral medicine, and oral pathology. 1994;78:718–26. doi: 10.1016/0030-4220(94)90086-8. [DOI] [PubMed] [Google Scholar]
- King MD, Reznik DA, O’Daniels CM, Larsen NM, Osterholt D, Blumberg HM. Human papillomavirus-associated oral warts among human immunodeficiency virus-seropositive patients in the era of highly active antiretroviral therapy: an emerging infection. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002;34:641–8. doi: 10.1086/338637. [DOI] [PubMed] [Google Scholar]
- Kowalkowski MA, Mims MP, Amiran ES, Lulla P, Chiao EY. Effect of immune reconstitution on the incidence of HIV-related Hodgkin lymphoma. PloS one. 2013;8:e77409. doi: 10.1371/journal.pone.0077409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Alberg AJ, Daniel R, Gravitt PE, Viscidi R, Garrett ES, Shah KV, Gillison ML. Oral human papillomavirus infection in adults is associated with sexual behavior and HIV serostatus. The Journal of infectious diseases. 2004;189:686–98. doi: 10.1086/381504. [DOI] [PubMed] [Google Scholar]
- Kreimer AR, Johansson M, Waterboer T, Kaaks R, Chang-Claude J, Drogen D, Tjonneland A, Overvad K, Quiros JR, Gonzalez CA, Sanchez MJ, Larranaga N, Navarro C, Barricarte A, Travis RC, Khaw KT, Wareham N, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, Panico S, Masala G, Grioni S, Tumino R, Vineis P, Bueno-de-Mesquita HB, Laurell G, Hallmans G, Manjer J, Ekstrom J, Skeie G, Lund E, Weiderpass E, Ferrari P, Byrnes G, Romieu I, Riboli E, Hildesheim A, Boeing H, Pawlita M, Brennan P. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013a;31:2708–15. doi: 10.1200/JCO.2012.47.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Pierce Campbell CM, Lin HY, Fulp W, Papenfuss MR, Abrahamsen M, Hildesheim A, Villa LL, Salmeron JJ, Lazcano-Ponce E, Giuliano AR. Incidence and clearance of oral human papillomavirus infection in men: the HIM cohort study. Lancet. 2013b;382:877–87. doi: 10.1016/S0140-6736(13)60809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krown SE, Lee JY, Dittmer DP, Consortium AM More on HIV-associated Kaposi’s sarcoma. The New England journal of medicine. 2008;358:535–6. doi: 10.1056/NEJMc072994. author reply 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarasamy N, Solomon S, Yesudian PK, Sugumar P. First Report Of Kaposi’s Sarcoma In An AIDS Patient From Madras, India. Indian Journal of Dermatology. 1996;41:23–25. [Google Scholar]
- Kura MM, Khemani UN, Lanjewar DN, Raghuwanshi SR, Chitale AR, Joshi SR. Kaposi’s sarcoma in a patient with AIDS. J Assoc Physicians India. 2008;56:262–4. [PubMed] [Google Scholar]
- Lewis JS, Jr, Thorstad WL, Chernock RD, Haughey BH, Yip JH, Zhang Q, El-Mofty SK. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. The American journal of surgical pathology. 2010;34:1088–96. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Li Y, Zhou Y, Zeng X. Focal epithelial hyperplasia (Heck’s disease) in two Chinese females. International journal of oral and maxillofacial surgery. 2012;41:1001–4. doi: 10.1016/j.ijom.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Lourenco AG, Motta AC, Figueiredo LT, Machado AA, Komesu MC. Oral lesions associated with HIV infection before and during the antiretroviral therapy era in Ribeirao Preto, Brazil. Journal of oral science. 2011;53:379–85. doi: 10.2334/josnusd.53.379. [DOI] [PubMed] [Google Scholar]
- Lowe SM, Katsidzira L, Meys R, Sterling JC, de Koning M, Quint W, Nathoo K, Munyati S, Ndhlovu CE, Salisbury JR, Bunker CB, Corbett EL, Miller RF, Ferrand RA. Acquired epidermodysplasia verruciformis due to multiple and unusual HPV infection among vertically-infected, HIV-positive adolescents in Zimbabwe. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;54:e119–23. doi: 10.1093/cid/cis118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SC, Hu J, Zhao J, Speight P. Typing human papilloma virus (HPV) infection in the warts of oral mucosa from HIV-positive patients. Hua Xi Kou Qiang Yi Xue Za Zhi. 2004;22:423–5. [PubMed] [Google Scholar]
- Marais DJ, Constant D, Allan B, Carrara H, Hoffman M, Shapiro S, Morroni C, Williamson AL. Cervical human papillomavirus (HPV) infection and HPV type 16 antibodies in South African women. Journal of clinical microbiology. 2008;46:732–9. doi: 10.1128/JCM.01322-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V, Parks T, Bagni R, Wang CD, Samols MA, Hu J, Wyvil KM, Aleman K, Little RF, Yarchoan R, Renne R, Whitby D. Conservation of virally encoded microRNAs in Kaposi sarcoma–associated herpesvirus in primary effusion lymphoma cell lines and in patients with Kaposi sarcoma or multicentric Castleman disease. The Journal of infectious diseases. 2007;195:645–59. doi: 10.1086/511434. [DOI] [PubMed] [Google Scholar]
- Marur S, D’Souza G, Westra WH, Forastiere AA. HPV-associated head and neck cancer: a virus-related cancer epidemic. The Lancet. Oncology. 2010;11:781–9. doi: 10.1016/S1470-2045(10)70017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbisa GL, Miley W, Gamache CJ, Gillette WK, Esposito D, Hopkins R, Busch MP, Schreiber GB, Little RF, Yarchoan R, Ortiz-Conde BA, Labo N, Whitby D. Detection of antibodies to Kaposi’s sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. Journal of immunological methods. 2010;356:39–46. doi: 10.1016/j.jim.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbulaiteye SM, Pfeiffer RM, Engels EA, Marshall V, Bakaki PM, Owor AM, Ndugwa CM, Katongole-Mbidde E, Goedert JJ, Biggar RJ, Whitby D. Detection of kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. The Journal of infectious diseases. 2004;190:1382–6. doi: 10.1086/424489. [DOI] [PubMed] [Google Scholar]
- Mertz GJ. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. The Journal of infectious diseases. 2008;198:1098–100. doi: 10.1086/591914. [DOI] [PubMed] [Google Scholar]
- Mesri EA, Cesarman E, Boshoff C. Kaposi’s sarcoma and its associated herpesvirus. Nat Rev Cancer. 2010;10:707–19. doi: 10.1038/nrc2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munawwar A, Sharma SK, Gupta S, Singh S. Seroprevalence and determinants of Kaposi sarcoma-associated human herpesvirus 8 in Indian HIV-infected males. AIDS research and human retroviruses. 2014;30:1192–6. doi: 10.1089/aid.2014.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz C. Role of human natural killer cells during Epstein-Barr virus infection. Critical reviews in immunology. 2014;34:501–7. doi: 10.1615/critrevimmunol.2014012312. [DOI] [PubMed] [Google Scholar]
- Mutalima N, Molyneux E, Jaffe H, Kamiza S, Borgstein E, Mkandawire N, Liomba G, Batumba M, Lagos D, Gratrix F, Boshoff C, Casabonne D, Carpenter LM, Newton R. Associations between Burkitt lymphoma among children in Malawi and infection with HIV, EBV and malaria: results from a case-control study. PloS one. 2008;3:e2505. doi: 10.1371/journal.pone.0002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabbanja J, Gitta S, Peterson S, Rwenyonyi CM. Orofacial manifestations in HIV positive children attending Mildmay Clinic in Uganda. Odontology / the Society of the Nippon Dental University. 2013;101:116–20. doi: 10.1007/s10266-012-0060-7. [DOI] [PubMed] [Google Scholar]
- Nagaraj M. Verruca vulgaris of the tongue. Journal of maxillofacial and oral surgery. 2013;12:329–32. doi: 10.1007/s12663-010-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SK, Hojen JF, Andersen KL, Kofod-Olsen E, Berg RK, Paludan SR, Ostergaard L, Jakobsen MR, Tolstrup M, Mogensen TH. Innate DNA sensing is impaired in HIV patients and IFI16 expression correlates with chronic immune activation. Clinical and experimental immunology. 2014;177:295–309. doi: 10.1111/cei.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Mde G, Azevedo-e-Silva M, Goncalves CP, Trope BM, Oliveira Ldo H, Ramos-e-Silva M. Human papillomavirus detection and typification in cutaneous and mucosal lesions of HIV-seropositive patients. International journal of STD & AIDS. 2008;19:611–6. doi: 10.1258/ijsa.2007.007224. [DOI] [PubMed] [Google Scholar]
- Nye MB, Leman AR, Meyer ME, Menegus MA, Rothberg PG. Sequence diversity in the glycoprotein B gene complicates real-time PCR assays for detection and quantification of cytomegalovirus. Journal of clinical microbiology. 2005;43:4968–71. doi: 10.1128/JCM.43.10.4968-4971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega KL, Ceballos-Salobrena A, Gaitan-Cepeda LA, Magalhaes MG. Oral manifestations after immune reconstitution in HIV patients on HAART. International journal of STD & AIDS. 2008;19:305–8. doi: 10.1258/ijsa.2007.007261. [DOI] [PubMed] [Google Scholar]
- Ortega KL, Vale DA, Magalhaes MH. Impact of PI and NNRTI HAART-based therapy on oral lesions of Brazilian HIV-infected patients. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2009;38:489–94. doi: 10.1111/j.1600-0714.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- Ortiz AP, Perez-Irizarry J, Soto-Salgado M, Suarez E, Perez N, Cruz M, Palefsky J, Tortolero-Luna G, Miranda S, Colon-Lopez V. Human papillomavirus-related cancers among people living with AIDS in Puerto Rico. Preventing chronic disease. 2014;11:E80. doi: 10.5888/pcd11.130361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagatsia Z, Jones J, Morgan P, Tappuni AR. Oral Kaposi sarcoma: a case of immune reconstitution inflammatory syndrome. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2009;108:70–5. doi: 10.1016/j.tripleo.2009.02.035. [DOI] [PubMed] [Google Scholar]
- Parisi SG, Cruciani M, Scaggiante R, Boldrin C, Andreis S, Dal Bello F, Pagni S, Barelli A, Sattin A, Mengoli C, Palu G. Anal and oral human papillomavirus (HPV) infection in HIV-infected subjects in northern Italy: a longitudinal cohort study among men who have sex with men. BMC infectious diseases. 2011;11:150. doi: 10.1186/1471-2334-11-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park NJ, Li Y, Yu T, Brinkman BM, Wong DT. Characterization of RNA in saliva. Clin Chem. 2006;52:988–94. doi: 10.1373/clinchem.2005.063206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton LL, McKaig R, Strauss R, Rogers D, Eron JJ., Jr Changing prevalence of oral manifestations of human immuno-deficiency virus in the era of protease inhibitor therapy. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 2000;89:299–304. doi: 10.1016/s1079-2104(00)70092-8. [DOI] [PubMed] [Google Scholar]
- Patton LL, Ranganathan K, Naidoo S, Bhayat A, Balasundaram S, Adeyemi O, Taiwo O, Speicher DJ, Chandra L. Oral lesions, HIV phenotypes, and management of HIV-related disease: Workshop 4A. Adv Dent Res. 2011;23:112–6. doi: 10.1177/0022034511400079. [DOI] [PubMed] [Google Scholar]
- Pickard RK, Xiao W, Broutian TR, He X, Gillison ML. The prevalence and incidence of oral human papillomavirus infection among young men and women, aged 18–30 years. Sexually transmitted diseases. 2012;39:559–66. doi: 10.1097/OLQ.0b013e31824f1c65. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amador VA, Espinosa E, Gonzalez-Ramirez I, Anaya-Saavedra G, Ormsby CE, Reyes-Teran G. Identification of oral candidosis, hairy leukoplakia and recurrent oral ulcers as distinct cases of immune reconstitution inflammatory syndrome. International journal of STD & AIDS. 2009;20:259–61. doi: 10.1258/ijsa.2008.008351. [DOI] [PubMed] [Google Scholar]
- Robledo-Sierra J, Mattsson U, Svedensten T, Jontell M. The morbidity of oral mucosal lesions in an adult Swedish population. Medicina oral, patologia oral y cirugia bucal. 2013;18:e766–72. doi: 10.4317/medoral.19286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rositch AF, Gravitt PE, Tobian AA, Newell K, Quinn TC, Serwadda D, Ssebbowa P, Kiggundu V, Gray RH, Reynolds SJ. Frequent detection of HPV before and after initiation of antiretroviral therapy among HIV/HSV-2 co-infected women in Uganda. PloS one. 2013;8:e55383. doi: 10.1371/journal.pone.0055383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said AK, Leao JC, Fedele S, Porter SR. Focal epithelial hyperplasia – an update. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2013;42:435–42. doi: 10.1111/jop.12009. [DOI] [PubMed] [Google Scholar]
- Salonen L, Axell T, Hellden L. Occurrence of oral mucosal lesions, the influence of tobacco habits and an estimate of treatment time in an adult Swedish population. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 1990;19:170–6. doi: 10.1111/j.1600-0714.1990.tb00819.x. [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Chen H, Secours R, Lee A, Webster-Cyriaque J, Ghannoum M, Evans S, Bernard D, Reznik D, Dittmer DP, Hosey L, Severe P, Aberg JA, Oral Hiv/Aids Research Alliance SotACTG High Accuracy of Common HIV-Related Oral Disease Diagnoses by Non-Oral Health Specialists in the AIDS Clinical Trial Group. PloS one. 2015;10:e0131001. doi: 10.1371/journal.pone.0131001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Sudenga SL, Smith JS, Bachmann LH, Wilson CM, Kempf MC. The impact of highly active antiretroviral therapy on prevalence and incidence of cervical human papillomavirus infections in HIV-positive adolescents. BMC infectious diseases. 2010;10:295. doi: 10.1186/1471-2334-10-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufiane M, Fadl TM, Nawfel M, Ouafae M, Kawtar Z, Afaf L, Jamal el FM, Hassan FM. Kaposi’s sarcoma: HIV-negative man with isolated penile localization. Indian J Pathol Microbiol. 2010;53:535–6. doi: 10.4103/0377-4929.68294. [DOI] [PubMed] [Google Scholar]
- Speicher DJ, Johnson NW. Comparison of salivary collection and processing methods for quantitative HHV-8 detection. Oral diseases. 2014;20:720–8. doi: 10.1111/odi.12196. [DOI] [PubMed] [Google Scholar]
- Speicher DJ, Nandagopal P, Saravanan S, Kumarasamy N, Rangananthan K, Johnson NW. Detection of Human Herpesviruses in HIV-positive patients from Southeast India; Seventh World Workshop on Oral Health & Disease in AIDS; Hyderabad, India. 2014a. p. 100. [Google Scholar]
- Speicher DJ, Sehu MM, Johnson NW, Shaw DR. Successful treatment of an HIV-positive patient with unmasking Kaposi’s sarcoma immune reconstitution inflammatory syndrome. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2013;57:282–5. doi: 10.1016/j.jcv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Speicher DJ, Sehu MM, Mollee P, Shen L, Johnson NW, Faoagali JL. Successful treatment of iatrogenic multicentric Castleman’s disease arising due to recrudescence of HHV-8 in a liver transplant patient. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2014b;14:1207–13. doi: 10.1111/ajt.12693. [DOI] [PubMed] [Google Scholar]
- Speicher DJ, Wanzala P, D’Lima M, Johnson KE, Johnson NW. Detecting DNA viruses in oral fluids: evaluation of collection and storage methods. Diagnostic microbiology and infectious disease. 2015a;82:120–7. doi: 10.1016/j.diagmicrobio.2015.02.013. [DOI] [PubMed] [Google Scholar]
- Speicher DJ, Wanzala P, D’Lima M, Njiru A, Chindia M, Dimba E, Johnson NW. Diagnostic challenges of oral and cutaneous Kaposi’s sarcoma in resource-constrained settings. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology. 2015b;44:842–9. doi: 10.1111/jop.12315. [DOI] [PubMed] [Google Scholar]
- Syrjanen S. Human papillomavirus infections and oral tumors. Medical microbiology and immunology. 2003;192:123–8. doi: 10.1007/s00430-002-0173-7. [DOI] [PubMed] [Google Scholar]
- Tappuni AR. Immune reconstitution inflammatory syndrome. Adv Dent Res. 2011;23:90–6. doi: 10.1177/0022034511399915. [DOI] [PubMed] [Google Scholar]
- Tappuni AR, Shiboski CH. Overview and Research Agenda Arising from the 7th World Workshop on Oral health and Disease in AIDS. Oral diseases. 2016 doi: 10.1111/odi.12459. [DOI] [PubMed] [Google Scholar]
- Taylor MM, Chohan B, Lavreys L, Hassan W, Huang ML, Corey L, Ashley Morrow R, Richardson BA, Mandaliya K, Ndinya-Achola J, Bwayo J, Kreiss J. Shedding of human herpesvirus 8 in oral and genital secretions from HIV-1-seropositive and -seronegative Kenyan women. The Journal of infectious diseases. 2004;190:484–8. doi: 10.1086/421466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugizov SM, Herrera R, Chin-Hong P, Veluppillai P, Greenspan D, Michael Berry J, Pilcher CD, Shiboski CH, Jay N, Rubin M, Chein A, Palefsky JM. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology. 2013;446:378–88. doi: 10.1016/j.virol.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaishnani JB, Bosamiya SS, Momin AM. Kaposi’s sarcoma: a presenting sign of HIV. Indian J Dermatol Venereol Leprol. 2010;76:215. doi: 10.4103/0378-6323.60542. [DOI] [PubMed] [Google Scholar]
- Videla S, Darwich L, Canadas MP, Coll J, Pinol M, Garcia-Cuyas F, Molina-Lopez RA, Cobarsi P, Clotet B, Sirera G, Group H-HS Natural history of human papillomavirus infections involving anal, penile, and oral sites among HIV-positive men. Sexually transmitted diseases. 2013;40:3–10. doi: 10.1097/OLQ.0b013e31827e87bd. [DOI] [PubMed] [Google Scholar]
- Vieira VC, Soares MA. The role of cytidine deaminases on innate immune responses against human viral infections. BioMed research international. 2013;2013:683095. doi: 10.1155/2013/683095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster-Cyriaque J, Duus K, Cooper C, Duncan M. Oral EBV and KSHV infection in HIV. Adv Dent Res. 2006;19:91–5. doi: 10.1177/154407370601900118. [DOI] [PubMed] [Google Scholar]
- Webster-Cyriaque J, Middeldorp J, Raab-Traub N. Hairy leukoplakia: an unusual combination of transforming and permissive Epstein-Barr virus infections. Journal of virology. 2000;74:7610–8. doi: 10.1128/jvi.74.16.7610-7618.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzensen N, Schwartz L, Zuna RE, Smith K, Mathews C, Gold MA, Allen RA, Zhang R, Dunn ST, Walker JL, Schiffman M. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res. 2012;18:4154–62. doi: 10.1158/1078-0432.CCR-12-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiley DM, Lambert SB, Bialasiewicz S, Goire N, Nissen MD, Sloots TP. False-negative results in nucleic acid amplification tests-do we need to routinely use two genetic targets in all assays to overcome problems caused by sequence variation? Critical reviews in microbiology. 2008;34:71–6. doi: 10.1080/10408410801960913. [DOI] [PubMed] [Google Scholar]
- Wittekindt C, Gultekin E, Weissenborn SJ, Dienes HP, Pfister HJ, Klussmann JP. Expression of p16 protein is associated with human papillomavirus status in tonsillar carcinomas and has implications on survival. Advances in oto-rhino-laryngology. 2005;62:72–80. doi: 10.1159/000082474. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhou H, He Y, Chen Z, He B, He M. The immune pathogenesis of immune reconstitution inflammatory syndrome associated with highly active antiretroviral therapy in AIDS. AIDS research and human retroviruses. 2014;30:1197–202. doi: 10.1089/aid.2014.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler JL, Newton R, Katongole-Mbidde E, Mbulataiye S, De Cock K, Wabinga H, Mugerwa J, Katabira E, Jaffe H, Parkin DM, Reeves G, Weiss R, Beral V. Risk factors for Kaposi’s sarcoma in HIV-positive subjects in Uganda. AIDS. 1997;11:1619–26. doi: 10.1097/00002030-199713000-00011. [DOI] [PubMed] [Google Scholar]


