Abstract
Background:
The most important risk that threatens the skin wounds is infections. Therefore, fabrication of a membrane as a wound dressing with the ability of antibiotic delivery in a proper delivery rate is especially important.
Materials and Methods:
Poly(glycerol sebacate) (PGS) was prepared from sebacic acid and glycerol with 1:1 ratio; then, it was added to gelatin in the 1:3 ratio and was dissolved in 80% (v/v) acetic acid, and finally, ciprofloxacin was added in 10% (w/v) of polymer solution. The gelatin/PGS membrane was fabricated using an electrospinning method. The membrane was cross-linked using ethyl-3-(3-dimethylaminopropyl) carbodiimide ethyl-3-(3-dimethylaminopropyl)carbodiim (EDC) and N-hydroxysuccinimide (NHS) in different time periods to achieve a proper drug release rate. Fourier-transform infrared (FTIR) spectroscopy was being used to manifest the peaks of polymers and drug in the membrane. Scanning electron microscopy (SEM) was used to evaluate the morphology, fibers diameter, pore size, and porosity before and after crosslinking process. Ultraviolet (UV)-visible spectrophotometry was used to show the ciprofloxacin release from the cross-linked membrane.
Results:
FTIR analysis showed the characteristic peaks of gelatin, PGS, and ciprofloxacin without any added peaks after the crosslinking process. SEM images revealed that nanofibers’ size increased during the crosslinking process and porosity was higher than 80% before and after crosslinking process. UV-visible spectrophotometry showed the proper rate of ciprofloxacin release occurred from cross-linked membrane that remaining in EDC/NHS ethanol solution for 120 min.
Conclusion:
The obtained results suggest that this recently developed gelatin/PGS membrane with controlled release of ciprofloxacin could be a promising biodegradable membrane for wound dressing.
Keywords: Ciprofloxacin, gelatin, membrane, poly(glycerol sebacate), wound dressing
Introduction
Wound dressing, which is a protective barrier used to help many aspects of the healing process, should have properties to control fluid loss, infections, contracture, and scarring.[1]
In comparison to typical bandages, which do not provide all the requirements of wound care, electrospun fiber mats could potentially provide an ideal environment for healing owing to their capability of closely mimicking the structure of the native environment of the cells.[2] Furthermore, the rate of drugs release from a mat can be controlled by selecting polymers or through electrospinning parameters, or on the surface of the fibers. Thus, there has been an increase in research interest in developing electrospun nanofiber mats that accelerate wound healing and prevent bacterial infections.[3]
Typical antimicrobials are the most effective for early-burnt wound care as the lack of blood supply within the scar decreases the efficacy of systemic antibiotics. Silver-based treatments have been widely investigated in wound healing applications for their ability to provide antimicrobial benefits against both Gram-positive and Gram-negative bacteria,[4] whereas the use of silver nanoparticles has raised concerns as these particles can penetrate into the stratum corneum of skin or can even diffuse into the cellular plasma membrane, which causes a delay in the healing process. Furthermore, these dressing materials require successive changes of application twice a day, which is costly and painful to the patients; hence, bioresorbable antibiotic-loaded dressing may successfully eliminate this deficiency.[5,6]
Another important parameter involved in fabricating a membrane is choosing the proper polymer constituent. Gelatin has been used extensively for various tissue engineering applications. Gelatin is a denatured protein derived from the triple helix of collagen and it is a biodegradable and nonantigenic polymer which provides hemostasis and facilitates cell adhesion and proliferation during the healing process. However, poor mechanical properties, low elasticity, poorly shaped stability, and low thermal stability limit its use.[7] One of the plausible ways to modify imperfections of this polymer is combining it with other polymers. Based on some studies, combining this polymer with synthesis polymers could be a promising option.[8]
Poly(glycerol sebacate) (PGS) is an elastomeric polymer that has been produced from oxidation of glycerol and sebacic acid.[9] This colorless polyester is biocompatible, biodegradable, and inexpensive and generally has soft and flexible mechanical properties which can be tailored to match the requirements of the intended applications.[10] Furthermore, it is bioresorbable which means that it can degrade and resorb in vivo. Degradation kinetics of PGS can be controlled; thus, it can be expanded to drug delivery. Its products are nontoxic and well tolerated in the body; therefore, the US Food and Drug Administration has approved them for medical applications.[11,12] In vivo applications of PGS in nerve, vascular, skin, and myocardial tissue engineering constantly show little or mild foreign body response in acute and chronic inflammations.[13]
Ciprofloxacin hydrochloride, a second-generation fluoroquinolone, is one of the most efficient antimicrobials used for a variety applications such as skin, eye, nose, and ear infections.[14] It is indeed one of the most widely used antibiotics in wound healing[15] because of its low minimal inhibitory concentration for both Gram-positive and Gram-negative bacteria that cause wound infections; the frequency of spontaneous resistance to ciprofloxacin is very low.
It also has immense spectrum activity on both Gram-positive and Gram-negative bacteria and many microorganisms.[16,17]
Several methods, including spectrophotometry, spectrofluorometry, high-performance liquid chromatography (HPLC) with fluorescence detection, HPLC with ultraviolet (UV) detection, liquid chromatography/mass spectroscopy, capillary electrophoresis, and capillary electrophoresis, have been reported for ciprofloxacin estimation in pharmaceutical and biological samples. Four rapid, simple, accurate, and precise spectrophotometric methods were used for the ciprofloxacin determination in the presence of its acidic degradation product.[18]
Extensive studies have been performed to develop biocompatible electrospun nanofibrous membrane for wound dressing applications because electrospun fiber mats could potentially provide an excellent environment for a proper healing process through generating high levels of porosity, gas permeation, and offering a high surface-to-volume ratio. These properties promote skin regeneration, cell respiration, moisture retention, removal of exudates, and hemostasis.[19] An electrospun nanofiber membrane containing antibiotic agents has been used as a barrier to prevent the postwound infections. The combination of both of these properties can result in a perfect wound dressing material in comparison to the one being used in typical bandages.
The present investigation aims to fabricate gelatin/PGS blend membrane with controlled release of ciprofloxacin for wound dressing applications. Ciprofloxacin was added to the polymer solution and the gelatin/PGS membrane was fabricated through electrospinning method. The membrane was cross-linked using EDC/N-hydroxysuccinimide (NHS) in different time periods to achieve a proper drug release rate.
Materials and Methods
Materials
The sebacic acid powder (molecular weight [Mw] = 258.35 g/mol, chemical abstracts service [CAS] number = 110-40-7) and glycerol (Mw = 92.1 g/mol, CAS number = 56-81-5) were supplied by Merck Co., Germany, to prepare PGS. Ciprofloxacin was obtained from Alborz Darou Co., Iran.
Gelatin powder (CAS number = 9000-70-8) and its cross-linkers N, N-(3-dimethylaminopropyl)-N0-ethyl-carbodiimide hydrochloride (EDC) (Mw = 191.7 g/mol, CAS number = 25952-53-8) and NHS (Mw = 115.09 g/mol, CAS number = 6066-82-6) as well as acetic acid (CAS number = 100062) were purchased from Merck Co., Germany.
Fabrication of nanofibrous gelatin: Poly(glycerol sebacate) membrane
Gelatin: PGS nanofibrous membrane with 3:1 ratio compositions was prepared using electrospinning techniques. At first, PGS prepolymer was synthesized according to the previously reported procedures.[20]
A stoichiometric proportion of sebacic acid and glycerol with 1:1 M ratio was reacted at 120°C under nitrogen gas and high vacuum (nearly 50 mTorr) for 24 h to synthesize PGS prepolymer.[20] To define a proper membrane for wound healing, we immersed 25% (w/v) of polymers in 80% (v/v) acetic acid. For the electrospinning process, a syringe pump was used to inject the polymer solution into a 5 mL syringe having a 23-gauge blunted stainless steel needle. The flow rate was set to 0.5 mL/h. The distance between the collector and needle was set to 12 cm, while the voltage was kept constant at 18 kV during the electrospinning process.
Crosslinking process
For crosslinking process, the electrospun membrane was cut into dimensions 2 cm × 2 cm and immersed in 90% (v/v) ethanol solution containing N, N-(3-dimethylaminopropyl)-N0-ethyl-carbodiimidehydrochloride (EDC) and NHS at 4°C for different lengths of time (15, 30, 60, 120, and 240 min). Therefore, five samples of each drug and nondrug loaded membrane were prepared for different lengths of crosslinking time (n = 3). EDC concentration was optimized at 75 mM and the molar ratio of EDC/NHS, which is less cytotoxic compared to other crosslinkers such as glutaraldehyde, was selected to be 2.5:1 for gelatin crosslinking.[21] The membrane was then washed three times so that the residual of crosslinking agents by phosphate-buffered saline (PBS) could be removed.
Characterization of nanofibrous membrane
Fourier-transform infrared (FTIR) spectroscopy (JASCO, FT/IR-6300, Japan) was performed over a range of 500–4000/cm to verify the chemical composition of the membrane and to study the specific interactions between gelatin, PGS, and ciprofloxacin
The surface morphologies, fiber diameter, porosity, and interconnectivity of the electrospun membrane, before and after the crosslinking process, were characterized using scanning electron microscopy (SEM) images (Seron, Korea AIS2300C [20 kV]). Samples were broken in liquid nitrogen, and a thin coating of gold was applied on the surface of the samples to create a conductive surface
UV-visible spectrophotometry (Shimadzu UVmini 1240, Japan) was set to measure the ciprofloxacin release.
Results
Fourier-transform infrared spectroscopy test
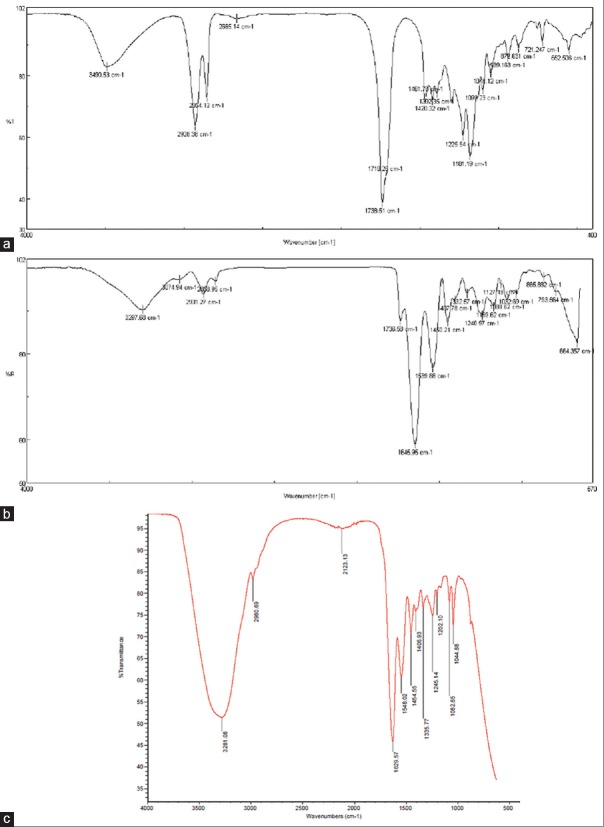
FTIR spectroscopy result was used to study the gelatin/PGS/ciprofloxacin blend interactions between the polymers and the drug. It is obvious that the peak at 1738/cm is caused by stretching vibration of the functional group of C–O and the peak at 3490/cm is attributed to the stretching vibration of functional group of O–H and also the peaks at 930/cm and 2978/cm represent the stretching vibration of functional group of –CH2 of PGS. All the results showed the characteristic peaks of PGS according to Yan et al. [Figure 1a].[22]
Figure 1.
Fourier-transform infrared spectrum of (a) poly(glycerol sebacate) in 1:1 ratio of sebacic acid and glycerol (b) gelatin/poly(glycerol sebacate) in 3:1 ratio and (c) cross-linked gelatin/poly(glycerol sebacate)/ciprofloxacin membrane
Attenuated total reflection FTIR spectroscopy analysis showed the peaks of amide bonds in gelatin nanofibers at 1631/cm and 1527/cm. In the hybrid mat, these peaks moved slightly and were detectable at 1736/cm for carbon-oxygen bonds and at 1645/cm and 1539/cm for amide bonds [Figure 1b].[23]
The remaining amine groups of the electrospun membrane significantly decreased as the crosslinking process progressed,[24] the lower intensity ratios of COOH and -OH groups of PGS within the cross-linked blended membrane compared to pure PGS prepolymer film (A[-COOH]/A[-OH]) indicated the formation of carbonyl (C = O) groups and involvement of PGS during the crosslinking process, and it was shown that strong absorption bands at 4000–2500/cm were due to stretching vibrations between hydrogen and some other atoms. The stretching O-H and N-H frequencies detected in the 3700–2500/cm region with various intensities showed the existence of ciprofloxacin [Figure 1c].[21,24]
Scanning electron microscopy test
SEM images explicitly demonstrated that crosslinking process resulted in changes mainly in morphology of fibers which were curled and fused together at multiple junctions [Figure 2].
Figure 2.
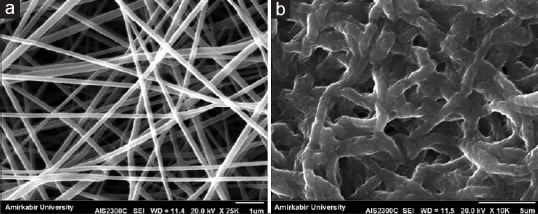
Fiber size and morphology of gelatin/poly(glycerol sebacate) membrane; (a) scanning electron microscopy images of uncross-linked fibrous membrane, (b) scanning electron microscopy images of cross-linked fibrous membrane
Measuring porosity and interconnectivity with MATLAB software version 7.0.1 (Math Soft ware Co., Math Works, USA) showed more than 80% porosity and proper interconnectivity in both cross-linked and uncross-linked membranes before and after the crosslinking process.
Release of ciprofloxacin
The rate of drug release was measured after reading the data with spectrophotometry method using the standard curve of ciprofloxacin release.
Crosslinking time is one of the effective parameters which influence drug release after the crosslinking process. Our results showed the fast and completed ciprofloxacin release after soaking membranes in PBS for the samples by 15, 30, and 60 min cross-linked time. This could be due to lack of enough time to knot the fibers although it may demonstrate that the samples which cross-linked 240 min had no more drug after crosslinking because they may take a long time to soak in the crosslinking solvent [Figure 3].
Figure 3.
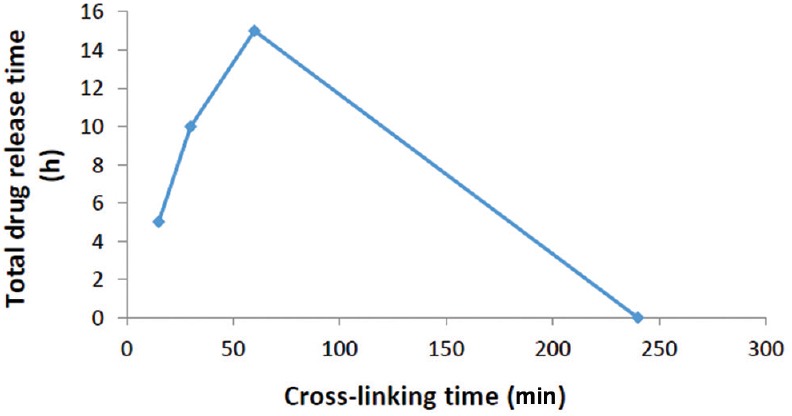
Comparison of crosslinking time of membranes on the total release time of ciprofloxacin from membranes
The sample which cross-linked 120 min showed the measurable rate of drug release; it had the 50% release in the first 24 h and another 20% in 72 h [Figure 4].
Figure 4.
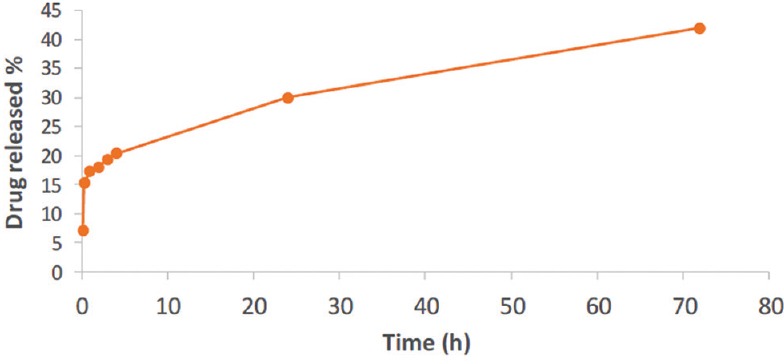
Ciprofloxacin release profile from membranes cross-linked for 120 min
Discussions
Fourier-transform infrared spectroscopy analysis
Glycerol is a simple polyol compound which hydroxyl groups along with carboxyl groups produce chemically in sebacate acid; thereby, glycerol and sebacic acid polycondense to form a unitary polymer. However, because the esterization was caused by three hydroxyl groups of glycerol and two carboxyl groups of sebacic acid, different ratios of glycerol and sebacic acid influenced the esterization degree, which resulted in different characterizations of the polymer.[25] In PGS curve, the strong peak at 3416/cm, 3548/cm, and 3615/cm can be ascribed to the stretching vibration of O-H band.[26]
The main absorption bands at characteristic peaks correspond to amide I, amide II, and amide III. Bands gather together around 3500/cm. This broadband may be attributed to bond free O-H and N-H groups that may form hydrogen bonding with the carbonyl group of the peptide linkage settled in the protein. As shown in [Figure 1a and b], outputs accommodate Guerrero et al.'s result.[27]
In numerous studies, it has been found that amide I absorption band is almost entirely because of C-O stretching vibrations of peptide linkages and is useful for the determination of the secondary structure of proteins. Strong bands located between 1500/cm and 1600/cm are known as amide II and are present as a result of C-N extension and angular deformation of the N-H ligation. Amide II bands are related to hydration.[28]
Gelatin crosslinking process within all membranes was confirmed through shifting the characteristic bands of gelatin peptide chain (i.e., amide I, amide II, and amide III) to lower wavenumbers along with reduced intensity ratio of amide I/amide II which is matched with reports of Kharaziha et al.[8] These factors are basically due to the reaction of carboxyl groups of amino acids originated from aspartic acid as well as the formation of the new covalent C-N bond.
As expected, increasing the molar ratio of EDC to gelatin ε-amino groups increased the ε-amino groups participating in the crosslinking, and therefore, it increased the extent and percentage of crosslinking similar to what occurred in Mwangi and Ofner's studies.[21]
Furthermore, the sebacic acid bands of PGS at 930/cm and 1300/cm disappeared and the intensity of carbonyl (C = O) band shifted to a lower wavenumber, confirming that PGS polymer was involved in the crosslinking process.[29]
These changes in FTIR bands, which were observed for amide I, II, and III, provide evidence with respect to increase or decrease in polypeptide chains interactions, thereby leading to toughening or weakening the textile, respectively.[28]
In FTIR spectra of ciprofloxacin, one distinguished characteristic peak was found between 3500/cm and 3450/cm, which was attributed to stretching vibration of OH groups and intermolecular hydrogen bonding. Another band at 3000–2950/cm represented alkene and aromatic C-H stretching, originally C-H. The 1950–1450/cm region exhibited FTIR absorption from a broad variety of double-bonded functional groups. The band at 1750–1700/cm represented the carbonyl C = O stretching.[24] Overall, the recorded spectrum is indicative of ciprofloxacin in gelatin/PGS textile which other researchers reported the same conclusion.[24,30]
Scanning electron microscopy analysis
Suitable membrane in the field of tissue engineering and drug delivery should provide a high surface area generated by the fibers, mimicking the natural extracellular matrix. Two main parameters that can affect the analysis of nanofibrous membrane are fiber diameter and porosity which both of them can obtained by SEM images.[31]
Figure 2 illustrates the increase in fiber size after crosslinking from 178 ± 60 nm to 872 ± 90 nm, which might be due to fibers swelling during the crosslinking process, and therefore, the shape of fibers and pore sizes changed. Our results which were determined by MATLAB software showed the higher 80% porosity in both cross-linked and uncross-linked membranes. Moreover, it was showed that porosity in membrane was interconnected before and after the crosslinking process which is completely suitable for wound dressing and controlled release of antibiotic drugs.
In other studies, Salehi et al.[32] fabricationed electrospun PGS/poly(ϵ-caprolactone) (PCL) blend to mimicking corneal stroma in 300–550 nm fibers diameter as well as the result of Vatankhah et al.[33] studies on electrospun gelatin/PCL scaffolds which revealed that fibers diameter are 415–688 nm. By comparing the obtained results with previous investigations, our fiber size without crosslinking was 178 ± 60 nm which is less than other studies, and after crosslinking process, we observed the increasing in fiber size up to 872 ± 90 nm which is nearly to results of Kharaziha et al.’s[8] study.
Ultraviolet-visible spectrophotometry analysis
Ciprofloxacin release from electrospun gelatin/PGS membrane was determined in vitro. Ciprofloxacin was loaded onto the membranes before the electrospinning process, and kinetics release was measured at 37°C by shaking gently. There was 60 μg/ml of ciprofloxacin in 1 cm × 1 cm dimension of cross-linked membrane which was determined using spectrophotometry method and the standard cure of ciprofloxacin release. The results showed that the membrane which cross-linked 15 min, released all the drugs in <15 min after soaking in PBS which might be due to lack of enough time to make strong cross-linked textile to tie up the drug properly; further, samples which cross-linked 30 and 60 min showed nearly the same result. In other words, we observed that all the 60 μg/ml of drug loaded in these textiles, released in <30 min soaking in PBS.
The membrane which cross-linked 240 min was out of drug release in PBS because of its complete release during the crosslinking process; thus, the kinetics release was very dependent on the length of crosslinking time.
The optimum conditions were obtained when the membrane was cross-linked for 120 min. Our measurements showed that the rate of releasing was gentle in the first 4 h soaking in PBS, which was 15.3, 17.2, 18, 19.25, and 20.25 μg/ml of ciprofloxacin released in 0.5, 1, 2, 3, and 4 h, respectively.
Eventually, 30 μg/ml of ciprofloxacin released after 24 h and further 12 μg/ml of the drug released after 72 h. In other words, in 120 min cross-linked membrane, we gained 50% of drug release after 24 h and 70% of the whole release after 72 h which is a suitable rate of drug release for wound dressing and prevention of the infections.
In other studies which cross-linked their blend scaffolds, decreased in the drug releasing rate observed by long crosslinking time. Han et al.'s[15] study on alginate/chitosan demonstrated that long crosslinking time lead to decrease ciprofloxacin release from the scaffold, so it seems necessary to choose the proper crosslinking time appropriate to membranes biomaterial.
Conclusion
The basic aim of this study is an initial evaluation of interaction between PGS, gelatin, and ciprofloxacin besides the releasing rate of mentioned drug. Based on the results, measured porosity was upper than 80% before and after crosslinking process, while the average fiber diameter increased by crosslinking from 178 ± 60 nm to 872 ± 90 nm. FTIR outcomes confirmed the presence of each two biomaterials and the drug after crosslinking and washing PBS. Considering UV-visible results, the most impressive drug delivery belongs to 120 min cross-linked membrane. Overall, results of this study demonstrated that novel electrospun gelatin/PGS cross-linked membrane with controlled release of ciprofloxacin could be a promising biodegradable membrane for wound dressing; however, more studies on assessing the optimum crosslinking process are suggested.
Financial support and sponsorship
This research was supported by Vice-Chancellery for Research and Technology of Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kamel RA, Ong JF, Eriksson E, Junker JP, Caterson EJ. Tissue engineering of skin. J Am Coll Surg. 2013;217:533–55. doi: 10.1016/j.jamcollsurg.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 2.Braghirolli DI, Steffens D, Pranke P. Electrospinning for regenerative medicine: A review of the main topics. Drug Discov Today. 2014;19:743–53. doi: 10.1016/j.drudis.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Rieger KA, Birch NP, Schiffman JD. Designing electrospun nanofiber mats to promote wound healing – A review. J Mater Chem B. 2013;1:4531–41. doi: 10.1039/c3tb20795a. [DOI] [PubMed] [Google Scholar]
- 4.Mohiti-Asli M, Pourdeyhimi B, Loboa EG. Novel, silver-ion-releasing nanofibrous scaffolds exhibit excellent antibacterial efficacy without the use of silver nanoparticles. Acta Biomater. 2014;10:2096–104. doi: 10.1016/j.actbio.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsner JJ, Berdicevsky I, Zilberman M. In vitro microbial inhibition and cellular response to novel biodegradable composite wound dressings with controlled release of antibiotics. Acta Biomater. 2011;7:325–36. doi: 10.1016/j.actbio.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Roy DC, Tomblyn S, Isaac KM, Kowalczewski CJ, Burmeister DM, Burnett LR, et al. Ciprofloxacin-loaded keratin hydrogels reduce infection and support healing in a porcine partial-thickness thermal burn. Wound Repair Regen. 2016;24:657–68. doi: 10.1111/wrr.12449. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal T, Narayan R, Maji S, Behera S, Kulanthaivel S, Maiti TK, et al. Gelatin/carboxymethyl chitosan based scaffolds for dermal tissue engineering applications. Int J Biol Macromol. 2016;93(Pt B):1499–506. doi: 10.1016/j.ijbiomac.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Kharaziha M, Nikkhah M, Shin SR, Annabi N, Masoumi N, Gaharwar AK, et al. PGS: Gelatin nanofibrous scaffolds with tunable mechanical and structural properties for engineering cardiac tissues. Biomaterials. 2013;34:6355–66. doi: 10.1016/j.biomaterials.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Ameer GA, Sheppard BJ, Langer R. A tough biodegradable elastomer. Nat Biotechnol. 2002;20:602–6. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 10.Moorhoff C, Li Y, Cook WD, Braybrook C, Chen QZ. Characterization of the prepolymer and gel of biocompatible poly (xylitol sebacate) in comparison with poly (glycerol sebacate) using a combination of mass spectrometry and nuclear magnetic resonance. Polym Int. 2015;64:668–88. [Google Scholar]
- 11.Rai R, Tallawi M, Grigore A, Boccaccini AR. Synthesis, properties and biomedical applications of poly (glycerol sebacate)(PGS): A review. Progr Polym Sci. 2012;37:1051–78. [Google Scholar]
- 12.Steyaert I, Rahier H, van Vlierberghe S, Olijve J, De Clerck K. Gelatin nanofibers: Analysis of triple helix dissociation temperature and cold-water-solubility. Food Hydrocoll. 2016;57:200–8. [Google Scholar]
- 13.Chen Q, Liang S, Thouas GA. Elastomeric biomaterials for tissue engineering. Prog Polym Sci. 2013;38:584–671. [Google Scholar]
- 14.Kevadiya BD, Rajkumar S, Bajaj HC, Chettiar SS, Gosai K, Brahmbhatt H, et al. Biodegradable gelatin-ciprofloxacin-montmorillonite composite hydrogels for controlled drug release and wound dressing application. Colloids Surf B Biointerfaces. 2014;122:175–83. doi: 10.1016/j.colsurfb.2014.06.051. [DOI] [PubMed] [Google Scholar]
- 15.Han F, Dong Y, Song A, Yin R, Li S. Alginate/chitosan based bi-layer composite membrane as potential sustained-release wound dressing containing ciprofloxacin hydrochloride. Appl Surf Sci. 2014;311:626–34. [Google Scholar]
- 16.Attia KA, Nassar MW, El-Zeiny MB, Serag A. Zero order and signal processing spectrophotometric techniques applied for resolving interference of metronidazole with ciprofloxacin in their pharmaceutical dosage form. Spectrochim Acta A Mol Biomol Spectrosc. 2016;154:232–6. doi: 10.1016/j.saa.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Jiang JQ. Reaction kinetics and oxidation products formation in the degradation of ciprofloxacin and ibuprofen by ferrate(VI) Chemosphere. 2015 Jan 31;119:S95–100. doi: 10.1016/j.chemosphere.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Attia KA, Nassar MW, El-Zeiny MB, Serag A. Stability-indicating methods for the analysis of ciprofloxacin in the presence of its acid induced degradation product: A comparative study. Spectrochim Acta A Mol Biomol Spectrosc. 2016;159:219–22. doi: 10.1016/j.saa.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 19.Unnithan AR, Barakat NA, Pichiah PB, Gnanasekaran G, Nirmala R, Cha YS, et al. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym. 2012;90:1786–93. doi: 10.1016/j.carbpol.2012.07.071. [DOI] [PubMed] [Google Scholar]
- 20.Masoumi N, Jean A, Zugates JT, Johnson KL, Engelmayr GC., Jr Laser microfabricated poly(glycerol sebacate) scaffolds for heart valve tissue engineering. J Biomed Mater Res A. 2013;101:104–14. doi: 10.1002/jbm.a.34305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mwangi JW, Ofner CM., 3rd Crosslinked gelatin matrices: Release of a random coil macromolecular solute. Int J Pharm. 2004;278:319–27. doi: 10.1016/j.ijpharm.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Yan W, Liu D, Tan D, Yuan P, Chen M. FTIR spectroscopy study of the structure changes of palygorskite under heating. Spectrochim Acta A Mol Biomol Spectrosc. 2012;97:1052–7. doi: 10.1016/j.saa.2012.07.085. [DOI] [PubMed] [Google Scholar]
- 23.Amjadian S, Seyedjafari E, Zeynali B, Shabani I. The synergistic effect of nano-hydroxyapatite and dexamethasone in the fibrous delivery system of gelatin and poly(l-lactide) on the osteogenesis of mesenchymal stem cells. Int J Pharm. 2016;507:1–11. doi: 10.1016/j.ijpharm.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 24.Sahoo S, Chakraborti C, Behera P, Mishra S. FTIR and Raman spectroscopic investigations of a norfloxacin/carbopol934 polymeric suspension. J Young Pharm. 2012;4:138–45. doi: 10.4103/0975-1483.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo XL, Lu XL, Dong DL, Sun ZJ. Characterization and optimization of glycerol/sebacate ratio in poly(glycerol-sebacate) elastomer for cell culture application. J Biomed Mater Res A. 2014;102:3903–7. doi: 10.1002/jbm.a.35066. [DOI] [PubMed] [Google Scholar]
- 26.Yang CX, Lei L, Zhou PX, Zhang Z, Lei ZQ. Preparation and characterization of poly (AA co PVP)/PGS composite and its application for methylene blue adsorption. J Colloid Interface Sci. 2015;443:97–104. doi: 10.1016/j.jcis.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 27.Guerrero P, Beatty E, Kerry J, de la Caba K. Extrusion of soy protein with gelatin and sugars at low moisture content. J Food Eng. 2012;110:53–9. [Google Scholar]
- 28.Clarke D, Molinaro S, Tyuftin A, Bolton D, Fanning S, Kerry JP. Incorporation of commercially-derived antimicrobials into gelatin-based films and assessment of their antimicrobial activity and impact on physical film properties. Food Control. 2016;64:202–11. [Google Scholar]
- 29.Sun ZJ, Wu L, Huang W, Zhang XL, Lu XL, Zheng YF, et al. The influence of lactic on the properties of poly (glycerol–sebacate–lactic acid) Mater Sci Eng C. 2009;29:178–82. [Google Scholar]
- 30.Singh B, Dhiman A, Kumar A. Slow release of ciprofloxacin from β-cyclodextrin containing drug delivery system through network formation and supramolecular interactions. Int J Biol Macromol. 2016;92:390–400. doi: 10.1016/j.ijbiomac.2016.07.060. [DOI] [PubMed] [Google Scholar]
- 31.Širc J, Hobzová R, Kostina N, Munzarová M, Juklícková M, Lhotka M, et al. Morphological characterization of nanofibers: Methods and application in practice. J Nanomater. 2012;2012:121. [Google Scholar]
- 32.Salehi S, Fathi M, Javanmard SH, Bahners T, Gutmann JS, Ergün S, et al. Generation of PGS/PCL blend nanofibrous scaffolds mimicking corneal stroma structure. Macromol Mater Eng. 2014;299:455–69. [Google Scholar]
- 33.Vatankhah E, Semnani D, Prabhakaran MP, Tadayon M, Razavi S, Ramakrishna S. Artificial neural network for modeling the elastic modulus of electrospun polycaprolactone/gelatin scaffolds. Acta Biomater. 2014;10:709–21. doi: 10.1016/j.actbio.2013.09.015. [DOI] [PubMed] [Google Scholar]