Abstract
Vascular calcification is one of the leading causes of death in chronic kidney disease (CKD) patients. Klotho protein encoded by the anti-aging gene, Klotho, and intermedin1–53 have both been shown to protect the vasculature from calcification in CKD. The study by Chang and colleagues links Klotho and intermedin1–53 in prevention of vascular calcification in CKD by showing that intermedin1–53 increased renal, vascular, and plasma Klotho, and reduced vascular calcification in CKD rats. Intermedin1–53 may be a novel therapeutic agent for CKD.
Medial artery calcification (referred to hereafter simply as vascular calcification) is one type of vascular calcification in CKD. It is also known as Mönckeberg sclerosis, in which amorphous mineral forms circumferentially along or within 1 or more elastic lamellae of the medial layer in vasculature. Vascular calcification is one of the leading causes of death in CKD. Apart from traditional risk factors, several novel factors such as perturbation of mineral metabolism, FGF23 excess, and Klotho deficiency make major contributions to the initiation and progression of vascular calcification in CKD.
Klotho was originally identified as an anti-aging gene encoding for Klotho protein in 1997 by Kuro-o et al.1 Shortly after its discovery, 2 more paralogs, (β-Klotho and γ-Klotho, were identified; then the Klotho gene was referred to as αKlotho to distinguish it from the other 2 paralogs. In this article, αKlotho is simply referred to as Klotho. The striking features of homozygous Klotho-deficient mice include massive calcification in the vasculature and other soft tissues, high serum FGF23, and short lifespan.1 Klotho is a single-transmembrane protein. The large extracellular part of membrane Klotho has 2 repeated internal domains, Kl1 and Kl2, which are structurally similar to (β-glucosidase. The extracellular domain of Klotho protein can be shed by secretases and released into the circulation.2 This is the main functional form in the circulation. Although the expression of Klotho in the vasculature is still controversial, there are good data supporting that high circulating Klotho is associated with low levels of vascular calcification. Animal experiments clearly confirmed that Klotho protects the vasculature from calcification in CKD.3
Vascular calcification is active extra-osseous calcification in vasculature due to the loss of endogenous inhibitors of mineralization. In addition to Klotho, several other inhibitors of vascular calcification have been identified, both inorganic substances such as pyrophosphate and magnesium, and peptidic substances such as matrix γ-carboxyglutamic acid protein (MP), fetuin-A, osteoprotegerin, bone morphogenetic protein-7, and intermedin (IMD)1–53. In 1993, adrenomedullin protein was initially isolated and identified from pheochromocytoma, a tumor in the adrenal medulla. Another similar peptide named adrenomedullin 2 was reported in rats in 2004 that exhibits function similar to that of adrenomedullin. Adrenomedullin 2 is also known as IMD.4 IMD1–53 is generated from prepro-intermedin by proteolytic cleavage via a yet-to-be-identified enzyme. In human, mouse, and rat, IMD1–53 is found in a variety of tissues and organs including the brain, kidney, heart, skin, pancreas, lung, spleen, thymus, gastrointestinal tract, and ovary, but not in the testis or adrenal gland.4,5 IMD1–53 protein in plasma is presumably released from those tissues and organs.
It has been shown that IMD1–53 expression in the heart is upregulated by hypertensive rats, whereas its expression in the kidney was reduced in hypertensive rats and subtotal-nephrectomized rats, indicating the discrepancy of IMD1–53 response to insults between the heart and kidney. Interestingly, ischemia upregulates IMD1–53 in both the heart and kidney, implying that the response of IMD1–53 may be tissue- and insult-specific. In this case, Chang and colleagues6 (2016) show the reduction of IMD1–53 in the plasma, aorta, and kidney. IMD1–53 deficiency conceivably renders aorta more prone to vascular calcification and kidney more prone to fibrosis in CKD.
Calcitonin receptor–like receptor and receptor activity–modifying proteins are required to transduce its biologic effects.4,5 Emerging data showed that IMD1–53 serves as a guard for the cardiovascular system to protect against ischemia- and hypertension-induced cardiac damage, and to prevent vascular calcification.4,5 Interestingly, the biologic effects of IMD1–53 are very similar to those of Klotho in retarding CKD progression and protecting from cardiovascular disease in CKD.7
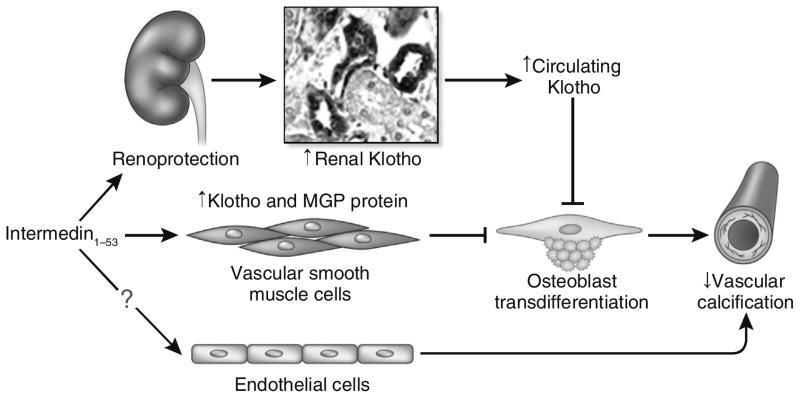
Cai et al. showed that IMD1–53 inhibited vascular calcification by increasing the level of MGP in vascular smooth muscle cells.5 The study by Chang et al. (2016) proposes a novel mechanism behind the suppression of IMD1–53 on vascular calcification, which is an MGP-independent mechanism.6 They demonstrated that IMD1–53 administration attenuated vascular calcification, suppressed osteoblast-like cell formation, and increased Klotho expression in the aorta of CKD rats. To directly examine the effect of IMD1–53 on Klotho and calcification in vascular smooth muscle cells, IMD1–53 was directly applied to cultured vascular smooth muscle cells. Interestingly and more importantly, IMD1–53 increased in vitro Klotho protein level in calcified vascular smooth muscle cells. Klotho knockdown blocked the inhibitory effect of IMD1–53 on calcification in vascular smooth muscle cells and transformation of vascular smooth muscle cells into osteoblast-like cells.7 Taken together, the in vivo and in vitro findings indicate that IMD1–53 functions as stimulator to upregulate renal, vascular, and plasma Klotho protein levels (Figure 1).
Figure 1. Schematic model of the effects of intermedin (IMD)1–53 on the suppression of vascular calcification in chronic kidney disease (CKD).
IMD1–53 protects the vasculature against calcification in CKD by direct and indirect actions. Indirect prevention of vascular calcification comes from the role of IMD1–53 in slowing down the progression of CKD, better maintaining renal Klotho expression, and consequently increasing the level of plasma Klotho in CKD (top). In addition, IMD1–53 directly upregulates Klotho and matrix γ-carboxyglutamic acid protein (MGP) protein expression in vascular smooth muscle cells (middle). The elevation of Klotho blocks the transdifferentiation of vascular smooth muscle cells into the osteoblast and inhibits vascular calcification. Whether IMD1–53 can directly protect endothelium against uremic toxin, high phosphate, or both, and prevent vascular calcification, are to be explored (bottom).
Although the protective effect of Klotho on vascular calcification has been well established, the presence of Klotho in vascular endothelial cells or smooth muscle cells is yet to be determined.8 Chang et al.’s new findings that Klotho was reduced in calcified vasculature and that this reduction in calcified vascular smooth muscle cells was attenuated by IMD1–53 support the concept that Klotho protein is expressed in vascular smooth muscle cells. Interestingly, IMD1–53 did not only alleviate kidney damage and improve renal function, but also elevated renal and plasma Klotho in CKD rats.6 Thus the beneficial effect of elevated Klotho in the circulation on vascular calcification in CKD rats cannot be ignored. However, there was a missing experiment in Chang et al.’s study examining the effect of IMD1–53 on the upregulation of renal, vascular, and plasma Klotho in normal rats. Therefore, whether IMD1–53 upregulates Klotho in the kidney and vasculature that in turn slows down CKD progression and reduces vascular calcification is still unknown.
Another interesting finding of this study is that blocking calcitonin receptor–like receptor and receptor activity–modifying protein complex signaling activity and inhibiting protein kinase A pathway effectively abolished the effects of IMD1–53 on the suppression of calcification and on the stimulation of Klotho expression in cultured vascular smooth muscle cells.6 Those results suggested that IMD1–53 attenuated vascular calcification by upregulating Klotho expression in vascular smooth muscle cells through calcitonin receptor–like receptor and receptor activity–modifying protein complex and cyclic adenosine monophosphate/protein kinase A signaling pathway. However, whether this signaling pathway is also present in the kidney is yet to be explored.
The therapeutic efficacy of Klotho in kidney disease has been unequivocally demonstrated in animal models. On one hand, the administration of exogenous Klotho has a favorable effect on CKD animals by improving renal function and attenuating cardiovascular calcification and cardiac hypertrophy. On the other hand, when local Klotho-producing cells, for instance in the kidney and vasculature, have not been entirely damaged in early stages of kidney disease or when exogenous Klotho protein for clinical administration is not available, derepression of local Klotho expression with Klotho inducers is an alternative approach to restore the plasma Klotho levels in CKD. Several studies have shown that endogenous Klotho inducers can upregulate plasma or vascular Klotho protein to prevent or reduce vascular calcification in CKD. Thus, stimulation of peroxisome proliferator–activated receptor-γ, angiotensin II inhibition, 3-hydroxy-3-methylglutaryl CoA reductase inhibition, and treatment with vitamin D derivatives and antioxidants have been shown to increase endogenous Klotho expression. The study of Chang et al. offers one more option to increase endogenous Klotho expression in CKD with administration of IMD1–53.6
Blood vessels are composed of endothelial cells, mural cells (smooth muscle cells and pericytes), their shared basement membrane, and extracellular matrix. Normal cross-talk between vascular smooth muscle cells and endothelial cells is required to maintain the integrity of the endothelial barrier. Endothelial dysfunction is recognized as a relevant factor for cardiovascular morbidity and mortality in CKD. Vascular endothelium was shown to be a source of osteoprogenitor cells in vascular calcification and also a stimulator for vascular smooth muscle cells to initiate or participate in vascular calcification in CKD. It has been shown that Klotho could act on both vascular smooth muscle cells and endothelial cells9 to protect the vasculature. On the other hand, emerging evidence indicated that IMD1–53 maintains vascular endothelial integrity and controls angiogenesis by modulating vascular endothelial growth factor signaling pathway, and also protects against vascular injury induced by oxidative stress, ischemia, inflammation, and cytokines.4,5 Those effects are presumably associated with inhibition of vascular calcification. Whether IMD1–53 suppresses vascular calcification by downregulating other promoters of calcification (Wnt, transforming growth factor-β, bone morphogenetic protein-2/4/6, or osteocalcin), upregulating other inhibitors of calcifications (osteopontin, osteoprotegerin, fetuin-A, bone morphogenetic protein-7, or pyrophosphate), or doing both, is to be examined. Furthermore, the effect of IMD1–53 on the endothelium in preventing vascular calcification in CKD is worth investigating (Figure 1).
In conclusion, the current study conducted by Chang and colleagues6 has added new elements to the modulation of Klotho expression and its role in the prevention of vascular calcification in CKD by identifying IMD1–53 as both a potent inducer of Klotho expression and a novel suppressor of vascular calcification in CKD (Figure 1). Meanwhile, several exciting questions arise as to whether, for instance, IMD1–53 upregulates Klotho under baseline conditions or only protects against Klotho downregulation in uremic state, and how IMD1–53 prevents CKD progression. Whatever the answers to these questions, IMD1–53 may be a novel therapeutic target for the retardation of CKD progression and the prevention or attenuation of cardiovascular complication in CKD.
Acknowledgments
The author is in part supported by the American Heart Association (0865235F), the National Institutes of Health (R01-DK091392 and R01-DK092461), the Pak Center Innovative Research Support, and the Pak-Seldin Center for Metabolic Research and Clinical Research.
Footnotes
DISCLOSURE
The author declared no competing interests.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu MC, Shi M, Zhang J, et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong Y, Hay DL, Quirion R, et al. The pharmacology of adrenomedullin 2/intermedin. Br J Pharmacol. 2012;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai Y, Xu MJ, Teng X, et al. Intermedin inhibits vascular calcification by increasing the level of matrix gamma-carboxyglutamic acid protein. Cardiovasc Res. 2010;85:864–873. doi: 10.1093/cvr/cvp366. [DOI] [PubMed] [Google Scholar]
- 6.Chang JR, Guo J, Wang Y, et al. Intermedin1–53 attenuates vascular calcification in rats with chronic kidney disease by upregulation of α-Klotho. Kidney Int. 2016;89:586–600. doi: 10.1016/j.kint.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 7.Hu MC, Shiizaki K, Kuro-o M, et al. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–533. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mencke R, Harms G, Mirkovic K, et al. NIGRAM Consortium. Membrane-bound Klotho is not expressed endogenously in healthy or uraemic human vascular tissue. Cardiovasc Res. 2015;108:220–231. doi: 10.1093/cvr/cvv187. [DOI] [PubMed] [Google Scholar]
- 9.Six I, Okazaki H, Gross P, et al. Direct, acute effects of Klotho and FGF23 on vascular smooth muscle and endothelium. PLoS One. 2014;9:e93423. doi: 10.1371/journal.pone.0093423. [DOI] [PMC free article] [PubMed] [Google Scholar]