Abstract
Inflammation plays a crucial role in acute kidney injury (AKI). The current study was designed to analyze the influence of prednisolone treatment on the inflammatory reaction during the first 96 h after AKI induction in a rat model. AKI was induced by unilateral clipping of the renal vessels. The treatment group received prednisolone 5 mg/kg s.c. daily. Infiltration rates of macrophages, leukocytes, and T-cells (24, 96 h) as well as plasma concentrations of the inflammatory markers intercellular adhesion molecule, interleukin-1 beta (IL-1β), IL-18, IL-6, and tumor necrosis factor-alpha (0, 6, 24, 96 h) were determined by fluorescence-activated cell sorting (FACS) analysis only. Ninety-six hours after AKI induction, the prednisolone group demonstrated significantly lower creatinine concentrations compared to the control group (P < 0.05). Twenty-four hours after induction of AKI, a significantly higher rate of infiltrating leukocytes was detectable with FACS analysis in the control group (P < 0.01) with a corresponding significantly higher rate of macrophages after 96 h (P < 0.01). IL-6 and IL-1β demonstrated a peak after 6 h with a significantly higher release in the control group (IL-6: P < 0.01; IL-1β: P < 0.05). In contrast to the control group, the prednisolone group demonstrated no further incline of IL-18 after 24 h. The results demonstrate the importance of stretching the observation period in an ischemia-reperfusion-induced AKI setting beyond the first 24 h. Despite the demonstrated protective effects of a continuous prednisolone application, it seems that this single anti-inflammatory agent will not be able to completely suppress the inflammatory response after an ischemia-reperfusion-induced AKI.
Keywords: Acute kidney injury, fluorescence-activated cell sorting, inflammation, prednisolone, rats
Introduction
Acute kidney injury (AKI) is a complex disorder with a wide variety of etiologies and corresponding risk factors.[1,2,3,4] The common causes for AKI include renal ischemia, systemic inflammatory processes/sepsis, hemodynamic insufficiency, and nephrotoxicity.[5] The current study was designed to analyze the course of the inflammatory response during the first 96 h after ischemia-reperfusion-induced AKI as well as the impact of a continuous corticosteroid application on this response. Furthermore, we analyzed the feasibility and efficiency of using a fluorescence-activated cell sorting (FACS) analysis for the plasma and tissue samples instead of the conventional analyzing methods (polymerase chain reaction/Western Blotting, ELISA, and immunohistological staining) to simplify the analyzing process.
Materials and Methods
All procedures were performed according to the Guide for the Care and Use of Laboratory Animals published by the National Academy of Sciences and were approved by the local authorities (Regional Council Karlsruhe, Germany [G162/13]).
Experimental animal model
AKI was induced by unilateral clipping of the renal vessels in Lewis rats for 45 min. After reperfusion of the clipped kidney, the contralateral kidney was removed. The body weight was determined before surgery and before termination.
Treatment protocol
Group 1 – untreated control group (n = 9): Blood sampling before surgery and 6 and 24 h after removing the clip. Rats were sacrificed for organ retrieval after 24 h
Group 2 – prednisolone treated (n = 10): Blood sampling before surgery and 6 and 24 h after removing the clip. Rats were sacrificed for organ retrieval after 24 h
Group 3 – untreated control group (n = 10): Blood sampling before surgery and 6, 24, and 96 h after removing the clip. Rats were sacrificed for organ retrieval after 96 h
Group 4 – prednisolone treated (n = 8): Blood sampling before surgery and 6, 24, and 96 h after removing the clip. Rats were sacrificed for organ retrieval after 96 h
Group 5 – sham (n = 6): Surgery without performing a clamping or nephrectomy. Blood sampling before surgery and 6, 24, and 96 h after removing the clip. Rats were sacrificed for organ retrieval after 96 h.
Sample analysis
Blood samples were taken before surgery and 6, 24 h, and in group 3–5 96 h after induction of AKI. Renal function was assessed in all animals by serum (s)-creatinine and serum-urea measurements using the dry-slide technology (Fuji-Dry Chem 4000 Autoanalyzer). The concentration of rat intercellular adhesion molecule (ICAM)/CD54, rat interleukin-1 beta (IL-1β), rat IL-18, rat IL-6, and rat tumor necrosis factor-alpha (TNF-α) in plasma was determined by a Multiplex FACS analysis using xMAP technology with the Luminex Performance Assay rat cytokine premixed kit (R&D Systems Inc., #FCST06). The leukocyte, macrophage, and T-cell infiltration rates were analyzed in kidney tissue samples using a cytomics FC500 flow cytometer.
Fluorescence-activated cell sorting-analysis
FACS is a tool to measure and analyze cell surface molecules of cells which flow in a stream through a beam of laser light to detect the fluorescence of the cells. FACS can be applied to determine immunological processes in the peripheral blood and organ tissues, which can be achieved by producing single-cell suspensions of the tissue with subsequent staining of relevant markers.
Fluorescence-activated cell sorting-renal tissue analysis
Kidneys were cut in 1–2 mm3 pieces and incubated in 5 ml Dulbecco's Modified Eagle's medium containing 2 mg/mL collagenase Type I for 1 h at 37°C. Kidney pieces were gently disrupted with a 5 mL serological pipette and filtered through a 70 μm cell strainer to produce a single-cell suspension into a colonial tube. After centrifugation at 300 ×g for 10 min, pellets were resuspended in 2 mL Versalyse for erythrocyte removal and incubated for further 10 min at room temperature (RT). Cells were centrifuged once more as described and pellets were resuspended in 2 mL Dulbecco's phosphate-buffered saline (DPBS)/3% fetal calf serum (FCS). For the staining of leukocytes and macrophages/monocytes, 1 × 106 cells were resuspended in 100 μl DPBS/1% BSA. After the addition of 5 μl RP-1 antibody (BD Pharmingen #550002), the cells were incubated for 30 min at RT. Then, 2 ml of DPBS/3% FCS were added and centrifuged at 300 ×g for 5 min. Supernatant was discarded and cells were resuspended in 100 μl of Leucoperm Reagent A (AbD Serotec #BUF09). The incubation time at RT for 15 min was followed by the addition of 3 ml DPBS/3% FCS. Again, supernatant was discarded after centrifugation. Afterward, cells were resuspended in 100 μl Leucoperm Reagent B. Tested markers of inflammatory cells involved CD45+ for leukocytes, CD68+ for macrophages, and CD3+ for T-cells.
Ten microliters CD68 antibody (AbD Serotec #MCA341A700) was added. Following the incubation time of 30 min at RT, cells were washed with 2 ml DPBS/3% FCS and resuspended in 200 μl sheath fluid and transferred to a microtiter plate. 1 × 105 cells were measured in a cytomics FC500 flow cytometer (Beckman Coulter).
For the staining of T-cells, 1 × 106 cells were resuspended in 100 μl DPBS/3% FCS. Afterward, 5 μl CD3 antibody (BD Pharmingen #557354), 10 μl CD45 antibody (BD Pharmingen #559135) and 1 μl of CXCR4 antibody (Bioss Antibodies # bs-1011R) were added to the cells with subsequent incubation for 30 min at RT. Then, cells were washed with 2 ml DPBS/3% FCS, resuspended in 200 μl sheath fluid, and transferred to a microtiter plate. As before 1 × 105 cells were measured in a cytomics FC500 flow cytometer.
Fluorescence-activated cell sorting-multiplex analysis of plasma samples
The concentration of rat ICAM/CD54, rat IL-1β, rat IL-18, rat IL-6, and rat TNF-α in plasma was determined using xMAP technology with the Luminex Performance Assay rat cytokine premixed kit (R&D Systems Inc., #FCST06). Standard and samples were reconstituted and diluted as described in the manual of the manufacturer. 5 × 104 cells were measured in a cytomics FC500 flow cytometer.
Statistical analysis
Data are expressed as mean ± standard deviation. For body weight, renal edema, renal function, and FACS-analysis, statistical analysis was performed using the Mann–Whitney U-test. A P < 0.05 was considered statistically significant.
Results
Induction of acute kidney injury
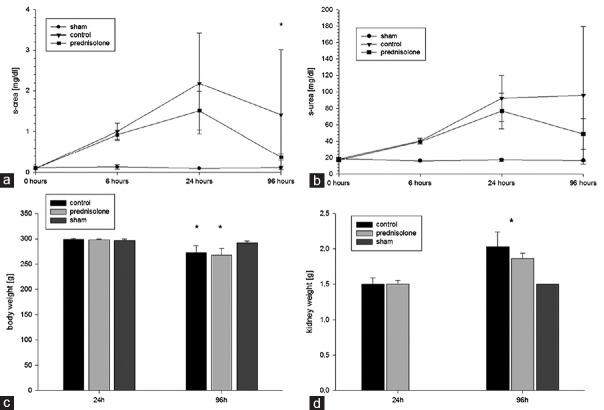
An ischemic period of 45 min was sufficient to induce a consistent AKI without any animal loss. The control group demonstrated an increase of the serum-creatinine concentrations above 2 mg/dl after 24 h, representing a 10–20-fold increase of the initial serum-creatinine values. Measurement of serum-urea resulted in a 4.5-fold increase after 24 h in the control group. During the observation period, loss of body weight was approximately 10% after 96 h in all AKI groups (P < 0.05). All AKI groups demonstrated an increasing renal weight throughout the observation period. After 96 h, a significant renal weight gain was detectable within the two AKI groups (day 1 vs. day 4: control group: 1.49 ± 0.09 g vs. 2.03 ± 0.21 g, P < 0.001, prednisolone group: 1.5 ± 0.04 g vs. 1.86 ± 0.09 g, P < 0.001). However, no significant differences were detectable between the control and the prednisolone group although the untreated group demonstrated a stronger tendency of renal weight gain compared to the prednisolone-treated group (control vs. prednisolone: +36% vs. +24%, P = 0.068). The renal weight of the sham group showed a significantly lower mean renal weight compared to the AKI groups after 96 h (P < 0.001) [Figure 1].
Figure 1.
Acute kidney injury induction: (a and b) shows the decline of renal function after acute kidney injury and the significantly faster recovery of serum-creatinine in the prednisolone group (a) and a trend for a faster recovery of serum-urea due to prednisolone treatment (b). All acute kidney injury groups revealed a significant body weight loss (approximately 10%) (c) and a significant increase of renal weight/renal edema with a trend for less weight gain in the treatment group (d)
Renal function
A continuous decline of renal function was detectable during the first 24 h with a significant deterioration as early as 6 h after AKI induction. After 96 h, a recovery of renal function was seen in all groups with a significantly better recovery in the prednisolone-treated group (control vs. prednisolone: 1.41 ± 1.61 vs. 0.38 ± 0.08, P < 0.05). The serum-urea concentration also demonstrated a more pronounced decline in the prednisolone group after 96 h but without reaching a significant level compared to the control group [Figure 1].
Local inflammatory response in renal tissue (fluorescence-activated cell sorting-analysis)
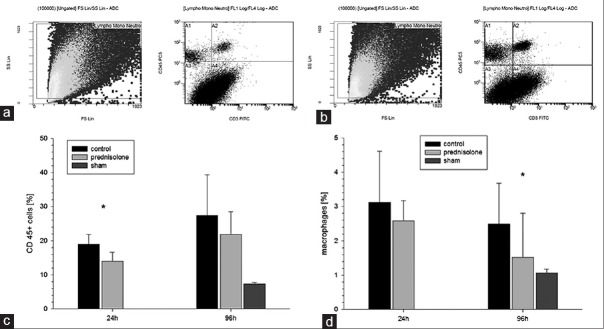
The rate of leukocyte infiltration rose significantly in both AKI groups within 96 h (24 h vs. 96 h; P < 0.001). However, 24 h after induction of AKI, the amount of infiltrated leukocytes was significantly higher in the control group compared to the prednisolone group (19.01% ±2.88% vs. 13.99% ±2.66%, P < 0.01). A loss of significance was seen between those groups after 96 h, while both AKI groups still presented significantly more infiltrating leukocytes compared to the sham group.
Similarly, a significant increase of infiltrating macrophages was observed in both AKI groups compared to the sham group 24 h after AKI induction (P < 0.05), whereas a nonsignificant trend for less infiltration in the prednisolone group could be observed. After 96 h, a decline of infiltrating macrophages could be observed in the AKI groups with a significant higher rate of infiltrating macrophages in the control group compared to the prednisolone group (2.5% ±1.78% vs. 1.52% ±1.28%, P < 0.01). The rate of infiltrating macrophages after 96 h was significantly lower in the sham kidneys compared to the AKI groups.
T-cell infiltration in renal tissue revealed a significant increase within both AKI groups after 24 h (P < 0.01) with significantly elevated infiltration rates in both AKI groups compared to the sham group after 96 h. No significant differences could be observed between the AKI groups [Figure 2].
Figure 2.
Renal inflammation: (a and b) shows leukocyte infiltration presented by fluorescence-activated cell sorting analysis. Twenty-four hours after induction of acute kidney injury the amount of infiltrated leukocytes was significantly higher in the control group compared to the prednisolone group (P < 0.01) (c). A significant increase of infiltrating macrophages was observed in both acute kidney injury groups 24 h after acute kidney injury induction. After 96 h, a decline of infiltrating macrophages could be observed in the acute kidney injury groups with a significant higher rate of infiltrating macrophages in the control group compared to the prednisolone group (P < 0.01) (d)
Systemic inflammatory response in plasma (fluorescence-activated cell sorting-multiplex analysis)
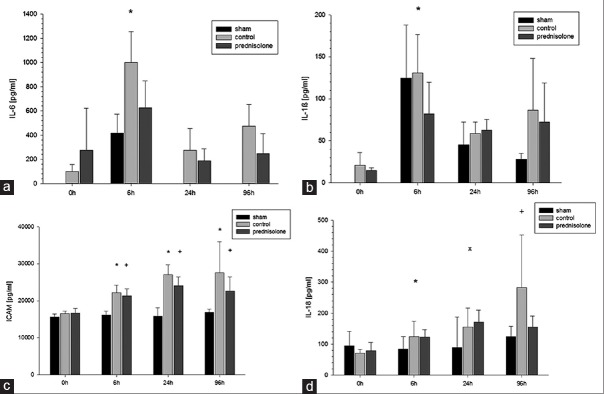
IL-6 demonstrated an early peak already 6 h after reperfusion followed by a sharp decline after 24 h with returning to physiological levels within 96 h. After 6 h, the control group demonstrated a significantly higher IL-6 release compared to the prednisolone group (1000.06 ± 255.1 pg/ml vs. 625.95 ± 223.18 pg/ml, P < 0.01) and the sham group (418.62 ± 152.89 pg/ml, P < 0.01).
The IL-1β release also reached an early peak in both AKI groups after 6 h, which was statistically significant within the AKI groups (P < 0.01) with significantly higher levels in the control group compared to the prednisolone group at this time point (130.95 ± 43.48 pg/ml vs. 81.92 ± 37.80 pg/ml, P < 0.05). Despite a continuous decline thereafter, the IL-1β levels of the AKI groups did not return to a physiological level within the observation period.
IL-18 release revealed a significant increase after 6 h, 24 h, and 96 h within the AKI groups compared to the baseline levels (P < 0.05) while no significant differences could be detected within the sham group during the first 96 h. While the control group demonstrated a continuous increase of the IL-18 level from day 0 to day 4, the prednisolone group reached its maximum peak after 24 h without any further incline thereafter. Nevertheless, those differences did not reach a significant level [Figure 3].
Figure 3.
Inflammatory markers in plasma: (a) The control group demonstrated a significantly higher interleukin-6 release after 6 h (P < 0.01). (b) The interleukin-1 beta release reached an early peak after 6 h with significantly higher levels in the control group (P < 0.05) with a continuous decline thereafter. (c) Intercellular adhesion molecule-1 measurements revealed significantly elevated levels in the acute kidney injury groups compared to the baseline levels at all times (P < 0.01) (d) while the control group demonstrated a continuous increase of the interleukin-18 level, the prednisolone group reached its maximum peak after 24 h without any further incline thereafter
TNF-α was released with some delay compared to the other investigated cytokines. No changes could be noted within the first 6 h. During the following 18 h, a trend for an increasing level could be detected in all groups but actually reached statistical significance only in the control group (P < 0.05). The TNF-α levels finally returned to physiological levels in all groups after 96 h.
ICAM-1 measurements revealed statistical significant higher levels after 6, 24, and 96 h within the AKI groups compared to the baseline levels (P < 0.01). A trend for decline was detected in the prednisolone group after 96 h in contrast to the control group without reaching statistical significance. No significant changes in ICAM-1 expression could be observed in the sham group with significantly lower ICAM-1 levels after 6, 24, and 96 h compared to the AKI groups (P < 0.01) [Figure 3].
Discussion
The main finding of our study is the attenuated inflammatory reaction in the prednisolone group demonstrated by significantly lower infiltration rates of leukocytes after 24 h and especially macrophages after 96 h. Moreover, significantly lower IL-1β and IL-6 plasma levels were found in the prednisolone group. These insights were gained by FACS analysis only which proved to be a feasible, comfortable, and very time efficient analyzing method to measure inflammatory reactions in the AKI setting.
The selection of the inflammatory markers in the current study was based on previous findings. ICAM-1 is an adhesion molecule which is required for leukocyte adhesion during the inflammation process and seems to play an essential role during AKI. The administration of monoclonal ICAM-1 antibodies as well as an ICAM-1 deficiency proved to be protective in AKI mouse models.[6,7] Furthermore, human as well as animal AKI models demonstrated a caspase-1-mediated increase of IL-1β and IL-18.[8] Caspase-1 deficient mice seem to be functionally and histologically protected against ischemic AKI while this effect seems to be mainly associated with a decreased conversion of IL-18 precursors to the mature form in the kidney.[9] Among others, TNF-α and IL-1β induce chemokines which are a large subgroup of cytokine-like molecules that play a major role in the recruitment process of leukocytes during the inflammation process and in the regulation of T helper-1/T helper-2 immune responses and therefore seem to be an important part of the AKI-induced inflammatory reaction.[10] IL-6 is also a pro-inflammatory cytokine that seems to mediate in the inflammatory process during ischemic AKI.[11,12] The exact mechanism of the IL-6 influence on ischemic AKI is so far not fully understood but seems to be related to trans-signaling and signal transducer and activator of transcription 3 (STAT3) activation in renal tubular cells.[13]
The effectiveness of subcutaneously admitted prednisolone in a dosage of 5 mg/kg was demonstrated previously in a cisplatin-induced AKI setting.[14]
The FACS method is routinely used for cell sorting in blood samples and in vitro cell lines but also seems to be a feasible tool when analyzing solid tissue samples.[15,16] The main benefit of using the FACS-multiplex method is the option to analyze different molecules simultaneously in a single step. The transfer of this interesting technical approach in the analysis of the inflammatory process during AKI was part of the current study.
AKI is a frequent complication in critically ill patients with a wide variety of etiologies and risk factors with inflammation playing a crucial role in this context.[17,18] Renal inflammation is associated with endothelial cell activation, vascular disintegration, and increased vascular permeability. This in turn facilitates leukocyte recruitment in the renal parenchyma through endothelial expression of adhesion molecules and chemokines.[13,19]
The influence of steroids in clinical AKI-settings was so far mainly analyzed in coronary surgery settings. In those patients, AKI was identified as a serious medical complication which seems to be associated with an increased postoperative mortality. It was demonstrated that steroids are able to reduce the overall postinterventional early inflammatory process in cardiac bypass settings measured by capillary permeability, subsequent edema formation, leukocyte migration, and pro-inflammatory cytokine expression[20,21,22] while the timing of steroid administration seems to play a crucial role. Schurr et al. who applied a single-shot intravenous methylprednisolone presurgically were not able to detect any benefit while Whitlock et al. who applied methylprednisolone intrasurgically and Weis et al. who applied stress doses of hydrocortisone after cardiac surgery in a high-risk patient collective were able to demonstrate an improved outcome by attenuation of the systemic inflammatory response.[23,24,25] The latter findings are in line with our results concerning the systemic anti-inflammatory effect of prednisolone treatment in a transient renal ischemia setting. In a previously performed meta-analysis, which analyzed anti-inflammatory strategies to reduce the AKI during cardiac surgery, steroid administration failed to demonstrate a positive effect on postoperative kidney dysfunction.[26] One reason why no benefit could be detected in this meta-analysis might be the inhomogeneity and high rate of comorbidities in those patient collectives which makes it very difficult to analyze the benefit of a single drug in such a complex setting. Experimental in vivo studies are therefore an inevitable supplementary tool when trying to evaluate the impact of a single drug on a multifactorial clinical complication. While most previous in vivo studies focused on the time frame between 24 and 48 h after AKI induction,[27,28,29,30] there is only little data available concerning the inflammatory reactions at the very early and later stages of AKI.
Williams et al. demonstrated a decline of renal function as early as 6 h after AKI induction.[31] In line with these findings, the current results demonstrate an early decline of renal function with significantly higher serum-creatinine/urea values as early as 6 h after AKI induction which reached a pathological level after 24 h and remained pathological during the complete observation period with a significant improvement after prednisolone treatment.
Corresponding to the early decline of renal function after 6 h, an early peak of the IL-1β and IL-6 plasma concentrations could be observed, which could be mitigated significantly by prednisolone treatment. Previous studies identified IL-1β and IL-6 as highly active pro-inflammatory cytokine.[32,33] Furthermore, an increased IL-6 plasma level seems to be associated with an increased mortality rate in patients with acute renal failure.[34] The TNF-α level, which has been identified as a key regulator of the inflammatory response,[35] reached with some delay to IL-1β and IL-6 a significantly elevated level after 24 h in the control group only. The ICAM-1 expression reached a peak after 24 h and remained at that level till 96 h after AKI induction in both groups. An ICAM-1 upregulation is one of the several mechanisms that contribute to leukocyte-mediated reperfusion injury. The impact of ICAM-1 in ischemia-reperfusion-induced renal injury was demonstrated by experiments using ICAM-1 antibodies.[7] The IL-18 expression demonstrated a continuous incline from 6 h to 96 h after AKI induction in the control group while the IL-18 expression remained stable after 24 h in the prednisolone group. This finding seems reasonable since IL-18 was previously characterized as a reliable marker for acute renal tubular necrosis in humans.[36] Furthermore, prednisolone treatment was able to reduce the infiltration of leukocytes as well as macrophages and therefore also demonstrated its local anti-inflammatory potency in the course of the AKI.
In summary, it could be shown that prednisolone attenuates the inflammatory response after induction of AKI and mitigates renal dysfunction. Moreover, the current data demonstrate that the feasibility of using FACS analysis for blood as well as tissue samples in such an experimental setting. FACS analysis seems to represent a reliable time and workforce saving tool to give a quick and detailed immunological survey when analyzing AKI.
Conclusion
Our results demonstrate the importance of stretching the observation period in an ischemia-reperfusion-induced AKI setting beyond the first 24 h. Despite the demonstrated protective effects of a continuous prednisolone application, it seems that this single anti-inflammatory agent will not be able to completely suppress the inflammatory response after an ischemia-reperfusion-induced AKI. Understanding the inflammatory course and the timing of treatment seems to play an essential role when trying to optimize the immunomodulatory therapy after ischemia-reperfusion-induced AKI. The FACS method seems to represent a feasible and time efficient tool to give a quick and detailed survey on AKI-induced inflammatory processes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, et al. Spectrum of acute renal failure in the Intensive Care Unit: The PICARD experience. Kidney Int. 2004;66:1613–21. doi: 10.1111/j.1523-1755.2004.00927.x. [DOI] [PubMed] [Google Scholar]
- 2.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM. Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol. 2006;17:1143–50. doi: 10.1681/ASN.2005091017. [DOI] [PubMed] [Google Scholar]
- 3.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA. 2005;294:813–8. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 4.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL. Epidemiology and outcomes of acute renal failure in hospitalized patients: A national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 5.Glodowski SD, Wagener G. New insights into the mechanisms of acute kidney injury in the Intensive Care Unit. J Clin Anesth. 2015;27:175–80. doi: 10.1016/j.jclinane.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, et al. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–63. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A. 1994;91:812–6. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Masood H, Che R, Zhang A. Inflammasomes in the pathophysiology of kidney diseases. Kidney Dis (Basel) 2015;1:187–93. doi: 10.1159/000438843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, et al. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J Clin Invest. 2001;107:1145–52. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segerer S, Nelson PJ, Schlöndorff D. Chemokines, chemokine receptors, and renal disease: From basic science to pathophysiologic and therapeutic studies. J Am Soc Nephrol. 2000;11:152–76. doi: 10.1681/ASN.V111152. [DOI] [PubMed] [Google Scholar]
- 11.Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, et al. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol. 2005;16:3315–25. doi: 10.1681/ASN.2003090757. [DOI] [PubMed] [Google Scholar]
- 12.Nechemia-Arbely Y, Barkan D, Pizov G, Shriki A, Rose-John S, Galun E, et al. IL-6/IL-6R axis plays a critical role in acute kidney injury. J Am Soc Nephrol. 2008;19:1106–15. doi: 10.1681/ASN.2007070744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akcay A, Nguyen Q, Edelstein CL. Mediators of inflammation in acute kidney injury. Mediators Inflamm. 2009;2009:137072. doi: 10.1155/2009/137072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill A, Wortham K, Costa D, Davis W, Ticho B, Whalley E. Protective effect of tonapofylline (BG9928), an adenosine A1 receptor antagonist, against cisplatin-induced acute kidney injury in rats. Am J Nephrol. 2009;30:521–6. doi: 10.1159/000248762. [DOI] [PubMed] [Google Scholar]
- 15.Harris DA, Zhao Y, LaPar DJ, Emaminia A, Steidle JF, Stoler M, et al. Inhibiting CXCL12 blocks fibrocyte migration and differentiation and attenuates bronchiolitis obliterans in a murine heterotopic tracheal transplant model. J Thorac Cardiovasc Surg. 2013;145:854–61. doi: 10.1016/j.jtcvs.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J, Zhu F, Wang X, Yao W, Wang M, Pei G, et al. Continuous AMD3100 treatment worsens renal fibrosis through regulation of bone marrow derived pro-angiogenic cells homing and T-cell-related inflammation. PLoS One. 2016;11:e0149926. doi: 10.1371/journal.pone.0149926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonventre JV, Zuk A. Ischemic acute renal failure: An inflammatory disease? Kidney Int. 2004;66:480–5. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–91. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 19.Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens. 2003;12:85–90. doi: 10.1097/00041552-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Hall RI, Smith MS, Rocker G. The systemic inflammatory response to cardiopulmonary bypass: Pathophysiological, therapeutic, and pharmacological considerations. Anesth Analg. 1997;85:766–82. doi: 10.1097/00000539-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Kawamura T, Inada K, Nara N, Wakusawa R, Endo S. Influence of methylprednisolone on cytokine balance during cardiac surgery. Crit Care Med. 1999;27:545–8. doi: 10.1097/00003246-199903000-00033. [DOI] [PubMed] [Google Scholar]
- 22.Toledo-Pereyra LH, Lin CY, Kundler H, Replogle RL. Steroids in heart surgery: A clinical double-blind and randomized study. Am Surg. 1980;46:155–60. [PubMed] [Google Scholar]
- 23.Weis F, Beiras-Fernandez A, Schelling G, Briegel J, Lang P, Hauer D, et al. Stress doses of hydrocortisone in high-risk patients undergoing cardiac surgery: Effects on interleukin-6 to interleukin-10 ratio and early outcome. Crit Care Med. 2009;37:1685–90. doi: 10.1097/CCM.0b013e31819fca77. [DOI] [PubMed] [Google Scholar]
- 24.Whitlock RP, Young E, Noora J, Farrokhyar F, Blackall M, Teoh KH. Pulse low dose steroids attenuate post-cardiopulmonary bypass SIRS; SIRS I. J Surg Res. 2006;132:188–94. doi: 10.1016/j.jss.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Schurr UP, Zünd G, Hoerstrup SP, Grünenfelder J, Maly FE, Vogt PR, et al. Preoperative administration of steroids: Influence on adhesion molecules and cytokines after cardiopulmonary bypass. Ann Thorac Surg. 2001;72:1316–20. doi: 10.1016/s0003-4975(01)03062-4. [DOI] [PubMed] [Google Scholar]
- 26.Scrascia G, Guida P, Rotunno C, de Luca Tupputi Schinosa L, Paparella D. Anti-inflammatory strategies to reduce acute kidney injury in cardiac surgery patients: A meta-analysis of randomized controlled trials. Artif Organs. 2014;38:101–12. doi: 10.1111/aor.12127. [DOI] [PubMed] [Google Scholar]
- 27.Semedo P, Palasio CG, Oliveira CD, Feitoza CQ, Gonçalves GM, Cenedeze MA, et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Int Immunopharmacol. 2009;9:677–82. doi: 10.1016/j.intimp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 28.McBride WT, Allen S, Gormley SM, Young IS, McClean E, MacGowan SW, et al. Methylprednisolone favourably alters plasma and urinary cytokine homeostasis and subclinical renal injury at cardiac surgery. Cytokine. 2004;27:81–9. doi: 10.1016/j.cyto.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Yao Y, Xiao F, Lan X, Yu C, Zhang Y, et al. Administration of dexamethasone protects mice against ischemia/reperfusion induced renal injury by suppressing PI3K/AKT signaling. Int J Clin Exp Pathol. 2013;6:2366–75. [PMC free article] [PubMed] [Google Scholar]
- 30.Acar G, Akcay A, Sayarlioglu M, Sokmen A, Sokmen G, Koroglu S, et al. Assessment of atrial conduction time in patients with familial Mediterranean fever. Pacing Clin Electrophysiol. 2009;32:308–13. doi: 10.1111/j.1540-8159.2008.02237.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams P, Lopez H, Britt D, Chan C, Ezrin A, Hottendorf R. Characterization of renal ischemia-reperfusion injury in rats. J Pharmacol Toxicol Methods. 1997;37:1–7. doi: 10.1016/s1056-8719(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–147. [PubMed] [Google Scholar]
- 33.Jones SA. Directing transition from innate to acquired immunity: Defining a role for IL-6. J Immunol. 2005;175:3463–8. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 34.Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int. 2004;65:1357–65. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 35.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–60. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 36.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis. 2004;43:405–14. doi: 10.1053/j.ajkd.2003.10.040. [DOI] [PubMed] [Google Scholar]