Abstract
Background:
Prevalence statistics are essential for cancer control in addition to incidence and mortality data. As we know, there is no published report regarding lung cancer (LC) prevalence in Iran. Herein, we provide model-based estimates of limited time LC prevalence in Iran, 2015.
Materials and Methods:
Incidence numbers of LC were extracted from Iranian National Cancer Registry reports for 2003–2009. Trends were analyzed by joinpoint regression, assuming a logarithmic poisson model. Incidence numbers were projected up to 2015, using linear regression models which were trained by corrected annual percentage changes. A Monte Carlo-based model was generated, and absolute survival rates, number of incident cases, and incompleteness of Iranian cancer registry for LC were included into it. Limited-time prevalence (within 1, 2–3, and 4–5 years from diagnosis) and its respective 95% uncertainty level (UL) were estimated by age, gender, and histopathological type.
Results:
Five-year prevalence was estimated to be 4.21 (95% UL: 3.37–5.38) per 100,000 adult person, with a male:female ratio of 2.01. Estimated number of patients within 1, 2–3, and 4–5 years from diagnosis were 1871 (1497–2392), 993 (770–1285), and 420 (322–550), respectively. Most prevalent form of LC were squamous cell carcinoma (802; 579–999) and adenocarcinoma (319; 230–389) in males and females, respectively.
Conclusion:
According to our results, the most plausible estimates of number of alive LC patients within initial treatment, clinical follow-up, and cure phases were 2392, 1285, and 550 cases in Iran in 2015.
Keywords: Histopathologic, Iran, lung cancer, prevalence
Introduction
Lung cancer (LC) is common cancer and the most common cause of cancer mortality worldwide.[1] It is estimated to be one of the most common cancers and the second cause of cancer mortality in Iran.[2] However, lack of a representative National Cancer Registry in Iran leads to an alarming dark corridor of LC control decision-making.
Despite of the absence of a national population-based cancer registry, there is a limited body of evidence on national or subnational LC incidence, mortality and survival,[2,3,4,5,6,7] but incidence, mortality and survival statistics, even based on a representative timely national registry, could not provide adequate information for policymaking regarding cancer survivor population and its medical and social needs.[8] As we know, there is no national report on the LC prevalence in Iran but only two regional reports from Kerman and Isfahan.[9,10]
Prevalence estimation, in one approach, requires information on the vital status of all previously diagnosed cancer patients in a region, what that requires a robust cancer registry.[11] Therefore, scarcity of data regarding cancer prevalence in Iran is also a consequence of the absence of a long-time representative population-based cancer registry. Another approach requires number of cancer incident cases and survival rates. This approach would also provide robust estimates as long as number of incident cases and survival rates be robust.[12]
Iranian National Pathology Based Cancer Registry (INPBCR) reported annual number of cancer incidence from 2003 to 2009. There are also relatively robust estimates of survival rate for LC patients in low- and middle-income countries such as Iran. Accordingly, the former approach of cancer prevalence estimation is applicable to estimate LC prevalence in Iran, although some correction factors are necessary.
Herein, limited-time prevalence (patients within 1, 2–3, and 4–5 years from diagnosis) of LC in Iran was estimated, based on corrected and projected the number of LC incident cases reported by INPBCR.
Materials and Methods
LC incidence data were extracted from INPBCR reports for 2003–2009. Extracted incidence data were manipulated into three sections. In the first section, incidence numbers were entered in Joinpoint regression program 4.0.1 by year, age group (15–39, 40–44, 45–49, 50–54, 55–59, 60–64, 65–69, 70–74, and 75+), gender, and histologic type. Cases with unspecified cell type (28–25% of annual registered LC cases) were redistributed according to the proportion of known histologic types. Possible change points were identified, and annual percent changes (APCs) were estimated after removing the effect of the major registration improvement (at 2007–2009). To do this, we interpolated incidence counts between 2006 and 2012, assuming a linear trend during this period. Incidence counts for 2012, we extracted from GLOBOCAN 2012.[13] As case finding from sources other than pathological laboratories failed at 2010–2011, and incidence rates decreased to the expected pathological-based rates at 2011 and further years.
In the second section, using linear regression models which were trained by the estimated APCs for the last segment of trends lines, incidence numbers were predicted up to 2015. We used the estimated APCs for the last segment of trend lines to prevent overestimation of projected incidence rates as overall trend lines are influenced by the improvement of registration methods and consequently are artificially higher than reality.
In the third section, prevalence (the number of alive patients at 1, 2–3, and 4–5 years after cancer diagnosis) of LC was estimated by age groups, genders, and considering phases of cancer care. To do this, projected incidence numbers for 2010–2015 were entered in a Monte Carlo-based model (consisting 5000 scenarios), in which prevalence estimates and respective 95% uncertainty levels (ULs) were estimated, considering a 40–80% (with a normal distribution) of incompleteness of case ascertainment at INPBCR,[14,15,16] and a 12.5 (95% confidence intervals [CIs]: 15, 10) and 2.5 (95% CI: 0.0, 5.0) percent of 1 and 5 year-survival rates (with a normal distribution) for LC patients.[6,17]
Two, three, and four years survival rates and respective 95% CIs were interpolated assuming an exponential distribution for survival from diagnosis. Calculations and modeling were done by using MS Excel software.
Results
A total Number of projected LC Incidence for 2010, 2011, 2012, 2013, 2014, and 2015 were estimated to be 4888, 5132, 5389, 5658, 5941, and 6238 cases, respectively. Accordingly, total number of alive LC patients within 5 years from diagnosis was estimated to be 3285 (95% UL: 2632, 4172) for both genders. Estimated number of patients within initial treatment and clinical follow-up phases were 1871 (95% UL: 1497, 2392) and 993 (95% UL: 770, 1285), respectively. The prevalence of cured patients was estimated to be at 420 (95% UL: 322, 550) cases [Table 1].
Table 1.
Number of lung cancer prevalence cases in Iran, 2015

Percentage of female LC patients within 5 years from diagnosis were 32.4% (95% CI: 30.8, 34.0), and male:female ratio was 2.01. Male prevalent cases were more than females in all age groups except for 15–39 years group. A number of estimated alive LC patients were increased by age in both males and females (P value for trend < 0.05). Five years prevalence in population aged 15–39 and 75 years and upper were estimated to be 0.41 (95% UL: 0.33, 0.51) and 20.9 (95% UL: 16.84, 26.56) per 100,000 population, respectively. Patients within initial treatment phase were the most prevalent cases in all age groups and both genders. Patients within cure phase were less prevalent than who were within other phases [Figure 1].
Figure 1.
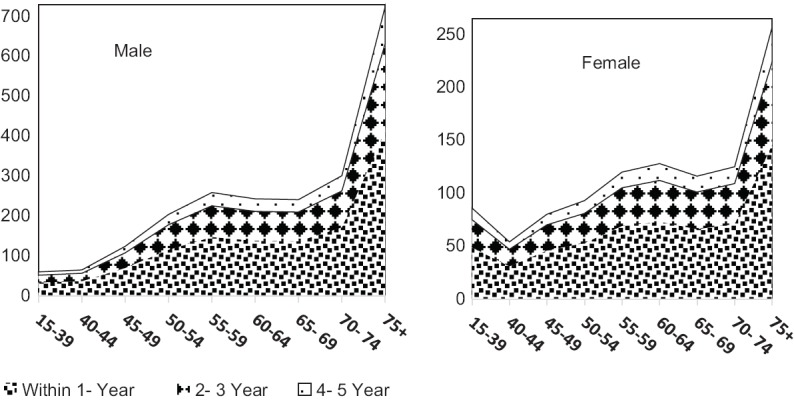
Age-distribution of prevalence of lung cancer in Iran, 2015 by gender
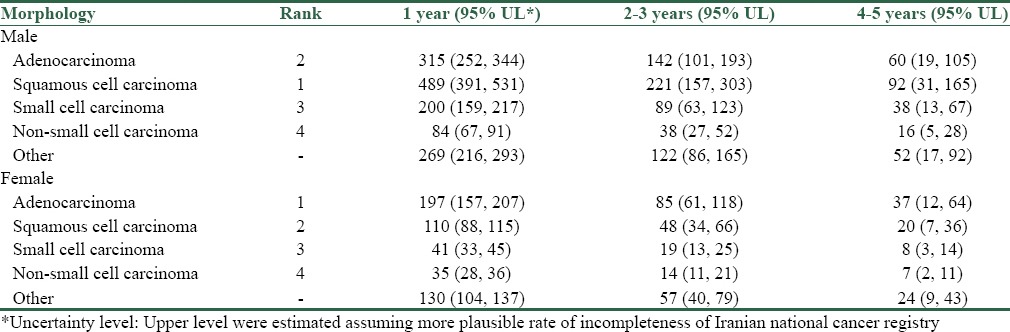
Most prevalent histopathologic types were squamous cell carcinoma (Within 5-year prevalence, 802; 95% UL, 579–999), adenocarcinoma (517; 95% UL, 372–642), and small cell carcinoma (327; 95% UL, 235–407) in males and adenocarcinoma (319; 95% UL, 230–389), squamous cell carcinoma (178; 95% UL, 129–227), and small cell carcinoma (68; 95% UL, 49–84) in females [Table 2].
Table 2.
Lung cancer prevalence in Iran, 2015, according to histopathologic type and gender (counts)
Discussion
We modeled available incidence and survival statistics to estimate limited-time prevalence of LC in Iran. Within 5-year prevalence of LC was estimated at 4.21 (95% UL: 3.37–5.38) cases per 100,000 adult person. This estimate could be considered as a part of LC overview in Iran as well as incidence and mortality statistics.
Our estimated 5-year prevalence for 2015 was relatively more than estimates by GLOBOCAN for 2012 (4015 cases in Iran).[18] This may be due to increasing trend of LC incidence in Iran in recent years or unlikely due to the improvement of survival rates for patients, or an underestimation of the incidence of LC in Iran by GLOBOCAN. However, GLOBOCAN estimates fall in our estimated 95% UL. It could be considered as an evidence of relatively proper modeling approach, which we used.
Moradpour and Fatemi reported a 5-year prevalence of 11.5 per 100,000 in Isfahan.[10] It may be due to higher risk of incidence of LC in Isfahan province. Another reasoning would be appeared when one considers survival rate of LC in Iran,[7] this prevalence is possible if only incidence rate had been more than 25 per 100,000, which is not plausible incidence estimate of LC in Iran.
Our current estimates are relatively less than our previously estimates for Kerman province.[9] Although we used a different method in the previous study, but believe that higher incidence of LC in Kerman leads to a higher prevalence for it and could explain this inconsistency.
Surprisingly, our results shown that in 15–39 years old females, prevalence of LC in any phases of diseases was more than males. It is inconsistent with overall pattern of LC incidence and prevalence worldwide.[11] It may be due to more tobacco use in younger females in Iran in recent decades and more smoking cessation in males.[19,20]
The proportion of estimated number of patients within initial treatment phase was 57% (95% CI: 55.2, 58.6) of total number of within 5-year prevalent cases. This proportion was roughly true throughout all age groups. Considering high-fatality rate of LC,[21] this finding could be explained.
Age-specific prevalence estimates for 60–64 years population were more than 65–69 years population in females and also for 55–59 years population compared with 60–64 years in males. This pattern was against the overall increasing trend of LC prevalence by age. It may be a result of cohort or period effect or more likely due to variations in coverage of registration and care-seeking behaviors in different ages.
According to our results, the predominant histologic type of LC in males was squamous cell carcinoma. This finding is consistent with international patterns.[22,23] Again, in agreement with international pattern of LC in females, the most form of LC in female cases was adenocarcinoma.[24] However, even after redistribution of cases with unspecified cell type, number of both squamous cell and adenocarcinoma cases were around 60% of registered cases. More than 20% of cases were not squamous cell, adeno, small cell, or non-small cell carcinoma. This rate of rare histopathological type could be an area for further research.
In this study, we presented the estimated number of alive LC patients and 95% ULs. In a practical point of view, the upper level of presented ULs for our estimates may be considered as the best estimates. It is more plausible because of a growing body of evidence regarding the rate of incompleteness at INPBCR, which had been reported to be < 50% for LC cases.[14,15,16]
We have some limitations in our study. First, because of the absence of national data on the survival rate of LC in Iran, we have to use survival provided for low- and middle-income countries. Our results may be insignificantly affected by this. Second, we did not find any age-specific survival rates for LC patients and consequently, we assumed uniform relative survival rates across age groups. Accordingly, variations in age-specific prevalence are only due to variations in incidence.
Conclusion
According to this study, 5-year LC prevalence was 5.38 per 100,000. More than half of the alive LC patients were estimated to be in the initial treatment phase. Therefore, Iranian policymakers and care providers should focus on supplying initial treatments for a maximum number of 2392 patients, and clinical follow-ups for 1285 patients, and social supports for a number of 550 patients, throughout the country. Adenocarcinoma and squamous cell carcinoma are the first two prevalent histopathologic types in Iran.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. Global Burden of Disease Cancer Collaboration. The global burden of cancer 2013. JAMA Oncol. 2015;1:505–27. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20:556–63. doi: 10.1093/annonc/mdn642. [DOI] [PubMed] [Google Scholar]
- 3.Vahedi M, Pourhoseingholi MA, Baghestani A, Abadi A, Sobhi S, Fazeli Z. Bayesian analysis of lung cancer mortality in the presence of misclassification. Iran J Cancer Prev. 2013;6:1–5. [Google Scholar]
- 4.Somi MH, Farhang S, Mirinezhad SK, Naghashi S, Seif-Farshad M, Golzari M. Cancer in East Azerbaijan, Iran: Results of a population-based cancer registry. Asian Pac J Cancer Prev. 2008;9:327–30. [PubMed] [Google Scholar]
- 5.Babaei M, Mousavi S, Malek M, Tosi G, Masoumeh Z, Danaei N, et al. Cancer occurrence in Semnan Province, Iran: Results of a population-based cancer registry. Asian Pac J Cancer Prev. 2005;6:159–64. [PubMed] [Google Scholar]
- 6.Zahir ST, Mirtalebi M. Survival of patients with lung cancer, Yazd, Iran. Asian Pac J Cancer Prev. 2012;13:4387–91. doi: 10.7314/apjcp.2012.13.9.4387. [DOI] [PubMed] [Google Scholar]
- 7.Abazari M, Gholamnejad M, Roshanaei G, Abazari R, Roosta Y, Mahjub H. Estimation of survival rates in patients with lung cancer in west Azerbaijan, the northwest of Iran. Asian Pac J Cancer Prev. 2015;16:3923–6. doi: 10.7314/apjcp.2015.16.9.3923. [DOI] [PubMed] [Google Scholar]
- 8.Carpenter WR, Yeh WS, Wobker SE, Godley PA. Getting cancer prevalence right: Using state cancer registry data to estimate cancer survivors. Cancer Causes Control. 2011;22:765–73. doi: 10.1007/s10552-011-9749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardanjani HM, Baneshi MR, Haghdoost A. Total and partial prevalence of cancer across Kerman Province, Iran, in 2014, using an adapted generalized network scale-up method. Asian Pac J Cancer Prev. 2015;16:5493–8. doi: 10.7314/apjcp.2015.16.13.5493. [DOI] [PubMed] [Google Scholar]
- 10.Moradpour F, Fatemi Z. Estimation of the projections of the incidence rates, mortality and prevalence due to common cancer site in Isfahan, Iran. Asian Pac J Cancer Prev. 2013;14:3581–5. doi: 10.7314/apjcp.2013.14.6.3581. [DOI] [PubMed] [Google Scholar]
- 11.Bray F, Ren JS, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 12.Pisani P, Bray F, Parkin DM. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer. 2002;97:72–81. doi: 10.1002/ijc.1571. [DOI] [PubMed] [Google Scholar]
- 13.GLOBOCAN 2012, Islamic Republic of Iran, Estimated Incidence and Prevalence, Adult Population: Both Sexes. France: IARC; 2013. [Last cited on 2016 Feb 14]. Available from: http://www.globocan.iarc.fr/old/summary_table_pop_prev.asp?selection=91364&title=Iran%2C+Islamic+Republic+of&sex=0&window=1&sort=0&submit=%C2%A0Execute . [Google Scholar]
- 14.Zendehdel K, Sedighi Z, Hassanloo J, Nahvijou A. Audit of a nationwide pathology-based cancer registry in Iran. Basic Clin Cancer Res. 2011;3:7–13. [Google Scholar]
- 15.Marzban M, Haghdoost AA, Dortaj E, Bahrampour A, Zendehdel K. Completeness and underestimation of cancer mortality rate in Iran: A report from Fars Province in Southern Iran. Arch Iran Med. 2015;18:160–6. [PubMed] [Google Scholar]
- 16.Molavi Vardanjani H. Compeleness of case acertainment at Kerman cancer registry. Kerman: Kerman University of Medical Sciences; 2015. [Google Scholar]
- 17.Gelband H, Sankaranarayanan R, Gauvreau CL, Horton S, Anderson BO, Bray F, et al. Costs, affordability, and feasibility of an essential package of cancer control interventions in low-income and middle-income countries: Key messages from Disease Control Priorities, 3rd edition. Lancet. 2016;387:2133–44. doi: 10.1016/S0140-6736(15)00755-2. [DOI] [PubMed] [Google Scholar]
- 18.Estimated incidence and prevalence, adult population Islamic Republic of Iran. France: IARC; 2013. [Last cited on 2016 Feb 14]. Available from: http://www.globocan.iarc.fr/old/summary_table_pop_prev.asp?selection=91364&title=Iran%2C+Islamic+Republic+of&sex=0&window=1&sort=0&submit=%C2%A0Execute . [Google Scholar]
- 19.Gallus S, Lugo A, La Vecchia C, Boffetta P, Chaloupka FJ, Colombo P, et al. Pricing policies and control of tobacco in Europe (PPACTE) project: Cross-national comparison of smoking prevalence in 18 European countries. Eur J Cancer Prev. 2014;23:177–85. doi: 10.1097/CEJ.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 20.Meysamie A, Ghaletaki R, Haghazali M, Asgari F, Rashidi A, Khalilzadeh O, et al. Pattern of tobacco use among the Iranian adult population: Results of the national survey of risk factors of non-communicable diseases (SuRFNCD-2007) Tob Control. 2010;19:125–8. doi: 10.1136/tc.2009.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jemal A. Lung cancer mortality. CA Cancer J Clin. 2003;53:5–26. [Google Scholar]
- 22.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histologic type: Male: female differences diminishing and adenocarcinoma rates rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 23.de Groot P, Munden RF. Lung cancer epidemiology, risk factors, and prevention. Radiol Clin North Am. 2012;50:863–76. doi: 10.1016/j.rcl.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Lortet-Tieulent J, Soerjomataram I, Ferlay J, Rutherford M, Weiderpass E, Bray F. International trends in lung cancer incidence by histological subtype: Adenocarcinoma stabilizing in men but still increasing in women. Lung Cancer. 2014;84:13–22. doi: 10.1016/j.lungcan.2014.01.009. [DOI] [PubMed] [Google Scholar]


