Abstract
Background:
Thromboembolic events are infrequent but serious complications of transcatheter aortic valve implantation (TAVI), occurring in 2.3–10% of the patients. However, the cause of post-TAVI stroke is unclear.
Case Description:
A 90-year-old female underwent transfemoral-TAVI for severe aortic stenosis. Ten days later, she presented with an ischemic stroke of the left middle cerebral artery territory due to new-onset atrial fibrillation (NOAF). She underwent emergent endovascular thrombectomy with good reperfusion approximately 6 hours after onset of symptoms. At hospital discharge, her National Institutes of Health Stroke Scale score was 11.
Conclusions:
Although NOAF is rare during the subacute phase of TAVI, in this patient it might be the cause of her stroke. This finding suggests that dual antiplatelet therapy alone may be insufficient in the prevention of stroke after TAVI. Nonetheless, this case demonstrates the efficacy and safety of endovascular thrombectomy in patients with acute ischemic stroke caused by NOAF after TAVI.
Keywords: Aortic stenosis, new-onset atrial fibrillation, thrombectomy, transcatheter aortic valve implantation
INTRODUCTION
Thromboembolic events are infrequent but serious complications after transcatheter aortic valve implantation (TAVI), occurring in 2.3–10% of the patients.[1,7,8,9] In addition, the mortality rate of post-TAVI stroke is high, reported to be 30–43%.[7,8,9] In most previous reports of TAVI, mean patient age was over 80 years; age is not a predictor for thromboembolic events according to TAVI.[7,8,9]
The occurrence of more than half of the thromboembolic events within the first post-procedural day suggests a procedure-related cause in these cases.[1,8,9] On the other hand, the risk of stroke is not limited to the procedure itself because roughly half of the strokes occur between days 2 and 30 post-TAVI.[1,8,9] The etiology of post-TAVI stroke is also poorly understood. Here, we present a case of a nonagenarian patient with acute ischemic stroke treated by aspiration thrombectomy 10 days after TAVI.
CASE DESCRIPTION
A 90-year-old female with a severe aortic stenosis was admitted to our institution after her first event of decompensated heart failure was treated at another hospital 4 months earlier. She had neither chest pain nor syncope and her functional status was NYHA class II. Her medical history included stage 4 chronic kidney disease. Preoperative transthoracic echocardiogram revealed severe aortic stenosis with an aortic valve area of 530 mm2 and a low-flow/low-gradient stenosis with a peak velocity of 3.84 m/s, a mean gradient of 36.3 mmHg, and a left ventricular ejection fraction of 41%. On the computed tomography (CT) scan, the aortic annulus measured 29 × 17 mm, with an area of 380 mm2. Based on a calculated logistic EuroSCORE and Society of Thoracic Surgeons risk score of 45.72% and 10.464%, respectively, surgery was considered to be of relatively high risk.
Transfemoral-TAVI was performed under general anesthesia. A 16-French (Fr) Edwards expandable eSheath (Edwards Lifesciences, Irvine, CA, USA) was inserted into the right femoral artery by surgical cut-down. Heparin was administered immediately after the eSheath insertion. Following balloon aortic valvuloplasty, a 23-mm Edwards Sapien XT valve (Edwards Lifesciences) was deployed under fluoroscopic guidance during rapid ventricular pacing. Transesophageal echocardiography (TEE) did not detect left atrial appendage (LAA) thrombus during the procedure nor was there any demonstration of atrial fibrillation (AF) on the electrocardiogram. Dual antiplatelet therapy (DAPT: aspirin 100 mg and clopidogrel 75 mg) was administered after the procedure. Diuretics and human atrial natriuretic peptide were added for 3 days because of worsening heart failure, but she had no AF during her stay in the intensive care unit.
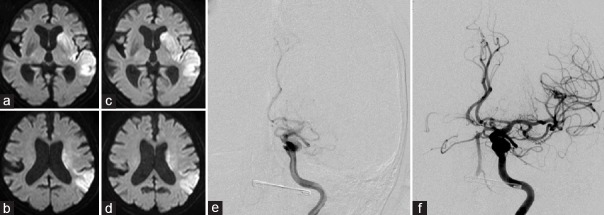
At 1:30 of day 10 after TAVI, the patient suffered aphasia and right hemiparesis with deteriorating consciousness. Her National Institutes of Health Stroke Scale (NIHSS) score was 19. Emergent brain CT was performed at 2:11 and magnetic resonance imaging (MRI) at 2:30. The latter showed an occlusion of the left middle cerebral artery (MCA) and acute ischemic lesions in its territory. Her diffusion-weighted imaging-Alberta Stroke Program early computed tomography score (DWI-ASPECTS) was 6 [Figure 1a and b]. The stroke onset time was considered to be day 9, at 23:00, when she was last known to be without new deficits. Endovascular thrombectomy was performed without intravenous tissue plasminogen activator. A 9-Fr sheath was inserted in the right femoral artery at 4:30. A 9-Fr Optimo device (Tokai Medical, Japan) was inserted into the left internal carotid artery. A Penumbra 5 Max system (Penumbra Inc., CA, USA) was guided to the thrombotic site using a microguidewire (Chikai 0.014 inch: ASAHI INTEC, Japan) coaxial to a Penumbra 3 Max system [Figure 1e]. A direct-aspiration first-pass technique was performed twice, leading to recanalization. Post-procedural angiography at 5:13, 373 min after stroke onset, demonstrated successful recanalization of the entire left MCA (thrombolysis in cerebral infarction scale: 3) [Figure 1f]. Post-procedural MRI showed no worsening of the cerebral infarction and no hemorrhagic changes [Figure 1c and d]. Anticoagulation therapy with heparin was added to DAPT for 8 days following thrombectomy. Subsequently, aspirin was withdrawn and oral anticoagulation with warfarin was started. After thrombectomy, TEE revealed a thrombus in the LAA that was presumed to be the cause of the stroke. AF was documented on day 12 post-TAVI. She was discharged on day 29 after thrombectomy with an NIHSS score of 11.
Figure 1.
(a and b) Pre-thrombectomy diffusion-weighted imaging (DWI) shows an acute ischemic lesion in left middle cerebral artery (MCA)-territory. The diffusion-weighted imaging-Alberta Stroke Program early computed tomography score is 6. (c and d) Post-thrombectomy DWI shows no worsening of the cerebral infarction. (e) Pre-thrombectomy digital subtraction angiography (DSA) shows occlusion of the left MCA. (f) Post-thrombectomy DSA shows complete recanalization, corresponding to thrombolysis in cerebral infarction grade 3
DISCUSSION
This case raises several issues. In the subacute phase of TAVI, new-onset AF (NOAF) is not a common cause of stroke,[2] although it seemed to be the cause in our patient. Therefore, DAPT alone may be insufficient for stroke prevention after TAVI. However, as demonstrated in this case, in a patient with acute ischemic stroke caused by NOAF after TAVI endovascular thrombectomy is an effective and safe treatment and may prevent a fatal outcome, even though the patient is very elderly.
The overall incidence of NOAF after TAVI is 4–32%,[5] and the NOAF frequently occurs in both acute and latent phases after TAVI. One reported that 81.8% of NOAF occurred within 3 days,[1] other reported the episodes increased after 30-day post-TAVI.[2] In any case, NOAF is not a common cause of stroke in the subacute phase of TAVI. Continuous ECG monitoring and routine echocardiography screening are effective methods for detecting NOAF or an LAA thrombus, and thus in preventing embolic stroke after TAVI. However, there is no consensus regarding the duration of ECG monitoring and follow-up echocardiography.
Despite the etiology of post-TAVI stroke is thought to be multifactorial,[4] DAPT is recommended empirically after TAVI although it may not adequately address the importance of NOAF. This case suggests that DAPT alone may be insufficient for stroke prevention after TAVI. Large studies comparing different anticoagulant or antiplatelet strategies are still lacking.
Recently, a meta-analysis has shown consistent benefit of endovascular thrombectomy even in elderly patients.[3] Moreover, in a report focusing on nonagenarian patients who underwent endovascular thrombectomy, there was no difference in the rate of modified Rankin Scale worsening from pre-stroke to 90 days between nonagenarians and younger patients.[6] Therefore, even though the patients were very elderly, endovascular thrombectomy may provide additional benefits when they had no or only mild pre-stroke functional deficits.
CONCLUSION
In the subacute phase of TAVI, NOAF is a rare cause of stroke, although it seemed to be the cause in our patient. Therefore, DAPT alone may be insufficient for stroke prevention after TAVI. However, as demonstrated in the present case, in a patient with acute ischemic stroke caused by NOAF after TAVI endovascular thrombectomy is an effective and safe treatment and may be preventing a fatal outcome, even though the patients are very elderly. To determine the most effective antiplatelet/anticoagulant strategy for stroke prevention, a clear understanding of the time occurrence and the pathophysiological mechanisms of stroke following TAVI is important.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare that they have no conflicts of interest. There was no grant support for this study.
Footnotes
Contributor Information
Kazuya Matsuo, Email: kkmatsuo@outlook.jp.
Atsushi Fujita, Email: afujita@med.kobe-u.ac.jp.
Jun Tanaka, Email: jun_flatout@yahoo.co.jp.
Tomoaki Nakai, Email: tomoakin@med.kobe-u.ac.jp.
Masaaki Kohta, Email: kohta@med.kobe-u.ac.jp.
Kohkichi Hosoda, Email: khosoda@med.kobe-u.ac.jp.
Toshiro Shinke, Email: shinke@med.kobe-u.ac.jp.
Ken-ichi Hirata, Email: hiratak@med.kobe-u.ac.jp.
Eiji Kohmura, Email: ekohmura@med.kobe-u.ac.jp.
REFERENCES
- 1.Amat-Santos IJ, Rodés-Cabau J, Urena M, DeLarochellière R, Doyle D, Bagur R, et al. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:178–88. doi: 10.1016/j.jacc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 2.Gargiulo G, Capodanno D, Sannino A, Barbanti M, Perrino C, Capranzano P, et al. New-onset atrial fibrillation and increased mortality after transcatheter aortic valve implantation: A causal or spurious association? Int J Cardiol. 2016;23:264–6. doi: 10.1016/j.ijcard.2015.10.133. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 4.Hassell ME, Hildick-Smith D, Durand E, Kikkert WJ, Wiegerinck EM, Stabile E, et al. Antiplatelet therapy following transcatheter aortic valve implantation. Heart. 2015;101:1118–25. doi: 10.1136/heartjnl-2014-307053. [DOI] [PubMed] [Google Scholar]
- 5.Jørgensen TH, Thygesen JB, Thyregod HG, Svendsen JH, Søndergaard L. New-onset atrial fibrillation after surgical aortic valve replacement and transcatheter aortic valve implantation: A concise review. J Invasive Cardiol. 2015;27:41–7. [PubMed] [Google Scholar]
- 6.Khan MA, Baird GL, Miller D, Patel A, Tsekhan S, Yaghi S, et al. Endovascular treatment of acute ischemic stroke in nonagenarians compared with younger patients in a multicenter cohort. J Neurointerv Surg. 2017;9:727–31. doi: 10.1136/neurintsurg-2016-012427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller DC, Blackstone EH, Mack MJ, Svensson LG, Kodali SK, Kapadia S, et al. Transcatheter (TAVR) versus surgical (AVR) aortic valve replacement: Occurrence, hazard, risk factors, and consequences of neurologic events in the PARTNER trial. J Thorac Cardiovasc Surg. 2012;143:832–43. doi: 10.1016/j.jtcvs.2012.01.055. [DOI] [PubMed] [Google Scholar]
- 8.Nombela-Franco L, Webb JG, de Jaegere PP, Toggweiler S, Nuis RJ, Dager AE, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041–53. doi: 10.1161/CIRCULATIONAHA.112.110981. [DOI] [PubMed] [Google Scholar]
- 9.Stortecky S, Windecker S, Pilgrim T, Heg D, Buellesfeld L, Khattab AA, et al. Cerebrovascular accidents complicating transcatheter aortic valve implantation: Frequency, timing and impact on outcomes. EuroIntervention. 2012;8:62–70. doi: 10.4244/EIJV8I1A11. [DOI] [PubMed] [Google Scholar]