Abstract
Membranous nephropathy (MN) is one of the common cause of nephrotic syndrome. The discrimination between primary MN (iMN) and secondary MN is essential because of treatment implications. Immunohistochemical (IHC) evaluation with the help of anti-phospholipase A2 receptor (PLA2R) antibody helps in tissue evaluation of iMN, which is an easy, cost-effective, and pathologist-friendly technique. The study included 82 cases of MN over a period of 3 years. IHC using PLA2R antibody was performed on iMN and secondary cases with adequate tissue. Cases of minimal change disease (MCD) were included as control. Granular staining along the basement membrane in the absence of staining of podocytes was considered positive. Medical records were verified for clinical information, baseline biochemical parameters, details of viral markers, connective tissue disease profile, and basic imaging workup. Of the 82 cases of MN, 51 were iMN and 31 secondary MN (sMN). Thirteen MCD cases were included as control. IHC with PLA2R antibody showed a sensitivity of 91.8% and specificity of 95.1%, positive predictive value of 95.7%, and negative predictive value of 90.7% in the diagnosis of iMN. The other parameters, either clinical or laboratory, did not show significant differences between iMN and sMN groups. The results of PLA2R staining by IHC were comparable with other studies and showed a higher sensitivity (91.8%) and specificity (95.1%). IHC with anti-PLA2R antibody can be considered as the standard diagnostic approach to identify iMN and offer scope for individualized treatment.
Keywords: Immunohistochemistry, membranous nephropathy, phospholipase A2 receptor
Introduction
Membranous nephropathy (MN) is a common cause of adult nephrotic syndrome (NS). The idiopathic/primary MN (iMN) forms the larger proportion of MN in adults. The secondary form has a wide variety of associated conditions ranging from autoimmune diseases to malignancies, drugs, and infections.[1] The difference in clinical features, basic biochemical parameters, and microscopic features between the two groups is nonspecific. Hence, iMN can be diagnosed only after exclusion of all the known secondary causes. Identification of M-type phospholipase A2 receptor (PLA2R1) as the target antigen on podocytes has been a breakthrough in the pathogenesis of iMN.[2] Identification of iMN cases with certainty offers scope for individualized treatment and also limits the necessity for exhaustive workup for identification of secondary causes. There are many reports on serological testing for PLA2R1 antibodies for detection of iMN cases with varying sensitivity and specificity.[2,3] Serologic diagnosis may not always be feasible and appropriate owing to fluctuations in titers.[4,5] Tissue expression of PLA2R1 on renal biopsies has been primarily studied by direct and indirect immunofluorescence technique on archival tissue blocks.[6] Hoxha et al. have published the first series of immunohistochemical (IHC) expression of PLA2R1 on renal biopsies.[7] In this study, we have analyzed the PLA2R1 expression on renal biopsies with IHC with respect to its sensitivity and specificity in the diagnosis of iMN.
Materials and Methods
This was a prospective and retrospective study undertaken in the Department of Pathology, Nizam's Institute of Medical Sciences including 82 cases of MN with 13 controls. This included 51 iMN and 31 sMN cases. The study was approved by the Institutional Ethics Committee. A known secondary cause was evaluated with appropriate history and investigations.
Inclusion criteria
Cases diagnosed as iMN and sMN with adequate tissue available for IHC.
Exclusion criteria
Other glomerulonephritis
Tissue inadequate for IHC.
Cases of minimal change disease (MCD) were taken as control.
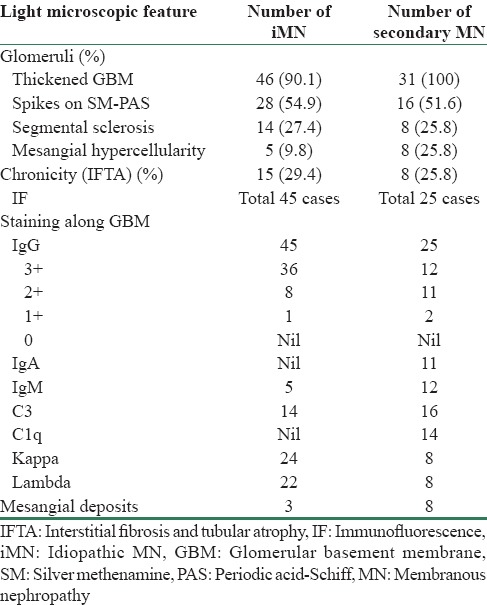
The light microscopy (LM) of renal biopsies was reviewed with the help of Hematoxylin and Eosin, periodic acid–Schiff, Masson Trichrome, and silver methenamine. The findings studied on LM were thickening of glomerular basement membrane (GBM), presence/absence of spikes, segmental sclerosis, mesangial hypercellularity, and chronic tubulointerstitial changes including tubular atrophy and interstitial fibrosis presented as % chronicity in the cortical core.
Immunohistochemistry
IHC using PLA2R antibody (HPAO12657, Sigma-Aldrich) was performed with horseradish peroxidase polymer technique and interpreted as follows:
PLA2R positive = Granular staining along basement membrane alone; absent in podocytes [Figure 1a and b]
PLA2R negative = Staining present in podocytes; absent along basement membrane [Figure 1c]
PLA2R equivocal = Staining present both along basement membrane as well as in podocytes [Figure 1d].
Figure 1.
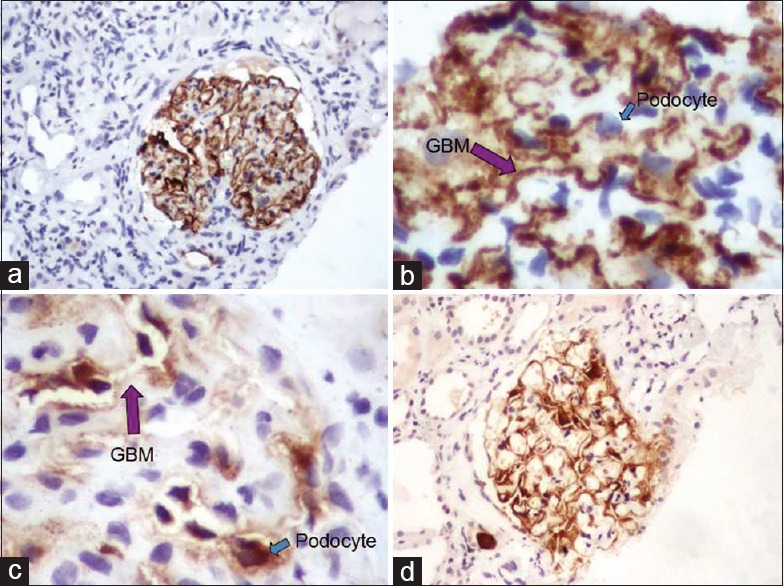
Immunohistochemical positivity of phospholipase A2 receptor in (a and b) idiopathic membranous nephropathy with granular positivity along the glomerular basement membrane (purple arrow) with absence of staining in podocytes (blue arrow). (c) Negative staining in secondary membranous nephropathy with expression only in podocytes (blue arrow). (d) Immunohistochemical expression along the basement membrane as well as podocytes in equivocal cases
Serum PLA2R levels were not available. Medical records were verified for clinical information and baseline biochemical parameters. Details of viral markers, connective tissue disease profile, and basic imaging workup were available in all the cases.
The details of possible secondary etiologies that could have surfaced after the diagnosis of iMN were also obtained from follow-up data.
Statistical analysis was performed using GraphPad Prism version 6. Demographic and relevant clinical and biochemical parameters are presented as mean ± standard deviation. The utility of PLA2R as a diagnostic marker of iMN was assessed by computing sensitivity, specificity, positive predictive value, and negative predictive value. The difference in various parameters between different groups was computed using unpaired t-tests, and the correlation between parameters was done using Pearson's correlation test/coefficient. P < 0.05 was considered statistically significant.
Results
There are a total of 82 cases of MN with 51 iMN and 31 sMN. Thirteen MCD cases are included as control. Among the secondary cases, systemic lupus erythematosus (SLE) accounted for the majority. The distribution of sMN is given in Chart 1.
Chart 1.
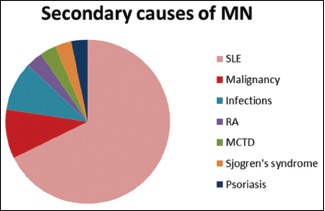
Distribution of secondary membranous nephropathy cases of which systemic lupus erythematosus was predominant
The patients were in the age range from 12 to 75 years with a mean of 39.07 years. The majority were in third and fourth decade with five patients below 18 years of age all of which are sMN cases including four SLE and one malaria.
LM and IF features of iMN and sMN are compared in Table 1.
Table 1.
Comparison of light microscopy and immunofluorescence features between idiopathic membranous nephropathy and secondary membranous nephropathy
The IHC positivity for PLA2R in different groups is given in Chart 2. IHC with PLA2R antibody showed sensitivity of 91.8% and specificity of 95.1%, positive predictive value of 95.7%, and negative predictive value of 90.7% in the diagnosis of iMN [Chart 3].
Chart 2.
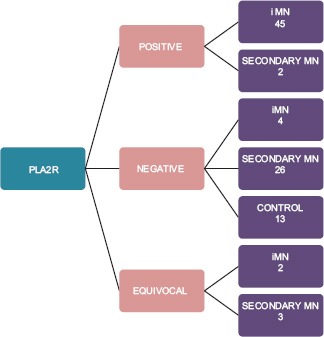
Immunohistochemistry results in different groups of membranous nephropathy
Chart 3.
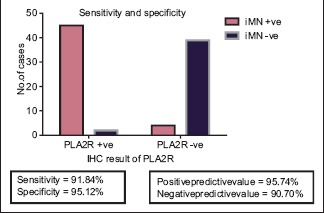
Statistical analysis of phospholipase A2 receptor in the diagnosis of idiopathic membranous nephropathy
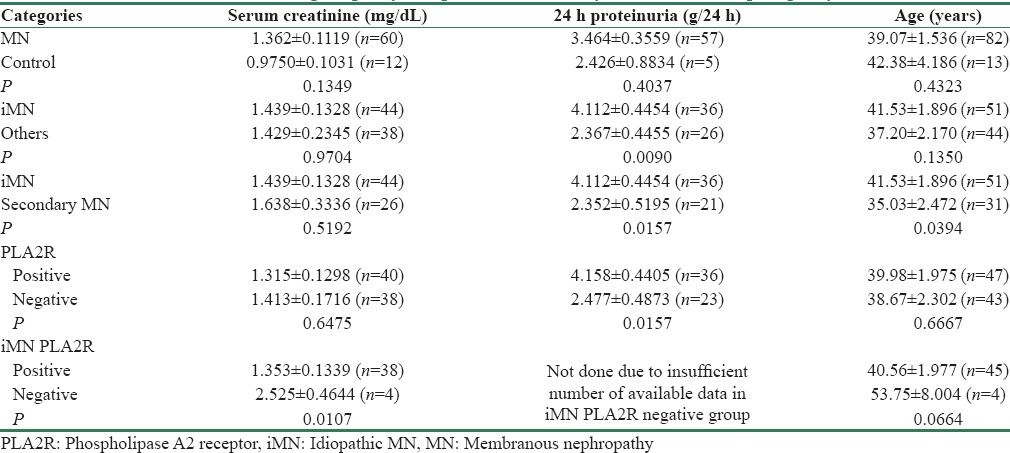
Table 2 gives the statistical analysis of comparison of 24 h proteinuria, serum creatinine at biopsy, and age between iMN, sMN, and control groups. Proteinuria and age were significantly higher in iMN (P = 0.01 and 0.03, respectively). Among PLA2R+ and PLA2R - cases, there was a significant difference in proteinuria with higher 24 h proteinuria in PLA2R+ cases (P = 0.01). Other differences were statistically not significant. Within iMN, there was significantly higher serum creatinine in PLA2R− group. Serum creatinine showed a positive correlation with interstitial fibrosis and tubular atrophy (IFTA) with P = 0.003. Summary of statistical analysis is given in Table 2.
Table 2.
Statistical analysis of comparison between different groups shows higher age and proteinuria in idiopathic membranous nephropathy compared to secondary membranous nephropathy
Discussion
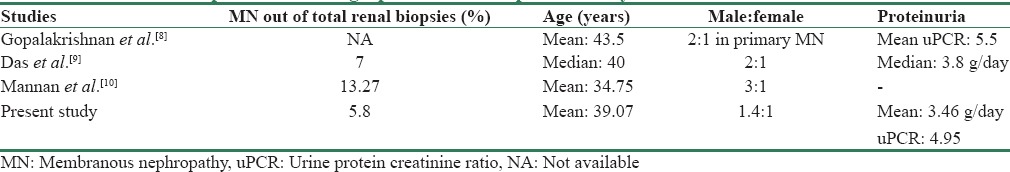
MN is one of the most common causes of NS in adults. About 40%–50% patients can progress to end-stage renal disease if not treated properly. The epidemiologic studies published about MN show variations in the baseline clinical and biochemical parameters worldwide. The demographic data of our study are comparable to other Indian studies in this respect with subtle differences [Table 3].
Table 3.
Comparison of demographic data of the present study with other similar Indian studies
MN is significantly rare in pediatric population and accounts for 3% of the biopsies done in pediatric patients.[11] Most of the pediatric MN have been found to be secondary.[12] The same was identified in our study where all the pediatric MN (n = 5) were due to known secondary causes and all iMN were seen in adult patients. The presence of associated mesangial hypercellularity has been shown to be more common in sMN. About 25% of the MN can develop secondary focal segmental glomerulosclerosis (FSGS) which further contributes to proteinuria.[13] We observed that 27% of our biopsies of MN showed FSGS at diagnosis. Tubulointerstitial chronicity, vascular sclerosis, and FSGS have been shown to have low creatinine clearance. In our study, the % IFTA showed statistically significant correlation with serum creatinine at biopsy while FSGS did not.
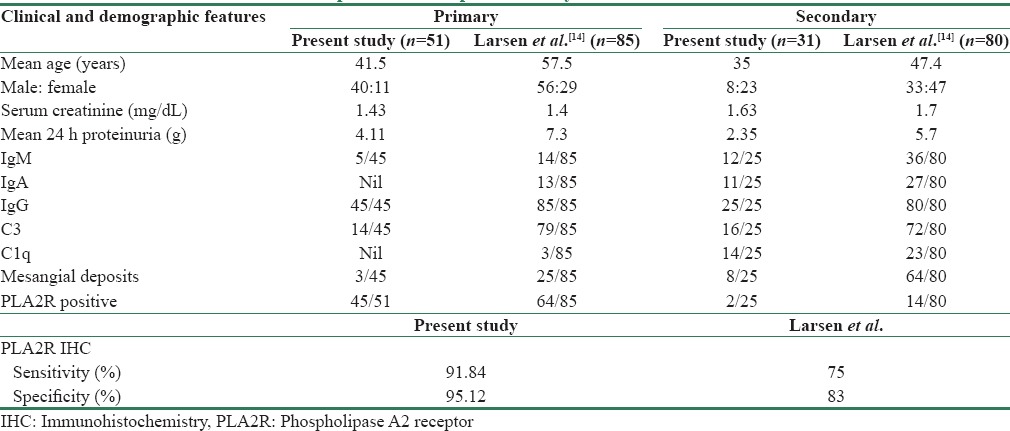
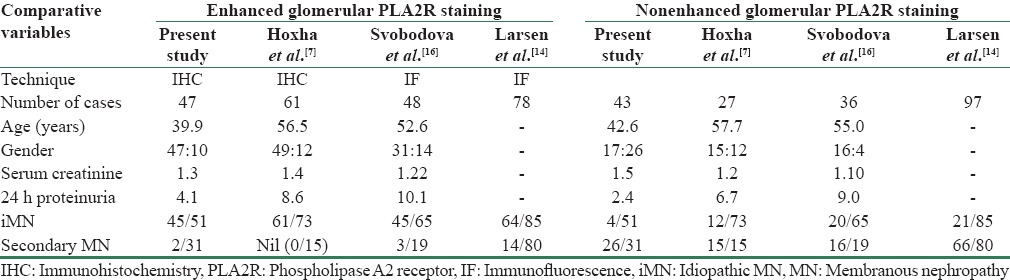
Table 4 gives comparison of age and demographic and microscopic features of the present study with that of Larsen et al.[14]
Table 4.
Comparison of the present study with that of Larsen et al.
The beginning of testing for anti-PLA2R antibodies started with estimation in serum. It was proposed that elevation of serum anti-PLA2R antibody could help avoid a percutaneous renal biopsy particularly in cases where it is contraindicated. Anti-PLA2R autoantibodies have been found to be present in >75% of individuals with iMN.[14] Antibodies disappear with remission of disease while recurrence of antibodies indicates relapse. The antibodies may not be detectable always due to various reasons such as fluctuations in titers, immunological remission, or administration of immunosupressants.[4,5]
Debiec and Ronco have clearly demonstrated that the absence of circulating PLA2R antibody at the time of kidney biopsy and proteinuria was probably not sufficient to rule out a diagnosis of PLA2R related MN.[15] Diagnosing iMN on renal tissue offers promising scope as the material is available at the same time as the diagnosis of MN. The study by Svobodova et al.[16] was the first one to highlight the utility of testing PLA2R on archival renal biopsies in patients who did not have serum antibody levels at diagnosis. They conclude that PLA2R antigen in biopsy specimens is more sensitive than the serological test alone for the diagnosis of PLA2R-related MN in case of delayed serum sampling. They found that PLA2R on tissue was more useful in distinguishing sMN due to SLE as compared to other secondary causes of MN. None of the lupus cases in their study showed positivity for PLA2R; however, three secondary cases (two of hepatitis B and one sarcoidosis) were positive for PLA2R. Similarly, two of our secondary cases (SLE and Sjogren's) were positive for PLA2R. The similar case has been demonstrated by other studies. PLA2R+ MN has been described in patients with sarcoidosis, hepatitis C, Sjogren's disease, malignancy, hepatitis B virus (HBV), lupus, and hematopoietic stem cell transplant.[3,14,16,17,18,19,20,21] It could be possible that the association is not relevant between the secondary cause and MN. Follow-up studies would perhaps give better insight if the MN resolves with treatment of underlying cause. We do not have follow-up details in our two patients of sMN showing positive PLA2R. Qin et al.[3] have shown that proteinuria did not normalize in HBV positive patients after antiviral therapy. One of the studies also mentions that occurrence of malignancy is higher in PLA2R-negative patients.[22]
Studies on testing PLA2R in renal tissue using IF are also very few in the Indian context[8,23] with results similar to that of western studies. The sensitivity of PLA2R has been shown to be higher on tissue sections as compared to serum. Debiec et al. have reported sensitivities of the serum and biopsy tests to be 57% and 74%, respectively. Hofstra and Wetzels[22] have beautifully analyzed the sensitivity of PLA2R by IF and Western blot studies and found that it ranges from 52% to 78% in various studies published in literature. Tissue testing is generally more sensitive than serologic testing.[15,16,24]
A study by Hoxha et al.[7] was the first elaborate expression of PLA2R IHC on renal biopsies. They found a sensitivity of 82% in diagnosis of iMN. We have observed higher sensitivity and specificity in comparison to literature. Although an attempt was made to rule out the secondary causes of MN in our patients, a longer follow-up is perhaps necessary to look for a late occurrence of a possible secondary etiology. There are no studies published yet on IHC using antibodies to PLA2R on renal tissue in India.
The majority of the tissue based studies about PLA2R are direct immunofluorescence assays performed on paraffin blocks. However, these studies have shown to be technically cumbersome with requirement of specialized equipment like a confocal microscope. Assandri et al. in their review on PLA2R have mentioned the limitations of IF studies for PLA2R including difficulties in standardization and interpretative errors.[6] In comparison, IHC is more pathologist-friendly technique which is widely available across the globe in majority of the laboratories. Hence, studies of PLA2R can be made easily available by carrying out IHC on tissues also on archival blocks.
Almost all the studies done on PLA2R on serum or tissue have shown that there is no significant difference in laboratory parameters in PLA2R positive versus negative patients. The similar was identified in our study. Table 5 gives a comparative analysis of the present study and other studies with respect to clinical and lab parameters. In our study, five cases showed equivocal PLA2R staining, i.e. with combined positivity in podocytes as well as along GBM. In such cases, further correlation with serum PLA2R antibodies will give true judgment of either a positive or negative result.
Table 5.
Comparative analysis of present study and other studies with respect to clinical and lab parameters
Conclusion
We have observed high sensitivity and specificity of PLA2R by IHC on renal biopsies for the diagnosis of iMN. IHC is a reliable technique to differentiate between iMN and sMN if serum levels are not available at diagnosis. Negative IHC expression of PLA2R should warrant necessary investigations for a secondary cause with close clinical follow-up. To the best of our knowledge, this is the first study in India about IHC expression of PLA2R in MN.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Schwartz MM. Membranous glomerulonephritis. In: Jennette JC, Heptinstall RH, editors. Heptinstall's Pathology of the Kidney. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 205–52. [Google Scholar]
- 2.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qin W, Beck LH, Jr, Zeng C, Chen Z, Li S, Zuo K, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–43. doi: 10.1681/ASN.2010090967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–50. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck LH, Jr, Salant DJ. Membranous nephropathy: Recent travels and new roads ahead. Kidney Int. 2010;77:765–70. doi: 10.1038/ki.2010.34. [DOI] [PubMed] [Google Scholar]
- 6.Assandri R, Montanelli A, Monari M, Podestà MA, Graziani G. Anti-phospholipase A2 receptor antibodies in membranous nephropathy: From bench to the patient. J Nephrol Ther. 2014;4:155. [Google Scholar]
- 7.Hoxha E, Kneißler U, Stege G, Zahner G, Thiele I, Panzer U, et al. Enhanced expression of the M-type phospholipase A2 receptor in glomeruli correlates with serum receptor antibodies in primary membranous nephropathy. Kidney Int. 2012;82:797–804. doi: 10.1038/ki.2012.209. [DOI] [PubMed] [Google Scholar]
- 8.Gopalakrishnan N, Abeesh P, Dineshkumar T, Murugananth S, Sakthirajan R, Raman GS, et al. Prevalence of serum anti M-type phospholipase A2 receptor antibody in primary membranous nephropathy: A single center experience. Indian J Nephrol. 2016;26:257–61. doi: 10.4103/0971-4065.160334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das U, Dakshinamurty KV, Prayaga A. Pattern of biopsy-proven renal disease in a single center of South India: 19 years experience. Indian J Nephrol. 2011;21:250–7. doi: 10.4103/0971-4065.85482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mannan R, Bhasin TS, Singh PA, Misra V, Manjari M. The pattern of glomerulonephritis in the North Indian gangetic plain-A 13-year epidemiological study. J Clin Diagn Res. 2012;6:855–88. [Google Scholar]
- 11.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: Evaluation, classification, and stratification. Pediatrics. 2003;111(6 Pt 1):1416–21. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 12.Menon S, Valentini RP. Membranous nephropathy in children: Clinical presentation and therapeutic approach. Pediatric Nephrology (Berlin, Germany) 2010;25:1419–28. doi: 10.1007/s00467-009-1324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Troyanov S, Roasio L, Pandes M, Herzenberg AM, Cattran DC. Renal pathology in idiopathic membranous nephropathy: A new perspective. Kidney Int. 2006;69:1641–8. doi: 10.1038/sj.ki.5000289. [DOI] [PubMed] [Google Scholar]
- 14.Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–15. doi: 10.1038/modpathol.2012.207. [DOI] [PubMed] [Google Scholar]
- 15.Debiec H, Ronco P. PLA2R autoantibodies and PLA2R glomerular deposits in membranous nephropathy. N Engl J Med. 2011;364:689–90. doi: 10.1056/NEJMc1011678. [DOI] [PubMed] [Google Scholar]
- 16.Svobodova B, Honsova E, Ronco P, Tesar V, Debiec H. Kidney biopsy is a sensitive tool for retrospective diagnosis of PLA2R-related membranous nephropathy. Nephrol Dial Transplant. 2013;28:1839–44. doi: 10.1093/ndt/gfs439. [DOI] [PubMed] [Google Scholar]
- 17.Kanigicherla D, Gummadova J, McKenzie EA, Roberts SA, Harris S, Nikam M, et al. Anti-PLA2R antibodies measured by ELISA predict long-term outcome in a prevalent population of patients with idiopathic membranous nephropathy. Kidney Int. 2013;83:940–8. doi: 10.1038/ki.2012.486. [DOI] [PubMed] [Google Scholar]
- 18.Van Damme B, Tardanico R, Vanrenterghem Y, Desmet V. Adhesions, focal sclerosis, protein crescents, and capsular lesions in membranous nephropathy. J Pathol. 1990;161:47–56. doi: 10.1002/path.1711610109. [DOI] [PubMed] [Google Scholar]
- 19.Timmermans SA, Damoiseaux JG, Heerings-Rewinkel PT, Ayalon R, Beck LH, Jr, Schlumberger W, et al. Evaluation of anti-PLA2R1 as measured by a novel ELISA in patients with idiopathic membranous nephropathy: A cohort study. Am J Clin Pathol. 2014;142:29–34. doi: 10.1309/AJCP8QMOY5GLRSFP. [DOI] [PubMed] [Google Scholar]
- 20.Knehtl M, Debiec H, Kamgang P, Callard P, Cadranel J, Ronco P, et al. A case of phospholipase A2 receptor-positive membranous nephropathy preceding sarcoid-associated granulomatous tubulointerstitial nephritis. Am J Kidney Dis. 2011;57:140–3. doi: 10.1053/j.ajkd.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Huang X, Qin W, Zhang M, Zheng C, Zeng C, Liu Z. Detection of anti-PLA2R autoantibodies and IgG subclasses in post-allogeneic hematopoietic stem cell transplantation membranous nephropathy. Am J Med Sci. 2013;346:32–7. doi: 10.1097/MAJ.0b013e318267b5cd. [DOI] [PubMed] [Google Scholar]
- 22.Hofstra JM, Wetzels JF. Anti-PLA2R antibodies in membranous nephropathy: Ready for routine clinical practice? Neth J Med. 2012;70:109–13. [PubMed] [Google Scholar]
- 23.Ramachandran R, Kumar V, Kumar A, Yadav AK, Nada R, Kumar H, et al. PLA2R antibodies, glomerular PLA2R deposits and variations in PLA2R1 and HLA-DQA1 genes in primary membranous nephropathy in South Asians. Nephrol Dial Transplant. 2016;31:1486–93. doi: 10.1093/ndt/gfv399. [DOI] [PubMed] [Google Scholar]
- 24.Hayashi N, Akiyama S, Okuyama H, Matsui Y, Adachi H, Yamaya H, et al. Clinicopathological characteristics of M-type phospholipase A2 receptor (PLA2R)-related membranous nephropathy in Japanese. Clin Exp Nephrol. 2015;19:797–803. doi: 10.1007/s10157-014-1064-0. [DOI] [PubMed] [Google Scholar]


