Abstract
Background:
Magnesium (Mg) is an essential mineral required to regulate body temperature, nucleic acid, and protein synthesis with an important role in maintaining nerve and muscle cell electrical potentials. It may reduce fetal growth restriction and preeclampsia as well as increase birth weight. This study aimed to assess the effects of consuming Mg supplementation during pregnancy on pregnancy outcomes.
Materials and Methods:
This is a randomized controlled trial with three sixty populated groups of pregnant women. Participants were randomized to treatment or control groups through random table numbers. Participants with Mg serum levels more than 1.9 mg/dl considered as control group A randomly. They just received one multimineral tablet once a day until the end of pregnancy participants with hypomagnesemia consider as Group B and C. Participants in Group B received one multimineral tablet daily until the end of pregnancy. Participants in Group C received 200 mg effervescent Mg tablet from Vitafit Company once daily for 1 month, and also they consumed one multimineral tablet from Alhavi Company, which contains 100 mg Mg, once a day until the end of pregnancy. Intrauterine growth retardation, preterm labor, maternal body mass index, neonatal weight, pregnancy-induced hypertension, preeclampsia, gestational diabetes mellitus, cramps of the leg Apgar score were compared between three groups.
Results:
In all pregnancy outcomes, Group C that received effervescent Mg tablet plus multimineral showed a better result than other groups, and frequency of complications of pregnancy was fewer than the other two groups and showed a significant difference.
Conclusion:
Mg supplement during pregnancy likely decrease probability occurrence of many complications of pregnancy.
Keywords: Magnesium Supplement, Pregnancy, Outcomes
Introduction
Magnesium (Mg) is one of the essential minerals needed by humans in substantial large amounts. Mg work with many enzymes to regulate body temperature, synthesis nucleic acids, and proteins as well as maintaining electrical potentials in nerves and muscle membranes.[1,2]
Mg also has an important role in modulating vasomotor tone and cardiac excitability. Mg is used widely in many foods; dairy products, breads and cereals, legumes, vegetables, and meats are all good sources.[3] It is, therefore, not surprising that frank Mg deficiency has never been reported to occur in healthy individuals who eat varied diets. However, processing of the above foods can lead to high depletion of Mg.[4,5]
Common causes of Mg deficiency include inadequate dietary intake or gastrointestinal absorption, increased losses through the gastrointestinal or renal systems, and increased the requirement for Mg, such as in pregnancy.[6,7]
There are many important complications during pregnancy (gestational diabetes mellitus [GDM], pregnancy-induced hypertension [HTN], leg cramps, and preeclampsia), and a lot of them will occur the first time in pregnancy. Many of these complications are due to pregnancy or risk of occurrence that will increase during pregnancy so there are many diagnosis criteria such as Apgar score, birth weight, intrauterine growth retardation [IUGR], and preterm birth.
A study measuring serum Mg during low-risk pregnancies reported that both ionized and total serum Mg were significantly reduced after the 18th week of gestation compared to measurements before this time.[8]
Dietary intake studies during pregnancy consistently demonstrate that many women, especially those from disadvantaged backgrounds, have intakes of Mg below the recommended levels.[9]
Although present in grains, green vegetables, and seeds, insufficient Mg intake is common, especially in low-income regions. Adolescents and women are more prone to Mg deficiency.[10] It is recommended that women consume 280 mg of Mg/day,[11] increasing in pregnancy.[12] Most Mg (99%) is intracellular, such that serum levels have a low accuracy for Mg deficiency.[13] Total and ionized Mg++ are inversely associated with gestational age in pregnancy.[14] Mg deficiency in pregnancy has been associated with a higher risk of chronic HTN, preeclampsia, placental dysfunction, and premature labor.[15]
More recent data suggest that maternal Mg supplementation in pregnancy may have other perinatal benefits. Treatment was begun after 22 weeks’ gestation in most women and was sustained for a mean of about 28 days in both groups. The risk of the primary outcome of hypoxic-ischemic encephalopathy was nonsignificantly lower in the Mg++ group, but the overall event rate was lower than expected in both groups. Interestingly, the risk of third trimester stillbirth was lower in Mg group.[16]
In a retrospective study of medical records reported that Mg supplementation during pregnancy was associated with a reduced risk of fetal growth retardation and preeclampsia.[17]
In addition, there are many evidence that Mg supplementation during pregnancy may lead to prevent some pregnancy complications and improved many health indicators and pregnancy outcomes;[18,19,20,21,22,23] but as stated by others, there is not enough high-quality evidence to show that Mg supplementation during pregnancy is beneficial.[24]
Thus, according to the above mentioned, Mg supplementation during pregnancy may be able to reduce growth restriction of the fetus and preeclampsia (high blood pressure and protein in the urine during pregnancy) and increase birth weight. This study aimed to assess the effects of Mg supplementation on pregnancy outcome by randomized controlled trial with this dosage of Mg, In the previous studies, they use different dosage.
Materials and Methods
In this randomized controlled trial study, the required sample size was calculated as 60 patients in each group (totally 180 patients) using sample size formula to compare the two means’ value at 95% confidence level, the power of 80% and considering the standard deviation (SD) of serum Mg levels in the normal population which is about 1.5 mg/dl and also taking into account the significant differences between the two groups as (d = 0.75).
To continue the study, 180 pregnant women were recruited into three randomized groups. Maternal serum Mg levels of pregnant women with gestational age 12–14 weeks were measured and those whose serum Mg levels were < 1.9 mg/dl selected. Participants were randomized to treatment or control groups through random table numbers. Participants with Mg serum levels more than 1.9 mg/dl considered as control Group A randomly. They just received one multimineral tablet once a day until the end of pregnancy (N = 60). Participants with Mg levels < 1.9 consider as Group B and C. Participants in Group B received one multimineral tablet daily until the end of pregnancy (N = 60). Participants in Group C received 200 mg effervescent Mg tablet from Vitafit company once daily for 1 month, and also they consumed one multimineral tablet from Alhavi Company, that contains 100 mg Mg, once a day until the end of pregnancy (N = 60) [Chart 1].
Chart 1.
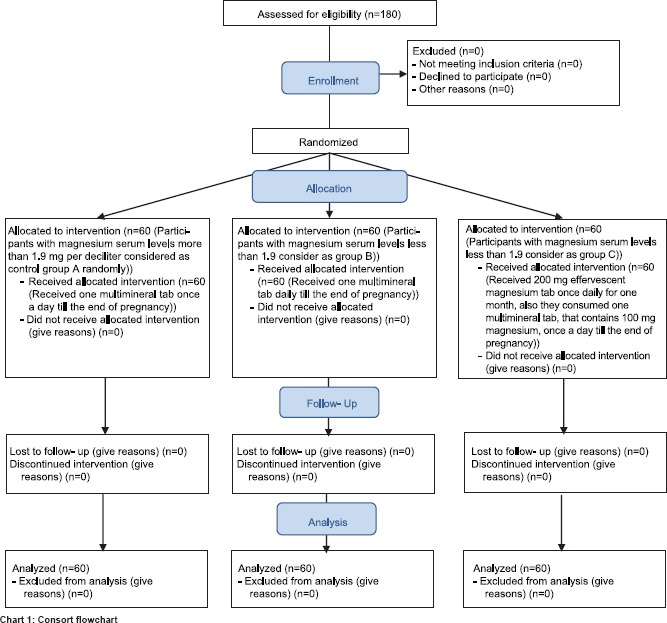
Consort flowchart
We compared the outcomes of pregnancy, including IUGR, preterm labor, maternal body mass index (BMI), neonatal weight, pregnancy-induced HTN, preeclampsia, GDM, cramps of the leg, and Apgar score between three groups.
Inclusion criteria were: have tendency to involve in this study and sign the agreement; single pregnancy; gestational age 12–14 weeks; lack of acute renal disease; hypomagnesemia in interventional group and normal Mg; lack of history chronic HTN; lack of history of overt diabetes; lack of history of severe anemia; lack of history of diagnostic heart disease; lack of anomaly in this pregnancy and previous ones; lack of use cigarette and alcohol; lack of molar pregnancy; lack of acute pancreatitis; multiparty.
Exclusion criteria were: withdrawal the study; inappropriate use of Mg; intractable vomiting.
Participants selected from the population that referred to hospital clinics. Individuals who fulfill the inclusion criteria invited to participate in this study. The trial protocol was in accordance with the Declaration of Helsinki and was approved by the Research and Ethics Committee of Isfahan University of Medical Sciences.
The trial was registered under trial registry code IRCT2015121925611N1 at the National Registry for Clinical Trials, which is a member of the World Health Organization. After providing detailed information, informed consent was signed by the participant, and oral assent from participants was obtained.
The Statistical Package for Social Sciences (SPSS) version 20.0 for Windows (SPSS, Chicago, IL, USA) was used for statistical analysis. Descriptive data were expressed as mean ± SD. Frequency tables were generated for relevant variables. Proportions were compared with the Pearson Chi-square test. A low Apgar score at 1 or 5 min was defined as a score of 7 or less. For comparisons of continuous variables in three groups, we used one-way analysis of variance (ANOVA). P < 0.05 was considered statistically significant.
Results
The baseline characteristics of multiparous pregnant women in three groups were shown in Table 1. As shown in Table 1, no significant difference in all variables of the characteristics of the study population, except for a history of diabetes that was significantly different between three groups (P = 0.006).
Table 1.
Baseline characteristics of study population
Table 2 shows the standard weight gain according to BMI. For each group, we calculated difference weight in early of pregnancy and weight in delivery. Then, for all category of BMI in three groups, calculate the excess weight gain. Chi-square test did not show a significance difference between three groups, although the percentage of excess weight gain had different in three groups. The Figure 1 shown the excess weight gain in three groups of the study. Type of delivery and sex of neonate have been shown in Table 3.
Table 2.
Standard weight gain according to body mass index

Figure 1.
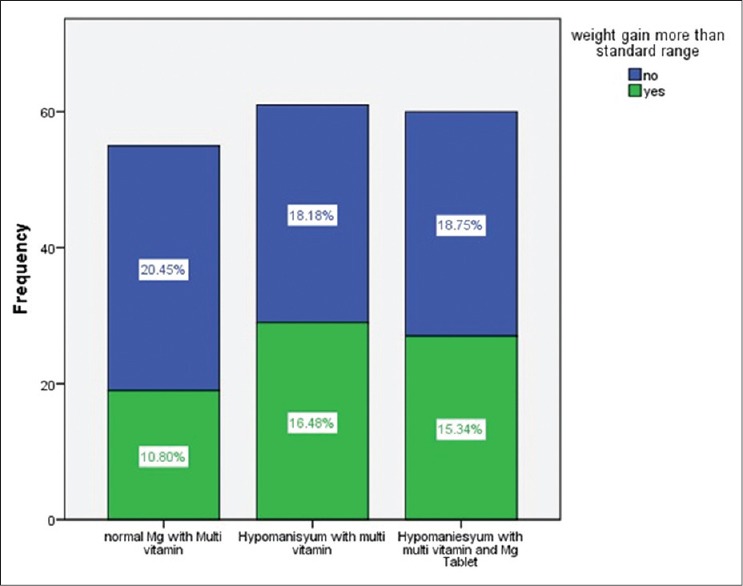
Excess weight gain in three groups of study
Table 3.
Type of delivery and sex of neonate
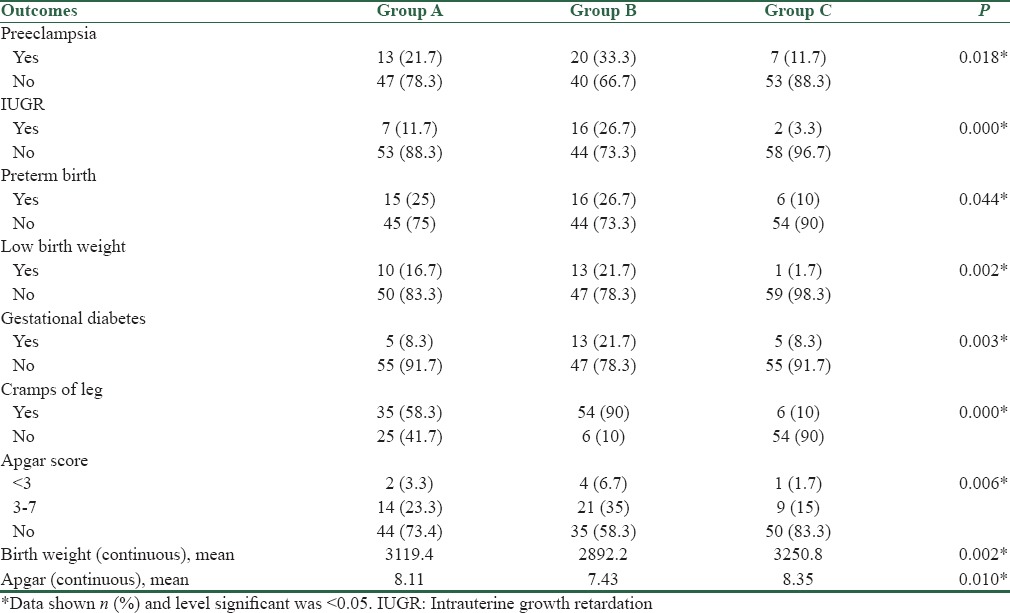
Table 4 shows the frequency of pregnancy outcomes between groups. We compared the frequency of IUGR, preterm birth, low birth weight (LBW), preeclampsia, GDM, cramps of the leg, Apgar score, stillbirth, and premature rupture of membranes with Pearson Chi-square test between three groups. A low Apgar score at 1 or 5 min was defined as a score of 7 or less. Furthermore, the mean weight of neonate between groups was assessed by one-way ANOVA. As shown in Table 4, in all pregnancy outcome, Group C that received effervescent Mg tablet plus multimineral tablet showed a better result than other groups and frequency of pregnancy complications as said above was fewer than the other two groups and demonstrated a significant difference.
Table 4.
Frequency of pregnancy outcomes between groups
Discussion
The present study showed the preventive effect of oral Mg tablet in the many of pregnancies with difficulty. Moreover, because this study set a normal group without hypo Mg, we found that hypo Mg may be a risk factor for some pregnancies with complications.
The results of the present study showed that Mg supplement administered to mothers’ antenatal leads to a reduction in many bad pregnancy outcomes. Many studies have examined the therapeutic or preventive effect of Mg in pregnancy outcomes, and some of them supported our findings. Czeizel et al., in agreement with our result, found that effect of periconceptional multivitamin on 5502 pregnant females was positive and increase fertility.[25] In addition, our results showed that IUGR was considerably reduced in oral Mg supplement groups compared with other two groups. Likewise, Roman et al. showed that maternal oral Mg supplementation reduced pregnancy-induced IUGR by 64% and suppressed cytokine/chemokine levels in the individual amniotic fluid and placentas.[26]
Today, the importance of Mg during pregnancy has been more understanding. Various studies focus on the effect of Mg on prevention or treatment of numerous pregnancy complications or pathological condition in pregnancy period.[24] Mg has an important role in homeostasis, enzyme system, and bone calcium stability.[27] As we found the beneficial effect of Mg on our oral mg supplement group such as decrease preeclampsia (P = 0.018), lower preterm birth (P = 0.044) as well as lower rate of LBW (P = 0.002), in the various clinical studies which were reviewed by Duley in 2010[28] and McDonald in 2012,[29] it has also been declared that efficiency of Mg during pregnancy is noticeable, and Mg prevents many bad effects in pregnant women, and Mg component like MgSO4 found to be efficient for preeclampsia and eclampsia.
Our evaluation demonstrated that all pregnancy outcomes such as preeclampsia (P = 0.018), IUGR (P < 0.001), preterm birth (P = 0.044), LBW (P = 0.002), GDM (P = 0.003), cramps of leg (P < 0.001), Apgar score (under 7) (P = 0.006), birth weight (continuous) (P = 0.002), and Apgar (continuous) (P = 0.01) was significantly better in Mg received group than control and multimineral tablet used groups. In accordance with our results, Shaikh et al. in his observational study found that in many pregnancy outcomes such as toxemia of pregnancy, preterm birth, Intra Uterine Growth Restriction (IUGR), and leg cramps, and pregnant women with hypo Mg have more frequent complications than normal groups.[30] Another investigation done by Doyle et al. demonstrated that Mg intakes of 513 women toward the end of the first trimester of pregnancy (300 mg/day) was associated with optimum birth weight, length, and head circumference.[31] We used 300 mg Mg pill for our Mg received group same as Doyle et al. did[31] although in the other studies they used various doses.[32] In addition, it would be beneficial for control group to administrate low-dose Mg.[32] We believe that the standard methodological usage should be created to use worldwide as the standard pattern as it recently has been noticed and discussed comprehensively.[33]
Mg is pivotal element for preventing some diseases during whole pregnancy. Mg has been stablished to be used for avoiding some unwanted condition. It could be used by food intake among pregnant women.[34,35,36] Furthermore, Mg has various physiological benefits. Numerous studies assessed the effect of Mg supplement in preventing of preeclampsia in pregnant women. Bullarbo et al. in a clinical trial designed study concluded that Mg supplementation prevents increasing of diastolic blood pressure during the last weeks of pregnancy.[37] Rudnecki et al. in a double-blind, randomized controlled study found that using Mg chloride until the end of pregnancy has a positive effect on decreasing of blood pressure during pregnancy and delivery time.[38] In agreement with them, we also found oral mg supplement group had lower rate of preeclampsia (P = 0.018). In addition, in a study by Dawson, the result showed that preeclampsia women had lower range of Mg compared to the nonpreeclampsia women.[18]
Our assessments revealed that Mg had a beneficial effect on preventing of LBW (P = 0.002). Similar to our findings, in a double-blind, randomized controlled study which was done on 985 pregnant women, using of Mg aspartate was beneficial for preventing of preterm light weight children and also preterm deliveries.[39] Other investigations also proved our result in term of low-weight delivery.[40]
Conclusion
Mg has a pivotal role in various body homeostasis, especially pregnancy period. Undoubtedly, the functioning of Mg in many different organs has already established. According to our result and other researchers’ findings, it should be said that Mg supplement during pregnancy could decrease the probability of occurrence of many complications of pregnancies. We believe that using the right dose of Mg plays a crucial role in the treatment of unwanted pregnancy disorders as well as preventing of preterm weight, LBW, and preeclampsia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Makrides M, Crosby DD, Bain E, Crowther CA. Magnesium supplementation in pregnancy. Cochrane Database Syst Rev. 2014;(4):C:CD000937. doi: 10.1002/14651858.CD000937.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNamara HC, Crowther CA, Brown J. Different treatment regimens of magnesium sulphate for tocolysis in women in preterm labour. Cochrane Database Syst Rev. 2015;12:CD011200. doi: 10.1002/14651858.CD011200.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaloga GP. Interpretation of the serum magnesium level. Chest. 1989;95:257–8. doi: 10.1378/chest.95.2.257. [DOI] [PubMed] [Google Scholar]
- 4.Mepba H, Eboh L, Banigo D. Effects of processing treatments on the nutritive composition and consumer acceptance of some Nigerian edible leafy vegetables. Afr J Food Agric Nutr Dev. 2007;7:1–18. [Google Scholar]
- 5.Rock CL, Lovalvo JL, Emenhiser C, Ruffin MT, Flatt SW, Schwartz SJ. Bioavailability of beta-carotene is lower in raw than in processed carrots and spinach in women. J Nutr. 1998;128:913–6. doi: 10.1093/jn/128.5.913. [DOI] [PubMed] [Google Scholar]
- 6.Seelig MS. Magnesium Deficiency in the Pathogenesis of Disease: Early Roots of Cardiovascular, Skeletal, and Renal Abnormalities. Newyourk, Springer Science and Business Media. 1980 [Google Scholar]
- 7.Hurley L. Magnesium-deficiency in pregnancy and its effects on the fetus. Magnes Bull. 1981;3:202–8. [Google Scholar]
- 8.Arikan G, Guecer F, Schoell W, Weiss P. Preterm labour during oral magnesium supplementation in uncomplicated pregnancies. Geburtshilfe Frauenheilkd. 1997;57:491–5. [Google Scholar]
- 9.Luke B. Nutrition During Pregnancy: Part I, Weight Gain; Part II, Nutrient Supplements. JAMA. 1991;265(2):281–2. [Google Scholar]
- 10.King DE, Mainous AG, 3rd, Geesey ME, Woolson RF. Dietary magnesium and C-reactive protein levels. J Am Coll Nutr. 2005;24:166–71. doi: 10.1080/07315724.2005.10719461. [DOI] [PubMed] [Google Scholar]
- 11.Becker W, Lyhne N, Pedersen AN, Aro A, Fogelholm M, Phorsdottir I, et al. Nordic nutrition recommendations 2004-integrating nutrition and physical activity. Scand J Nutr. 2004;48:178–87. [Google Scholar]
- 12.Young VR. Dietary Reference Intakes: For Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Washington (DC): National Academies Press; 1997. [PubMed] [Google Scholar]
- 13.Mittendorf R, Dambrosia J, Dammann O, Pryde PG, Lee KS, Ben-Ami TE, et al. Association between maternal serum ionized magnesium levels at delivery and neonatal intraventricular hemorrhage. J Pediatr. 2002;140:540–6. doi: 10.1067/mpd.2002.123283. [DOI] [PubMed] [Google Scholar]
- 14.Arikan GM, Panzitt T, Gücer F, Scholz HS, Reinisch S, Haas J, et al. Course of maternal serum magnesium levels in low-risk gestations and in preterm labor and delivery. Fetal Diagn Ther. 1999;14:332–6. doi: 10.1159/000020952. [DOI] [PubMed] [Google Scholar]
- 15.Wynn A, Wynn M. Magnesium and other nutrient deficiencies as possible causes of hypertension and low birthweight. Nutr Health. 1988;6:69–88. doi: 10.1177/026010608800600201. [DOI] [PubMed] [Google Scholar]
- 16.Harrison V, Fawcus S, Jordaan E. Magnesium supplementation and perinatal hypoxia: Outcome of a parallel group randomised trial in pregnancy. BJOG. 2007;114:994–1002. doi: 10.1111/j.1471-0528.2007.01409.x. [DOI] [PubMed] [Google Scholar]
- 17.Conradt A, Weidinger H, Algayer H. On the role of magnesium in fetal hypotrophy, pregnancy induced hypertension, and pre-eclampsia. Magnes Bull. 1984;6:68–76. [Google Scholar]
- 18.Dawson EB, Evans DR, Kelly R, Van Hook JW. Blood cell lead, calcium, and magnesium levels associated with pregnancy-induced hypertension and preeclampsia. Biol Trace Elem Res. 2000;74:107–16. doi: 10.1385/BTER:74:2:107. [DOI] [PubMed] [Google Scholar]
- 19.Dasgupta S, Ghosh D, Seal SL, Kamilya G, Karmakar M, Saha D. Randomized controlled study comparing effect of magnesium sulfate with placebo on fetal umbilical artery and middle cerebral artery blood flow in mild preeclampsia at=34 weeks gestational age. J Obstet Gynaecol Res. 2012;38:763–71. doi: 10.1111/j.1447-0756.2011.01806.x. [DOI] [PubMed] [Google Scholar]
- 20.Crowther CA, Brown J, McKinlay CJ, Middleton P. Magnesium sulphate for preventing preterm birth in threatened preterm labour. Cochrane Database Syst Rev. 2014;4:CD001060. doi: 10.1002/14651858.CD001060.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ariza AC, Bobadilla N, Fernández C, Muñoz-Fuentes RM, Larrea F, Halhali A. Effects of magnesium sulfate on lipid peroxidation and blood pressure regulators in preeclampsia. Clin Biochem. 2005;38:128–33. doi: 10.1016/j.clinbiochem.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Alves JG, de Araújo CA, Pontes IE, Guimarães AC, Ray JG. The BRAzil MAGnesium (BRAMAG) trial: A randomized clinical trial of oral magnesium supplementation in pregnancy for the prevention of preterm birth and perinatal and maternal morbidity. BMC Pregnancy Childbirth. 2014;14:222. doi: 10.1186/1471-2393-14-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul MA, Nasir UI, Khan N, Yusuf MD. Low-dose magnesium sulphate in the control of eclamptic fits: A randomized controlled trial. Arch Gynecol Obstet. 2013;287:43–6. doi: 10.1007/s00404-012-2523-z. [DOI] [PubMed] [Google Scholar]
- 24.Spatling LU, Spatling GA. Magnesium supplementation in pregnancy. A double-blind study. Br J Obstet Gynaecol. 1988;95(2):120–5. [PubMed] [Google Scholar]
- 25.Czeizel AE, Dudás I, Métneki J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report. Arch Gynecol Obstet. 1994;255:131–9. doi: 10.1007/BF02390940. [DOI] [PubMed] [Google Scholar]
- 26.Roman A, Desai N, Rochelson B, Gupta M, Solanki M, Xue X, et al. Maternal magnesium supplementation reduces intrauterine growth restriction and suppresses inflammation in a rat model. Am J Obstet Gynecol. 2013;208:383.e1–7. doi: 10.1016/j.ajog.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 27.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: Implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 28.Duley L, Gülmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;11:CD000025. doi: 10.1002/14651858.CD000025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald SD, Lutsiv O, Dzaja N, Duley L. A systematic review of maternal and infant outcomes following magnesium sulfate for pre-eclampsia/eclampsia in real-world use. Int J Gynaecol Obstet. 2012;118:90–6. doi: 10.1016/j.ijgo.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 30.Shaikh K, Das CM, Baloch GH, Abbas T, Fazlani K, Jaffery MH, et al. Magnesium associated complications in pregnant women. World Appl Sci J. 2012;17:1074–8. [Google Scholar]
- 31.Doyle W, Crawford MA, Wynn AH, Wynn SW. Maternal magnesium intake and pregnancy outcome. Magnes Res. 1989;2:205–10. [PubMed] [Google Scholar]
- 32.Franz KB. Magnesium intake during pregnancy. Magnesium. 1987;6:18–27. [PubMed] [Google Scholar]
- 33.Liu FL, Zhang YM, Parés GV, Reidy KC, Zhao WZ, Zhao A, et al. Nutrient intakes of pregnant women and their associated factors in eight cities of China: A cross-sectional study. Chin Med J (Engl) 2015;128:1778–86. doi: 10.4103/0366-6999.159354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoenaker DA, Soedamah-Muthu SS, Mishra GD. The association between dietary factors and gestational hypertension and pre-eclampsia: A systematic review and meta-analysis of observational studies. BMC Med. 2014;12:157. doi: 10.1186/s12916-014-0157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sibai BM, Villar MA, Bray E. Magnesium supplementation during pregnancy: A double-blind randomized controlled clinical trial. Am J Obstet Gynecol. 1989;161:115–9. doi: 10.1016/0002-9378(89)90246-9. [DOI] [PubMed] [Google Scholar]
- 36.Rylander R, Vormann J. Magnesium intervention studies-methodological aspects. Magnes Res. 2015;28:75–8. doi: 10.1684/mrh.2015.0385. [DOI] [PubMed] [Google Scholar]
- 37.Bullarbo M, Ödman N, Nestler A, Nielsen T, Kolisek M, Vormann J, et al. Magnesium supplementation to prevent high blood pressure in pregnancy: A randomised placebo control trial. Arch Gynecol Obstet. 2013;288:1269–74. doi: 10.1007/s00404-013-2900-2. [DOI] [PubMed] [Google Scholar]
- 38.Rudnicki M, Frölich A, Rasmussen WF, McNair P. The effect of magnesium on maternal blood pressure in pregnancy-induced hypertension. A randomized double-blind placebo-controlled trial. Acta Obstet Gynecol Scand. 1991;70:445–50. doi: 10.3109/00016349109007158. [DOI] [PubMed] [Google Scholar]
- 39.Kovács L, Molnár BG, Huhn E, Bódis L. Magnesium substitution in pregnancy. A prospective, randomized double-blind study. Geburtshilfe Frauenheilkd. 1988;48:595–600. [PubMed] [Google Scholar]
- 40.Zarcone R, Cardone G, Bellini P. Role of magnesium in pregnancy. Panminerva Med. 1994;36:168–70. [PubMed] [Google Scholar]


